
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 17 April 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1078076
This article is part of the Research TopicCase Reports in Gastrointestinal Cancers : 2022View all 33 articles
Patients with metastatic pancreatic cancer have limited treatment options and a dismal prognosis. While RET fusion is rare (0.6%) in pancreatic cancer, the efficacy of RET-targeted treatment in patients with TRIM33-RET fusion has not been previously reported. Herein, we presented a case of a 68-year-old man with pancreatic cancer harboring TRIM33-RET fusion who responded remarkably to pralsetinib despite being intolerant to chemotherapy. To our knowledge, this is the first report on the clinical value of a single TRIM33-RET fusion in pancreatic cancer, which may benefit from the targeted therapy.
Pancreatic cancer has a high degree of malignancy and rapid progression. According to the latest statistics from the China National Cancer Center, the annual incidence of pancreatic cancer in China is approximately 4.29/100,000, a considerable increase from 15 years ago (1). For all stages combined, the 5-year survival rate was 5%–10% (2, 3). Chemotherapy remains the primary treatment for pancreatic cancer. However, the progression-free survival (PFS) for first-line chemotherapy in advanced pancreatic cancer patients is typically approximately 3–6 months (4). The advancement of targeted therapy has increased the number of potential benefits. Olaparib has been approved by the Food and Drug Administration (FDA) as a first-line maintenance treatment for metastatic pancreatic cancer patients with germline BRCA1/2 mutations based on the improvement in progression-free survival demonstrated in a randomized phase III POLO trial (5). Larotrectinib and entrectinib have been approved as agnostic treatments for solid malignancies with NTRK fusion (6, 7). Patients harboring the NRG1 gene fusion are sensitive to Zenocutuzumab (8). The activity of adagrasib and sotorasib in KRAS G12C pancreatic cancer also provides new hope for KRAS-mutant pancreatic cancer patients (9, 10). However, these drug-targeted genetic mutations only account for a low percentage of pancreatic cancer cases. Therefore, it is essential to search for precision therapies based on genetic alterations for pancreatic cancer patients.
The proto-oncogene RET encodes a membrane receptor tyrosine kinase involved in many cellular processes, including the development of the central nervous system, peripheral nervous system, and kidney (11, 12). RET fusions are activated in a ligand-independent manner, promoting cancer cell proliferation and survival (13). As a result, RET fusion proteins have become an attractive target for precision medicine. RET inhibitors, such as selpercatinib and pralsetinib, have demonstrated efficacy in patients with RET fusion-positive tumors. The incidence of RET fusion in pancreatic cancer is 0.6% (14).
In this case, a TRIM33-RET fusion was detected through next-generation sequencing (NGS) in a patient with pancreatic ductal adenocarcinoma (PDAC) who responded well to pralsetinib.
A 68-year-old man was admitted to our hospital on 20 May 2021 due to persistent upper abdominal pain. The patient had no personal or family history of malignancy, pancreatitis, or liver disease. His serum CA 19-9 level was above 10,000 U/ml (Figure 1A), and his carcinoembryonic antigen (CEA) level was 68.7 U/ml. A CT scan revealed a 4.1 × 2.0-cm mass in the pancreatic uncinate process and a 3.8 × 2.4-cm mass in the liver (Figures 2A, B). Pathological evaluation of the tissue samples with liver biopsy indicated PDAC. Immunohistochemical (IHC) staining revealed that the cells were positive for CA19-9 and CK7 while negative for p53, CK20, AFP, and c-erbb-2. Ki-67 exhibited a 70% proliferative rate. To explore precision treatment options, a biopsy tissue sample from the patient was sent for NGS analysis using a 733-gene panel. The test was performed by a laboratory (3D Medicine Inc., Shanghai, China) certified by the College of American Pathologists (CAP), Clinical Laboratory Improvement Amendments (CLIA), and China National Accreditation Service for Conformity Assessment (CNAS). The tumor mutational burden (TMB) was 4.47 mutations/Mb, and the microsatellite status was stable. Meanwhile, a somatic RET fusion (TRIM33-RET) was detected (Figure 1B). Moreover, other pathogenic or likely to be pathogenic variations were detected, including somatic TGFBR1 (p.I66Yfs*9, 23.94%) mutation, amplification of BCORL1 (copy number = 6), and germline RAD50 mutation (RAD50, p.K722Nfs*6). In addition, the patient was found to have wild-type variants in HER2, BRCA1/2, and RAS/RAF.
