
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 May 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1076428
This article is part of the Research Topic Updates on Radiation-induced Lymphopenia View all 10 articles
Background: Previous studies have shown that systemic inflammation indicators could predict the survival outcomes of patients with malignant tumors receiving various treatments. Radiotherapy, as a crucial treatment modality, effectively alleviates discomfort in patients with bone metastasis (BM) and greatly improves the quality of life for them. This study aimed to investigate the prognostic value of systemic inflammation index in hepatocellular carcinoma (HCC) patients with BM treated with radiotherapy.
Methods: We retrospectively analyzed clinical data collected from HCC patients with BM who received radiotherapy in our institution between January 2017 and December 2021. The pre-treatment neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) were derived to determine their relationship with overall survival (OS) and progression-free survival (PFS), using the Kaplan-Meier survival curves. The optimal cut-off value of the systemic inflammation indicators for predicting prognosis was assessed by receiver operating characteristic (ROC) curves. Univariate and multivariate analyses were performed to ultimately evaluate the factors associated with survival.
Results: The study included 239 patients with a median 14-month follow-up. The median OS was 18 months (95% confidence interval [CI] = 12.0-24.0) and the median PFS was 8.5 months (95% CI = 6.5-9.5). The optimal cut-off values for the patients were determined by ROC curve analysis as follows: SII =395.05, NLR=5.43 and PLR = 108.23. The area under the receiver operating characteristic curve values for SII, NLR and PLR in disease control prediction were 0.750, 0.665 and 0.676, respectively. Elevated systemic immune-inflammation index (SII>395.05) and higher NLR (NLR>5.43) were independently associated with poor OS and PFS. In multivariate analysis, Child-Pugh class (P = 0.038), intrahepatic tumor controlled (P = 0.019), SII (P = 0.001) and NLR (P = 0.007) were independent prognostic factors of OS and Child-Pugh class (P = 0.042), SII (P < 0.001) and NLR (P = 0.002) were independently correlated with PFS.
Conclusion: NLR and SII were associated with poor prognosis in HCC patients with BM receiving radiotherapy and might be considered reliable and independent prognostic biomarkers for HCC patients with BM.
Hepatocellular carcinoma (HCC), one of the most common cancers worldwide, is an aggressive tumor, which is prone to extrahepatic metastasis that occurs in 25.5-38.5% of patients (1–3). In recent years, with the continuous prolongation of the survival period of liver cancer and the gradual progression of imaging diagnosis technology, the positive diagnosis rate of bone metastasis (BM) among HCC patients has increased significantly (4–8). Those patients often suffer pain, pathological fractures, spinal cord compression, hypercalcemia and other skeletal-related events (SRE), seriously damaging their quality of life (9). Thus, having a method for determining the survival in HCC patients with BM can us anticipate the development of detrimental symptoms stated above and prepare treatments preemptively to help mitigate them.
Recently, accumulating studies have confirmed that peripheral blood markers have prognostic significance in patients with malignant tumors (10–15). NLR, defined as neutrophil-to-lymphocyte count ratio, and PLR, defined as platelet-to-lymphocyte count ratio, are proved to be applicable biomarkers for patient prognostic evaluation and therapeutic decision-making (16, 17). SII is a comprehensive parameter, defined as the absolute platelet count multiplied by the neutrophil-to-lymphocyte count ratio (18, 19). The NLR, PLR, and SII are sensitive inflammatory markers in peripheral blood that can predict poor outcomes and prognosis for HCC patients who underwent surgical resection (20–22), liver transplantation (23, 24), stereotactic ablative radiation therapy (25), transarterial chemoembolization (26) or sorafenib treatment (27).
However, there are still much to learn about the prognostic potential of systemic inflammation biomarkers on HCC patients treated with palliative radiation therapy for bone metastases. Therefore, investigating the clinical significance of systemic Immune-Inflammation markers in those patients can further deepen our understanding of tumor inflammation and help us manage the wellbeing of our patients better.
In this retrospective study, our main purpose is to investigate the prognostic value of inflammatory indexes (NLR, PLR, and SII) before radiotherapy for predicting survival outcomes in HCC patients with BM.
Patients who were diagnosed with HCC with bone metastases between January 2017 and December 2021 and received radiotherapy for bone metastases at Zhongshan Hospital, Fudan University were retrospectively identified. The eligibility criteria were as follows: (1) Clinical diagnosis or pathologically confirmed hepatocellular carcinoma and no coinciding other malignancy; (2) Computed tomography (CT), magnetic resonance imaging (MRI), or bone scan evidence of bone metastasis at the index site; (3) Child-Turcotte-Pugh (CTP) class A or B liver function; (4) over the age of 18. The exclusion criteria were as follows: (1) pregnant or lactating women; (2) combined with other serious complications; (3) with serious infection or bleeding disease; (4) using immunosuppressive or anti-inflammatory drugs before treatment; (5) incomplete or absent follow-up. Ethics approval for the use of human subjects was obtained from the research ethics committee of Zhongshan Hospital, and informed consent was obtained from each patient.
Demographic information and tumor variables of all patients were collected, including gender, age, Eastern Cooperative Oncology Group performance status (ECOG PS), HCC etiologic history, liver functionality, sites and number of bone metastases, other distant metastatic sites, serum ALP and AFP and blood cell counts. Among them, blood information such as platelet(P), neutrophil (N), and lymphocyte (L) were collected from reports of routine blood samples performed within one week before the radiotherapy for bone metastases.
