
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 February 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1071268
This article is part of the Research Topic 50 Years of BMT: Long-Term Outcomes and Late Complications After Transplantation View all 5 articles
Background: Post-transplant cyclophosphamide (PTCy) and anti-thymocyte globulin (ATG) are both common graft-versus-host disease (GVHD) prophylaxis strategies in allo-HSCT from unrelated donors. However, no consensus has reached on which regimen is optimal. Although several studies concerning this topic exist, the outcomes of different studies still conflict with each other. Therefore, an overall comparison of the two regimens is urgently needed to help make informed clinical decisions.
Methods: Studies comparing PTCy and ATG regimens in unrelated donor (UD) allo-HSCT were searched in four critical medical databases from inception to April 17, 2022. The primary outcome was grade II-IV aGVHD, grade III-IV aGVHD and chronic GVHD (cGVHD), and the secondary outcomes included overall survival (OS), relapse incidence (RI), non-relapse mortality (NRM), and several severe infectious complications. The quality of articles was assessed by the Newcastle-Ottawa scale (NOS), and data were extracted by two independent investigators and then analyzed by RevMan 5.4.
Results: Six out of 1091 articles were eligible for this meta-analysis. Compared with the ATG regimen, prophylaxis based on PTCy achieved a lower incidence of grade II-IV aGVHD incidence (RR=0.68, 95% CI 0.50-0.93, P=0.010, I2 = 67%), grade III-IV aGVHD (RR=0.32, 95% CI 0.14-0.76, P=0.001, I2 = 75%), NRM (RR=0.67, 95% CI 0.53-0.84, P=0.17, I2 = 36%), EBV-related PTLD (RR=0.23, 95% CI 0.09-0.58, P=0.85, I2 = 0%) and better OS (RR=1.29, 95% CI 1.03-1.62, P=0.0001, I2 = 80%). The cGVHD, RI, CMV reactivation and BKV-related HC showed no significant difference between the two groups (RR=0.66, 95% CI 0.35-1.26, P<0.00001, I2 = 86%; RR=0.95, 95% CI 0.78-1.16, P=0.37, I2 = 7%; RR=0.89, 95% CI 0.63-1.24, P=0.07, I2 = 57%; RR=0.88, 95% CI 0.76-1.03, P=0.44, I2 = 0%).
Conclusion: In the setting of unrelated donor allo-HSCT, prophylaxis based on PTCy can lower the incidence of grade II-IV aGVHD, grade III-IV aGVHD, NRM and EBV-related complication, achieve better OS compared to ATG-based regimen. And cGVHD, RI, CMV reactivation and BKV-related HC were comparable in the two groups.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established curative treatment for various hematologic malignancies that provides hematopoietic reconstruction as well as the graft-versus-leukemia (GVL) effect (1). However, acute GVHD (aGVHD) is the leading cause of poor prognosis and thus restrain the implementation of allo-HSCT. The risk of GVHD is associated with the degree of human leukocyte antigen (HLA) match (2, 3). Unrelated donors are the main alternative for patients who lack HLA matched siblings. Furthermore, the high incidence of GVHD and non-relapse mortality (NMR) are reported in unrelated donors, especially in the setting of mismatched unrelated donors (MMUDs).
For unrelated donors, anti-thymocyte globulin (ATG)-based protocols is recommended as the standard GVHD prophylaxis, in combination with calcineurin inhibitors, and either methotrexate (MTX) or mycophenolate mofetil (MMF), which has been proven efficiently overcome the HLA disparity (4, 5). Nevertheless, ATG-based regimen is associated with more infections, especially viral ones (6–8). Recent years, Luznik et al. introduced posttransplant cyclophosphamide (PTCy) into haploidentical transplantation and reported relatively low GVHD and NRM (9). Gradually, PTCy with or without other immunosuppressive agents were administrated in other transplantation settings, including matched sibling donor (MSD), matched unrelated donor (MUD) and even MMUD (9–11). In study of Battipaglia et al., PTCy was demonstrated more favorable in GVHD prophylaxis and likely to provide better outcomes in the long run (12).
Although both PTCy- and ATG-based regimens have displayed efficacy in aGVHD prophylaxis, no consensus on which protocol is more effective has been reached. Although several studies compare the two strategies in setting of unrelated donors, their results contradict each other. Therefore, a comprehensive comparison between these two regimens in unrelated donors is in urgent need. Moreover, not only the incidence of GVHD but also infection is one of the major causes of NRM after transplantation (13). Up to now, comparisons of infection complications between these two strategies are still lacking. Gao et al. have already performed a meta-analysis to compare PTCy and ATG as GVHD prophylaxis regimens in terms of OS, NRM, relapse incidence (RI), leukemia-free survival (LFS), cGVHD, and aGVHD (14). However, the previous comparison just simply pooled results from various donor types together, thus making the outcome less convincing. In order to minimize the confounding results caused by different donor types, we only included MUD and MMUD for analysis. Furthermore, with the studies and articles emerging in the last two years, a new comparison between PTCy and ATG is necessary.
