- 1GI and HPB Services, Department of Surgical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai, India
- 2Department of Interventional Radiology, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai, India
- 3Department of Medical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai, India
AIM: Complimentary use of Liver directed therapies (LDTs) with systemic chemotherapy has improved oncologic outcomes in colorectal liver metastasis (CRLM). We analysed institutional results of multimodality management.
Methods: Retrospective analysis of prospectively maintained database of CRLM patients managed with LDT including surgical resection, Ablation, Transarterial chemoembolization (TACE) or Transarterial radioembolization (TARE) between November 2011 to March 2020. Management plan was decided in multidisciplinary meeting. Resectable tumours underwent surgical resection or ablation or both in some cases. Borderline resectable or unresectable disease was treated with down staging chemotherapy or TACE/TARE followed by resection or ablation. All patients received adjuvant chemotherapy. Factors influencing survival were analysed.
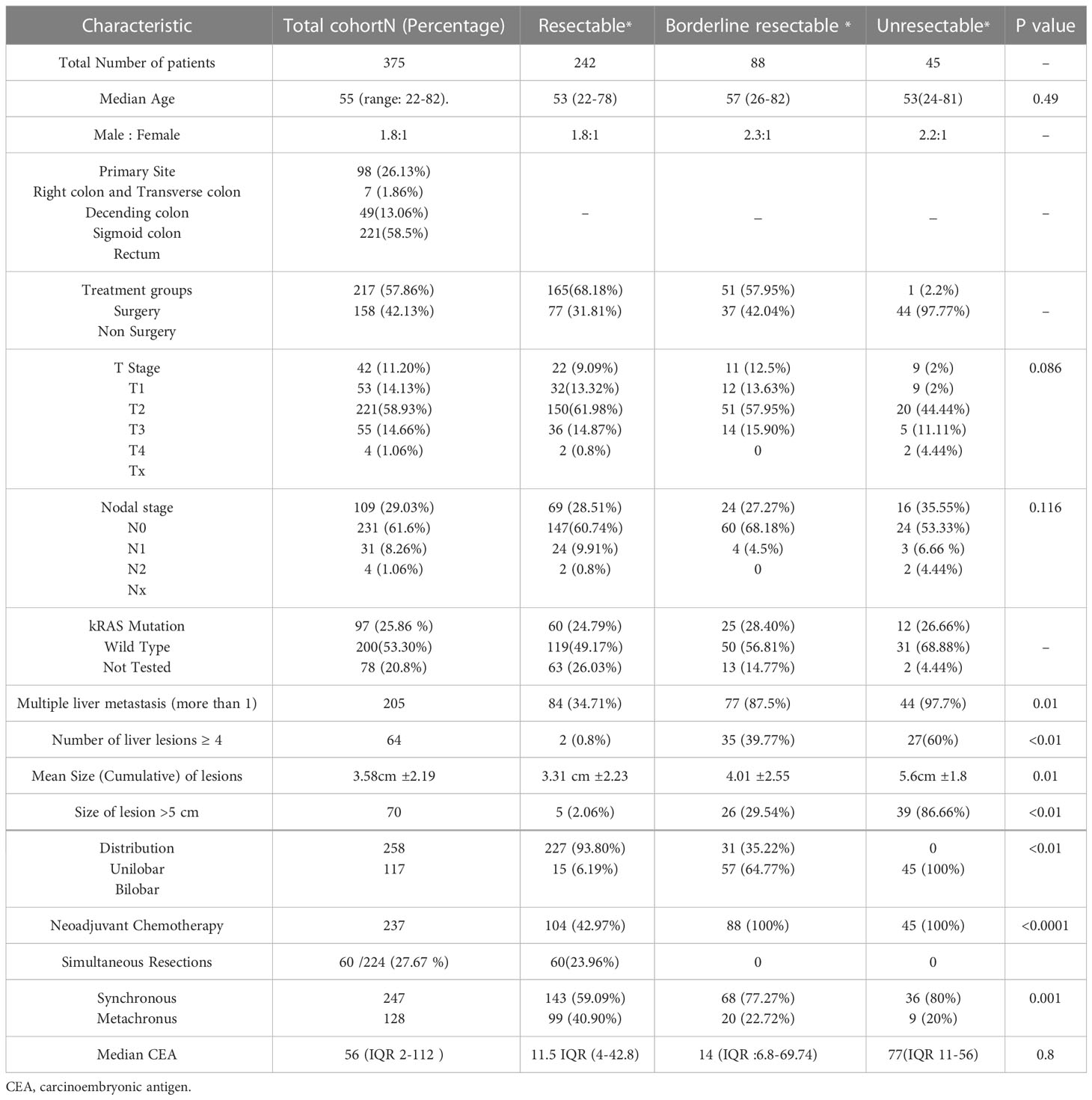
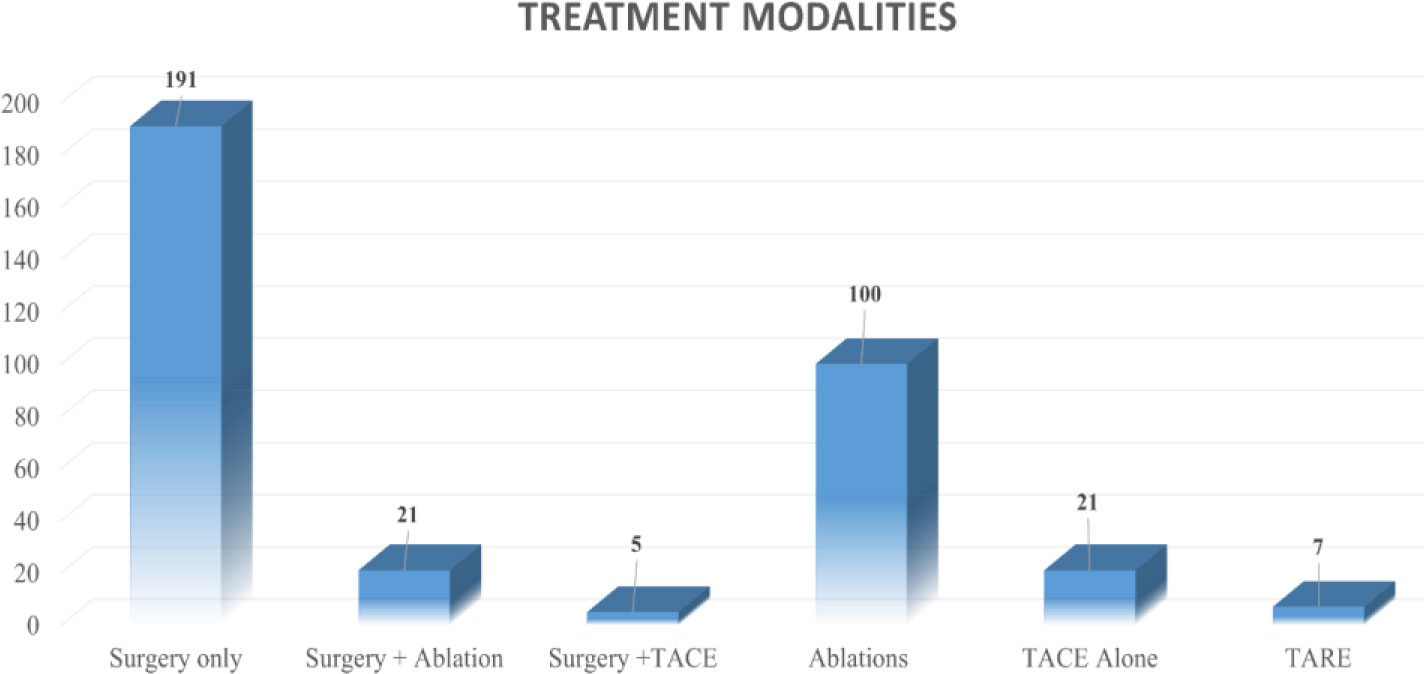
Results: Out of total 375 patients, surgery alone was done in 191 (50.93%) patients while surgery with other LDT in 26 patients (6.93%). Ablation alone was done in 100 (26.66%) whereas TACE/TARE were done as standalone treatment in 21 (5.6%) and 7 (1.86%) patients respectively. TACE + ablation was done in 28 (7.46%) and TARE + ablation was done in 2(0.53%) patients.5-year Overall Survival(OS) was 49.8% while Event free survival(EFS) was 21.4%. The median OS and EFS for surgical group was significantly better than non-surgical group (78 V/s 39 months; p<0.05 and 20 V/s 15 months p <0.005). The resectable (78 months) group had better median OS as compared to borderline resectable and Unresectable group (39 months and 29 months). Male gender, resectable disease and surgical intervention were associated with improved OS.
