
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 February 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1068469
This article is part of the Research Topic Advances in Molecular Biology Knowledge of Rectal Cancer and Forthcoming Role of Liquid Biopsy View all 10 articles
 Jia Jiang1†
Jia Jiang1† Anjie Li2†
Anjie Li2† Xiaolian Lai3†
Xiaolian Lai3† Hanqun Zhang4†
Hanqun Zhang4† Chonghong Wang3
Chonghong Wang3 Huimin Wang3
Huimin Wang3 Libo Li4
Libo Li4 Yuncong Liu4
Yuncong Liu4 Lu Xie1
Lu Xie1 Can Yang1
Can Yang1 Cui Zhang5
Cui Zhang5 Shuoyan Lu3*
Shuoyan Lu3* Yong Li4*
Yong Li4*Colorectal cancer is a common malignancy, and the incidence and mortality rates continue to rise. An important factor in the emergence of inflammation-induced colorectal carcinogenesis is elevated cyclooxygenase-2. Prostaglandin E2 (PGE2) over-production is frequently equated with cyclooxygenase-2 gene over-expression. PGE2 can be assessed by measuring the level of prostaglandin’s main metabolite, PGE-M, in urine. Colorectal adenoma is a precancerous lesion that can lead to colorectal cancer. We conducted research to evaluate the association between urinary levels of the PGE-M and the risk of colorectal adenomas. In a western Chinese population, we identified 152 cases of adenoma and 152 controls patients without polyps. Adenoma cases were categorized into control, low-risk and high-risk groups. There was no significant change in PGE-M levels, between the control group and the low-risk adenoma group. In the high-risk group, the PGE-M levels were 23% higher than the control group. When compared to people with the lowest urine PGE-M levels (first quartile), people with greater urinary PGE-M levels had a higher chance of developing high-risk colorectal adenomas, with an adjusted odds ratio (95% CI) of 1.65 (0.76-3.57) in the fourth quartile group, (p= 0.013). We conclude urinary PGE-M is associated with the risk of developing high-risk adenomas. Urinary PGE-M level may be used as a non-invasive indicator for estimating cancer risk.
Colorectal cancer incidence around the world has increased in tandem with an increase in respective Human Development Indices. This disease has the third highest incidence rate and the second highest fatality rate, globally (1).Similarly, colorectal cancer is becoming more common in China (2). Numerous investigations on the adenoma-carcinoma sequence have conclusively shown that between 60% and 90% of sporadic colorectal cancers result from adenomas that have undergone malignant transformation (3, 4). Therefore, the risk of cancer can be decreased by identifying risk factors for adenomas and preventing their growth (5).
Increasing evidence points to the possibility that inflammation increases the vulnerability of developing colorectal malignancies (6, 7). The enzyme cyclooxygenase-2 (COX-2), mediates the relationship between cancer and inflammation and is 50-85% more abundant in patients with colorectal malignancies (8, 9). COX-2 is a key rate-limiting enzyme for the conversion of arachidonic acid to prostaglandins (PG), and when COX-2 gene expression is elevated, more prostaglandin E2(PGE2) is produced (10, 11). Clinical studies have demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) lower the chance of developing adenoma and colorectal tumors, and their effects are linked to the suppression of prostaglandin 2 and cyclooxygenase-2 (12–15). The pro-inflammatory mediator PGE2 has been shown to be able to support colorectal tumor progression through a variety of mechanisms. PGE2’s primary effects include inhibiting apoptosis, promoting angiogenesis, and encouraging epithelial cell proliferation、 survival、 migration、invasion、repair and regeneration (16–20).It is the most abundant prostaglandin found in colorectal cancer patients (21).
