- 1Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, National Clinical Research Center for Digestive Disease, Beijing, China
- 2Department of Pathology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background: Collision cancer, a rare tumor, rarely occurs in the esophagus. Most reported cases of esophageal collision cancers are advanced cancers that can only be treated with surgery or palliative chemoradiotherapy. Here, we report a rare case of collisional squamous cell carcinoma (SqCC) and adenoid cystic carcinoma (AdCC) that was detected in the early stages by endoscopy.
Case summary: A 66-year-old man presented with retrosternal pain after swallowing and underwent endoscopy. Pathological biopsy showed high-grade squamous intraepithelial neoplasia. The lesion was removed by endoscopic submucosal dissection (ESD) after magnification and endoscopic ultrasonography. Postoperative pathology proved that the lesion was collision cancer comprising SqCC and AdCC. After six months of postoperative follow-up, there was no recurrence of esophageal cancer.
Conclusions: We provided a case report related to the diagnosis and treatment of esophageal collision cancer, especially early collision cancer. More research is needed to provide insights into the management of collision cancers.
Introduction
Collision cancer refers to a tumor that occurs at the same site but originates from two tissues that infiltrate each other but do not migrate to each other (1). Collision cancer has been reported in many sites of the human body, including the skin, crania, lung, bladder and uterus (2–4). In the digestive system, collision cancer mostly occurs in large digestive glands, such as the liver and pancreas (5, 6). Approximately 2.0% to 3.6% of collision cancers occur in the liver, and approximately 0.06% to 0.2% occur in the pancreas (7, 8). In contrast, this type of tumor is rarer in the digestive tract. A previous review reported 53 cases of collision cancers of the esophagus, stomach, small intestine and large intestine (9).
To date, 16 cases of esophageal collision cancer have been reported in the English literature (10–19). However, most previous reports on esophageal collision cancer described advanced cancer with tumor tissue invading the muscle layer, and all previously reported cases of esophageal collision cancer have been treated with radical surgical resection or palliative chemoradiotherapy. The collision of squamous cell carcinoma (SqCC) and small cell carcinoma (SmCC) was the most common combination.
Here, we report a rare case of a 66-year-old Chinese man with collisional cancer of SqCC and adenoid cystic carcinoma (AdCC), which was detected in the early stages by endoscopy. This is the first case of early esophageal collision cancer that was removed by endoscopic submucosal dissection (ESD) and had not recurred at 6 months of follow-up.
Case description
The patient was a 66-year-old man who was admitted to Beijing Friendship Hospital mainly because of retrosternal pain during swallowing for more than six months. The main symptom was a stabbing pain in the chest behind the breastbone when swallowing solid food, which was relieved after swallowing. He did not report dysphagia, acid regurgitation, heartburn, nausea, vomiting, or melena. There was no significant change in body weight in the past six months. In terms of past history, the patient had a smoking history of more than 30 years, approximately 30 cigarettes per day, and had quit smoking for 4 years. The patient had no other underlying diseases and no family history of cancer. Physical examination revealed no obvious abnormality.
The patient underwent electronic endoscopy in a local hospital on February 22, 2022. Flaky erosions were observed 29 cm away from the incisors, and they were approximately 0.8*1 cm in size with surfaces covered with white hair. A biopsy was taken from the erosion site, and the local hospital’s pathology suggested high-grade squamous intraepithelial neoplasia.
After admission, the patient’s routine blood, liver and kidney function, electrolytes, myocardial enzymes and other laboratory tests showed no abnormalities. Only the tumor marker prostate-specific antigen was increased. Enhanced chest computed tomography showed no esophageal space-occupying lesions or swollen lymph nodes around the esophagus.