Conclusion: Although surgery remains the mainstay of treatment, complementary use of non-surgical LDT with systemic therapy offers possibility of good outcomes in advanced liver limited disease. Our experience highlights the impact of multidisciplinary care in optimizing CRLM treatment.
1 Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related deaths in the world with 50%–60% of patients with CRC developing liver metastasis at some point in the disease course (1, 2). Approximately 15%–20% of CRC patients have synchronous colorectal liver metastasis (CRLM) at the time of diagnosis of primary, whereas 30%–40% of patients will develop liver metastasis on follow-up (3). The management of CRLM is challenging, with only 10%–25% of patients having resectable CRC at the time of diagnosis (4, 5). Surgical resection has been considered to be the only curative option for resectable CRLM without extrahepatic disease with a 5-year survival of 40%–50% (6, 7).
Increased use and availability of various treatment modalities in multidisciplinary settings have led to aggressive management of potentially resectable as well as unresectable CRLM with better oncologic outcomes (4, 5, 8, 9). Systemic chemotherapy along with targeted agents has gained an important place in the armamentarium for the management of CRLM (8). Combined use of these liver-directed therapies (LDTs) along with systemic therapy with or without surgery could lead to better overall survival in advanced liver metastatic disease (4, 9). India has a low incidence of CRC compared to the West, but with the increasing availability of diagnostic modalities, an increasing number of cases are being detected (10). The available literature on CRLM from India and South Asia is extremely scarce, and available studies do not focus on the management of CRLM (11, 12). This is one of the earliest experiences from South Asia highlighting the multidisciplinary management of CRLM and the oncologic outcomes.
2 Methods
2.1 Patient cohort
This study is a retrospective analysis of a prospectively maintained database of all patients undergoing multimodality treatment for CRLM in the form of surgery or other non-surgical LDTs at an eminent tertiary referral centre in India. The period of the study included all patients with CRLM treated from November 2011 to December 2020. Only the patients with a follow-up period of 6 months after the intervention for CRLM were included in the study. The study included all patients with CRLM managed with liver-directed therapies, i.e., surgical resection, radiofrequency ablation (RFA), transarterial chemoembolization (TACE), or transarterial radioembolization (TARE) with or without systemic chemotherapy. Patients treated with palliative intent with only systemic therapy at the index diagnosis as well as patients with extrahepatic metastasis were excluded from the study.
2.2 Multidisciplinary treatment
All potential patients of CRLMs were discussed in a multidisciplinary “Liver Clinic” meeting comprising surgical, medical, and radiation oncologists along with interventional radiologists. Demographic parameters and clinico-radiologic data were recorded. Characteristics of the primary tumour, viz., location, stage, treatment modalities, tumour differentiation, and serum carcinoembryonic antigen (CEA) levels, were also noted.
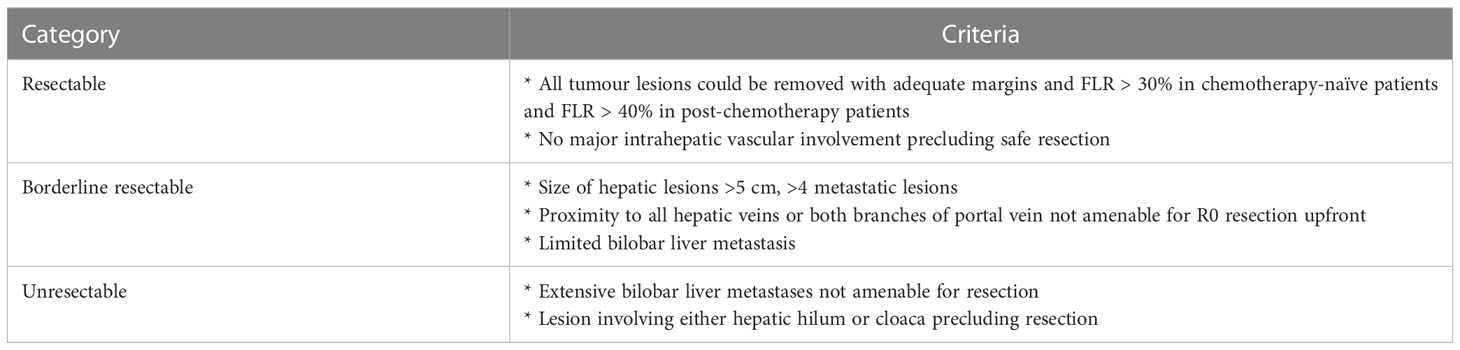
Contrast-enhanced computed tomography (CECT) scan of the thorax, abdomen, and pelvis or whole-body positron emission tomography and computed tomography (PET-CT) was performed to stage and characterize the liver metastases such as size, distribution, i.e., unilobar versus bilobar, and the number of lesions. Depending upon the extent of the disease, tumour location, and liver function, patients were categorized as resectable, borderline resectable, or unresectable (Table 1).
Surgery was performed with curative intent to obtain a margin negative (R0) resection for patients with resectable disease. For superficial lesions, a non-anatomical resection was performed. For lesions situated deep in the liver parenchyma, a formal liver resection such as segmentectomy or hepatectomy was performed as per the International Hepatopancreatobiliary Association Brisbane 2000 Classification (13).
Synchronous liver metastases were managed with simultaneous resection or a staged approach as per discretion of the surgical team depending on the extent of the disease, the site of primary, and the nature of the liver resection that would be required. The metachronous liver lesions were confirmed as colorectal metastasis by immunohistochemistry.
Some patients, though deemed resectable, underwent ablation, as the tumour location would have entailed the loss of a large amount of normal liver parenchyma if resected. Some were treated with a combined approach of surgery with intraoperative or perioperative ablations.