Consequently, in this meta-analysis, we updated the previous work by adding several current articles, including several infectious complications, to the primary outcomes. We also restricted the donor type to unrelated donors (MUD and MMUD) to provide more specific and comprehensive evidence for clinical decision-making.
We systematically searched PubMed, Embase, Web of Science, and the Cochrane library to select relevant articles up to April 17, 2022. We applied no language restrictions. We used the following combined text and MeSH terms: “ATG,” “anti-thymocyte globulin,” “GVHD,” “graft-versus-host disease,” “PTCy,” “posttransplant cyclophosphamide,” and “allogeneic transplantation.” The complete search used for PubMed is provided in Supplementary Data 1. We considered all potentially eligible studies for analysis, irrespective of the primary outcome or language.
After excluding duplications, two independent investigators reviewed the study titles and abstracts. All studies that satisfied the inclusion criteria were retrieved for full-text assessment. Two investigators analyzed trials selected for detailed analysis and data extraction with an agreement value (κ) of 96.5%; a third investigator resolved disagreements.
The criteria for eligible articles were as follows: (1) original human study; (2) patients diagnosed with hematological malignancies receiving allo-HCT; (3) receive MUD or MMUD; (4) comparison between PTCy and ATG; and (5) at least one of the following outcomes: incidence of grade II-IV and III-IV aGVHD, cGVHD, OS, RI, NRM, CMV reactivation, BKV reactivation, and EBV reactivation.
Criteria for exclusion: (1) duplicate studies, conference reports, letters, or other articles without available full text; (2) cell or animal studies; (3) receive other donor types: haploidentical donor or matched sibling donors; and (4) studies using PTCy and ATG in the same group.
Data extracted from all eligible articles included the first author, year of publication, journal, sample size, number of patients in the PTCy and ATG groups, patient age, patient sex, graft source, disease type, donor type, conditioning regimen, and follow-up time, and all these characteristics are provided in Supplementary Table 1.
In addition, primary clinical outcomes (grade II-IV and III-IV aGVHD, cGVHD, overall survival (OS), relapse incidence (RI), non-relapse mortality (NRM)), and infections (cytomegalovirus (CMV) reactivation and CMV disease, Epstein−Barr virus (EBV)-related posttransplant lymphoproliferative disease (PTLD), BKV-related hemorrhagic cystitis (HC)) were independently extracted from each article for aggregation and analysis. Two authors worked separately on a quality assessment by the Newcastle−Ottawa Scale (NOS) according to the NOS system’s three components (selection, comparability, and outcome). The range of scores is from 0 to 9, and a study with a score of 5 or higher is considered high quality (Supplementary Table 2).
This meta-analysis was performed with Review Manager (RevMan) software version 5.4. A two-sided P value ≤ 0.05 was considered statistically significant. All outcomes were counted as dichotomous variables, and events and total numbers were aggregated to compare the efficacy between the PTCy and ATG groups. This method was also adopted in subgroup analysis. We graphically displayed the results by forest plot. Heterogeneity was checked by the chi-square-based Q test and I2 statistic (I2 > 50%, P ≤ 0.1 indicated high heterogeneity). If heterogeneity was considered not obvious, a fixed-effect model was employed for calculation; otherwise, a random-effect model was adopted.
We identified 1091 articles, 206 from PubMed, 247 from Embase, 595 from Web of Science, and 43 from the Cochrane Library. After eliminating 189 duplications electronically, 902 potentially relevant publications were screened. Of these records, 855 citations were removed by reviewing the titles and abstracts. Full text or further details were retrieved for the remaining 47 citations. Finally, six eligible studies were included in the meta-analysis (12, 15–19). The flow diagram of the search and selection process is shown in Figure 1.
The six studies were published between 2016 and 2021 and were of relatively high quality (NOS scores higher than 5). All were retrospective studies, with a total of 2379 patients, of which 472 received PTCy and 1907 were treated with ATG.
The average age of patients in the six included studies ranged from 31 to 62. The median follow-up time varied substantially between groups, namely, 12 to 26 months in the PTCy group and 17 to 78 months in the ATG group. Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are the most common diseases. Among these six studies, there were three studies whose number of male subjects outweighed the number of female ones, only one study has more female patients, while another two did not mention the female-to-male ratio. The PTCy group had 472 unrelated donors, including 297 (62.9%) matched unrelated donors (MUDs) and 175 (37.1%) mismatched unrelated donors (MMUDs); while the ATG group included 1607 (84.3%) MUDs and 300 (15.7%) MMUDs. Of all the patients, 1093 (45.9%) subjects received myeloablative conditioning (MAC), including 201 (18.4%) in the PTCy group and 892 (81.6%) in the ATG group. The remaining 1286 (54.1%) patients were treated with nonmyeloablative or reduced-intensity conditioning (RIC), with 271 (21.1%) in the PTCy group and 1015 (78.9%) in the ATG group. For the graft source, 43 (9.1%) in the PTCy group were transplanted with bone marrow (BM) and the rest 429 (90.9%) received peripheral blood (PB) stem cells. In the ATG group, the number of patients who chose BM or PB as the graft source was 165 (8.7%) and 1742 (91.3%), respectively.