The patient underwent endoscopy in our hospital on March 29, 2022. A type 0-IIa lesion, approximately 1*1 cm in size, was located in the middle of the esophagus and 28-29 cm from the incisors (Figure 1A). The lesion mucosa was red and rough, with good extension. The lesion boundary was clear under white light observation, and the lesion mucosa did not stain with 1.25% iodine staining. Blue laser imaging magnifying endoscopy (BLI-ME) revealed positive background staining (Figures 1B, C). The Japan esophageal society (JES) type was B1, and the avascular area (AVA) type was small AVA. Endoscopic ultrasonography suggested that the five-layer structure of the esophageal wall at the lesion was clear, and the mucosal layer was slightly thickened (Figure 1D). ESD was performed to remove the diseased mucosa, and 18*14-mm esophageal mucosal tissue was obtained (Figures 1E, F). There were no short-term complications, such as bleeding, perforation or infection, after endoscopic surgery.
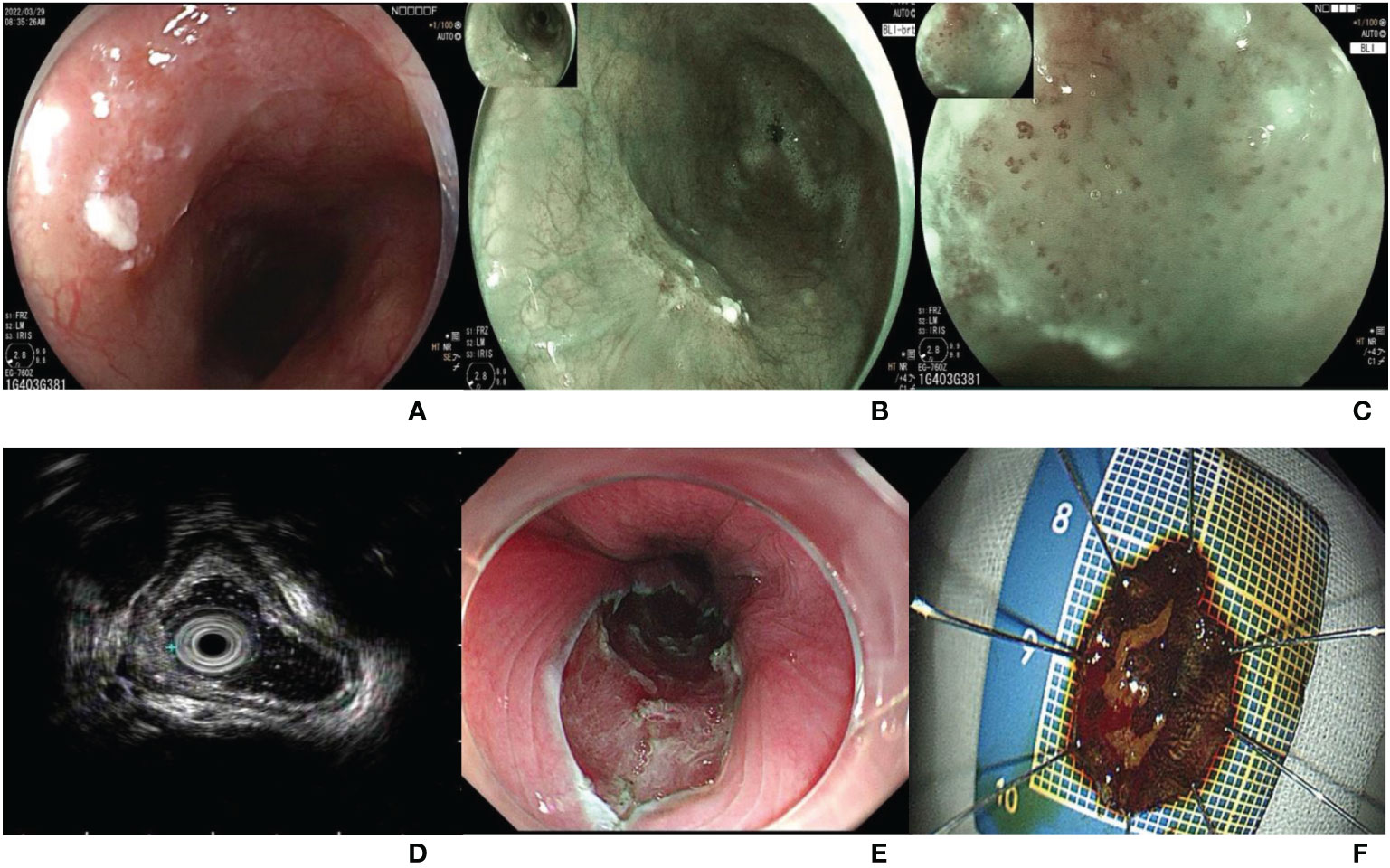
Figure 1 (A) A type 0-IIa lesion under white light endoscopy, approximately 1*1 cm in size, located in the middle of the esophagus and 28-29 cm from the incisors; (B) Esophageal lesion under blue laser endoscopy; (C) The background staining was positive under blue laser imaging magnifying endoscope. The JES type was B1, and the AVA type was small AVA. (D) Five-layer structure of the esophageal wall at the lesion was clear, and the mucosal layer was slightly thickened under ultrasound endoscope. (E) Esophageal wound after ESD. (F) An 18*14 mm esophageal mucosal tissue.