Patients with borderline resectable and unresectable diseases were treated with downsizing conversion chemotherapy (in combination with other LDTs in few cases) followed by curative intent surgery if adequate downsizing was achieved by chemotherapy to facilitate R0 resection. Those who were still unresectable after chemotherapy received non-surgical LDTs with systemic chemotherapy.
Ablation by RFA or microwaves was performed in few resectable tumours as described above and for unresectable diseases or if patients were either unfit or refused surgery. TACE was used in patients with unresectable liver disease as a means of disease control and also as a downsizing tool in few patients. TARE using yttrium-90 (a pure beta emitter)-impregnated microspheres to the hepatic tumours via the tumour arterial feeders were used mainly for those patients who had progressive but liver-limited unresectable disease post-chemotherapy.
2.3 Response and follow-up
For patients undergoing ablation, response assessment was performed using a regional PET scan 4–6 weeks and 3 months from the day of ablation using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (14). Patients treated with TACE and TARE were followed up with PET-CT (with triphasic CECT) after 4 weeks of the procedure. Treatment response was assessed using Positron Emission Tomography Response Criteria in Solid Tumours (PERCIST) criteria and RECIST criteria (14, 15).
All patients were offered post-intervention adjuvant chemotherapy and followed up every 3 months with serum CEA and routine blood investigations and an ultrasound examination of the abdomen with a chest X-ray. A CECT scan of the thorax, abdomen, and pelvis was performed every 6 months for the first 2 years and annually thereafter or when there was suspicion of recurrence. The last follow-up was performed telephonically.
The data of the present study were collected in the course of common clinical practice, and accordingly, signed informed consent was obtained from each patient. Data collection was in accordance with the Declaration of Helsinki. (16) As per institutional protocol, a formal board review was not taken, as it is a retrospective analysis.
2.4 Statistical analysis
SPSS version 25 was used for statistical analysis. Student’s t-test was used for quantitative data. The chi-square test was used for categorical variables. Overall survival (OS) and event-free survival (EFS) were calculated using the Kaplan–Meier curves. OS was calculated from the diagnosis date till death due to any cause or last follow-up, and EFS was calculated from the date of intervention for CRLM to either recurrence or progression of the disease. Univariate and multivariate analyses for prognostic variables were performed using the Cox regression analysis.
3 Results
3.1 Demographics and population details
A total of 627 cases of CRLM were discussed in the multidisciplinary meeting. Out of these, 252 cases were excluded, as they had extrahepatic metastasis or were treated with palliative chemotherapy without any LDT. Finally, approximately 375 eligible patients undergoing multimodality treatment for CRLM including surgical or non-surgical LDTs were included in the study (Figure 1). The total cohort consisted of 245 (65.3%) male patients and 130 (34.7%) female patients with a male-to-female ratio of 1.8:1. The median age of the population was 55 (range, 22–82) years.
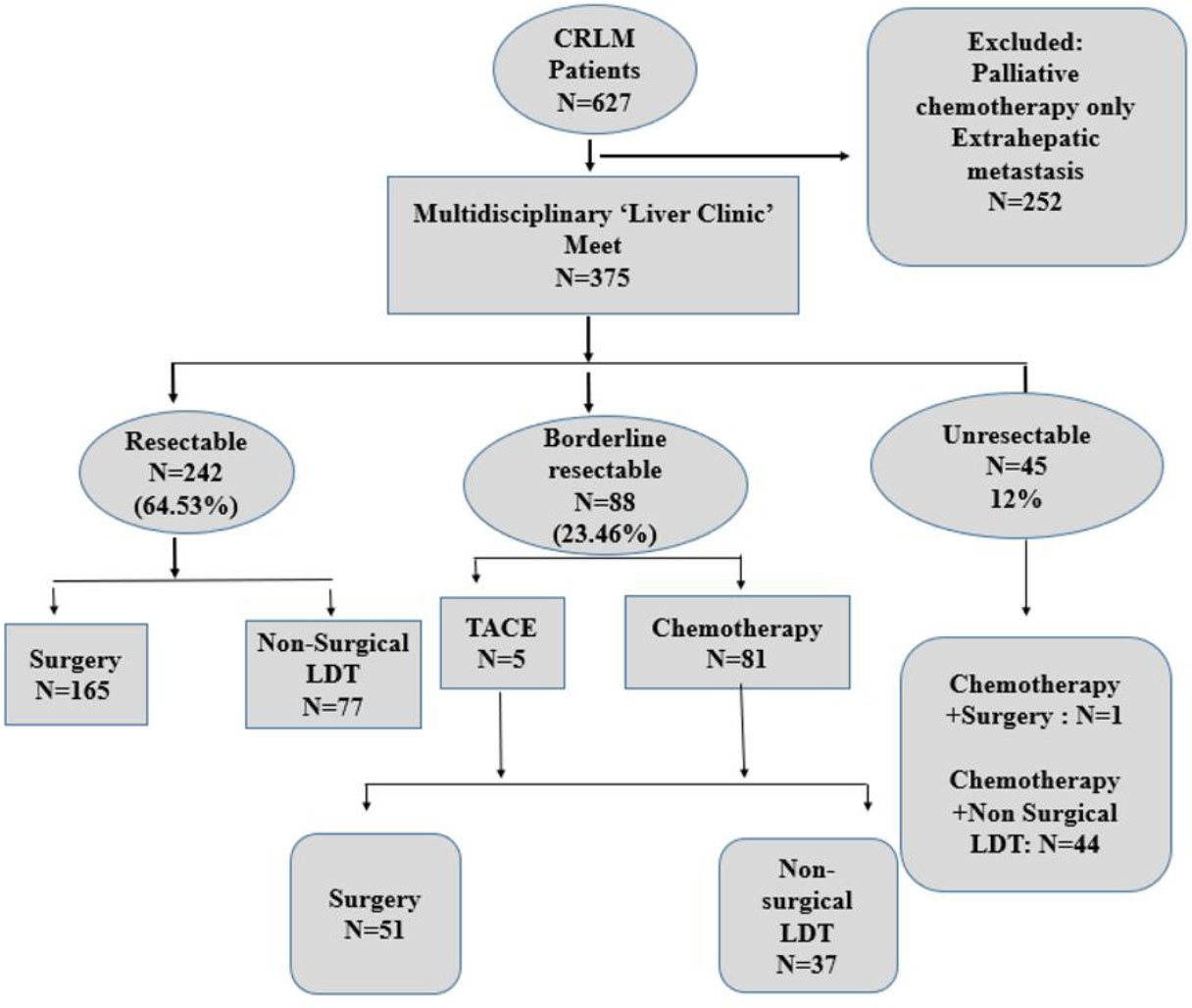
Figure 1 CONSORT diagram depicting the subgroups as per resectability criteria and treatment received.
The primary cancer was addressed with surgical resection in all but four (1.06%) patients who either had progressive metastatic disease even after undergoing liver-directed treatment along with chemotherapy (n = 3) or refused surgery for primary disease (n = 1). The rectum was the most prevalent primary site, with 221 (58.9%) patients. More than four metastatic lesions were seen in 64 (17.1%) patients. Table 2 describes the characteristics of the primary tumour and liver metastatic disease.