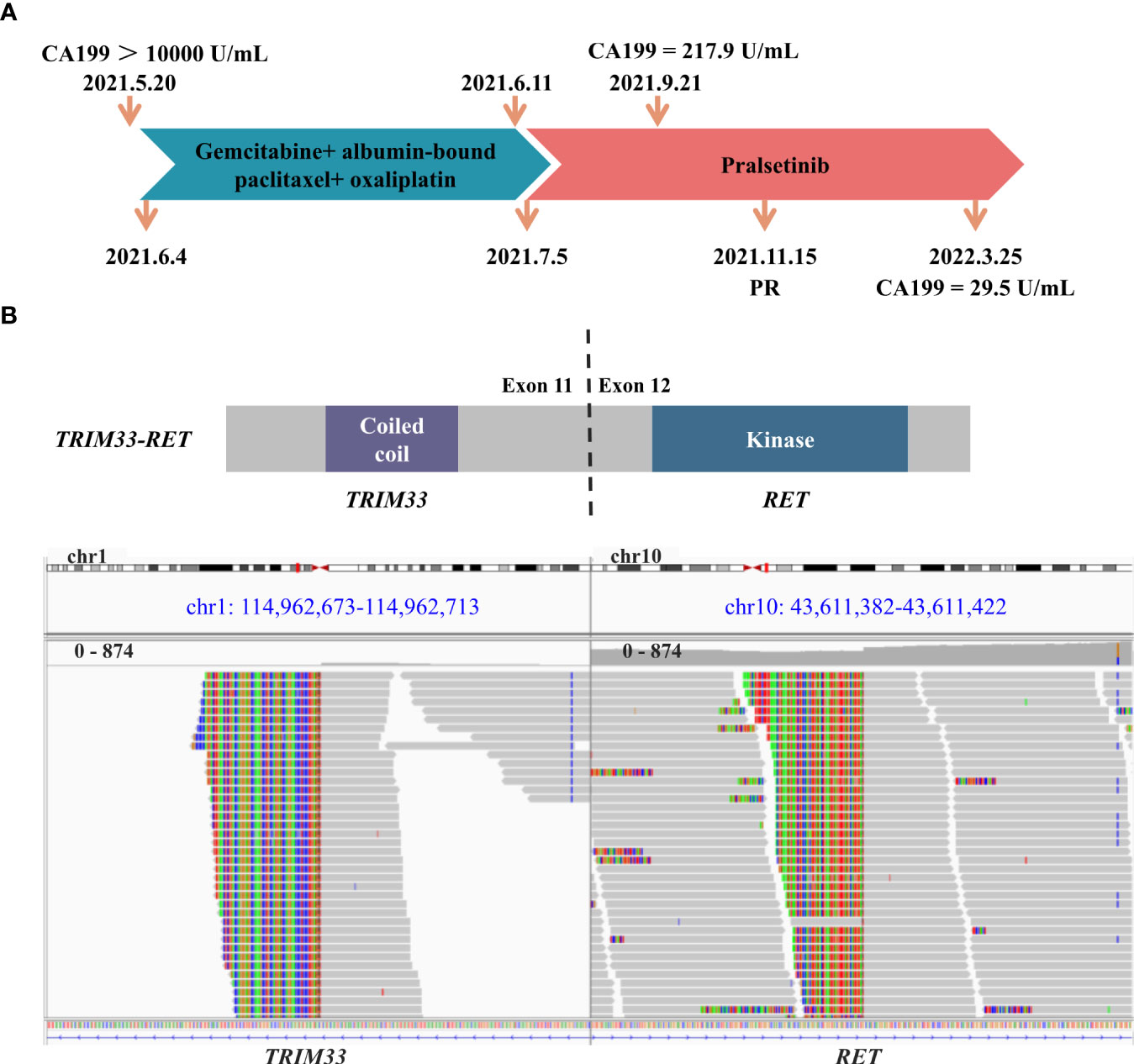
Figure 1 Schematic of treatment history and next-generation sequencing (NGS)‐detected RET fusion. (A) The timeline of treatment and corresponding CA199 levels. (B) The schematic diagram and identification of the TRIM33-RET fusion. Sequencing reads of TRIM33 and RET are visualized by the Integrative Genomics Viewer (IGV).