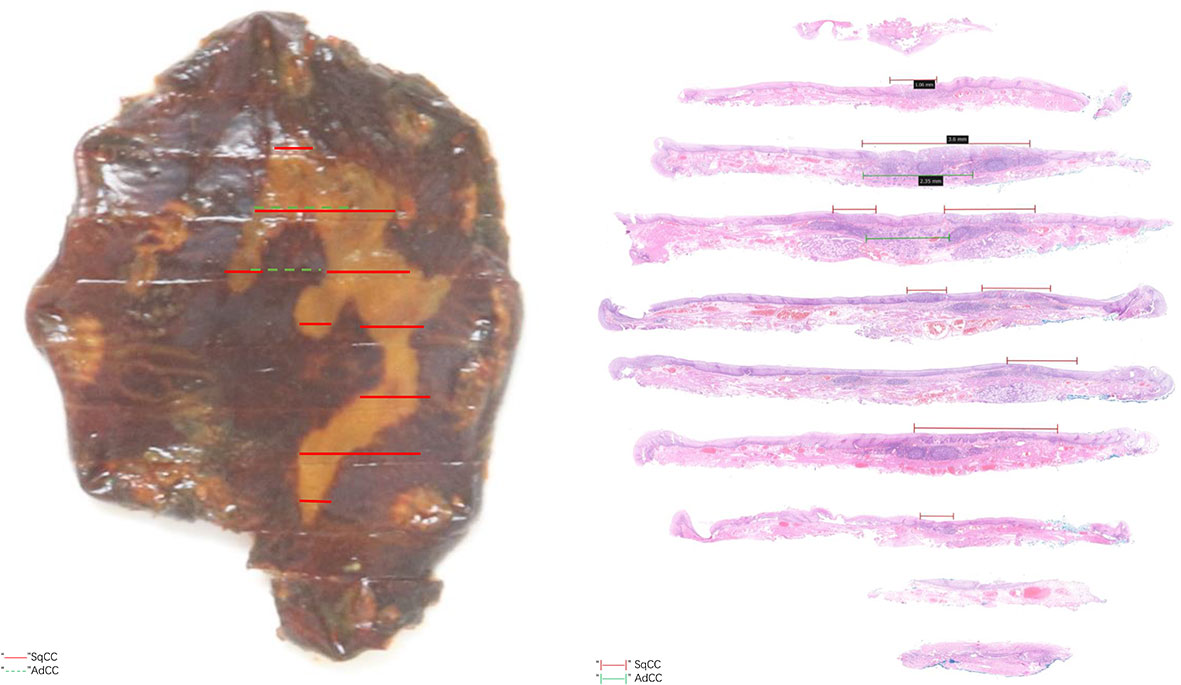
The postoperative pathological results showed that there were two malignant tumor components in the tissue submitted for examination (Figures 2, 3A). And microscopy revealed an abrupt transition between these two components that developed adjacently but never intermingled (Figure 3B). Combined with preoperative evaluation that failed to confirm any primary lesions that had metastasized to the esophagus, this patient was unequivocally diagnosed with an esophageal collision tumor. One of them was SqCC (approximately 4*2 cm in area) (Figure 3C). The cancer tissue infiltrated the lamina propria (pT1a-LPM). Another cancerous tissue was tubular and had cribriform structures with variably solid components (Figure 3D), which was located in the lamina propria and submucosa, with an infiltration depth of 60 μm into the submucosa (pT1b-SM1). Subsequent immunohistochemistry of this component yielded positive CD 117, S-100, p63, and CK8 staining (Figures 3E, F). Using the World Health Organization’s classification of tumors of the digestive system 2019, we diagnosed this component as an AdCC (14*4 mm in area) (20). The horizontal resection margin was clean, and the tumor was approximately 20 microns away from the nearest vertical resection margin. This lesion was free of lymphovascular invasion.