Synchronous liver metastasis was seen in 247 (65.9%) patients, while 128 (34.1%) developed metachronous liver metastasis. The median time interval between primary surgery and identification of metachronous lesion was 15 months (range, 8 to 123 months). Amongst patients presenting with the synchronous disease, the most common primary site was the rectum at 122/247 (49.34%), followed by the ascending and transverse colon (87; 35.22%) and the left colon (38; 15.38%). Of the patients, 64 (17.1%) developed extrahepatic disease during the follow-up period with the lung (28 patients; 43.75%) as the most common site.
A total of 237 (58.7%) patients received some form of neoadjuvant chemotherapy before any surgical or non-surgical LDT intervention. Most of them were fluoropyrimidine-oxaliplatin-based regimens. Four cycles of chemotherapy were administered prior to surgery in most cases. All patients received adjuvant chemotherapy post-intervention. In addition, based on K-RAS status, targeted cetuximab was given in the later part of the study.
3.2 LDTs
Surgical resection was performed in 217 (57.9%) patients as a definitive modality for the CRLM, and 158 (42.1%) patients underwent non-surgical LDTs. Out of 217 patients undergoing surgical modality, 191 (88%) patients underwent only surgery, whereas 21 (9.7%) patients were treated by a combination of surgical resection and post-operative ablation by either RFA or microwave energy. Five (2.3%) patients underwent surgery after being downsized by TACE. Of the total, 92 (42.3%) patients underwent parenchyma-preserving non-anatomical liver resections, with 125 (57.6%) patients requiring a formal hepatic resection.
In the surgical cohort, median blood loss was 750 ml (range, 30–13,000 ml). The median hospital stay was 7 days (range, 3–65 days). The major (Clavien–Dindo Grade III and above) post-operative morbidity was 9.8%, and mortality was 1.8%. Post-operative liver insufficiency and bile leaks were the commonest complications occurring in 6.4% (n = 14) and 5.5% (n = 12) patients, respectively. A total of 60 patients had simultaneous resection of primary tumour along with liver metastasis and had similar post-operative major morbidity rates as compared to staged resection. All patients received chemotherapy after surgery.
Amongst patients receiving non-surgical LDT, 100 (63.29%) patients received ablation alone. Of the patients, 49 received TACE either as a standalone treatment option [n = 21; (13.92%)] or in combination with ablation [n = 28 (17.77%)]. Seven (4.4%) patients were managed by TARE as a standalone treatment option, while two were managed with a combination of TARE with ablation. There was no major complication or mortality in the non-surgical group with a median hospital stay of 2.5, 3, and 2.8 days for those undergoing ablation, TACE, and TARE, respectively (Figure 2).
3.3 Follow-up and survival
At a median follow-up period of 39 (range, 6–163) months, the median OS for the entire study population was 52 months (range, 6–163) months. At the last follow-up, 106 (28.3%) patients were alive and disease free, whereas 70 (18.7%) patients were alive with disease. Of the patients, 158 (42.1%) died due to the disease, and 30 were lost to follow-up (8%).
The median EFS was 16 months (range, 0–138 months). The 5-year overall survival for the cohort was 49.8%, whereas the 5-year EFS was 21.4% (refer to Figures 3A, 4A).
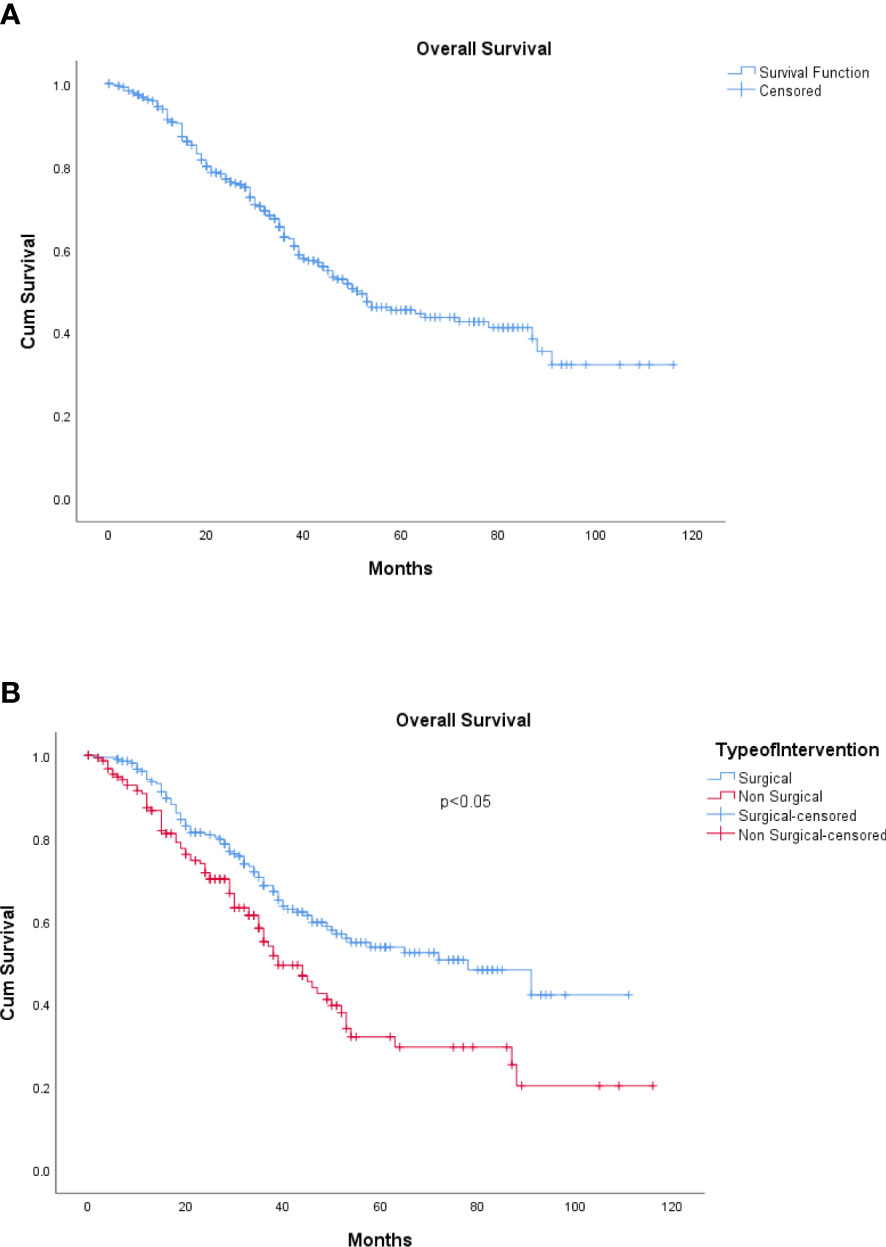
Figure 3 (A) Overall survival. (B) Overall survival: surgical and non-surgical LDT. LDT, liver-directed therapy.
