
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 03 April 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1071792
This article is part of the Research TopicCase Reports in Neuro-Oncology and Neurosurgical Oncology : 2022View all 32 articles
 Ali S. Haider1*
Ali S. Haider1* Chibawanye I. Ene1
Chibawanye I. Ene1 Paolo Palmisciano2
Paolo Palmisciano2 Maryam Haider3
Maryam Haider3 Ganesh Rao4
Ganesh Rao4 Leomar Y. Ballester5
Leomar Y. Ballester5 Gregory N. Fuller5
Gregory N. Fuller5Isocitrate dehydrogenase (IDH) mutations are cornerstone diagnostic features in glioma classification. IDH mutations are typically characterized by mutually exclusive amino acid substitutions in the genes encoding for the IDH1 and the IDH2 enzyme isoforms. We report our institutional case of a diffuse astrocytoma with progression to secondary glioblastoma and concurrent IDH1/IDH2 mutations. A 49-year-old male underwent a subtotal resection of a lobular lesion within the right insula in 2013, revealing a WHO grade 3 anaplastic oligoastrocytoma, IDH1 mutated, 1p19q intact. Symptomatic tumor progression was suspected in 2018, leading to a surgical tumor biopsy that demonstrated WHO grade 4 IDH1 and IDH2 mutant diffuse astrocytoma. The patient subsequently underwent surgical resection followed by medical management and finally died in 2021. Although concurrent IDH1/IDH2 mutations have been rarely reported in the current literature, further study is required to better define their impact on patients’ prognoses and their response to targeted therapies.
Isocitrate dehydrogenase (IDH) mutations were first identified in diffuse gliomas in 2009 and have since played a crucial role in glioma classification (1, 2). The 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System incorporated IDH classification as a cornerstone diagnostic feature, which has continued in the 2021 update (3, 4). IDH mutations typically result in mutually exclusive amino acid substitutions in genes encoding for the IDH1 (cytoplasmic isoform) or IDH2 (mitochondrial isoform) enzyme (5). Histopathological confirmation, IDH mutation status, and 1p/19q codeletion status allows the molecular stratification of diffuse gliomas into 3 subgroups with significant survival implications: 1) IDH mutant, 1p/19q codeleted oligodendrogliomas; 2) IDH mutant, 1p/19q non-codeleted astrocytoma; 3) IDH wildtype gliomas (3, 6, 7). Though IDH1 and IDH2 mutations are usually mutually exclusive, we report a case seen at our institution of diffuse astrocytoma with progression to secondary glioblastoma and concurrent IDH1/IDH2 mutations.
A 49-year-old right-handed asymptomatic male presented to our institution in 2013 after magnetic resonance imaging (MRI) demonstrated an extensive lobular region of T2 signal hyperintensity within the right insula and perisylvian region. He initially underwent resection in 2007 and was diagnosed with grade 2 oligoastrocytoma, IDH1 mutant at an outside institution. The IDH1 mutation was determined by immunostaining. We do not have access to additional clinical information about the patient from that time since they were at an outside institution. He underwent a subtotal resection which revealed WHO grade 3 anaplastic oligoastrocytoma, IDH1 mutated, 1p19q intact. The 2013 diagnosis at our institution was determined by next generation sequencing (NGS) 50-gene panel showing IDH1 mutation (c.395G>A p.R132H), no IDH2 mutation, and TP53 mutation (c.817C>T p.R273C). ATRX was not included in the 50-gene panel, thus not tested. The patient then received 200mg/m2 per day temozolomide (TMZ) as chemotherapy for 8 cycles, followed by intensity-modulated radiation therapy (IMRT) to a total of 57 Gy in 30 fractions. In 2018, the patient underwent a surgical biopsy for suspected tumor progression that demonstrated WHO grade 4 IDH1 and IDH2 mutant diffuse astrocytoma. Microscopic examination of hematoxylin and eosin (H&E) stained sections of the biopsy showed a densely cellular, highly mitotic diffuse glioma with vascular proliferation composed predominantly of cells with relatively uniform round nuclei, many of which had perinuclear halos (Figures 1A–E). Mitotic activity was quantified at 8 mitoses per 10 high-power fields (HPF) on H&E stained sections, and at 17 mitoses per 10 HPF using phosphohistone H3 (pHH3). Computer-assisted automated quantification showed an elevated single field Ki67 antigen (MIB1) labeling index of 17.5%, with an average index of 10.3% over 4 hotspot fields. NGS identified the following somatic mutations: IDH1 R132H, IDH2 R172S, TP53 R273C, and ATRX c3565del p.L1189* (Figure 2). The 2018 diagnosis at our institution was determined using a 126-gene NGS panel which also included ATRX. The IDH1 and TP53 mutations identified were identical to those identified in 2013. The patient subsequently underwent surgical resection and was then managed with radiation via volumetric modulated arc therapy (VMAT) of 40 Gy in 20 fractions followed by 150mg/m2 per day of TMZ chemotherapy for 8 cycles. He died in 2021 (Figure 3).