Six studies with 2379 patients all reported the incidence of grade II-IV aGVHD. We calculated each study’s events and total number as a dichotomous variable. Pooling the data of these studies showed that the PTCy has an advantage over ATG in grade II-IV aGVHD prevention. (RR=0.68, 95% CI 0.50-0.93, P=0.010, I2 = 67%; Figure 2). Considering the apparent heterogeneity, a sensitivity analysis and subgroup analysis were conducted. The studies were divided into two groups based on the sample size. Two studies were included into big sample group while the other four were in a small sample group. Patients in small sample size reported a lower incidence of grade II-IV aGVHD (RR=0.53, 95% CI 0.38-0.74, P=0.23, I2 = 31%; Figure 2) than large sample studies (RR=0.97, 95% CI 0.79-1.20, P=0.86, I2 = 0%; Figure 2), implying that the sample size of investigations might be the source of heterogeneity.
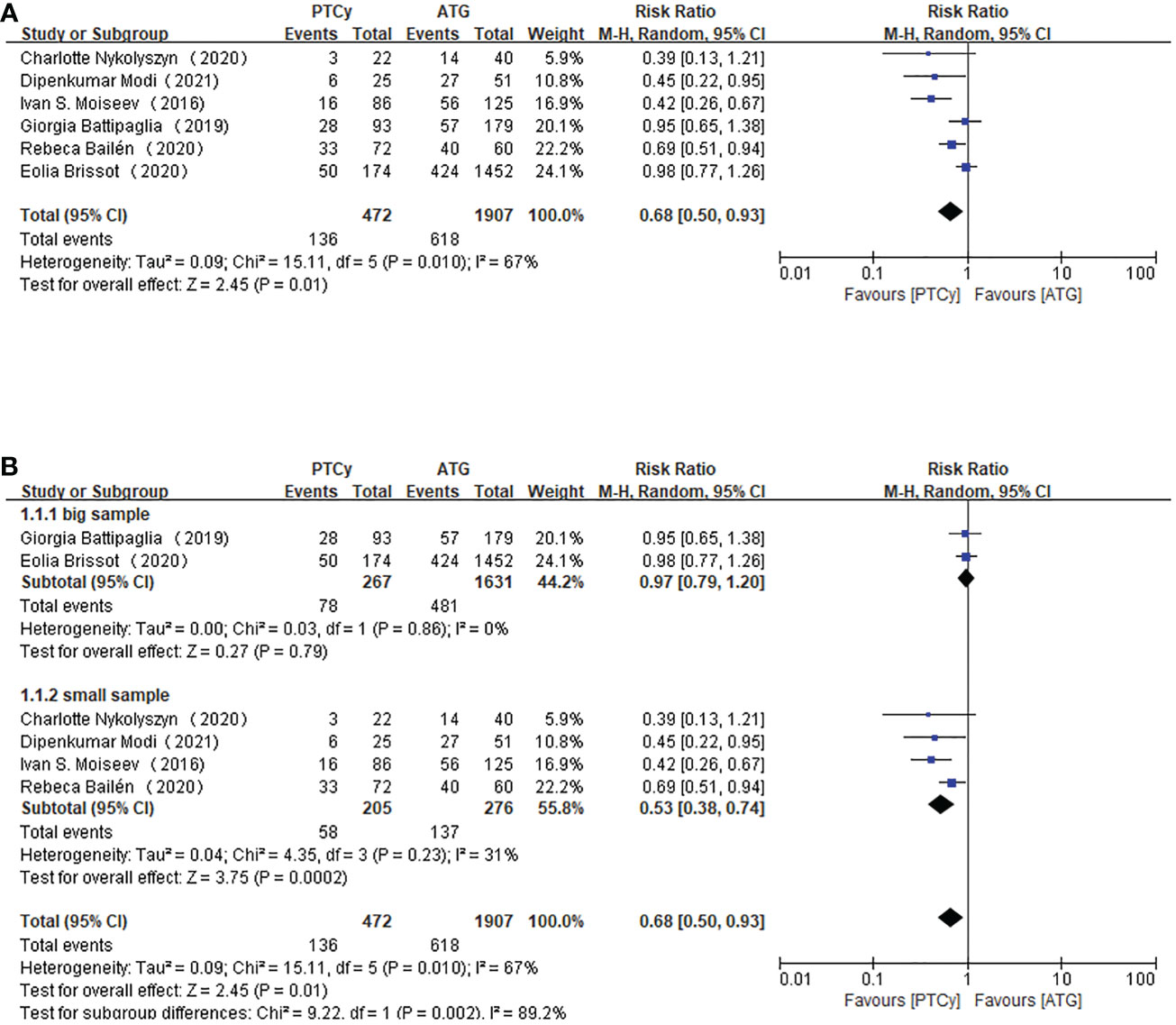
Figure 2 Forest plot of II-IV aGVHD (A) and Subgroup analysis of II-IV aGVHD according to the sample size (B).