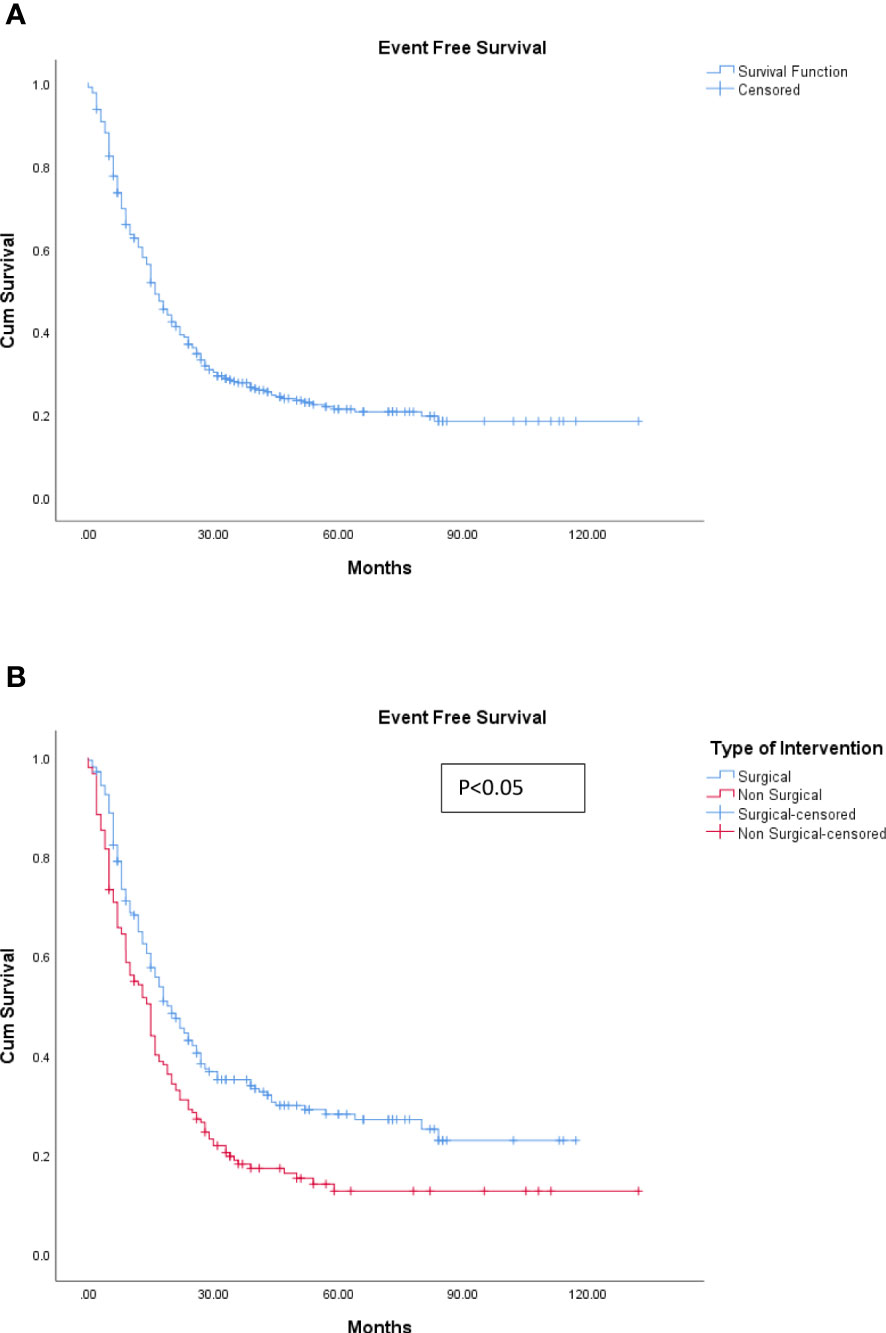
Figure 4 (A) Event-free survival. (B) Event-free survival: surgical and non-surgical LDT. LDT, liver-directed therapy.
The median OS for the patients undergoing surgical resection was 78 months (range, 6–163 months) compared to 39 months (range, 6–142) for those patients who underwent non-surgical LDTs, which was statistically significant (p < 0.05) (refer to Figure 3B). Similarly, the median EFS was significantly better in the surgical group (20 months) as compared with the non-surgical LDT group (15 months) (p < 0.005) (refer to Figure 4B).
Recurrence was seen in 133 patients out of the 217 undergoing surgical resection (61.2%), while 132 patients had disease progression (83.5%) after non-surgical LDT.
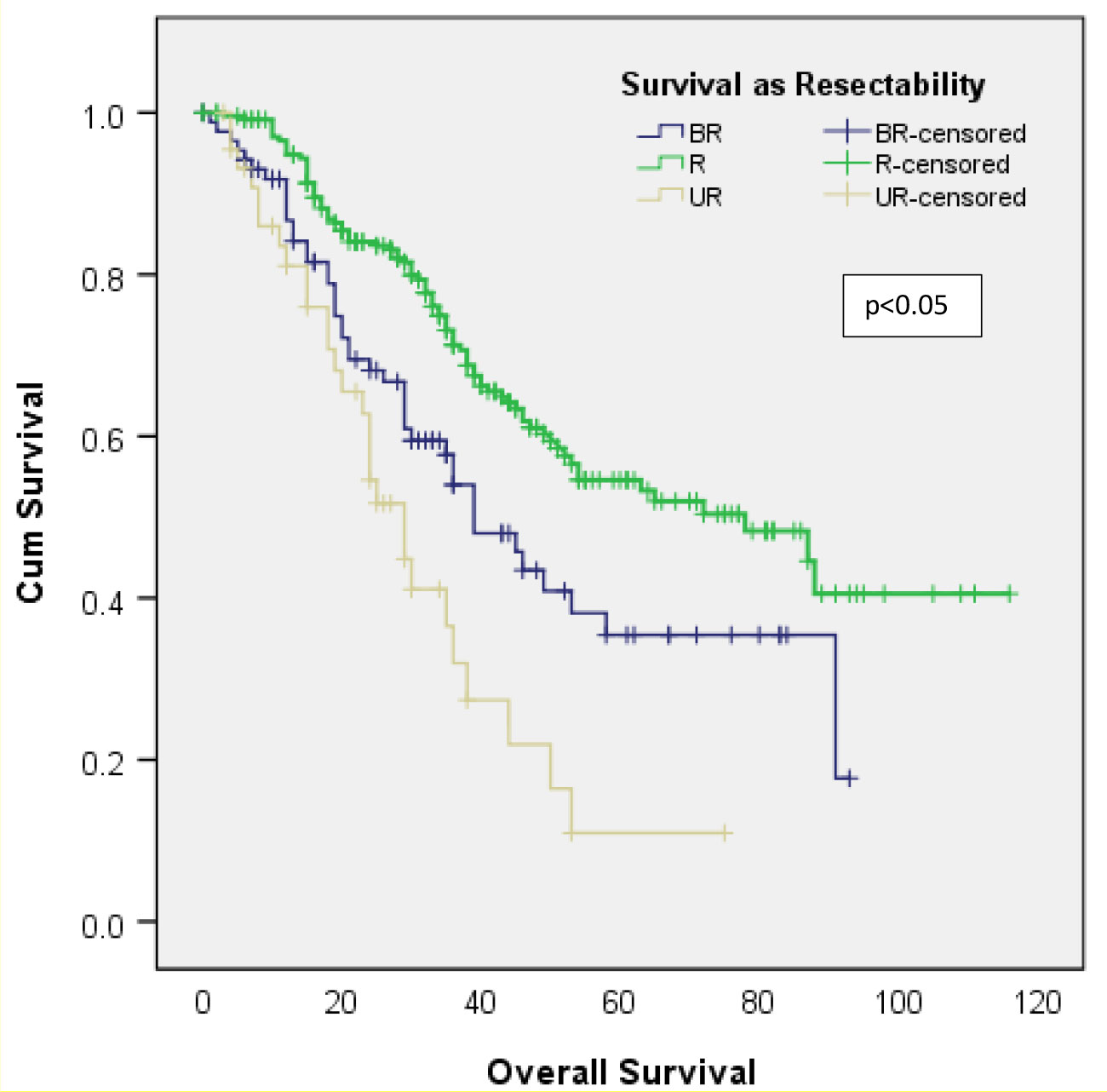
Better OS was observed in patients considered to have a resectable disease (median OS, 78 months) in the original multidisciplinary meeting as compared to patients with borderline resectable (median OS, 39 months) and unresectable disease (median OS, 29 months). The unresectable group had the worse survival (refer to Figure 5).