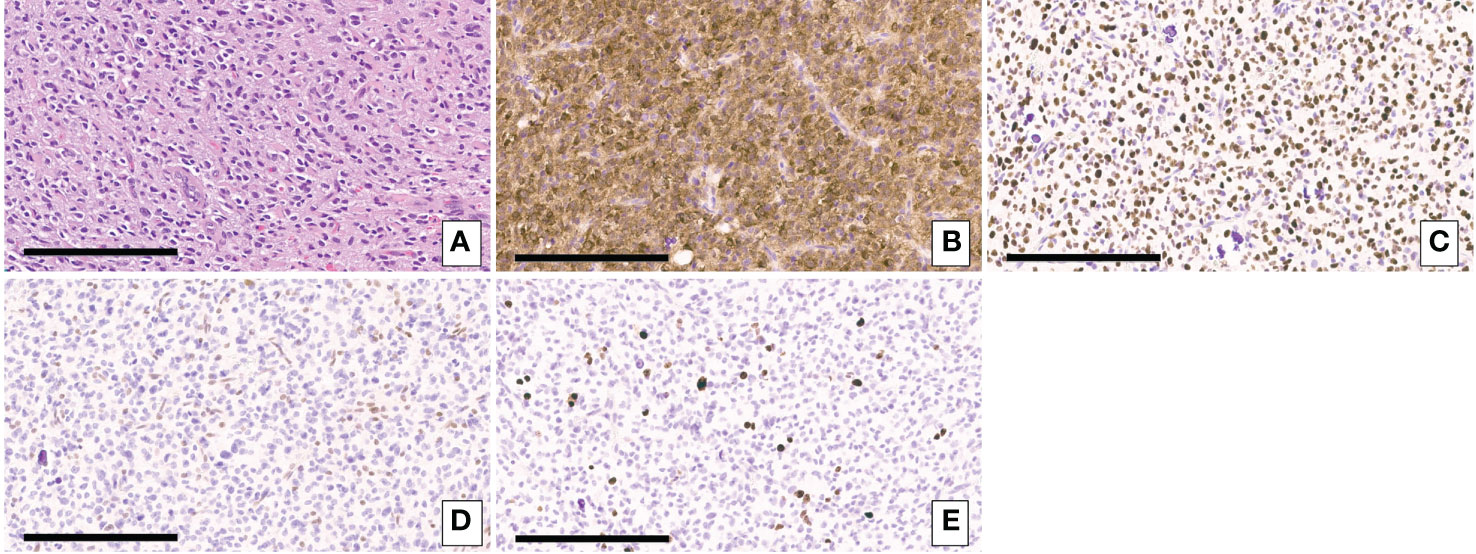
Figure 1 (A) H&E-stained sections of the biopsy showing a densely cellular diffuse glioma with vascular proliferation composed predominantly of cells with relatively uniform round nuclei, many of which had perinuclear halos. (B) Immunohistochemical staining for IDH1 p.R132H showing expression of the mutant protein in tumor cells. (C) Immunohistochemical staining for p53 showing strong nuclear staining in the majority of tumor cells consistent with the presence of a TP53 mutation. (D) Immunohistochemical staining for ATRX demonstrating loss of ATRX expression tumor cells but retained normal ATRX expression in endothelial cells. (E) Immunohistochemical staining for Ki67. Scale bar = 200um.