The treatments were administrated as described previously according to our institutional protocol (28). All patients underwent external beam radiotherapy with linear accelerator beam energies ranging from 6–15 megavolts (MV). Each radiation dose was administered using the ONCOR Avant-Garde Linear Accelerator (Siemens Medical Solutions, Inc. Oncology Care Systems Group). The types and modalities of radiation therapy were chosen based on the location and size of the lesions as well as the general condition of the patients. The bone metastatic lesions were scheduled the full radiation dosage at 28-60 Gy in 5-30 fractions.
All patients were assessed via blood examination, CT, MRI, and bone scan at 1 to 3 months after radiotherapy completion and every 3 months thereafter. Survival data was followed up by telephone and email 3-monthly until December 2021 to understand the patient’s survival status, tumor recurrence, or time to metastasis. Overall survival (OS) was defined as the time from the initiation of radiotherapy for bone metastases to death or the last follow-up, and progression-free survival (PFS) was calculated from the time from the first day of radiotherapy for bone metastases to recurrence and deterioration, death, or final follow-up.
The SII, NLR, and PLR were calculated as follows: SII = P ×N/L, NLR = N/L, and PLR = P/L. All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA). The continuous variables were presented as the median ± interquartile range (IQR). The categorical variables were described by numbers and percentages. Patient characteristics were examined using the χ2 test or Fisher exact test. OS and PFS were assessed with the Kaplan-Meier to analyze the survival probability, and Log–rank test was used to calculate the significance of differences. Cox proportional hazard model was applied for the univariate and multivariate analyses to calculate the hazard ratios (HRs) and 95% confidence intervals on survival outcomes. Variables with P values <0.1 in univariable analyses were selected for multivariable analyses. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off values of serum biomarkers in predicting patient survival based on the Youden index. The area under the curve (AUC) was calculated to evaluate the discriminatory power. A two-tailed P value less than 0.05 was considered statistically significant in the study.