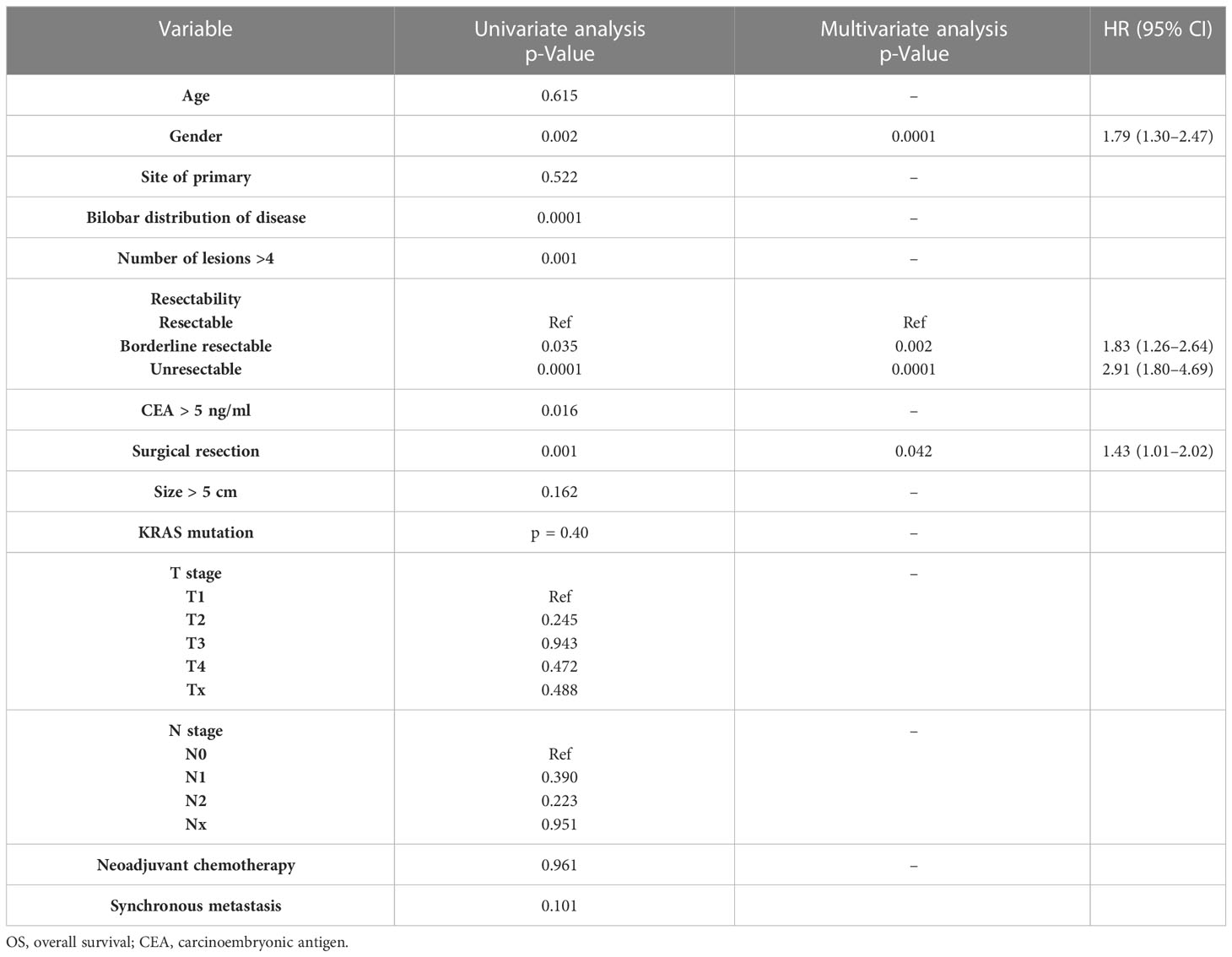
On univariate analysis, the OS was significantly worse in women as compared to men (0.002). Also, metastatic lesions more than 4 (p = 0.001) bilobar distribution of the metastatic lesions (p = 0.0001), borderline resectable (p = 0.039) as well as an unresectable disease at (p = 0.0001), elevated CEA levels more than 5 ng/ml (p = 0.016), and non-surgical LDT (p = 0001) were associated with poor OS (refer Table 3) Age, site of primary, size of tumour >5 cm, KRAS mutational status, synchronous or metachronous liver metastasis, neo-adjuvant or pre-procedural conversion chemotherapy, and T and N stage of disease did not affect OS.
A stepwise multivariate analysis, however, showed that only female gender (HR 1.79, 95% CI 1.30–2.47, p = 0.002), borderline resectable disease (HR 1.83 95% CI 1.26–2.64, p = 0.0001), unresectable disease (HR 2.91 95% CI 1.80 –4.69, p = 0.0001), and non-surgical LDTs as the modality of treatment (HR 1.43, 95% CI 1.01–2.02, p = 0.042) were independently associated with poor OS.
4 Discussion
Management of CRLM remains challenging despite the availability of multiple modalities for treatment. With the availability of effective chemotherapy and access to a variety of ablative and non-surgical modalities of LDTs, an increasing number of patients with CRLM are being managed through a combination of the different treatment modalities (17, 18). Though the multimodality approach has become an integral part of treating CRLM, literature exploring multimodality treatment outcomes is limited especially from the South Asian region (11, 12, 19–22). This is the largest series on multimodality liver-directed therapy in CRLM from the Indian sub-continent.
In the present study, the entire cohort has a median OS of 52 months and 5-year overall survival of 49.8%. Similar results for multimodal treatment were reported by other studies (9, 23). We have also found that acceptable disease control in terms of event-free survival could be achieved by complementary use of various surgical as well as non-surgical LDTs (Table 4). Extirpation/local control of disease with or without chemotherapy has been shown to have better survival than palliative chemotherapy alone, and our results convey similar findings (5, 9, 23).