Regarding grade III-IV aGVHD, all the included studies reported this outcome. The results demonstrated that prophylaxis based on PTCy was associated with a lower risk of developing grade III-IV aGVHD (RR=0.32, 95% CI 0.14-0.76, P=0.001, I2 = 75%; Figure 3). There was still heterogeneity, which could not be neglected. However, a sensitivity analysis showed that any separate study did not influence the heterogeneity. Therefore, subgroup analysis according to age was carried out. We took age 50 as the categorizing criterion. If the median age of both PTCy and ATG groups were lower than 50, the study would be categorized as younger subgroup; if not, the article would be placed in elder subgroup. The pooled results showed that the PTCy group tended to have a lower risk of III-IV aGVHD in the younger subgroup (RR=0.11, 95% CI 0.04-0.26, P=0.64, I2 = 0%; Figure 3) than elder subgroup (RR=0.64, 95% CI 0.37-1.12, P=0.22, I2 = 33%; Figure 3).
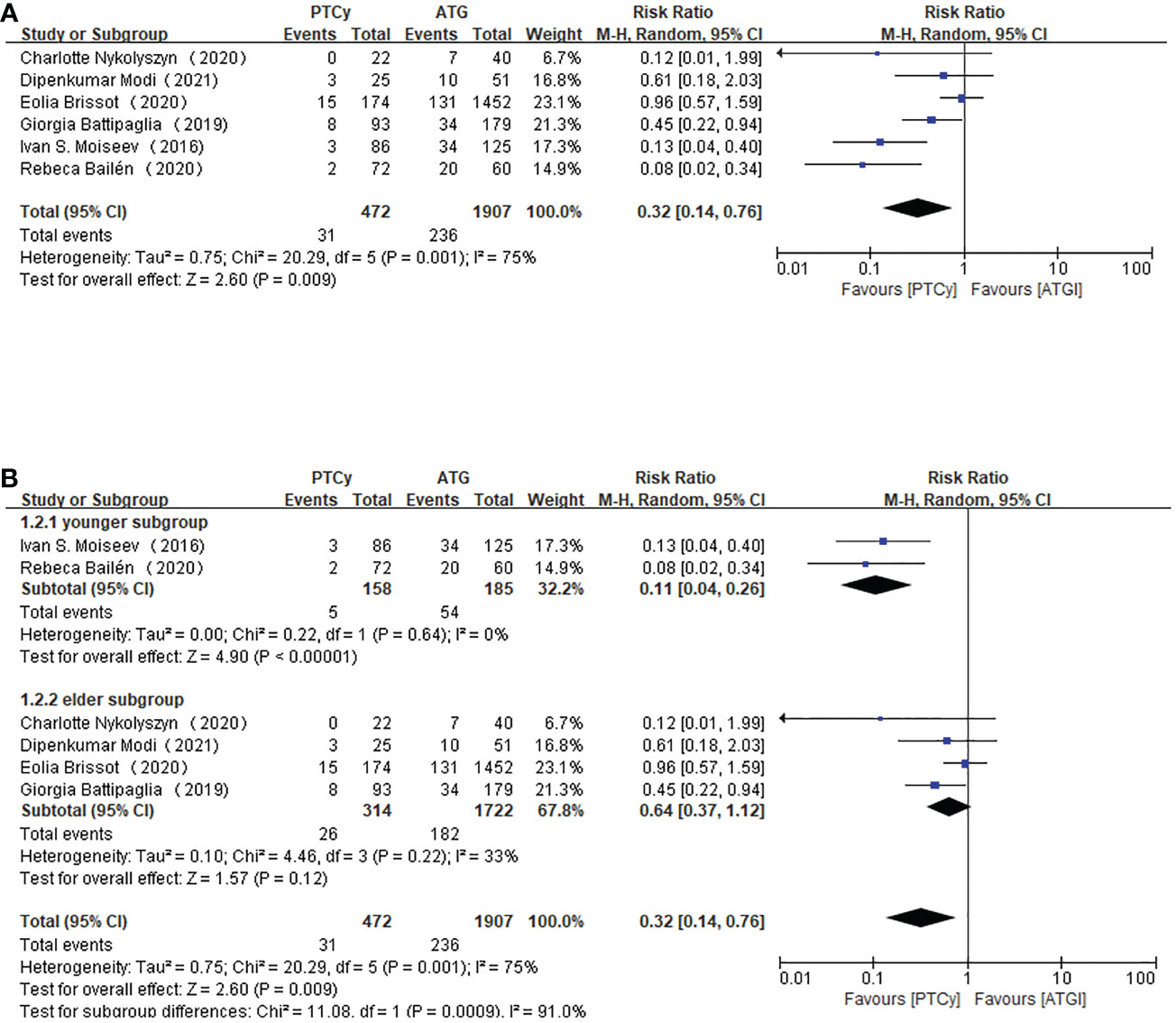
Figure 3 Forest plot of III-IV aGVHD (A) and subgroup analysis of III-IV aGVHD according to the age of the patients (B).