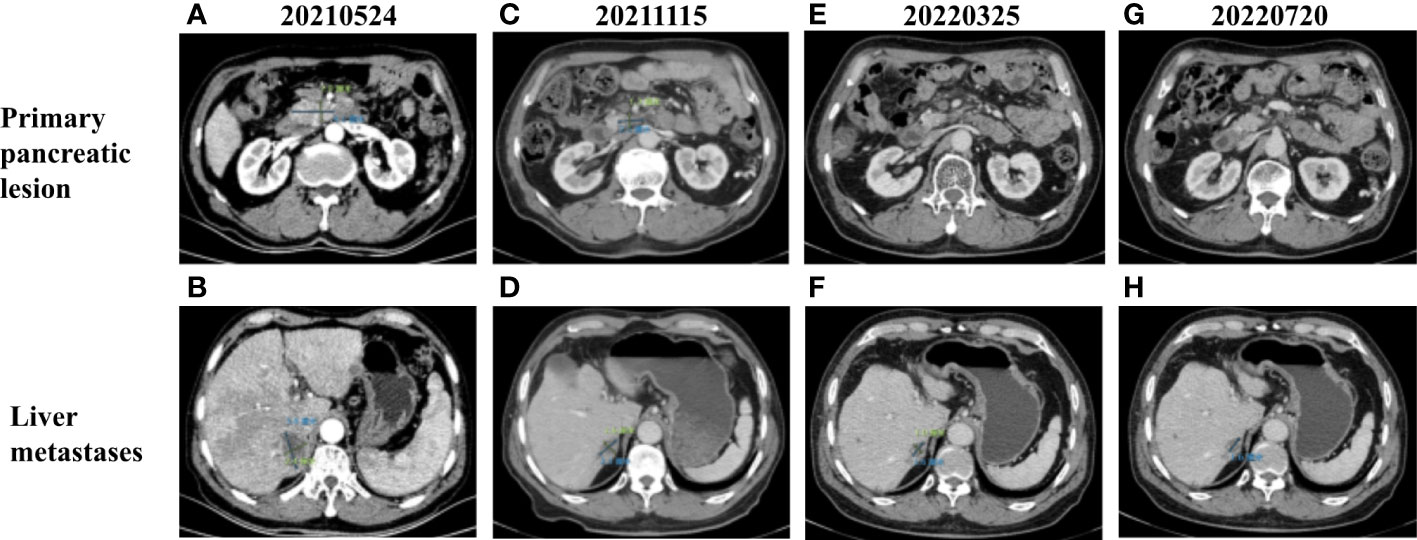
Figure 2 (A) CT images of primary pancreatic lesion with a size of 4.1 cm × 2 cm before treatment. (B) CT images of liver metastases with a size of 3.8 cm × 2.4 cm before treatment. Contrast-enhanced CT scan images completed in (C, D) November 2021, (E, F) March 2022, and (G, H) July 2022 demonstrating progressive decrease in the size of primary pancreatic and liver metastases.
Based on the previous clinical research results (15–18), the patient’s financial situation, genetic testing results, and guidelines, first-line chemotherapy (albumin-bound paclitaxel 200 mg/m2, oxaliplatin 85 mg/m2, and gemcitabine 1.4 g/m2) was administered on 4 June 2021. However, the patient rapidly developed significant gastrointestinal toxicity and myelosuppression. Due to intolerance, the patient only had one course of chemotherapy. Since 5 July 2021, the patient received 400 mg of pralsetinib daily. Four months later, the volume of the primary tumor decreased by approximately 39.0% (Figures 2A, C), and that of the metastases decreased by approximately 39.5% (Figures 2B, D) compared to that before treatment, and a partial response was confirmed. However, due to the adverse effect of anemia in the patient, the treatment dose was reduced accordingly. From 15 November 2021 to the present, the patient has been receiving 200 mg of pralsetinib daily for maintenance therapy. Also, the tumor biomarker cancer antigen CA199 dropped from a very high level (>10,000 U/ml) to 29.5 U/ml (Figure 1A). Currently, it was concluded that the patient reached a partial response. The patient’s primary tumor and metastases were still shrinking (Figures 2E–H), and the progression-free survival was at least 12 months.
The TRIM33-RET fusion protein contains a coiled-coil domain encoded by TRIM33 exons 1–11 and a complete kinase domain encoded by RET exons 12–20, which may result in the activation of the RET tyrosine kinase. RET fusion is a rare genomic alteration in the PDAC. TRIM33-RET fusion has previously been reported in non-small cell lung cancer and oncocytic intraductal carcinoma of salivary glands (19, 20). We reported on a case of advanced PDAC that responded to pralsetinib as second-line systemic therapy. The PFS has been more than 12 months.