Multiple lines of research indicate that COX-2-derived PGE2 has a role in the growth of colorectal adenomas and can predict the risk of developing colorectal cancer (22). However, epidemiological evidence directly linking urinary PGE2 levels to the risk of colorectal adenoma is lacking in China. Direct measurement of unstable PGE2, however, is unreliable. Currently the best method for measuring systemic PGE2 synthesis in vivo to assess the quick metabolism of PGE2 by 15-hydroxyprostaglandin oxidase to form stable 11 alpha-hydroxy-9,15dioxo-2,3,4,5-tetranorprostane-1,20-dioicacid (PGE-M) (23). A nested study design has shown a connection between Chinese women’s urinary PGE-M levels and their chance of developing colorectal carcinomatosis (24). By examining samples and data gathered from colorectal adenoma patients and a healthy control population in Guizhou Provence, the present study evaluates the relationship between urinary PGE-M levels and the risk of colorectal adenoma in a in western China population. This study also aims to provide new strategies and tools for implementing interventions in the early stages of tumors development.
Study participants were chosen from the Guizhou Cancer Center, Guizhou Provincial People’s Hospital, and Songtao Miao Autonomous County People’s Hospital in Guizhou province to screen for colorectal adenomas. Patients were initially seen at the endoscopy centers of the aforementioned hospitals. Participants were 18-75 years of age and in generally good health with no vital organ failures. According to WHO guidelines, a colorectal adenoma diagnosis was made, and the degree of neoplasia was assessed. The following criteria was used to diagnosis colorectal adenoma: ①Adenoma was verified by a pathological biopsy, ② villous adenoma or mixed adenoma with more than 25% villous-like features was identified, ③high-grade epithelial neoplasia was identified. Patients with colorectal adenoma who met the aforementioned diagnostic standards had a subsequent colonoscopy adenectomy, and histology was used to confirm the diagnosis. All specimens were examined by two or more experienced pathologists in the hospital’s pathology department. Exclusion criteria for our study included those with a history of familial adenomatous polyposis (FAP), inflammatory bowel disease, hereditary non-polyposis colorectal cancer (HNPCC), Turcot syndrome, severe cardiovascular disease, recurrent adenoma with a confirmed diagnosis, colorectal cancer, and tumors in other organs. Because NSAIDs affect PGE-M levels, subjects had used aspirin or any NSAID for at least 48 hours prior to colonoscopy. They were ineligible for analysis. Our study also excluded participants. Because they used any dose of NSAIDs, including aspirin, for 3 days or more in the 3 months prior to enrollment or 3 days per month or used NSAIDs for 36 days in the past year. Finally, a sample of 304 participants’ data was kept for analysis. The study protocol was approved by the Ethics Committee of Guizhou Provincial People’s Hospital. A signed informed consent was required for all study subjects.
There were 412 study participants who provided urine samples; however only 304 samples were usable, due to sample damage and inconvenience of follow-up during the novel coronavirus pandemic. There were 152 cases identified as negative controls. This meant they did not have any polyps at the endoscopic screening. Patients with single tubular adenomas with a maximum diameter of less than 1 cm were categorized as low-risk cases, whereas those with a maximum diameter of more than 1 cm and/or histology of tubular villi, villi, and any multiple adenomas were categorized as high-risk cases (22, 25, 26). Table 1 displays the characteristics of the three categories. To stop the oxidation of unstable metabolites, urine samples were taken in sterile cups containing 125 mg of ascorbic acid. Following collection, samples were kept chilled (at about 0 to 4°C) in a portable foam box with an ice pack before being processed within 6 hours for long-term storage at -80 ± 5°C. Each participant had a biospecimen collection form filled out at the time of sample collection, which listed the day and time of sample collection as well as any drug usage within the previous 48 hours of the colonoscopy.
We developed the questionnaire for this study based on the food frequency questionnaire (SQFFQ) used in the 2002 Chinese population dietary survey methodology, and made appropriate adjustments to incorporate the regional dietary habits of Guizhou. All participants completed the questionnaire at the time of study enrollment. The first section evaluates the annual average food consumption, including average intake, frequency of consumption, etc.; the second section tracks nutrient supplement usage, including the name of the supplements, dose, and regimen. The Chinese Food Composition Table (2nd edition) and Nutrition Calculator V2.7.3 were used to convert nutrient intakes, to evaluate the overall amount of calcium consumed through diet and dietary supplements. Information on medical history, drug usage, demographics, anthropometrics, daily food habits, physical activity, and other lifestyle factors were also included as added content to this questionnaire.