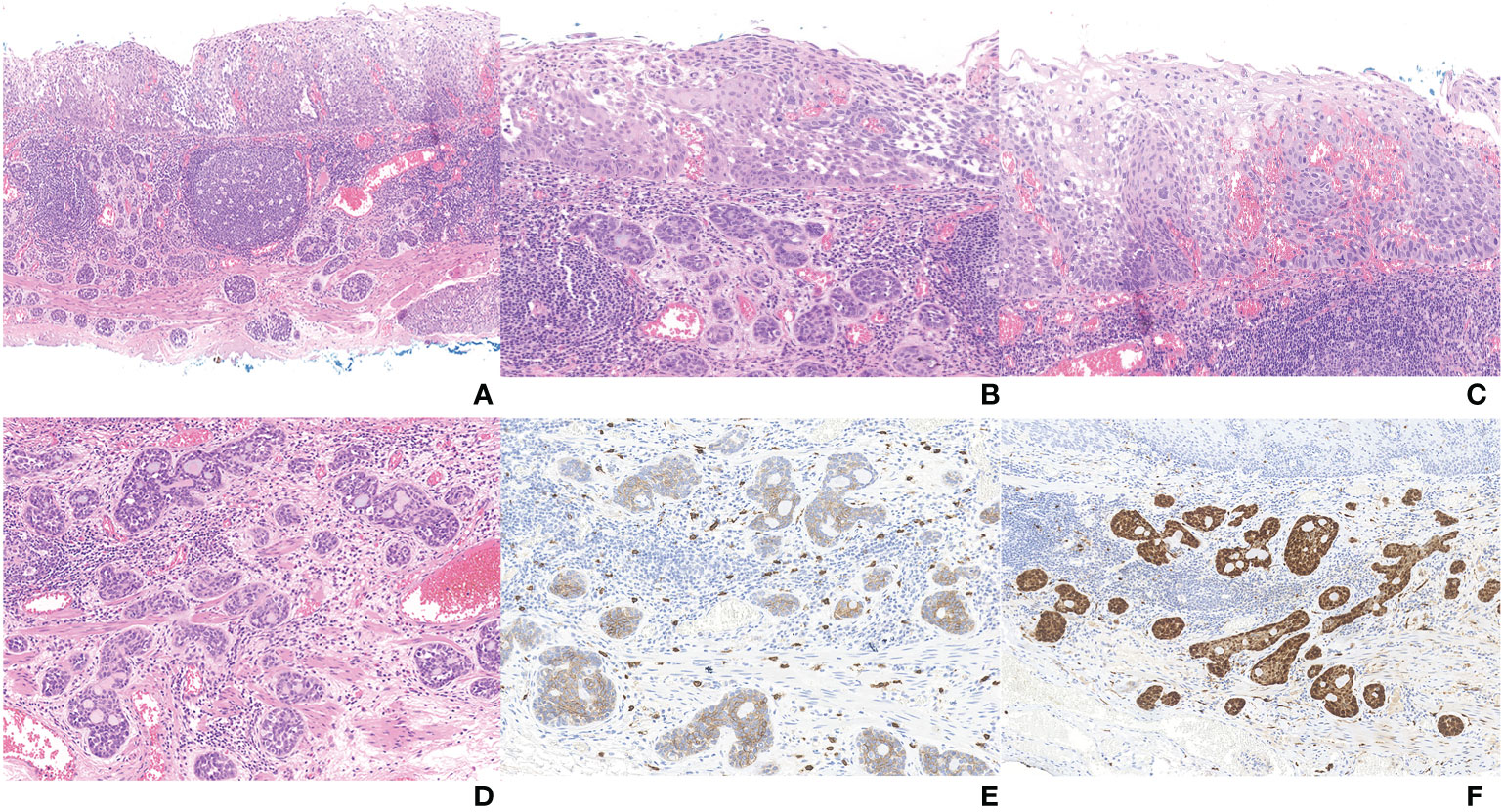
Figure 3 (A) Full view of collision carcinoma; (B) Area of collision between SqCC (up) and AdCC (down); (C) Area of SqCC; (D) Area of AdCC; (E) Immunohistochemical staining of AdCC (CD117); (F) Immunohistochemical staining of AdCC (S-100).