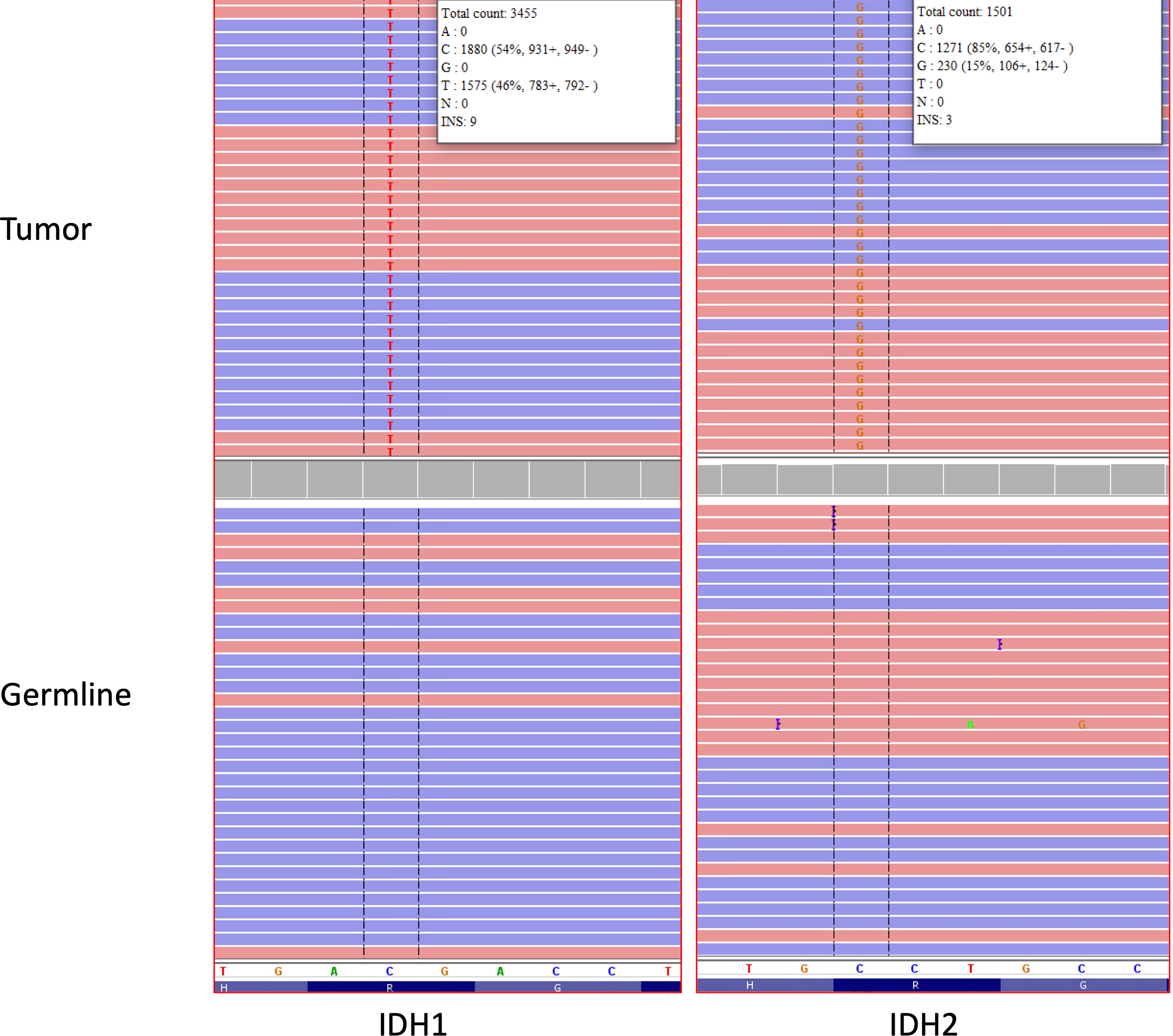
Figure 2 IGV traces showing somatic IDH1 p.R132H (C>T nucleotide change) and IDH2 p.R172S (C>G nucleotide change) mutations in the brain tumor tissue analyzed by NGS (Top). No IDH1 or IDH2 mutations were detected in DNA obtained from normal cells as a germline control (bottom). Red and blue bars represent forward and reverse sequencing reads, respectively. The reference nucleotide sequence for the depicted region of IDH1 and IDH2 is shown in the white bar in the lower portion of the figure. The “H”, “R” and “G” letters at the bottom of the figure represent the corresponding reference amino acid sequence for IDH1 and IDH2 (H = histidine, R= arginine, G=glycine).