PGE-M was measured using liquid chromatography/tandem mass spectrometry to determine the endogenous production of PGE2 in humans (27). Briefly, urine was placed in a 10 mL polypropylene tube at room temperature, and then a sample of 1.0 mL was acidified to pH=3 by adding 1.0 mol/L HCl. Next, endogenous PGE-M was converted to methoxime derivatives and treated with 1600 mg of methoxamine hydrochloride in 10 mL of 1.5 mol/L sodium acetate solution (pH=5). The methoxylated PGE-M was dissolved in 8 ml of water after 1 hour of greenhouse incubation, and the aqueous sample was then transferred to C-18 Sep-Pak that had been prepared with 5 ml of methanol and 5 ml of water (pH 3). Sep-Pak was then eluted with ethyl acetate. Thermo SCIENTIFIC Hypersil GOLD (1.9 µm, 2.1×50 mm) column linked to a TSQ-Altis, and Thermo Fisher mass spectrometry pump was then used for liquid chromatography. Heated electrospray ion source was used as the ionization technique. The mass to charge ratios (m/z) monitored were 385.3 ~ 336 and m/z 385.3 ~ 367 for endogenous PGE-M, in the selected response monitoring (SRM) mode. The ratio of the mass spectral peak regions of the m/z 336 and m/z 367 ions was used to calculate the amount of endogenous PGE-M. With a coefficient of variation of 4.1% between batches and 8.7% within batches, the lower limit of detection for PGE-M was set at 2.00 ng/ml. There were no incidents that compromised data integrity or quality throughout the experiment. The quality control samples’ identities and the status of the urine samples used in the study were both unknown to the laboratory staff. Additionally, Urinary creatinine was measured using a Sigma kit (Sigma Co., Inc., St. Louis, MO, USA). The levels of urinary creatinine were determined and reported as standardized PGE-M values, PGE-M (ng)/creatinine (mg).
Selected baseline characteristics for cases and controls were computed as means, standard deviations, and percentages. We compared the means of age, body mass index (BMI), and calcium intake data between case and control participants using analysis of variance. To compare categorical variables, we employed the chi-square test. Urinary PGE-M levels for each sample were normalized using the urinary creatinine level of the sample and expressed as ng/ml creatinine. The PGE-M data in urine were skewed to the right; therefore, the median, interquartile range, and geometric mean were estimated for descriptive statistics. After adjusting for age, sex, smoking status, alcohol use, education, and prior hypertensive diabetes mellitus, Wilcoxon rank-sum tests and log-transformed linear regression models were used to analyze differences in PGE-M levels between groups. The PGE-M concentrations in the control group’s quartile distribution served as the basis for establishing cut points for categorical variables. The odds ratio (OR) and 95% confidence interval (95% CI) between urine PGE-M levels and the risk of colorectal adenoma were calculated using logistic regression models. Trend p-values were derived by using categorical variables as continuous parameters of the model and passing the linear trend test. We also stratified associations by subgroups, such as BMI, sex and calcium intake level, in order to focus on these factors’ influence on the association of urinary PGE-M levels with the incidence of high-risk colorectal adenomas. We performed all analyses using SPSS 26.0 (SPSS Inc, Chicago, IL, USA), and P values ≤0.05 (two-sided probability) were interpreted as statistically significant for all analyses.
In this investigation, 304 patient urine samples were examined. We found that the sample group had 153 controls, 59 low-risk, and 93 high-risk cases. Table 1 displays the characteristics of the cases and the controls. The high-risk adenoma group had a greater prevalence of diabetes mellitus, hypertension, and a higher BMI when compared to the control group. Low-risk adenomas were more prevalent in people with lower education levels and low calcium intake. In contrast to controls, patients with adenomas were more likely to be male, smokers, and drinkers, although the difference was not statistically significant.
Table 2 shows the baseline urinary PGE-M levels. Urinary PGE-M levels in the low-risk adenoma patients’ group did not differ statistically from those in the control group. However, urinary PGE-M levels were higher in individuals with high-risk adenomas. patients with high-risk adenomas had urinary PGE-M levels 7% and 23.56% higher than patients with low-risk adenomas or control group patients, respectively (p=0.04).