Six studies were available for the analysis for moderate to severe cGVHD. The aggregated results showed no difference between PTCy and ATG regimens in cGVHD (RR=0.66, 95% CI 0.35-1.26, P<0.00001, I2 = 86%; Figure 4). A sensitivity analysis revealed that the obvious heterogeneity did not come from any included article. And subgroup analysis according to median age or the published year could not lower the heterogeneity.
As for OS, according to the pooled RR, the use of PTCy was associated with a higher overall survival (RR=1.29, 95% CI 1.03-1.62, P=0.0001, I2 = 80%; Figure 5). Considering the obvious heterogeneity, a sensitivity analysis was applied to find the source of it. But ruling out any separate study did not significantly influence the result. However, subgroup analysis based on published year found that studies published before 2020 reported higher overall survival in PTCy group (RR=1.59, 95% CI 1.32-1.91, P=0.41, I2 = 0%; Figure 5).
Among the six included studies, five of them reported the outcome of RI, while the rest one did not choose the relapse rate as primary outcome. There was no obvious heterogeneity among the included studies, therefore a fixed-effect model was used. The pooling data of RI showed no difference between the PTCy and ATG groups (RR=0.95, 95% CI 0.78-1.16, P=0.37, I2 = 7%; Figure 6).
For NRM, we gathered data from 2379 patients in all studies and adopted a fixed-effect model, which showed that, compared with ATG group, patients treated with PTCy is associated with lower NRM (RR=0.67, 95% CI 0.53-0.84, P=0.17, I2 = 36%; Figure 6). And the result was statistically significant as well.
The related data on infectious complications can be found in four articles. Four studies reported incidences of CMV reactivation and CMV disease. However, only three of them reported EBV-related PTLD and BKV-related HC. According to the aggregated RR value, we found that there was no significant difference in CMV reactivation and CMV disease between the PTCy group and ATG group (RR=0.89, 95% CI 0.63-1.24, P=0.07, I2 = 57%; Figure 7). Although apparent heterogeneity was observed, we did not conduct any sensitivity analysis or subgroup analysis due to the limited study data.
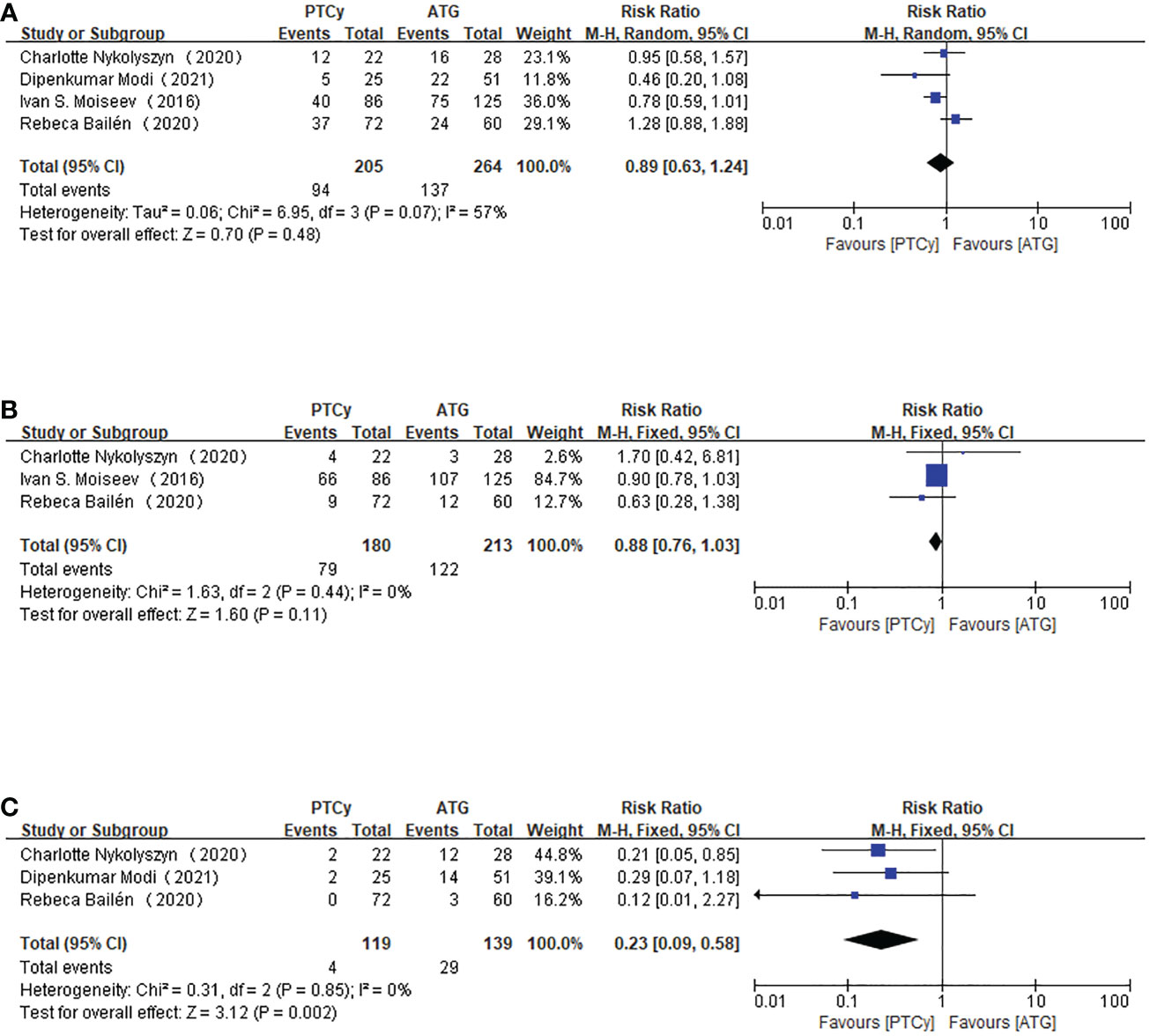
Figure 7 Forest plot of CMV reactivation (CMV disease) (A), BKV-related HC (B) and EBV-related PTLD (C).