Our case illustrates the complexity and variability regarding IDH mutations in gliomas. The normal role of IDH1 and IDH2 are to convert isocitrate to alpha-ketoglutarate. Mutations in IDH1/IDH2 result in the conversion of alpha-ketoglutarate to 2-hydroxyglutarate, which functions as an oncometabolite and is a key driver of gliomagenesis (2, 8). Glioma-specific mutations in IDH1 are known to most frequently affect codon 132, while the IDH2 mutations typically affect codon 172 (2, 9). Both mutations are known to be heterozygous and somatic, leading to amino acid substitutions (2, 5, 10). IDH1 and IDH2 enzymatically function in separate subcellular compartments, with IDH1 in cytosol and IDH2 in mitochondria. It has been shown that under hypoxia IDH1 mutated cells have a decreased ability to induce reductive carboxylation and instead rely on oxidative mitochondrial metabolism (11). In contrast, IDH2 mutated cells have shown the ability to maintain reductive carboxylation in a hypoxic state. Therefore, tumor maintenance and oncogenicity may be impacted by hypoxia depending on the mutation status of IDH1 and IDH2, however our understanding of this is limited since the metabolic communication between mutant IDH1 and IDH2 is not yet understood (11). There is a paucity in the literature regarding the different types and frequencies of IDH1/IDH2 mutations in gliomas. A search of the literature identified 4 cases of concurrent IDH1/IDH2 mutations in gliomas similar to our case (12). An additional case of concurrent IDH1 and IDH2 mutations in gliomas was found in the literature (13). This case was a WHO grade 3 astrocytoma and noted to have a prolonged time to recurrence. This case is similar to the 4 cases published by Hartmann et al. in that all are histologically WHO grade 3 (12, 13). In comparison, our case of concurrent IDH1 and IDH2 mutations was histologically WHO grade 4. It could be possible to study survival or outcomes in association with the detection of concurrent IDH1 and IDH2 mutation in gliomas, however we have limited information at this time and thus are unable to theorize further. A query of The Cancer Genome Atlas (TCGA) database of lower grade gliomas and glioblastoma (n=794 samples) identified 2 cases of concurrent IDH1/IDH2 mutations (tendency for mutual exclusivity, p<0.001).
IDH mutated gliomas are known to be a larger percentage of lower grade gliomas while comprise only a small minority of grade 4 gliomas in comparison to IDH wildtype gliomas (14). A more detailed and multi-institutional evaluation of this rare histopathological feature may have an impact on the development of future therapeutic approaches for patients with IDH-mutant gliomas. Although several targeted therapeutic options have been currently investigated for the management of tumors with IDH1 and/or IDH2 mutations, such as acute myeloid leukemias and cholangiocarcinomas, they have still a limited role for the treatment of gliomas (15). The co-presence of IDH1 and IDH2 mutations in patients with glioblastomas, as shown in our case, may suggest the occurrence of currently unknown epigenetic and/or molecular mechanisms whose discovery may likely lead to important advances in patient management and prognosis. As our understanding of concurrent IDH1 and IDH2 mutations in gliomas develops, this directly impacts clinical diagnostic testing standards. In current clinical practice, the immunohistochemical detection of IDH1 R132H in gliomas precludes the obligation to proceed to sequencing and any further information about IDH2 (16). Thus, it is likely that the current rate of concurrent IDH1 and IDH2 mutations in glioma is underestimated. This has further implications in assessing the effectiveness of novel therapeutics in development that target the IDH mutation, with further study necessary to understand the influence of concurrent IDH1 and IDH2 mutations on survival. In our case, both mutations were detected in the same tissue section. The lower VAF of the IDH2 mutation suggests the possibility that the IDH2 mutation is present only in a subset of the cells compared to the IDH1 mutation. At present, it is not possible for us to determine if the mutations were present in the same tumor cells or in distinct tumor cells within the same tissue section. In particular, the investigation of current inhibitors of IDH mutations, epigenetic therapies, and peptide vaccines may significantly benefit from the reports and analyses of these rare cases.