The Spearman correlation coefficients between urine PGE-M level and several lifestyle factors are shown in Table 3. The results showed a direct correlation between PGE-M and age, gender, and smoking status. We further analyzed urinary PGE-M levels and the risk of developing colorectal adenomas (Table 4). High-risk adenomas were more likely to occur in patients with higher urine PGE-M concentrations (p =0.013-0.016). The highest, fourth quartile, urine PGE-M levels were associated with a 1.65-fold higher incidence of high-risk colorectal adenoma compared to the lowest urinary PGE-M levels. Results did not indicate an association between urine PGE-M levels and an increased incidence of low-risk adenomas.
Urinary PGE-M levels were tested in relation to the incidence of high-risk colorectal adenoma, stratified by BMI, gender, and calcium intake (Table 5). Within the highest PGE level, fourth quartile, PGE level subgroup, women had a stronger association with the incidence of high-risk colorectal adenomas (adjusted OR, 3.72, 95% confidence interval, 0.54-25.45) than did males (adjusted OR, 1.75; 95% confidence range, 0.66-4.61). Although not statistically significant, the patient subgroup with BMI ≥25 (kg/m2) had OR changing from 1.0 to 3.46, to 1.50, to 2.30 for each increasing PGE-M quartiles respectively. Body weight may modify the association between urinary PGE-M and incidence of high-risk colorectal adenoma. The present study did not find a significant interaction between BMI, gender, calcium intake and PGE-M in the high-risk adenoma group (P for interaction >0.05).
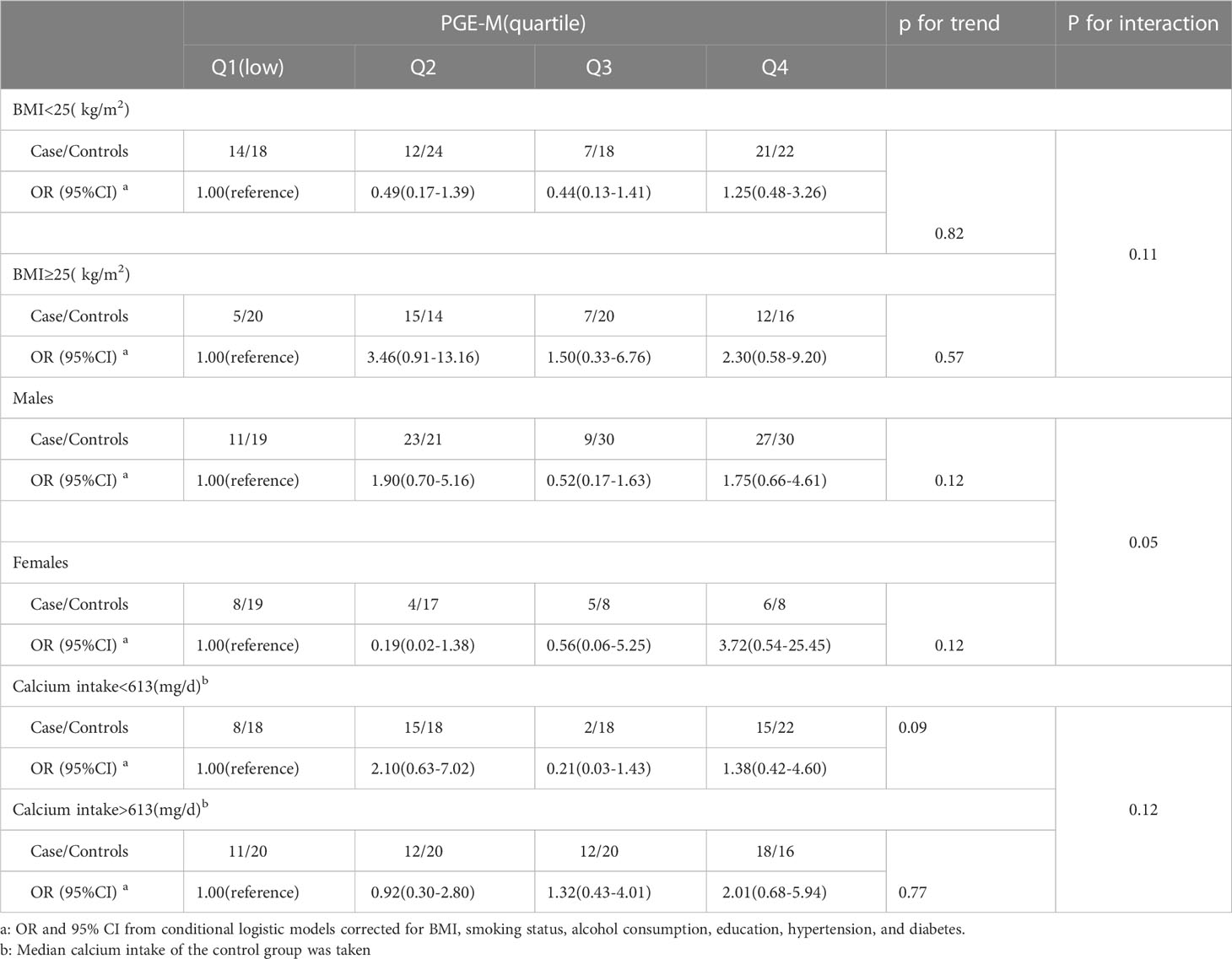
Table 5 Association of urinary PGE-M levels with incidence of high-risk colorectal adenoma stratified by BMI, gender, and calcium intake.