For BKV-related HC, we adopted the fixed-effect model because I2<50%. The aggregated RR indicated that PTCy-based protocol and ATG-based prophylaxis were comparable in developing BKV-related HC. The heterogeneity was not significant (RR=0.88, 95% CI 0.76-1.03, P=0.44, I2 = 0%; Figure 7).
EBV-related PTLD was only reported in three articles, and further analysis showed that the heterogeneity was not apparent; therefore, a fixed-effect model was used to assess the outcome. The results indicated that adopting the PTCy regimen was associated with a lower chance of developing EBV-related PTLD disease (RR=0.23, 95% CI 0.09-0.58, P=0.85, I2 = 0%; Figure 7), and the result was statistically significant.
Allo-HSCT is an essential treatment for patients with hematological malignancies. Nevertheless, the incidence of aGVHD remains the major obstacle to the full benefit of the transplant. Regimens based on PTCy or ATG were both used for aGVHD prophylaxis in setting of unrelated donors (9, 20–22). The main objective of this meta-analysis was to compare the two regimens in MUD and MMUD and determine which one provided better outcomes.
The results of this meta-analysis indicated that, compared with ATG, the administration of PTCy reduced the incidence of all grades aGVHD but did not alter the risk of developing cGVHD. This result of aGVHD was in line with many previous results, which showed that PTCy was more favorable for reducing the incidence of both grade II-IV and grade III-IV aGVHD (12, 16, 19, 23). The feasibility and efficacy of PTCy as a single agent for aGVHD prevention have been proven in a body of studies, especially when patients are transplanted with BM cells (9, 11). However, in peripheral blood stem cell (PBSC) graft settings, a single agent of Cy may not be enough to prevent severe aGVHD (24, 25). Considering that most of the patients in this meta-analysis received PTCy-based GVHD prophylaxis in the setting of PBSC and the incidence in aGVHD was acceptable, we speculated that other immunosuppressive agents used simultaneously might play a role. Carnevale-Schianca et al. used a combination of PTCy and tacrolimus-MMF to prevent GVHD in PBSC from HLA-matched donors and found that the incidence of aGVHD was hugely reduced (26). And low-dose ATG/PTCy combined with CsA/MMF as GvHD prophylaxis in MUD-PBSCT had promising activity (27). Additionally, the subgroup analysis in grade III-IV aGVHD based on the age of subjects illustrated that the administration of the PTCy agent might exert a better prophylactic effect in the younger subgroup. This can be easily understood because many adverse events after transplantation can be attributed to the confounding disadvantages of increasing age. The similar incidence of moderate to severe cGVHD in two regimens may be attributed to the cells targeted by ATG, not only donor T cells but also neutrophils, monocytes, NK- and B cells and non-immune cells such as endothelial cells, which are crucial in developing cGVHD (28, 29). However, all the results should be interpreted with caution for the high heterogeneity.
Concerning infections, the lower incidence of EBV-related PTLD and the comparable incidence of CMV reactivation and BKV-related HC are consistent with previous studies. Bailén et al. reported all these infectious complications between the two regimens, and the results demonstrated that the risk of EBV reactivation was higher in the ATG group. In contrast, CMV reactivation and HC were similar between groups (18). Moreover, Walker et al. and Finke et al. also found higher EBV reactivation in the ATG group in unrelated donor setting (20, 30). However, the incidence of BKV-related HC was considered lower in the PTCy group with statistical significance in the study of Ciurea et al., which disagreed with our findings (4). One possible explanation for the similar BKV-related HC in two regimens was that the low dose of ATG in the included studies. A higher dose of ATG is associated with a higher risk of infection or PTLD (20, 31) because ATG may negatively affect survival by increasing infection and relapse rate (18). But that should be interpreted with caution for the dose of ATG in several included studies was not available or not defined, thus limiting the possibility to explore its specific influence on the main transplantation outcomes. Moreover, this finding might also be related to insufficient infection data.