Advances in surgical technique and intra-operative management have rendered major hepatectomies safe and expanded the scope of extended resections for CRLMs with the only caveat being adequate functional remnant liver volume (24, 25). Major post-operative morbidity in our series was 9.8%, with 1.8% (n = 4) mortality in the surgery group, which is in keeping with the published literature (24, 25). The 5-year OS for surgically treated patients was 54.5%, which is comparable to that of published literature (5, 26, 27).
The current study has 65% of cases presenting as synchronous colorectal metastasis. This reflects the referral patterns in a developing country with few centres of excellence for the management of metastatic colorectal cancer and may not be representative of the population as a whole. Indeed, most patients of CRLM are referred to us on the diagnosis of liver metastasis for further management leading to skewing of data. Many patients received resection of primary before referral. For synchronous CRLM, the approach of resection of the primary tumour and liver metastasis whether performed simultaneously or staged has not been shown to affect perioperative outcomes (28, 29). In the present study, no difference in peri-operative outcomes was seen in simultaneously resected patients as compared to staged liver resection.
Though modern chemotherapy and biological agents have improved the median survival of advanced CRLM patients up to 15–30 months, the intention remains palliative with historical 5-year survival rates <10% (5, 30–32). In patients with unresectable hepatic disease, conversion chemotherapy is reported to downsize the metastases and convert them to potentially resectable disease (9, 32). The use of chemotherapy, with or without targeted therapy, has resulted in conversion rates to surgery in patients with unresectable liver metastases, but there are unanswered questions regarding the routine use of neoadjuvant therapy in patients with resectable disease. A recent meta-analysis showed the benefit of neoadjuvant chemotherapy (NACT) with increased disease-free survival, but it did not translate into overall survival benefit (33). Similarly, the European Organisation for Research and Treatment of Cancer (EORTC) trial showed increased progression-free survival at 3 years by 9.2% with NACT compared with surgery alone in patients undergoing liver resection (34).
In the present study, NACT was administered to 58.7% of patients prior to LDT. Neoadjuvant chemotherapy in resectable liver metastasis has been treated previously without NACT over the years, especially in the right colonic primary. The majority (242; 64.5%) of the patients in this cohort had resectable liver disease. Resectable liver metastasis has been treated previously without NACT over the years, especially in the right colonic primary. Our practice has evolved over the years from upfront resections to neoadjuvant chemotherapy. We found that, though the use of chemotherapy helped in downsizing and further resection or nonsurgical LDT being given to the patients, neoadjuvant chemotherapy failed to show benefit in improving OS on univariate and multivariate analyses in this study. This finding is consistent with the long-term results of the EORTC trial and other studies (34, 35). The potential benefit of chemotherapy needs to be weighed against the associated hepatotoxicity in evaluating patients with resectable CRLM.
Non-surgical LDTs have traditionally been used for the management of unresectable CRLM either alone or more often in conjunction with systemic chemotherapy. Most of the evidence available for non-surgical LDTs evaluates a single modality, i.e., RFA/ablations, and many of them are single-arm studies [36.37]. We have used non-surgical liver-directed interventions in patients with unresectable or borderline resectable CRLM or in resectable disease patients deemed unfit for surgery or small resectable CRLM, which would have entailed a formal hepatectomy due to their anatomical location.
The OS and EFS for non-surgical LDTs in the current study were significantly less than those for surgically treated patients due to inherent selection bias. The only phase II randomized trial comparing systemic chemotherapy alone versus combination with RFA has shown a clear benefit with a significantly longer progression-free survival as well as OS in the combination arm (9). We had 100 patients who were managed with only ablations and systemic chemotherapy. The median OS in this group was 53 months (6–142 months), which was comparable to that of the surgical group (p = 0.46). This explains the good OS in the non-surgical LDT group and correlates with previous studies (36, 37).
TARE with yttrium-90 (90Y)-loaded microspheres has emerged in the last decade as an effective method of local disease control in patients with chemorefractory disease. A phase III randomized trial has shown prolongation in time to liver progression in patients with unresectable chemorefractory CRLM receiving radioembolization with 90Y-resin microspheres (38). We used TARE in seven patients who had progressive but liver-limited unresectable disease post-chemotherapy and in two patients with disease progression after RFA. At 3 months’ follow-up, clinical benefit rate (CBR) was 83.33%, suggesting good local disease control.
Various prognostic factors affecting survival like age, gender, site of primary, and KRAS mutation have been described in the literature (39, 40). We found that factors such as male gender, resectable disease, and surgical intervention were associated with better outcomes. KRAS mutational status had no effect on survival in the present study, contradicting other studies (40). However, not all patients had mutational analysis, which could explain this lack of effect.
The current study is limited in its retrospective nature as well as non-uniform systemic chemotherapy received in neoadjuvant and adjuvant settings, which limits generalization. There is also some selection bias in the treatment offered based on the resectability and performance status of patients. Also, the study does not take into account the site of recurrence of CRC whether local or distant and only pertains to patients having liver metastasis without any extrahepatic disease, which may have bearing on the oncologic outcomes. In the future, a focussed prospective study accommodating multimodality treatment could overcome the shortcomings of this study. However, we believe this effort highlights the importance of the multidisciplinary team (MDT) approach reflecting real-world management of CRLM. We have found that extirpation/local control of disease with or without chemotherapy is better than historically shown survival by palliative chemotherapy alone and that acceptable outcomes can be achieved with multimodality treatment even in advanced liver-limited disease. We have also found that whenever feasible, LDT either alone or in combinations can be effectively used in carefully selected patients to achieve good disease control. However, their impact on improving long-term survival remains to be ascertained.
5 Conclusion
Aggressive multimodality treatment of CRLM could lead to good oncologic outcomes. Surgical resection remains the mainstay of treatment with better OS and EFS than other modalities. Complementary use of non-surgical options along with systemic therapy offers the possibility of good survival outcomes in unresectable advanced liver-limited diseases. The role of multidisciplinary meetings exploring all available options for CRLM patients is crucial for overall favourable outcomes.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [MG], upon reasonable request due to ethical and legal restrains.