In the current investigation, we discovered a positive correlation between high urine PGE-M levels and the incidence of high-risk colorectal adenomas, but no such correlation was found for single tubular adenomas smaller than 1 cm. These findings imply that urine PGE-M level may be a helpful non-invasive indicator of adenoma risk. Additionally, we discovered that individuals with above-average or low body weight had a strong correlation with PGE-M.
Numerous pieces of evidence point to colorectal adenoma as a prominent precancerous condition of the colorectum. Colorectal adenoma has a complicated etiology, involving a number of biological pathways, one of which has been demonstrated to be COX-2 (8, 9). Arachidonic acid is one of the fatty acid substrates that are converted by COX-2, an inducible isoform of COX, into pro-inflammatory prostaglandins (7). PGE2 is a crucial mediator of the proto-oncogenic actions of COX (28).Therefore, COX-2 inhibitor use lowers urine PGE2 levels, which lowers the incidence of colorectal cancers and adenomas (12, 13). The primary urinary PGE2 metabolite is PGE-M. Additionally, earlier research by others has demonstrated an association between the down-regulation of 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) expression and activity in colorectal cancer and the generation of the urinary PGE2 metabolite PGE-M (29–31).
It was found in a prospective trial of Chinese women that baseline urine PGE-M was most likely a urinary marker for early prediction of CRC and was linked to a high likelihood of advanced colorectal cancer diagnosis (24). In several earlier investigations, it was discovered that patients with advanced adenomas and numerous tubular adenomas had much greater PGE-M levels than the controls group patients (26, 32, 33). Therefore, based on the quantity, size, and complexity of the adenomas, we categorized the cases in this study into high-risk, low-risk and control groups. Our results confirmed this association between PGE-M and high-risk adenomas. We observed higher levels of urinary PGE-M in the western Chinese population in the high-risk of adenoma group, compared to the low-risk group. Calcium may control the inflammatory response affecting colorectal adenomas by affecting a variety of mechanisms including bile acid catabolism, immune regulation, and fatty acid metabolism (34). The present study also considered the influence of calcium intake. However, only the potential effect of calcium intake on correlation among US registered female nurses was assessed in the relevant study (p>0.05) (22). Our results are in line with those of earlier research, although the associations between PGE-M level and the low and high-risk groups was not significantly changed by calcium intake levels. This is the first epidemiological study of calcium intake levels, urinary PGE-M levels, and risk of colorectal adenoma in western China. Currently, calcium is still one of the most deficient nutrients among Chinese residents, and this situation may be even more serious in western China (35). We hypothesize that high levels of PGE2 may be determined by a combination of calcium deficiency and individual genetic susceptibility. Therefore, further studies are needed to explore and reveal the mechanisms and significance of calcium intake levels related to PGE2 and colorectal adenoma, and identify key genes for calcium re-absorption.