The clinical characteristics of all patients are shown in Table 1, who were diagnosed with HCC with bone metastases between January 2017 and December 2021 in Zhongshan Hospital, Fudan University (Shanghai, China). A total of 239 patients with a median age of 58 years were retrospectively identified; 89.1% were male (213/239) and 23.6% were female (26/239). Among them, 96.7% (231/239) had an ECOG PS score of 0–1, 77.8% (186/239) were positive for hepatitis B virus, and 3.8% (9/239) for hepatitis C virus, 95.8% (229/239) and 4.2% (10/239) patients were Child-Pugh class A and B, respectively. There were 20.5% (49/239) patients diagnosed with bone metastases at the same time of diagnosis of HCC. The median radiation dose was 40 Gy (IQR, 30-45 Gy), delivered in 10-20 fractions. In addition to bone metastases, 46% of patients (110/239) had other sites of distant metastases, such as lung and adrenal gland. The blood characteristics of all patients are shown in Table 2. The median SII, NLR, and PLR were 705.05(IQR, 298.28-783.23), 4.73 (IQR, 2.38-6.00) and 163.56 (IQR, 95.12-196.67).
In 239 patients, a total of 389 bone metastatic sites were identified. Sites of bone metastases for all patients were shown in Figure 1. The most common site of bone metastases was the spine (66%), followed by ribs (32%) and pelvis (29%). One hundred fifty-two patients (64%) had a single bone metastatic site, while the other patients (36%) had more than one bone lesion.
The optimal cut-off values for the patients were determined by ROC curve analysis (Figure 2) as follows: SII =395.05, NLR=5.43 and PLR = 108.23. In disease control prediction, the area under the receiver operating characteristic curve values for SII, NLR, and PLR were 0.750, 0.665 and 0.676, respectively. Consequently, patients were stratified into two groups (low and high groups) based on the optimal cut-off value of each index.
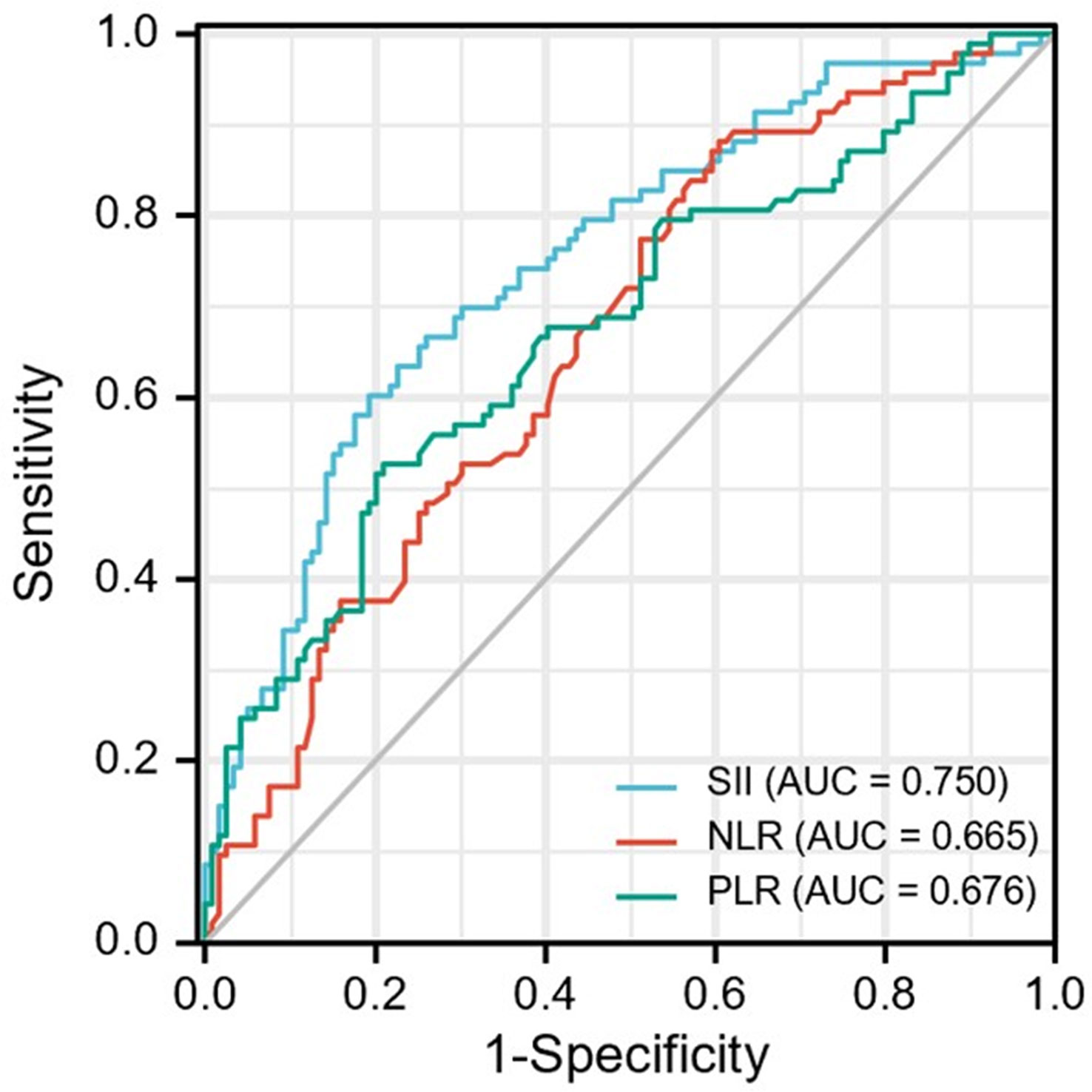
Figure 2 ROC curve analysis for optimal cut-off value of SII, NLR and PLR. ROC, receiver operating characteristic; SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
The median follow-up duration was 14 months. The median overall survival was 18 months (95%CI, 12.0-24.0). The 1-,2-,3-year OS rate was 58.9%, 44.6%, 42.1%, respectively.
Compared with the low SII group, the high SII group had inferior survival outcomes. The median OS of the low SII group was statistically higher than that of the high SII group (NR vs. 16 months, P = 0.015; Figure 3A). The median OS of the low NLR group was 29 months, significantly higher than the 9 months of the high NLR group (P = 0.011; Figure 3B). However, there was no significant difference between low PLR and high PLR group (P=0.083; Figure 3C).
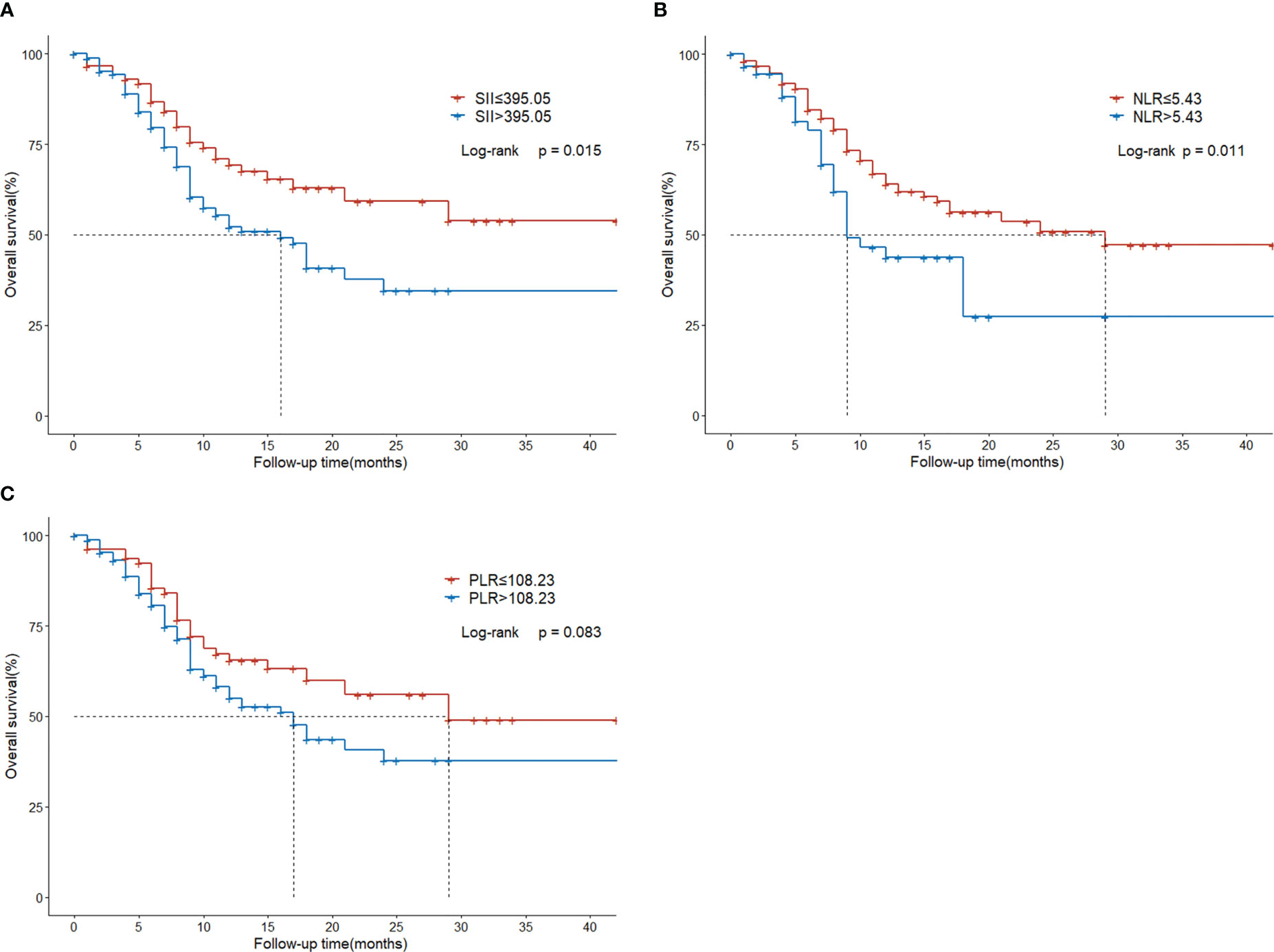
Figure 3 Overall survival in HCC patients with BM treated with radiotherapy based on their systemic immune-inflammation index (A) and neutrophil-to-lymphocyte ratio (B) and platelet-to-lymphocyte ratio (C).