IDH mutations are key diagnostic and prognostic indicators in the management of gliomas. These mutations vary in frequency and type, with little epidemiological data in the literature. Concurrent IDH1/IDH2 mutations are rare and require further study to better evaluate their impact on patients’ prognoses and their response to targeted therapies.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by University of Texas MD Anderson Cancer Center. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained for the publication of this case report.
All authors contributed to the manuscript writing and preparation of the figures. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med (2009) 360:765–73. doi: 10.1056/NEJMoa0808710
2. Picca A, Berzero G, Di Stefano AL, Sanson M. The clinical use of IDH1 and IDH2 mutations in gliomas. Expert Rev Mol Diagn (2018) 18:1041–51. doi: 10.1080/14737159.2018.1548935
3. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
4. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
5. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol (2013) 125:621–36. doi: 10.1007/s00401-013-1106-9
6. Labussière M, Idbaih A, Wang X-W, Marie Y, Boisselier B, Falet C, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology (2010) 74:1886–90. doi: 10.1212/WNL.0b013e3181e1cf3a
7. Tabouret E, Nguyen AT, Dehais C, Carpentier C, Ducray F, Idbaih A, et al. Prognostic impact of the 2016 WHO classification of diffuse gliomas in the French POLA cohort. Acta Neuropathol (2016) 132:625–34. doi: 10.1007/s00401-016-1611-8
8. Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S-H, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell (2011) 19:17–30. doi: 10.1016/j.ccr.2010.12.014
9. Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, Aldape KD, Yung WKA, Salama SR, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med (2015) 372:2481–98. doi: 10.1056/NEJMoa1402121
10. Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (2008) 321:1807–12. doi: 10.1126/science.1164382
11. Grassian AR, Parker SJ, Davidson SM, Divakaruni AS, Green CR, Zhang X, et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res (2014) 74:3317–31. doi: 10.1158/0008-5472.CAN-14-0772-T
12. Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol (2009) 118:469–74. doi: 10.1007/s00401-009-0561-9
13. Yuile A, Satgunaseelan L, Wei J, Kastelan M, Back MF, Lee M, et al. Implications of concurrent IDH1 and IDH2 mutations on survival in glioma–a case report and systematic review. Curr Issues Mol Biol (2022) 44:5117–25. doi: 10.3390/cimb44100348
14. Neff C, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS, Ostrom QT, et al. Molecular marker testing and reporting completeness for adult-type diffuse gliomas in the united states. Neuro-Oncology Pract (2023) 10:24–33. doi: 10.1093/nop/npac079
15. Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol (2021) 18:645–61. doi: 10.1038/s41571-021-00521-0
Keywords: glioblastoma, histopathology, isocitrate dehydrogenase, somatic mutation, tumor progression
Citation: Haider AS, Ene CI, Palmisciano P, Haider M, Rao G, Ballester LY and Fuller GN (2023) Concurrent IDH1 and IDH2 mutations in glioblastoma: A case report. Front. Oncol. 13:1071792. doi: 10.3389/fonc.2023.1071792
Received: 16 October 2022; Accepted: 20 March 2023;
Published: 03 April 2023.
Edited by:
David D. Eisenstat, Royal Children’s Hospital, AustraliaReviewed by:
Junya Fukai, Wakayama Medical University, JapanCopyright © 2023 Haider, Ene, Palmisciano, Haider, Rao, Ballester and Fuller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali S. Haider, YXJhbGFtMDlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.