Author contributions
Conceptulization: MG. Data collection: SP, AC, NS, KG, DC, SK. Data Analysis: AC, SP, AR, VO. Drafting of manuscript: All. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist (2012) 17(10):1225–39. doi: 10.1634/theoncologist.2012-0121
2. Van der Pool AE, Damhuis RA, Ijzermans JN, de Wilt JH, Eggermont AM, Kranse R, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Colorectal Dis (2012) 14(1):56–61. doi: 10.1111/j.1463-1318.2010.02539.x
3. LeGolvan MP, Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol (2010) 102(8):898–908. doi: 10.1002/jso.21817
4. Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: Is there a possibility of cure? J Clin Oncol (2009) 27(11):1829–35. doi: 10.1200/JCO.2008.19.9273
5. Blackham AU, Swett K, Levine EA, Shen P. Surgical management of colorectal cancer metastases to the liver: Multimodality approach and a single institutional experience. Colorectal Cancer (2013) 2(1):73–88. doi: 10.2217/crc.12.80
6. Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol (2012) 4:283–301. doi: 10.2147/CLEP.S34285
7. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer (2018) 18(1):78. doi: 10.1186/s12885-017-3925-x
8. Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC–a review and evidence-based algorithm. Nat Rev Clin Oncol (2015) 12(10):607–19. doi: 10.1038/nrclinonc.2015.129
9. Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst (2017) 109(9):djx015. doi: 10.1093/jnci/djx015
10. Patil PS, Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A, et al. Colorectal cancer in India: An audit from a tertiary center in a low prevalence area. Indian J Surg Oncol (2017) 8(4):484–90. doi: 10.1007/s13193-017-0655-0
11. Sharma V, Sharma A, Raina V, Dabkara D, Mohanti BK, Shukla NK, et al. Metastatic colo-rectal cancer: Real life experience from an Indian tertiary care center. BMC Cancer (2021) 21(1):630. doi: 10.1186/s12885-021-08398-z
12. Sharma A, Sharma A, Sharma V, Kumar S, Kumar A, Deo S, et al. Long-term survivors of metastatic colorectal cancer: A tertiary care centre experience. South Asian J Cancer (2021) 10(2):87–91. doi: 10.1055/s0041-1736343
13. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB (2000) 2:333–39. doi: 10.1080/136518202760378489
14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
15. Wahl RL, Jacene H, Kasamon Y, Lodge MA. RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med (2009) 50(suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307
16. Ashcroft RE, Emanuel EJ, Grady C, Crouch RA, Lie RK, Miller , et al. The declaration of helsinki. the Oxford textbook of clinical research ethics, Vol. 21. New York: Oxford University Press. (2008). pp. 141–8.
17. Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol (2003) 10(9):1059–69. doi: 10.1245/aso.2003.03.026
18. de Jong MC, van Vledder MG, Ribero D, Hubert C, Gigot JF, Choti MA, et al. Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: An international, multiinstitutional analysis. J Gastrointest Surg (2011) 15(2):336–44. doi: 10.1007/s11605-010-1391-8
19. Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases - the effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol (2009) 35(3):302–6. doi: 10.1016/j.ejso.2008.01.028
20. Viganò L, Langella S, Ferrero A, Russolillo N, Sperti E, Capussotti L. Colorectal cancer with synchronous resectable liver metastases: monocentric management in a hepatobiliary referral center improves survival outcomes. Ann Surg Oncol (2013) 20(3):938–45. doi: 10.1245/s10434-012-2628-4
21. Lan YT, Jiang JK, Chang SC, Yang SH, Lin CC, Lin HH, et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis (2016) 31(2):403–11. doi: 10.1007/s00384-015-2459-4
22. Basso M, Corallo S, Calegari MA, Zurlo IV, Ardito F, Vellone M, et al. The impact of multidisciplinary team management on outcome of hepatic resection in liver-limited colorectal metastases. Sci Rep (2020) 10(1):10871. doi: 10.1038/s41598-020-67676-1
23. Joharatnam-Hogan N, Wilson W, Shiu KK, Fusai GK, Davidson B, Hochhauser D, et al. Multimodal treatment in metastatic colorectal cancer (mCRC) improves outcomes-the university college London hospital (UCLH) experience. Cancers (Basel) (2020) 12(12):3545. doi: 10.3390/cancers12123545
24. González HD, Figueras J. Practical questions in liver metastases of colorectal cancer: General principles of treatment. HPB (Oxford) (2007) 9(4):251–8. doi: 10.1080/13651820701457992
25. Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Liver resection for colorectal cancer metastases. Curr Oncol (2013) 20(3):e255–65. doi: 10.3747/co.20.1341
26. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg (2008) 247(1):125–35. doi: 10.1097/SLA.0b013e31815aa2c2
27. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol (2007) 25(29):4575–80. doi: 10.1200/JCO.2007.11.0833
28. Martin RC 2nd, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg (2009) 208(5):842–52. doi: 10.1016/j.jamcollsurg.2009.01.031
29. Kelly ME, Spolverato G, Lê GN, Mavros MN, Doyle F, Pawlik TM, et al. Synchronous colorectal liver metastasis: A network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol (2015) 111(3):341–51. doi: 10.1002/jso.23819
30. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol (2008) 26(12):2013–9. doi: 10.1200/JCO.2007.14.9930
31. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. Jama (2017) 317(23):2392–401. doi: 10.1001/jama.2017.7105
32. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol (2016) 27(8):1386–422. doi: 10.1093/annonc/mdw235
33. Bosma NA, Keehn AR, Lee-Ying R, Karim S, MacLean AR, Brenner DR. Efficacy of perioperative chemotherapy in resected colorectal liver metastasis: A systematic review and meta-analysis. Eur J Surg Oncol (2021) 47(12):3113–22. doi: 10.1016/j.ejso.2021.07.024
34. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol (2013) 14(12):1208–15. doi: 10.1016/S1470-2045(13)70447-9
35. Araujo R, Gonen M, Allen P, Blumgart L, DeMatteo R, Fong Y, et al. Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol (2013) 20(13):4312–21. doi: 10.1245/s10434-013-3162-8
36. Tsitskari M, Filippiadis D, Zavridis P, Mazioti A, Vrachliotis T, Alevizos L, et al. Efficacy and safety of percutaneous computed tomography-guided microwave ablation for colorectal cancer, oligometastatic liver-only disease: A single center's experience. Ann Gastroenterol (2021) 34(1):61–7. doi: 10.20524/aog.2020.0545
37. Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD 3rd, Dorfman GS, et al. American Society of clinical oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol (2010) 28(3):493–508. doi: 10.1200/JCO.2009.23.4450
38. Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol (2010) 28(23):3687–94. doi: 10.1200/JCO.2010.28.5643
39. Donadon M, Lleo A, Di Tommaso L, Soldani C, Franceschini B, Roncalli M, et al. The shifting paradigm of prognostic factors of colorectal liver metastases: From tumor-centered to host immune-centered factors. Front Oncol (2018) 8:181. doi: 10.3389/fonc.2018.00181
Keywords: colorectal cancer, colorectal liver metastasis, liver directed surgery, liver resection, multimodality management
Citation: Patkar S, Chopde A, Shetty N, Kulkarni S, Gala KB, Chandra D, Ramaswamy A, Ostwal V and Goel M (2023) Multimodality liver directed treatment for colorectal liver metastasis: Array of complementary options can improve outcomes - A single centre experience from India. Front. Oncol. 13:1073311. doi: 10.3389/fonc.2023.1073311
Received: 18 October 2022; Accepted: 06 March 2023;
Published: 22 March 2023.
Edited by:
Wenqing Cao, New York University, United StatesReviewed by:
Satvinder Singh Mudan, The London Clinic, United KingdomProf Amit Gupta, All India Institute of Medical Sciences, Rishikesh, India
Copyright © 2023 Patkar, Chopde, Shetty, Kulkarni, Gala, Chandra, Ramaswamy, Ostwal and Goel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahesh Goel, ZHJtYWhlc2hnb2VsQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shraddha Patkar
Shraddha Patkar Amit Chopde
Amit Chopde Nitin Shetty
Nitin Shetty Suyash Kulkarni
Suyash Kulkarni Kunal Bharat Gala
Kunal Bharat Gala Daksh Chandra
Daksh Chandra Anant Ramaswamy
Anant Ramaswamy Vikas Ostwal3
Vikas Ostwal3 Mahesh Goel
Mahesh Goel