A total of 24 variables were applied for univariate Cox regression analysis, and P-values less than 0.1 were included in the multivariable analysis. For OS, univariate analysis indicated that ECOG performance status (P = 0.018), Child-Pugh class (P < 0.001), multiple intrahepatic tumors (P = 0.086), intrahepatic tumor controlled (P = 0.034), AFP level (P < 0.001), ALP level (P = 0.001), ALT level (P = 0.011), AST level (P = 0.007), SII (P =0.018), NLR (P = 0.014), and PLR (P = 0.092) were statistical prognostic factors. Multivariate analysis determined that Child-Pugh class (P = 0.038), intrahepatic tumor controlled (P = 0.019), SII (P = 0.001) and NLR (P = 0.007) were independent prognostic factors (Table 3).
The median progression-free survival was 8.5 months (95%CI, 6.5-9.5). The 1- and 2-year PFS rate was 36.8% and 21.2%, respectively.
Regarding survival outcomes of different groups, the median PFS of the low SII group was statistically higher than that of the high SII group (21 vs. 6 months, P < 0.001; Figure 4A). The median PFS of the low NLR group was 11.5 months, which was significantly extended than the 4.5 months of the high NLR group (P < 0.001; Figure 4B). The median PFS of the low PLR group was 13 months, significantly higher than the 6.5 months of the high PLR group (P < 0.001; Figure 4C).
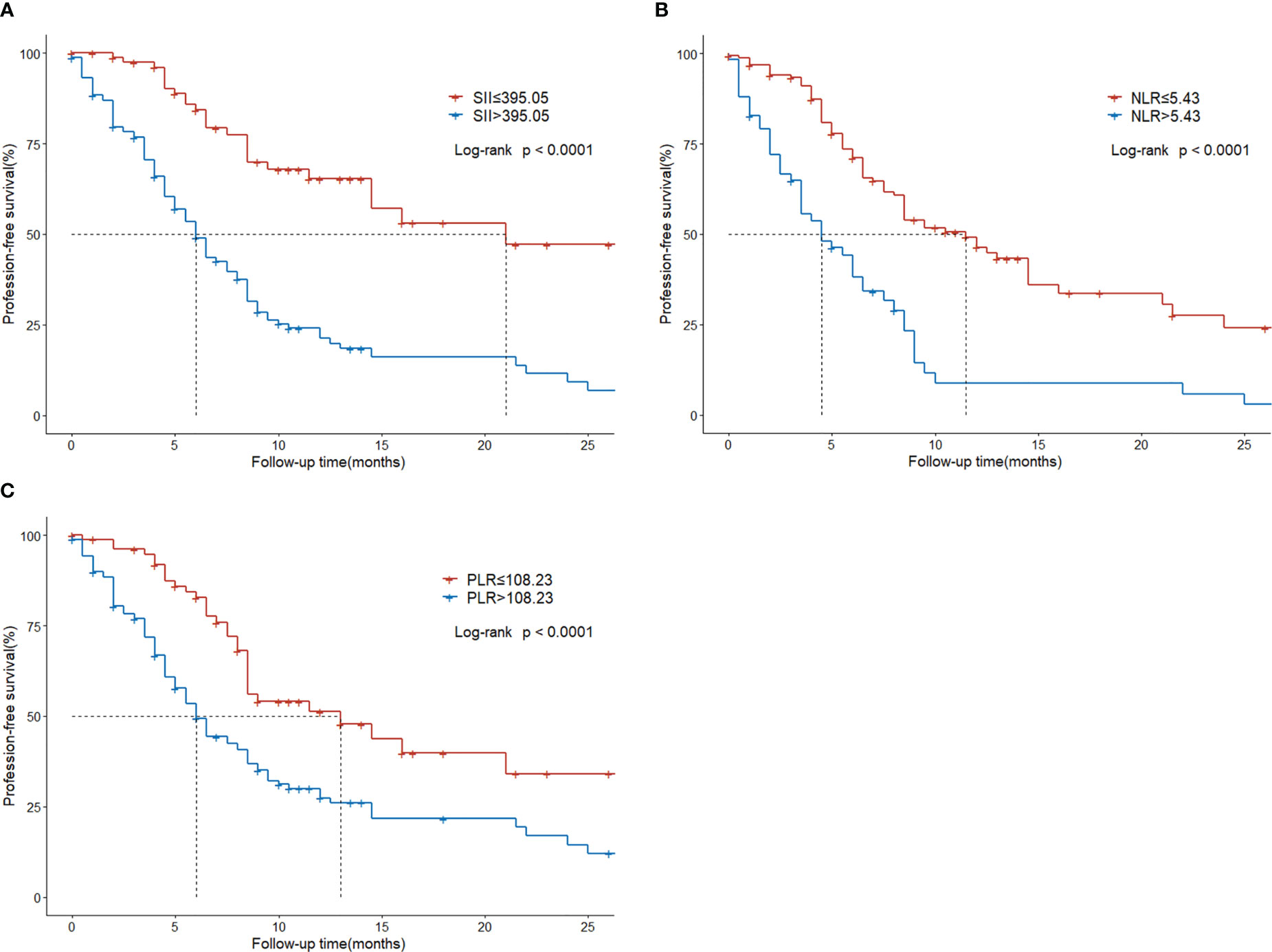
Figure 4 Progression-free survival in HCC patients with BM treated with radiotherapy based on their systemic immune-inflammation index (A) and neutrophil-to-lymphocyte ratio (B) and platelet-to-lymphocyte ratio (C).