Despite the concern about the high relapse rate with the administration of PTCy in haplo-HSCT for hematologic diseases, which is up to 50% (23, 32). However, in this meta-analysis, no differences were observed between the two groups concerning RI. And PTCy-based prophylaxis even achieves better OS and NRM. The difference in NRM can be partially boiled down to the higher infection rate in ATG group, especially viral infection. At the same time, a matched-pair analysis performed by the ALWP of the EBMT showed that PTCy was associated with better LFS and GVHD-free relapse-free survival (GRFS) in patients undergoing 9/10 UD-HSCT (12). And lower NRM in PTCy group may contribute to improved OS.
Undeniably, there are several unavoidable limitations of this analysis. First, almost all included studies were retrospective studies rather than prospective randomized controlled trials, which may result in irremovable bias. Furthermore, the heterogeneity in several outcomes was notable, probably due to the unmeasured factors caused by the retrospective nature of this analysis. However, sensitivity and subgroup analyses were conducted to identify the potential source of heterogeneity.
Collectively, in the absence of prospective, randomized data, the results of this meta-analysis indicated that compared with ATG, the administration of PTCy demonstrated better prevention for grade II-IV and grade III-IV aGVHD, achieve better OS and NRM, and was associated with a lower incidence of EBV-related PTLD without increasing other risks. Therefore, using PTCy as GvHD prophylaxis may be considered an optimal strategy for the prevention of GVHD in patients undergoing UD-HSCT.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All authors contributed to the study conception and design. JJ had the idea for the article. Literature search and data analysis were performed by LT, ZL, and TOL. TD, QW, TN, and TL critically revised the work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1071268/full#supplementary-material
aGVHD/cGVHD, Acute/chronic graft-versus-host disease; PTCy, posttransplant cyclophosphamide; ATG, anti-thymocyte globulin; OS, overall survival; RI, relapse incidence; NRM, non-relapse mortality; NOS, Newcastle−Ottawa scale; CMV, cytomegalovirus; BKV, BK virus; EBV, Epstein−Barr virus; PTLD, posttransplant lymphoproliferative disease; HC, hemorrhagic cystitis; GVL, graft-versus-leukemia; BM, Bone marrow; PB, Peripheral blood; HID, haploidentical donor; MSD, matched sibling donor; MUD, matched unrelated donor; MRD, matched related donor; LFS, leukemia-free survival; RevMan, Review Manager; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin leukemia; MMUDs, mismatched unrelated donors; UDs, unrelated donors; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; PBSC, peripheral blood stem cell; NK cells, natural killer cells; RR, Risk ratio; CIs, Confidence intervals.
1. Dekker L, Sanders E, Lindemans C, de Koning C, Nierkens S. Naive T cells in graft versus host disease and graft versus leukemia: Innocent or guilty? Front Immunol (2022) 13:893545. doi: 10.3389/fimmu.2022.893545
2. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol (2020) 7(2):e157–e67. doi: 10.1016/s2352-3026(19)30256-x
3. Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient hla matching contributes to the success of unrelated donor marrow transplantation. Blood (2007) 110(13):4576–83. doi: 10.1182/blood-2007-06-097386
4. Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2012) 18(12):1835–44. doi: 10.1016/j.bbmt.2012.07.003
5. Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative hla-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: Effect of hla disparity on outcome. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2010) 16(4):482–9. doi: 10.1016/j.bbmt.2009.11.011
6. Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-Cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood (2011) 117(25):6963–70. doi: 10.1182/blood-2011-01-332007
7. Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-Versus-Host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell depletion trial): A multi-centre, randomised phase ii-iii trial. Lancet (London England) (2005) 366(9487):733–41. doi: 10.1016/s0140-6736(05)66996-6
8. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia (2007) 21(7):1387–94. doi: 10.1038/sj.leu.2404683
9. Luznik L, Bolaños-Meade J, Zahurak M, Chen A, Smith B, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-Versus-Host disease. Blood (2010) 115(16):3224–30. doi: 10.1182/blood-2009-11-251595
10. Sanz J, Galimard J, Labopin M, Afanasyev B, Angelucci E, Ciceri F, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: A comparative study of the alwp ebmt. J Hematol Oncol (2020) 13(1):46. doi: 10.1186/s13045-020-00882-6
11. Kanakry C, O'Donnell P, Furlong T, de Lima M, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-Versus-Host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol (2014) 32(31):3497–505. doi: 10.1200/jco.2013.54.0625
12. Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in hla-mismatched unrelated donor transplantation. Blood (2019) 134(11):892–9. doi: 10.1182/blood.2019000487
13. Mohty R, Brissot E, Battipaglia G, Ruggeri A, Dulery R, Bonnin A, et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br J Haematol (2019) 187(3):e64–e8. doi: 10.1111/bjh.16189