Since the lesion had clean incisors and no vascular infiltration, additional surgery or chemoradiotherapy was not considered. After 6 months of follow-up, the patient did not complain of any discomfort. Three months after surgery, endoscopy revealed that there was a white scar approximately 28 to 29 cm away from the incisors in the middle esophagus, and esophageal stenosis and esophageal fistula were not observed. No abnormality was observed by narrowband light imaging (NBI), and no light staining was found after staining with 1.25% iodine solution.
Discussion
A collision tumor is a subtype of neoplasm consisting of two or more distinct cell populations, and some other types include composite tumors (no clear-cut interface or a transition zone between histological patterns) and carcinosarcomas (extensive intermingling between cell populations) (10). Furthermore, amphicrine neoplasms, one cell population exhibiting characteristics of both epithelial and sarcomatous cells, and cancer-to-cancer metastasis are also included in these rare neoplasms (21, 22). The diagnostic criteria of collision tumors proposed in the previous literature are as follows: a. two distinct topographically separate sites of origin for the two components must be present; b. there must be at least some separation of the two components so that, despite intimate mixing at points of juxtaposition, a dual origin can still be recognized; and c. at the areas of collision, in addition to intimate mixing of the two components, some transitional patterns may be seen (23). Combined with the results of immunohistochemistry, the pathological diagnosis of what we reported was that of an AdCC associated with an SqCC with features of a collision tumor.
According to the definition of collision cancer, the previous literature was strictly searched. As of August 31, 2022, a total of 16 cases (10–19) of esophageal collision cancer have been reported in the English literature (Table 1). Notably, most of the literature reports were from East Asia, and only 2 cases were from European and American countries. This may be related to the lower incidence of esophageal cancer in Western countries and the predominant pathological type of esophageal adenocarcinoma (24), while most esophageal collision tumors contain elements of squamous cell carcinoma. The incidence of esophageal collision cancer was higher in males than in females, with a male-to-female ratio of 13:3, mostly in the 60-70 years old age group. This distribution was similar to that of normal esophageal cancer.
The correct diagnosis of a collision tumor is difficult but crucial because individualized treatment and disease monitoring depend on the diagnosis. The medical history, clinical manifestations, and imaging findings of the collision tumor were not specific. The gold standard for routine tumor diagnosis (endoscopic pathological biopsy) yields the diagnosis of only one cancerous component in most cases; accordingly, our literature review yielded only 1 case (12) wherein a collision tumor was confirmed by endoscopic biopsy of the two components. Hence, it is important to examine multiple tumor biopsy sites to improve the efficacy of preoperative diagnosis. However, the difficulty of subsequent treatment due to fibrosis of the esophageal mucosa caused by multiple biopsies must be considered. Immunohistochemistry is a routine method for pathological diagnosis. If immunohistochemistry remains inconclusive, molecular genetic analysis may be an important supplementary method for the diagnosis of collision tumors (25). Fukui et al. used gene sequencing to identify collision tumors and compound tumors (26).