The result of the univariate analysis revealed that ECOG performance status (P = 0.052), etiology (P = 0.028), Child-Pugh class (P = 0.007), intrahepatic tumor controlled (P = 0.014), extraosseous metastases (P = 0.008), multiple bone metastases (P = 0.099), AFP level (P = 0.011), ALT level (P = 0.089), SII (P < 0.001), NLR (P < 0.001), and PLR (P < 0.001) were significant risk factors for PFS. Multivariate analysis determined that Child-Pugh class (P = 0.042), SII (P < 0.001) and NLR (P = 0.002) were independently associated with PFS (Table 4).
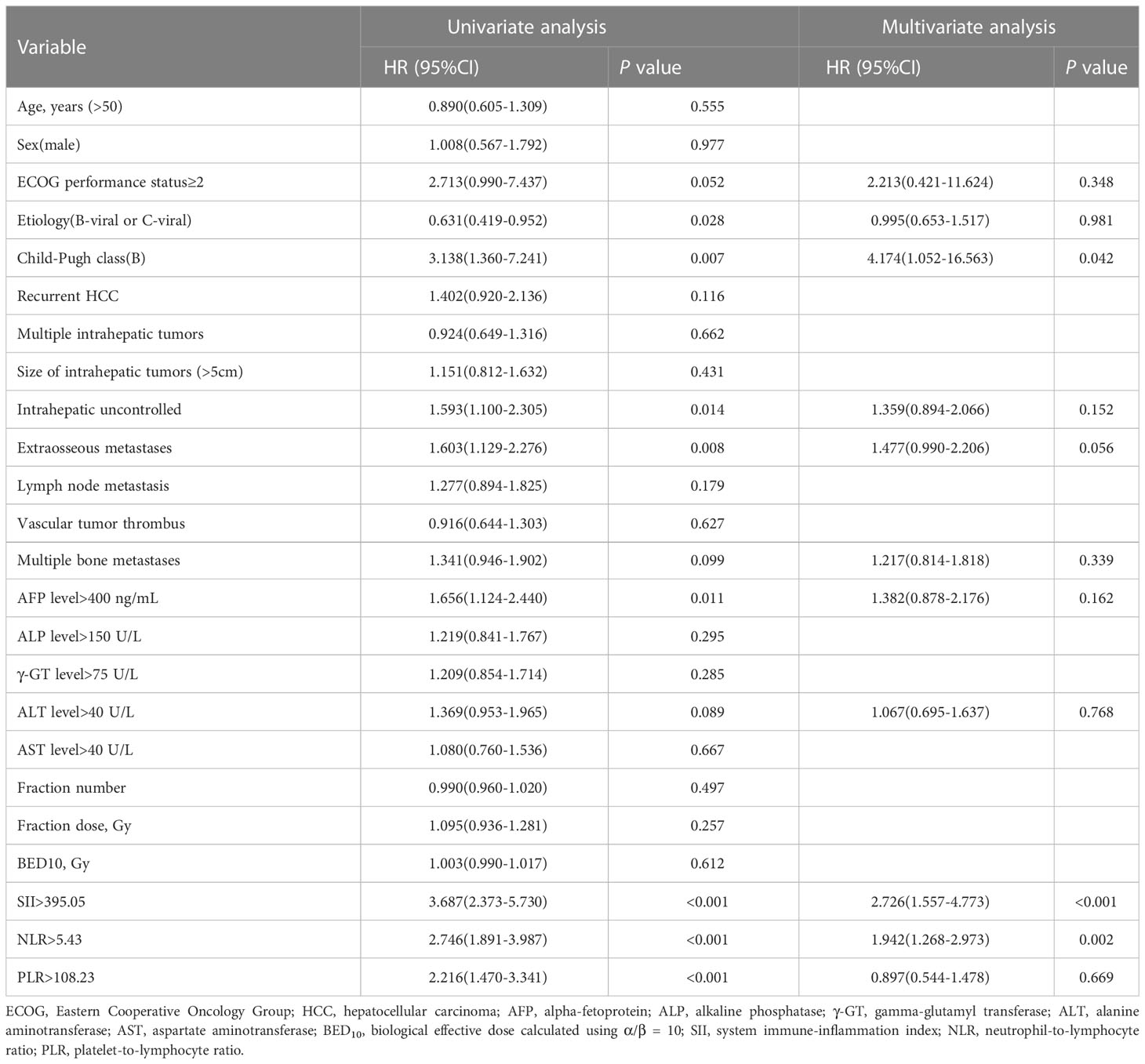
Table 4 Univariate and multivariate cox proportional hazards analysis for progression-free survival.