14. Gao F, Zhang J, Hu J, Lin L, Xu Y. Post-transplant cyclophosphamide versus antithymocyte globulin in allogeneic hematopoietic cell transplantation: A meta-analysis. Ann Hematol (2021) 100(2):529–40. doi: 10.1007/s00277-021-04399-x
15. Nykolyszyn C, Granata A, Pagliardini T, Castagna L, Harbi S, Bouabdallah R, et al. Posttransplantation cyclophosphamide vs. antithymocyte globulin as gvhd prophylaxis for mismatched unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant (2020) 55(2):349–55. doi: 10.1038/s41409-019-0682-2
16. Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-Versus-Host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant (2016) 22(6):1037–42. doi: 10.1016/j.bbmt.2016.03.004
17. Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 hla-matched unrelated donors. J Hematol Oncol (2020) 13(1):87. doi: 10.1186/s13045-020-00923-0
18. Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for gvhd prophylaxis compared to atg-based prophylaxis in unrelated donor transplantation. Ann Hematol (2021) 100(2):541–53. doi: 10.1007/s00277-020-04317-7
19. Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant cyclophosphamide versus thymoglobulin in hla-mismatched unrelated donor transplant for acute myelogenous leukemia and myelodysplastic syndrome. Transplant Cell Ther (2021) 27(9):760–7. doi: 10.1016/j.jtct.2021.06.018
20. Finke J, Bethge W, Schmoor C, Ottinger H, Stelljes M, Zander A, et al. Standard graft-Versus-Host disease prophylaxis with or without anti-T-Cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. The Lancet Oncol (2009) 10(9):855–64. doi: 10.1016/s1470-2045(09)70225-6
21. Chang Y, Wu D, Lai Y, Liu Q, Sun Y, Hu J, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: A multicenter, open-label, randomized controlled study. J Clin Oncol (2020) 38(29):3367–76. doi: 10.1200/jco.20.00150
22. Broers A, de Jong C, Bakunina K, Hazenberg M, van Marwijk Kooy M, de Groot M, et al. Posttransplant cyclophosphamide for prevention of graft-Versus-Host disease: Results of the prospective randomized hovon-96 trial. Blood Adv (2022) 6(11):3378–85. doi: 10.1182/bloodadvances.2021005847
23. Ruggeri A, Sun Y, Labopin M, Bacigalupo A, Lorentino F, Arcese W, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin as graft- versus-host disease prophylaxis in haploidentical transplant. Haematologica (2017) 102(2):401–10. doi: 10.3324/haematol.2016.151779
24. Holtick U, Chemnitz J, Shimabukuro-Vornhagen A, Theurich S, Chakupurakal G, Krause A, et al. Octet-cy: A phase ii study to investigate the efficacy of post-transplant cyclophosphamide as sole graft-Versus-Host prophylaxis after allogeneic peripheral blood stem cell transplantation. Eur J Hematol (2016) 96(1):27–35. doi: 10.1111/ejh.12541
25. Mielcarek M, Furlong T, O'Donnell P, Storer B, McCune J, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-Versus-Host disease after hla-matched mobilized blood cell transplantation. Blood (2016) 127(11):1502–8. doi: 10.1182/blood-2015-10-672071
26. Carnevale-Schianca F, Caravelli D, Gallo S, Coha V, D'Ambrosio L, Vassallo E, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination prevents graft-Versus-Host disease in allogeneic peripheral blood hematopoietic cell transplantation from hla-matched donors. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2017) 23(3):459–66. doi: 10.1016/j.bbmt.2016.12.636
27. Sun X, Yang J, Cai Y, Wan L, Huang C, Qiu H, et al. Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamide combined with cyclosporine and mycophenolate mofetil for prevention of graft-Versus-Host disease after hla-matched unrelated donor peripheral blood stem cell transplantation. Bone Marrow Transplant (2021) 56(10):2423–31. doi: 10.1038/s41409-021-01358-y
28. Shimony O, Nagler A, Gellman Y, Refaeli E, Rosenblum N, Eshkar-Sebban L, et al. Anti-T lymphocyte globulin (Atg) induces generation of regulatory T cells, at least part of them express activated Cd44. J Clin Immunol (2012) 32(1):173–88. doi: 10.1007/s10875-011-9599-2
29. Fang L, Fehse B, Engel M, Zander A, Kröger NJT. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation (2005) 79(3):369–71. doi: 10.1097/01.tp.0000150210.77543.1b
30. Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol (2016) 17(2):164–73. doi: 10.1016/s1470-2045(15)00462-3
31. Wang Y, Fu H, Liu D, Xu L, Zhang X, Chang Y, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: A randomized trial. Bone Marrow Transplant (2014) 49(3):426–33. doi: 10.1038/bmt.2013.191
Keywords: post-transplant cyclophosphamide, allogeneic hematopoietic stem cell transplantation, graft-versus-host disease, infectious complication, anti-thymocyte globulin, unrelated donors
Citation: Tang L, Liu Z, Li T, Dong T, Wu Q, Niu T, Liu T and Ji J (2023) Post-transplant cyclophosphamide versus anti-thymocyte globulin in allogeneic hematopoietic stem cell transplantation from unrelated donors: A systematic review and meta-analysis. Front. Oncol. 13:1071268. doi: 10.3389/fonc.2023.1071268
Received: 16 October 2022; Accepted: 01 February 2023;
Published: 16 February 2023.
Edited by:
Jean El Cheikh, American University of Beirut Medical Center, LebanonReviewed by:
Rémy Dulery, Hôpital Saint-Antoine, FranceCopyright © 2023 Tang, Liu, Li, Dong, Wu, Niu, Liu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Ji, amllamlAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.