In this study, we had several advantages. These included the random recruitment of participants in a large sample database at the Guizhou Cancer Center. This allowed us to collect patient urine samples before diagnosis and avoid selectivity bias. All participants received an endoscopy, and all adenomas excised from the case group underwent a pathological evaluation, which contributed to the accuracy of the groups. The Tennessee Colorectal Polyp Study in the United States has conducted a number of studies on PGE-M and the risk of acquiring colorectal adenomas (26, 32). To further these earlier findings, the present study was carried out in a population in western China. For the first time in China, we used liquid chromatography/tandem mass spectrometry to detect PGE-M levels. It must be acknowledged that our study also has many limitations. For example, our sample size was constrained after relevant cases were excluded due to individual differences or other factors like the use of NSAIDs. Second, the long-term association between urinary PGE-M levels and the risk of developing colorectal adenoma needs to be further studied because, further studied only measured the PGE-M levels from urine samples at one time point. Although we studied calcium intake level and PGE-M levels’ influence on the incidence of colorectal adenoma, we could not assess how calcium deficiency alters urinary PGE-M level and colorectal adenoma incidence. A more comprehensive understanding of colorectal adenoma development at the molecular and genetic level is needed.
In conclusion, this study evaluated the association of urinary PGE-M levels with increased incidence of high-risk colorectal adenomas in a western Chinese population. Because high-risk adenomas have the greatest likelihood of malignant development, it may be possible to intervene early and prevent cancer by using urine PGE-M levels as a non-invasive indicator for estimating cancer risk.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guizhou Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
(I) Conception and design: YoL, SL. (II) Administrative support: YoL, AL, HZ. (III) Provision of study materials or patients: HW, CW, LL. (IV) Collection and assembly of data: XL, JJ, YuL. (V) Data analysis and interpretation: XL, JJ, XL, CY, CZ. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
This project is supported by 1) the National Natural Science Foundation of China (Grant No.: 81960587); 2) Science and Technology Program of Guizhou Province (Contract No.: Guizhou Kehe Support [2021] General 067)
The authors acknowledge the support of the Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
4. Vacante M, Ciuni R, Basile F, Biondi A. Gut microbiota and colorectal cancer development: A closer look to the adenoma-carcinoma sequence. Biomedicines (2020) 8(11):489. doi: 10.3390/biomedicines8110489
5. Roncucci L, Mariani F. Prevention of colorectal cancer: How many tools do we have in our basket? Eur J Internal Med (2015) 26(10):752–6. doi: 10.1016/j.ejim.2015.08.019
6. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
7. Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA: Cancer J Clin (2006) 56(2):69–83. doi: 10.3322/canjclin.56.2.69
8. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology (1994) 107(4):1183–8. doi: 10.1016/0016-5085(94)90246-1
9. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer (2001) 1(1):11–21. doi: 10.1038/35094017
10. Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer metastasis Rev (2007) 26(3-4):525–34. doi: 10.1007/s10555-007-9096-5
11. Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer metastasis Rev (2011) 30(3-4):277–94. doi: 10.1007/s10555-011-9310-3
12. Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New Engl J Med (2000) 342(26):1946–52. doi: 10.1056/nejm200006293422603
13. Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. New Engl J Med (2003) 348(10):891–9. doi: 10.1056/NEJMoa021735
14. Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet (London England) (2007) 369(9573):1603–13. doi: 10.1016/s0140-6736(07)60747-8
15. Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology (2020) 158(2):368–88. doi: 10.1053/j.gastro.2019.06.047
16. Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res (1998) 58(2):362–6.
17. Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem (2001) 276(21):18075–81. doi: 10.1074/jbc.M009689200
18. Salcedo R, Zhang X, Young HA, Michael N, Wasserman K, Ma WH, et al. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood (2003) 102(6):1966–77. doi: 10.1182/blood-2002-11-3400
19. Gustafsson A, Andersson M, Lagerstedt K, Lönnroth C, Nordgren S, Lundholm K. Receptor and enzyme expression for prostanoid metabolism in colorectal cancer related to tumor tissue PGE2. Int J Oncol (2010) 36(2):469–78. doi: 10.3892/ijo_00000521
20. Jara-Gutiérrez Á., Baladrón V. The role of prostaglandins in different types of cancer. Cells (2021) 10(6):1487. doi: 10.3390/cells10061487
21. Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology (2015) 149(7):1884–1895.e1884. doi: 10.1053/j.gastro.2015.07.064
22. Bezawada N, Song M, Wu K, Mehta RS, Milne GL, Ogino S, et al. Urinary PGE-m levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory drugs. Cancer Prev Res (Philadelphia Pa) (2014) 7(7):758–65. doi: 10.1158/1940-6207.Capr-14-0120
23. Wang D, DuBois RN. Urinary PGE-m: A promising cancer biomarker. Cancer Prev Res (Philadelphia Pa) (2013) 6(6):507–10. doi: 10.1158/1940-6207.Capr-13-0153
24. Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, et al. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol Off J Am Soc Clin Oncol (2006) 24(31):5010–6. doi: 10.1200/jco.2006.06.4931
25. Johnson JC, Schmidt CR, Shrubsole MJ, Billheimer DD, Joshi PR, Morrow JD, et al. Urine PGE-m: A metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol (2006) 4(11):1358–65. doi: 10.1016/j.cgh.2006.07.015
26. Bezawada N, Song M, Wu K, Mehta RS, Milne GL, Ogino S, et al. Urinary PGE-m levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory DrugsPGE-m and risk of colorectal adenoma. Cancer Prev Res (Phila). (2014) 7(7):758–65. doi: 10.1158/1940-6207.CAPR-14-0120
27. Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: Determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Analytical Biochem (2004) 334(2):266–75. doi: 10.1016/j.ab.2004.08.019
28. Cha YI, DuBois RN. NSAIDs and cancer prevention: Targets downstream of COX-2. Annu Rev Med (2007) 58:239–52. doi: 10.1146/annurev.med.57.121304.131253
29. Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem (2005) 280(5):3217–23. doi: 10.1074/jbc.M411221200
30. Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis, proceedings of the national academy of sciences of the united states of America. Proc Natl Acad Sci U S A. (2006) 103(32):12098–102. doi: 10.1073/pnas.0603235103
31. Fink SP, Yamauchi M, Nishihara R, Jung S, Kuchiba A, Wu K, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Trans Med (2014) 6(233):233re232. doi: 10.1126/scitranslmed.3008481
32. Shrubsole MJ, Cai Q, Wen W, Milne G, Smalley WE, Chen Z, et al. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prev Res (Philadelphia Pa) (2012) 5(2):336–42. doi: 10.1158/1940-6207.Capr-11-0426
33. Davenport JR, Cai Q, Ness RM, Milne G, Zhao Z, Smalley WE, et al. Evaluation of pro-inflammatory markers plasma c-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Mol carcinogenesis (2016) 55(8):1251–61. doi: 10.1002/mc.22367
34. Gibbs DC, Fedirko V, Baron JA, Barry EL, Flanders WD, McCullough ML, et al. Inflammation modulation by vitamin d and calcium in the morphologically normal colorectal mucosa of patients with colorectal adenoma in a clinical trial. Cancer Prev Res (Philadelphia Pa) (2021) 14(1):65–76. doi: 10.1158/1940-6207.Capr-20-0140
Keywords: colorectal adenoma, prostaglandin E2, PGE-M, Colorectal cancer, cancer risk, bio-markers
Citation: Jiang J, Li A, Lai X, Zhang H, Wang C, Wang H, Li L, Liu Y, Xie L, Yang C, Zhang C, Lu S and Li Y (2023) Correlation between Metabolite of Prostaglandin E2 and the incidence of colorectal adenomas. Front. Oncol. 13:1068469. doi: 10.3389/fonc.2023.1068469
Received: 13 October 2022; Accepted: 13 February 2023;
Published: 27 February 2023.
Edited by:
Francesca Negri, University Hospital of Parma, ItalyReviewed by:
Soumya Basu, Dr. D. Y. Patil Biotechnology & Bioinformatics Institute, IndiaCopyright © 2023 Jiang, Li, Lai, Zhang, Wang, Wang, Li, Liu, Xie, Yang, Zhang, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, bGl5b25nNzIyOTc3MUAxNjMuY29t; Shuoyan Lu, MjM1MjI5MDZAcXEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.