High NLR, PLR, and SII have been associated with poor survival in individuals with several solid tumors, including lung cancer, gastric cancer, colorectal cancer, and pancreatic cancer (12, 18, 29). High NLR and PLR correspond to worse OS and PFS in geriatric patients with HCC who underwent resection (29, 30). An elevated NLR and PLR independently predicted higher mortality in NSCLC patients treated with immunotherapy (11). NLR is an objective and valuable inflammatory marker that can predict survival outcomes and liver toxicity in HCC patients treated with SBRT. Likewise, post-PLR ≥263.0 was a prognostic factor of inferior PFS and OS in small hepatocellular carcinoma patients treated with SBRT (31). SII could be considered a combination of NLR and PLR and thus might be a better predictive biomarker, which has been proven to be an independent predictor in patients with HCC who received sequential therapy with sorafenib and regorafenib (32). In a meta-analysis comprising 2796 HCC patients, the results revealed that elevated pre-treatment SII was related to lower OS (HR:1.54, P < 0.001) and earlier time to recurrence (HR:1.77, P < 0.001) (33).
While previous studies have mainly focused on HCC patients receiving various other treatments, there are few reports on the prognostic role of these indicators in HCC patients with BM receiving radiotherapy. The development of bone metastasis is considered a multi-step process, including the displacement of cancer cells from the primary site, vascular invasion, distal capillary migration and attachment to bone, recruitment of inflammatory factors, and adjacent tissue invasion. Among them, systemic inflammation is an important accelerator in the proliferation, invasion, and metastasis of tumor cells. It plays an essential role in the tumor microenvironment, thus influencing cancer development and therapeutic response (34, 35). As one of the most common palliative treatments for patients with bone metastasis, radiotherapy is a crucial treatment modality, effectively alleviating discomfort and greatly improving quality of life (36). Previous studies revealed that systemic inflammation would inevitably impact radiotherapy’s efficacy (37, 38). In this study, we evaluated the association between several immune inflammatory parameters (NLR, PLR, SII) and clinical outcomes in HCC patients with BM. We demonstrated that NLR and SII were independently associated with survival outcomes in patients after radiotherapy. The optimal predictive potential of these biomarkers was determined based on the ROC curve, and the patients were divided into high- and low-value groups. Patients with NLR> 5.43 or SII >395.05 have poorer clinical outcomes. In the multivariate cox regression analyses, the results revealed that SII independently predicted OS (HR, 2.539; 95% CI, 1.439–4.481; P = 0.001) and PFS (HR, 2.726; 95% CI, 1.557-4.773; P < 0.001). NLR independently predicted OS (HR, 1.771; 95% CI, 1.171-2.679; P = 0.007) and PFS (HR, 1.942; 95% CI, 1.268-2.973; P = 0.002).
The molecular mechanisms of the prognostic significance of NLR, PLR, and SII for cancer patients may correlate with the function of platelets, neutrophils, and lymphocytes, reflecting inflammatory response and immune dysfunction. Beyond hemostasis and thrombosis, blood platelets also play a part in numerous pathways pivotal for cancer progression and metastasis. Research indicated that platelets can protect tumor cells from shear forces and assault of NK cells, and communicate with multiple growth factors, chemokines, inflammatory factors, and other immune cells, thereby inducing tumor cells proliferation and distant extravasation (39). Neutrophils, considered essential for the immune surveillance of tumor cells, exert multifaceted and sometimes opposing roles during cancer initiation, progression and dissemination (40). Neutrophils may be reprogrammed into a cancer-promoting state in the cancer microenvironment. They could produce some granule proteins (MMP-9 and ARG-1), subsequently degrading the extracellular matrix, and suppressing antigen-presentation and T lymphocyte activation, thereby resulting in immune escape, prompting cancer cells evasion and decreasing sensitivity to radiation treatment (41, 42). It is well established that as one of the most vital cells of the immune system, lymphocytes play a crucial role in tumorigenesis, cancer progression, metastatic seeding, and therapy resistance. Lymphocytes can directly interact with circulating tumor cells through the FAS-FASL axis or immune-checkpoint molecules, such as PD1-PDL1 and CTLA 4, which will induce immunosuppressive responses, leading to enhanced survival of the tumor cells (43, 44). Together with those findings, we can better understand the interactive functions of the immune inflammatory biomarkers with cancer progression and therapeutic response.
We considered several limitations to this study as follows. Firstly, this was a real-world retrospective study from only a single center, with a small sample size, and existing unavoidable in presence of objective biases. Therefore, it is reasonable to conduct additional large-scale and muti-center studies to validate the prognostic potential of immune-inflammatory indicators. Secondly, there were measurement biases because peripheral blood cell counts were performed only once. Inflammatory indicators in the peripheral blood can be influenced by infections, cirrhosis-associated hypersplenism, or medications including steroid. Lastly, HCC patients with bone metastases often sought treatment until significant symptoms had occurred, so we are unable to identify the exact number of patients with bone metastases who exhibited minor or no symptoms.
In conclusion, NLR and SII were associated with poor prognosis in HCC patients with BM receiving radiotherapy and might be considered reliable and independent prognostic biomarkers for HCC patients with BM. Furthermore, the systemic inflammation indexes are convenient and readily available during routine clinical practice, adding no additional financial burden to patients, so they are worthy of widespread use in clinical practice.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
The protocol was approved by the Ethics Committee of Zhongshan Hospital, and the requirement of informed consent was waived by the Institutional Review Board since this was a retrospective analysis.