To date, surgery remains the first-line treatment for patients with esophageal collision tumors, as is common for esophageal cancer. However, the presence of multiple components of collision tumors significantly alters treatment options, as it affects the adjuvant treatment options (10). No established guidelines are available. Some papers have argued that treatment should target the more aggressive component, while others consider that combined therapy targeting both tumor components can also be considered (27–29). More evidence is needed to determine the best individualized treatment for collisional tumors. The preoperative pathology of the case reported herein suggested high-grade intraepithelial neoplasia, which led us to use ESD. Postoperative pathological specimens were incidentally obtained as collision cancer. This contingency resulted in the current case being the only case of esophageal collision carcinoma that was removed endoscopically.
Notably, in this case, adenoid cystic carcinoma was one of the two cancerous components. This cancer is very rare in the esophagus, accounting for approximately 0.04%-0.16% of esophageal malignant tumors (30, 31). Due to the morphological similarity, the nomenclature of salivary tumors is adopted (32). AdCC is a type of submucosal tumor (SMT). The diagnosis of SMTs by endoscopic ultrasonography and the choice of ESD to remove submucosal lesions are controversial. He et al. (33) reported 224 upper gastrointestinal SMT patients detected with endoscopy who were further checked by EUS before receiving a series of ESD treatments; these patients also completed 3- and 12-month follow-up EUS detection. The accuracy rate of EUS in pathological diagnosis or the original layer was 82.6% (185/224) or 74.6% (167/224), respectively, and the ESD success rate was 92.9%. Residual tumors were detected with EUS in 3 patients (1.3%) at the 3-month follow-up, and no recurrence was observed during the 12-month follow-up period. Hence, endoscopic ultrasonography appears to be an effective routine follow-up for SMTs in the future, although the health and economic impacts of this measure remain unclear.
In summary, we described the clinical, histologic, and molecular features of a rare collision tumor comprising SqCC and AdCC, which was the first early esophageal collision tumor to be resected endoscopically. There was no recurrence after 6 months of follow-up. We provided more evidence for the diagnosis and treatment of esophageal collision cancer, especially early collision cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
QZ, PL, and SZ treated the patient. ZL, YW, and RX wrote the paper. All authors contributed to the article and approved the submitted version.
Acknowledgments
We wish to express our gratitude to the patient for allowing us to use and publish his photographs and for providing us with considerable information about this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AdCC, Adenoid cystic carcinoma; ASC, Adenosquamous carcinoma; ADC, adenocarcinoma; AVA, Avascular area; BLI-ME, Blue laser imaging magnifying endoscope; ESD, Endoscopic submucosal dissection; GIST, Gastrointestinal stromal tumor; NBI, Narrowband light imaging; SmCC, Small cell carcinoma; SMT, Submucosal tumor; SqCC, Squamous cell carcinoma.
References
1. Coca-Pelaz A, Triantafyllou A, Devaney KO, Rinaldo A, Takes RP, Ferlito A. Collision tumors of the larynx: A critical review. Am J Otolaryngol (2016) 37:365–8. doi: 10.1016/j.amjoto.2016.02.010
2. Syed S, Karambizi DI, Baker A, Groh DM, Toms SA. A comparative report on intracranial tumor-to-Tumor metastasis and collision tumors. World Neurosurg (2018) 116:454–463.e2. doi: 10.1016/j.wneu.2018.04.109
3. Bhangoo MS, Zhou JY, Ali SM, Madison R, Schrock AB, Costantini C. Objective response to mTOR inhibition in a metastatic collision tumor of the liver composed of melanoma and adenocarcinoma with TSC1 loss: A case report. BMC Cancer (2017) 17:197. doi: 10.1186/s12885-017-3167-y