New targeted drugs for RET fusion are constantly emerging in succession (21). Recently, selpercatinib and pralsetinib were approved by the FDA for the treatment of lung and thyroid cancers with RET gene mutations or fusions (22), (23). Although no targeted drug for RET fusion-positive PDAC has been approved, ongoing clinical studies of target drugs for RET fusion in more cancer types are underway. The ARROW study is a multi-cohort, open-label, phase 1/2 study designed to investigate pralsetinib for the treatment of RET-altered solid tumors, including four patients with pancreatic cancer (24). The results confirmed that response occurred in 57% of 23 evaluable patients (24). The most common grade 3–4 treatment-related adverse events (TRAEs) in the pre-treated population were neutropenia, anemia, and hypertension (24). Notably, JMJD1C-RET fusion and TRIM33-RET fusion were detected in a pancreatic cancer patient who achieved an ongoing complete response at a treatment duration of 33.1 months (24). The LIBRETTO-001 study, a phase 1/2 study of selpercatinib in participants with advanced solid tumors, RET fusion-positive solid tumors, and medullary thyroid cancer, had been reported. Forty-five patients with RET fusion had been enrolled, including 12 patients with pancreatic cancer. The overall response rate (ORR) was 43.9% in 41 efficacy-evaluable patients confirmed by an independent review committee (25). Many novel selective RET inhibitors have shown good efficacy and low off-target toxicity in clinical trials (e.g., BLU-667 and LOXO-292), which encourages the development and research of more selective RET inhibitors (10). This is the first case report of a patient with only the TRIM33-RET fusion, a single fusion gene, detected who has an ongoing partial response to pralsetinib in PDAC.
In conclusion, this is the first case report in which a patient with only the TRIM33-RET fusion, a single fusion gene, detected in PDAC had a remarkable response to pralsetinib. This suggests the importance of NGS testing for patients with PDAC, especially those intolerant to chemotherapy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The Local Ethics Review Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CJ, TZ, and HW followed up the patient and collected patient data. SZ contributed to the writing of the original draft. ZC contributed to the collection of CT image data. All authors contributed to the article and approved the submitted version.
The study was supported by the Shanghai Science and Technology Commission of Shanghai Municipality, No. 20Y11908600; the Shanghai Shenkang Hospital Development Center, No. SHDC2020CR5008; and Shanghai Municipal Health Commission, No. 20194Y0195.
We owe thanks to the patient and his family for their participation and cooperation.
Author SZ was employed by 3D Medicines Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol (2015) 21(26):7988–8003. doi: 10.3748/wjg.v21.i26.7988
2. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet (London England) (2016) 388(10039):73–85. doi: 10.1016/s0140-6736(16)00141-0
3. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health (2018) 6(5):e555–e67. doi: 10.1016/s2214-109x(18)30127-x
4. Hajatdoost L, Sedaghat K, Walker EJ, Thomas J, Kosari S. Chemotherapy in pancreatic cancer: a systematic review. Medicina (Kaunas Lithuania) (2018) 54(3):48. doi: 10.3390/medicina54030048
5. Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline brca-mutated metastatic pancreatic cancer. New Engl J Med (2019) 381(4):317–27. doi: 10.1056/NEJMoa1903387
6. Scott LJ. Larotrectinib: first global approval. Drugs (2019) 79(2):201–6. doi: 10.1007/s40265-018-1044-x
7. Marcus L, Donoghue M, Aungst S, Myers CE, Helms WS, Shen G, et al. Fda approval summary: entrectinib for the treatment of ntrk gene fusion solid tumors. Clin Cancer Res an Off J Am Assoc Cancer Res (2021) 27(4):928–32. doi: 10.1158/1078-0432.Ccr-20-2771
8. Schram AM, Odintsov I, Espinosa-Cotton M, Khodos I, Sisso WJ, Mattar MS, et al. Zenocutuzumab, a Her2xher3 bispecific antibody, is effective therapy for tumors driven by Nrg1 gene rearrangements. Cancer Discovery (2022) 12(5):1233–47. doi: 10.1158/2159-8290.Cd-21-1119
9. Bekaii-Saab TS, Spira AI, Yaeger R, Buchschacher GL, McRee AJ, Sabari JK, et al. (2022) Krystal-1: updated activity and safety of adagrasib (Mrtx849) in patients (Pts) with unresectable or metastatic pancreatic cancer (Pdac) and other gastrointestinal (Gi) tumors harboring a Krasg12c mutation. J Clin Oncol (2022) 40(4_suppl):519. doi: 10.1200/JCO.2022.40.4_suppl.519