JC, ZZ and JH designed the study. WH, XX and XL collected the data. JC, SF and QZ contributed to the data statistical analyses and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The research was supported by the major project in the basic research field of Shanghai Science and Technology Innovation Action Plan [22JC1402300].
The authors would like to thank all the patients who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Changes in the characteristics and survival rate of hepatocellular carcinoma from 1976 to 2000: analysis of 1365 patients in a single institution in Japan. Cancer (2004) 100(11):2415–21. doi: 10.1002/cncr.20289
2. Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer Pet/Ct imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med (2007) 48(6):902–9. doi: 10.2967/jnumed.106.036673
3. Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer (2011) 117(19):4475–83. doi: 10.1002/cncr.25960
4. He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer (2009) 115(12):2710–20. doi: 10.1002/cncr.24300
5. Harding JJ, Abu-Zeinah G, Chou JF, Owen DH, Ly M, Lowery MA, et al. Frequency, morbidity, and mortality of bone metastases in advanced hepatocellular carcinoma. J Natl Compr Canc Netw (2018) 16(1):50–8. doi: 10.6004/jnccn.2017.7024
6. Hu C, Yang J, Huang Z, Liu C, Lin Y, Tong Y, et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer (2020) 20(1):494. doi: 10.1186/s12885-020-06995-y
7. Huang Z, Wen J, Wang Y, Han S, Li Z, Hu X, et al. Bone metastasis of hepatocellular carcinoma: facts and hopes from clinical and translational perspectives. Front Med (2022) 16(4):551–73. doi: 10.1007/s11684-022-0928-z
8. Yuan X, Zhuang M, Zhu X, Cheng D, Liu J, Sun D, et al. Emerging perspectives of bone metastasis in hepatocellular carcinoma. Front Oncol (2022) 12:943866. doi: 10.3389/fonc.2022.943866
9. Oldenburger E, Brown S, Willmann J, van der Velden JM, Spalek M, van der Linden YM, et al. Estro acrop guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother Oncol (2022) 173:240–53. doi: 10.1016/j.radonc.2022.06.002
10. Yang L, He W, Kong P, Jiang C, Yang Q, Xie Q, et al. Clinical baseline and prognostic difference of platelet lymphocyte ratio (Plr) in right-sided and let-sided colon cancers. BMC Cancer (2017) 17(1):873. doi: 10.1186/s12885-017-3862-8
11. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (Nlr) and platelet-to-Lymphocyte ratio (Plr) as prognostic markers in patients with non-small cell lung cancer (Nsclc) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
12. Fu X, Li T, Dai Y, Li J. Preoperative systemic inflammation score (Sis) is superior to neutrophil to lymphocyte ratio (Nlr) as a predicting indicator in patients with esophageal squamous cell carcinoma. BMC Cancer (2019) 19(1):721. doi: 10.1186/s12885-019-5940-6
13. Tang X, Cao Y, Liu J, Wang S, Yang Y, Du P. Diagnostic value of inflammatory factors in pathology of bladder cancer patients. Front Mol Biosci (2020) 7:575483. doi: 10.3389/fmolb.2020.575483
14. Wang C, Zhao K, Hu S, Huang Y, Ma L, Song Y, et al. A predictive model for treatment response in patients with locally advanced esophageal squamous cell carcinoma after concurrent chemoradiotherapy: based on suvmean and nlr. BMC Cancer (2020) 20(1):544. doi: 10.1186/s12885-020-07040-8
15. Ruan GT, Ge YZ, Xie HL, Hu CL, Zhang Q, Zhang X, et al. Association between systemic inflammation and malnutrition with survival in patients with cancer sarcopenia-a prospective multicenter study. Front Nutr (2022) 8:811288. doi: 10.3389/fnut.2021.811288
16. Prabawa IPY, Bhargah A, Liwang F, Tandio DA, Tandio AL, Lestari AAW, et al. Pretreatment neutrophil-to-Lymphocyte ratio (Nlr) and platelet-to-Lymphocyte ratio (Plr) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev (2019) 20(3):863–8. doi: 10.31557/APJCP.2019.20.3.863
17. Lou C, Jin F, Zhao Q, Qi H. Correlation of serum nlr, plr and halp with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am J Transl Res (2022) 14(5):3240–6.