4. Montironi R, Santoni M, Goteri G, Mazzucchelli R, Lopez-Beltran A, Cheng L, et al. Pseudocarcinomatous hyperplasia associated with primary lymphoma in the urinary bladder: A case report. Hum Pathol (2015) 46:1040–4. doi: 10.1016/j.humpath.2015.03.004
5. Choi GH, Ann SY, Lee SI, Kim SB, Song IH. Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: Case report and review of the literature. World J Gastroenterol (2016) 22:9229–34. doi: 10.3748/wjg.v22.i41.9229
6. Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, et al. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: A case report and review of the literature. World J Surg Oncol (2017) 15:93. doi: 10.1186/s12957-017-1157-9
7. Cubilla AL, Fitzgerald PJ. Cancer of the exocrine pancreas: The pathologic aspects. CA Cancer J Clin (1985) 35:2–18. doi: 10.3322/canjclin.35.1.2
8. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: Demographic, clinical, and prognostic factors. Cancer (2002) 94:2040–6. doi: 10.1002/cncr.10392
9. Schizas D, Katsaros I, Michalinos A, Damaskos C, Garmpis N, Ntomi V, et al. Collision tumors of the gastrointestinal tract: A systematic review of the literature. Anticancer Res (2018) 38:6047–57. doi: 10.21873/anticanres.12955
10. Schizas D, Michalinos A, Alexandrou P, Moris D, Baliou E, Tsilimigras D, et al. A unique tripartite collision tumor of the esophagus: A case report. Medicine (Baltimore) 96, e8784. doi: 10.1097/MD.0000000000008784
11. Yao B, Guan S, Huang X, Su P, Song Q, Cheng Y. A collision tumor of esophagus Int J Clin Exp Pathol (2015) 8:15143–6.
12. Choe AR, Shim K-N, Lim J, Song EM, Tae CH, Jung S-A, et al. A collision tumor of the esophagus: Mixed squamous cell carcinoma and neuroendocrine carcinoma. Korean J Gastroenterol (2020) 75:207–11. doi: 10.4166/kjg.2020.75.4.207
13. Li J, Chen X, Shen Y, Hou Y, Zhang S, Wang H, et al. A rare collision tumor of squamous carcinoma and small cell carcinoma in esophagus involved with separate lymph nodes: A case report. J Thorac Dis (2013) 5:E203–206. doi: 10.3978/j.issn.2072-1439.2013.09.13
14. Adachi K, Tateno Y, Okada H. An esophageal collision tumor. Clin Gastroenterol Hepatol (2014) 12:e17–18. doi: 10.1016/j.cgh.2013.08.016
15. Qian T, Gao F, Chen M-Z, Meng F-H, Li X-J, Liu Y-J, et al. Collision tumor of the esophagus: report of a case with mixed squamous cell carcinoma and gastrointestinal stromal tumor. Int J Clin Exp Pathol (2014) 7:1206–11.
16. Wilson CI, Summerall J, Willis I, Lubin J, Inchausti BC. Esophageal collision tumor (Large cell neuroendocrine carcinoma and papillary carcinoma) arising in a Barrett esophagus. Arch Pathol Lab Med (2000) 124:411–5. doi: 10.5858/2000-124-0411-ECTLCN
17. Wang L, Zhan C, Ma J, Shi Y, Wang Q. Collision tumor of esophagus: Report of three cases. Ann Thorac Surg (2014) 97:1075–7. doi: 10.1016/j.athoracsur.2013.06.090
18. Kang MK, Kang DK. Esophageal neuroendocrine carcinoma with focal squamous cell carcinoma component. J Investig Med High Impact Case Rep (2020) 8:2324709620918245. doi: 10.1177/2324709620918245
19. Zhang G-C, Wang B-Z, Wang L-Y, Wang Y-L, Guo W, Sun N, et al. Collision tumor of the esophagus: a report of five cases. Chin Med J (Engl) (2020) 133:2386–8. doi: 10.1097/CM9.0000000000000982
20. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. WHO classification of tumours editorial board. The 2019 WHO classification of tumours of the digestive system. Histopathology (2020) 76:182–8. doi: 10.1111/his.13975
21. Majmudar B, Dillard R, Susann PW. Collision carcinoma of the gastric cardia. Hum Pathol (1978) 9:471–3. doi: 10.1016/s0046-8177(78)80032-x
22. Michalinos A, Constantinidou A, Kontos M. Gastric collision tumors: An insight into their origin and clinical significance. Gastroenterol Res Pract (2015) 2015:314158. doi: 10.1155/2015/314158
23. Spagnolo DV, Heenan PJ. Collision carcinoma at the esophagogastric junction: Report of two cases. Cancer (1980) 46:2702–8. doi: 10.1002/1097-0142(19801215)46:12<2702::AID-CNCR2820461228>3.0.CO;2-M
24. Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician (2017) 95:22–8. Available at: https://www.aafp.org/pubs/afp/issues/2017/0101/p22.html.