10. Strickler JH, Satake H, Hollebecque A, Sunakawa Y, Tomasini P, Bajor DL, et al. First data for sotorasib in patients with pancreatic cancer with kras P.G12c mutation: a phase I/Ii study evaluating efficacy and safety. J Clin Oncol (2022) 40(36_suppl):360490. doi: 10.1200/JCO.2022.40.36_suppl.360490
11. Ibáñez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol (2013) 5(2):a009134. doi: 10.1101/cshperspect.a009134
12. Takahashi M, Kawai K, Asai N. Roles of the ret proto-oncogene in cancer and development. Jma J (2020) 3(3):175–81. doi: 10.31662/jmaj.2020-0021
13. Regua AT, Najjar M, Lo HW. Ret signaling pathway and ret inhibitors in human cancer. Front Oncol (2022) 12:932353. doi: 10.3389/fonc.2022.932353
14. Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. Ret aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(8):1988–97. doi: 10.1158/1078-0432.Ccr-16-1679
15. Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, et al. Randomized phase iii trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the gip-1 study. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(10):1645–51. doi: 10.1200/jco.2009.25.4433
16. Yun W, GengYuan Z, ZhangJiang L, HanTeng Y. Clinical effect of nanoparticle albumin-bound paclitaxel combined with gemcitabine in treatment of advanced pancreatic cancer: a meta-analysis. J Clin Hepatol (2019) 35(5):1041. doi: 10.3969/j.issn.1001-5256.2019.05.021
17. Park W, Chen J, Chou JF, Varghese AM, Yu KH, Wong W, et al. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26(13):3239–47. doi: 10.1158/1078-0432.Ccr-20-0418
18. Jameson GS, Borazanci E, Babiker HM, Poplin E, Niewiarowska AA, Gordon MS, et al. Response rate following albumin-bound paclitaxel plus gemcitabine plus cisplatin treatment among patients with advanced pancreatic cancer: a phase 1b/2 pilot clinical trial. JAMA Oncol (2019) 6(1):125–32. doi: 10.1001/jamaoncol.2019.3394
19. Drilon A, Wang L, Hasanovic A, Suehara Y, Lipson D, Stephens P, et al. Response to cabozantinib in patients with ret fusion-positive lung adenocarcinomas. Cancer Discovery (2013) 3(6):630–5. doi: 10.1158/2159-8290.Cd-13-0035
20. Bishop JA, Nakaguro M, Whaley RD, Ogura K, Imai H, Laklouk I, et al. Oncocytic intraductal carcinoma of salivary glands: a distinct variant with Trim33-ret fusions and braf V600e mutations. Histopathology (2021) 79(3):338–46. doi: 10.1111/his.14296
21. Shabbir A, Kojadinovic A, Shafiq T, Mundi PS. Targeting ret alterations in cancer: recent progress and future directions. Crit Rev oncology/hematology (2023) 181:103882. doi: 10.1016/j.critrevonc.2022.103882
22. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. Fda approval summary: selpercatinib for the treatment of lung and thyroid cancers with ret gene mutations or fusions. Clin Cancer Res an Off J Am Assoc Cancer Res (2021) 27(8):2130–5. doi: 10.1158/1078-0432.Ccr-20-3558
23. Kim J, Bradford D, Larkins E, Pai-Scherf LH, Chatterjee S, Mishra-Kalyani PS, et al. Fda approval summary: pralsetinib for the treatment of lung and thyroid cancers with ret gene mutations or fusions. Clin Cancer Res an Off J Am Assoc Cancer Res (2021) 27(20):5452–6. doi: 10.1158/1078-0432.Ccr-21-0967
24. Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, et al. Pan-cancer efficacy of pralsetinib in patients with ret fusion-positive solid tumors from the phase 1/2 arrow trial. Nat Med (2022) 28(8):1640–5. doi: 10.1038/s41591-022-01931-y
25. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with ret fusion-positive solid tumours other than lung or thyroid tumours (Libretto-001): a phase 1/2, open-label, basket trial. Lancet Oncol (2022) 23(10):1261–73. doi: 10.1016/s1470-2045(22)00541-1
Keywords: pancreatic cancer, RET fusion, pralsetinib, target therapy, RET inhibitors
Citation: Zhang T, Wang H, Cai Z, Zhang S and Jiang C (2023) RET rearrangement-positive pancreatic cancer has remarkable response to pralsetinib: a case report. Front. Oncol. 13:1078076. doi: 10.3389/fonc.2023.1078076
Received: 24 October 2022; Accepted: 30 March 2023;
Published: 17 April 2023.
Edited by:
Giovanni Crisafulli, IFOM - The FIRC Institute of Molecular Oncology, ItalyReviewed by:
Alfonso De Stefano, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2023 Zhang, Wang, Cai, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chongyi Jiang, amlhbmd6aG9uZ3lpOUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.