18. Murthy P, Zenati MS, Al Abbas AI, Rieser CJ, Bahary N, Lotze MT, et al. Prognostic value of the systemic immune-inflammation index (Sii) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol (2020) 27(3):898–906. doi: 10.1245/s10434-019-08094-0
19. Ruiz-Ranz M, Lequerica-Fernandez P, Rodriguez-Santamarta T, Suarez-Sanchez FJ, Lopez-Pintor RM, Garcia-Pedrero JM, et al. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment. Front Immunol (2022) 13:941351. doi: 10.3389/fimmu.2022.941351
20. Qu Z, Lu YJ, Feng JW, Chen YX, Shi LQ, Chen J, et al. Preoperative prognostic nutritional index and neutrophil-to-Lymphocyte ratio predict survival outcomes of patients with hepatocellular carcinoma after curative resection. Front Oncol (2021) 11:823054. doi: 10.3389/fonc.2021.823054
21. Wu W, Wang Q, Han D, Li J, Nie Y, Guo D, et al. Prognostic value of preoperative inflammatory markers in patients with hepatocellular carcinoma who underwent curative resection. Cancer Cell Int (2021) 21(1):500. doi: 10.1186/s12935-021-02204-3
22. Ji F, Liang Y, Fu SJ, Guo ZY, Shu M, Shen SL, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (Nlr) combined with the aspartate Aminotransferase/Platelet count ratio index (Apri). BMC Cancer (2016) 16:137. doi: 10.1186/s12885-016-2189-1
23. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation Via inflammatory microenvironment. J Hepatol (2013) 58(1):58–64. doi: 10.1016/j.jhep.2012.08.017
24. Kong W, Qu E, Sheng N, Zhang J, Li X, Zheng J, et al. Prognostic significance of inflammation-based score in patients with hepatocellular carcinoma after liver transplantation. Eur J Gastroenterol Hepatol (2021) 33(1S Suppl 1):e282–e9. doi: 10.1097/MEG.0000000000002037
25. Wang S, Deng Y, Yu X, Zhang XW, Huo CL, Sun ZG, et al. Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram. Sci Rep (2021) 11(1):13814. doi: 10.1038/s41598-021-93289-3
26. Wang H, Lin C, Fan W, Zhang J, Zhang Y, Yao W, et al. Dynamic changes in the neutrophil-to-Lymphocyte ratio predict the prognosis of patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer Manag Res (2020) 12:3433–44. doi: 10.2147/CMAR.S245396
27. Sprinzl MF, Kirstein MM, Koch S, Seib ML, Weinmann-Menke J, Lang H, et al. Improved prediction of survival by a risk factor-integrating inflammatory score in sorafenib-treated hepatocellular carcinoma. Liver Cancer (2019) 8(5):387–402. doi: 10.1159/000492628
28. He J, Shi S, Ye L, Ma G, Pan X, Huang Y, et al. A randomized trial of conventional fraction versus hypofraction radiotherapy for bone metastases from hepatocellular carcinoma. J Cancer (2019) 10(17):4031–7. doi: 10.7150/jca.28674
29. Wang W, Tong Y, Sun S, Tan Y, Shan Z, Sun F, et al. Predictive value of nlr and plr in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol (2022) 12:936206. doi: 10.3389/fonc.2022.936206
30. Safcak D, Drazilova S, Gazda J, Andrasina I, Adamcova-Selcanova S, Balazova L, et al. Inflammatory indexes as prognostic factors of survival in geriatric patients with hepatocellular carcinoma: a case control study of eight Slovak centers. J Clin Med (2022) 11(14):4183. doi: 10.3390/jcm11144183
31. Zhuang Y, Yuan BY, Hu Y, Chen GW, Zhang L, Zhao XM, et al. Pre/Post-treatment dynamic of inflammatory markers has prognostic value in patients with small hepatocellular carcinoma managed by stereotactic body radiation therapy. Cancer Manag Res (2019) 11:10929–37. doi: 10.2147/CMAR.S231901
32. Hong YM, Yoon KT, Cho M. Systemic immune-inflammation index predicts prognosis of sequential therapy with sorafenib and regorafenib in hepatocellular carcinoma. BMC Cancer (2021) 21(1):569. doi: 10.1186/s12885-021-08124-9
33. Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (Sii) in hepatocellular carcinoma: a meta-analysis. Med (Baltimore) (2020) 99(1):e18571. doi: 10.1097/MD.0000000000018571
34. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
35. Sas Z, Cendrowicz E, Weinhauser I, Rygiel TP. Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci (2022) 23(7):3778. doi: 10.3390/ijms23073778
36. Meyer J, Singal AG. Stereotactic ablative radiotherapy for hepatocellular carcinoma: history, current status, and opportunities. Liver Transpl (2018) 24(3):420–7. doi: 10.1002/lt.24991
37. Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-Small-Cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer (2016) 17(1):39–46. doi: 10.1016/j.cllc.2015.07.007
38. Chen DJ, Qin HY, Deng GC, Wang Q, Wang HY, Liu XJ. Pre-radiotherapy systemic immune inflammation index associated with overall survival in patients with advanced egfr mutant non-small cell lung cancer receiving thoracic radiotherapy. Clin Transl Oncol (2022) 25(1):226–35. doi: 10.1007/s12094-022-02936-2
39. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol (2018) 11(1):125. doi: 10.1186/s13045-018-0669-2
40. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52
41. Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, et al. Vegf-a recruits a proangiogenic mmp-9-Delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood (2012) 120(23):4653–62. doi: 10.1182/blood-2012-04-421040
42. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol (2021) 14(1):173. doi: 10.1186/s13045-021-01187-y
43. Gruber I, Landenberger N, Staebler A, Hahn M, Wallwiener D, Fehm T. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res (2013) 33(5):2233–8.
44. Arnoletti JP, Reza J, Rosales A, Monreal A, Fanaian N, Whisner S, et al. Pancreatic ductal adenocarcinoma (Pdac) circulating tumor cells influence myeloid cell differentiation to support their survival and immunoresistance in portal vein circulation. PloS One (2022) 17(3):e0265725. doi: 10.1371/journal.pone.0265725
Keywords: hepatocellular carcinoma, bone metastasis, radiotherapy, systemic immune-inflammation index, prognostic value
Citation: Chen J, Huang W, Xu X, Fan S, Zhang Q, Li X, Zeng Z and He J (2023) Prognostic implications of systemic immune-inflammation index in patients with bone metastases from hepatocellular carcinoma treated with radiotherapy. Front. Oncol. 13:1076428. doi: 10.3389/fonc.2023.1076428
Received: 21 October 2022; Accepted: 26 April 2023;
Published: 12 May 2023.
Edited by:
Deniz Yuce, Hacettepe University, TürkiyeReviewed by:
Qiliang Peng, Second Affiliated Hospital of Soochow University, ChinaCopyright © 2023 Chen, Huang, Xu, Fan, Zhang, Li, Zeng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian He, aGVqaWFuNjJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.