25. Song M, Liu X, Liu K, Zhao R, Huang H, Shi Y, et al. Targeting AKT with oridonin inhibits growth of esophageal squamous cell carcinoma In vitro and patient-derived xenografts In vivo. Mol Cancer Ther (2018) 17:1540–53. doi: 10.1158/1535-7163.MCT-17-0823
26. Fukui H, Takada M, Chiba T, Kashiwagi R, Sakane M, Tabata F, et al. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours? Gut (2001) 48:853–6. doi: 10.1136/gut.48.6.853
27. La Rosa FG, Nawaz S. Fine needle aspiration cytology of intramammary lymph nodes in an HIV-1-positive patient. a case report. Acta Cytol (1997) 41:849–51. doi: 10.1159/000332715
28. Nishimaki T, Nakagawa S, Aizawa K, Suzuki T, Hatakeyama K, Watanabe H. Composite tumor of the esophagus with tripartite differentiation. Dig Dis Sci (1997) 42:1041–6. doi: 10.1023/a:1018897321851
29. Bibeau F, Chateau M-C, Guiu M, Assenat E, Azria D, Lavaill R, et al. Small cell carcinoma with concomitant adenocarcinoma arising in a barrett’s oesophagus: report of a case with a favourable behaviour. Virchows Arch (2008) 452:103–7. doi: 10.1007/s00428-007-0533-1
30. Cantù G. Adenoid cystic carcinoma. indolent but aggress tumour. Part A: aetiopathog to diagn Acta Otorhinolaryngol Ital (2021) 41:206–14. doi: 10.14639/0392-100X-N1379
31. Yoshikawa K, Kinoshita A, Hirose Y, Shibata K, Akasu T, Hagiwara N, et al. Endoscopic submucosal dissection in a patient with esophageal adenoid cystic carcinoma. World J Gastroenterol (2017) 23:8097–103. doi: 10.3748/wjg.v23.i45.8097
32. Nie L, Li W, Xue L, Wang L, Shen Y, Fan X. Submucosal gland neoplasms of the esophagus: an update and review. Esophagus (2020) 17:376–84. doi: 10.1007/s10388-020-00758-1
33. He G, Wang J, Chen B, Xing X, Wang J, Chen J, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: A prospective study of 224 cases in a single medical center. Surg Endosc (2016) 30:4206–13. doi: 10.1007/s00464-015-4729-1
Keywords: collision cancer, early esophageal cancer, esophageal squamous cell carcinoma, esophageal adenoid cystic carcinoma, endoscopic submucosal dissection
Citation: Liang Z, Wei Y, Li P, Xu R, Zhou Q and Zhang S (2023) Case report: First case of early adenoid cystic carcinoma and squamous cell carcinoma collision cancer treated by endoscopic submucosal dissection. Front. Oncol. 13:1072336. doi: 10.3389/fonc.2023.1072336
Received: 17 October 2022; Accepted: 23 January 2023;
Published: 02 February 2023.
Edited by:
Rupert Langer, University of Bern, SwitzerlandReviewed by:
Jean-Philippe Ratone, Institut Paoli-Calmettes (IPC), FranceGianni Lazzarin, Abano Terme Hospital, Italy
Copyright © 2023 Liang, Wei, Li, Xu, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shutian Zhang, emhhbmdzaHV0aWFuQGNjbXUuZWR1LmNu; Qiaozhi Zhou, emhvdXF6aEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Zheng Liang
Zheng Liang Yongqiu Wei
Yongqiu Wei Peng Li
Peng Li Rui Xu
Rui Xu Qiaozhi Zhou
Qiaozhi Zhou Shutian Zhang
Shutian Zhang