
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 03 March 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1067289
This article is part of the Research TopicAdvances in the Treatment of Hodgkin LymphomaView all 6 articles
Hodgkin lymphoma (HL) is a rare type of lymphoma with unique histologic, immunophenotypic, and clinical features. It represents approximately one-tenth of lymphomas diagnosed in the United States and consists of two subtypes: classical Hodgkin’s lymphoma (cHL), which accounts for majority of HL cases, and nodular lymphocyte predominant Hodgkin lymphoma represent approximately 5% of Hodgkin lymphoma cases. From this point, we will be focusing on cHL in this review. In general, it is considered a highly curable disease with first-line chemotherapy with or without the addition of radiotherapy. However, there are patients with disease that relapses or fails to respond to frontline regimens and the standard treatment modality for chemo sensitive cHL is high dose chemotherapy followed by autologous hematopoietic stem cell transplant (AHSCT). In recent years, targeted immunotherapy has revolutionized the treatment of cHL while many novel agents are being explored in addition to chimeric antigen receptor (CAR) T-cell therapy which is also being investigated in clinical trials as a potential treatment option.
Hodgkin lymphoma is derived primarily from B-cell lineage which consists of two subtypes, cHL and nodular lymphocyte predominant Hodgkin lymphoma. HL is a rare type of lymphoma and represents approximately 10% of the lymphomas in the UnitedStates with cHL accounting for nearly 95% of all HL cases (1), which is divided into nodular sclerosis, mixed cellularity, lymphocyte deplete variant, and lymphocyte rich variant (2). Reed-Sternberg cells are the pathognomonic malignant cell associated with cHL and drives continuous cell proliferation via NF-kB transcription factor expression (3, 4). cHL has a bimodal age distribution with a first peak around the age of 20-30 years and the second peak around the age 50-70 years which is more often associated with Epstein-Barr virus, and can occasionally occur in patients aged ≥75 years (5, 6). While cHL is considered highly curable with combination chemotherapy with or without the addition of radiotherapy (7), there are a small proportion of patients who do not respond or relapse after treatment with these therapies, and often high dose chemotherapy (HDC) and AHSCT can be curative in the 2nd line setting (8–11).
For a select group of patients who have disease relapse after AHSCT or disease that is not chemo sensitive, allogeneic HSCT can provide a curative therapeutic option and the use of lower doses of chemo- or radiotherapy (reduced intensity conditioning, RIC) has significantly reduced toxicity while maintaining good outcomes (12–16). Prior to transplant, the goal of reducing disease burden is achieved with salvage chemotherapy, radiotherapy, or targeted agents (such as brentuximab vedotin or checkpoint inhibitors) and more frequently with a combination of chemotherapy agents or chemotherapy and immunotherapy. There are no randomized trials that directly compare salvage chemotherapy for relapsed HL. Response rates for salvage regimens in various phase II studies ranged from 60 to 85 percent (17). In this review, we will discuss the available novel therapeutic options and their efficacy and safety in the frontline and relapsed setting.
The therapeutic approach to cHL depends on stage at presentation, clinical prognostic factors and comorbidities. Staging is assessed by the Ann Arbor staging system (18). Treatment for early-stage (I-IIA) HL initially consisted of extended field radiation as the standard therapy. In the modern era, due to high relapse rates and significant long-term complications, extended field radiation therapy is no longer used (19). The current standard treatment for early-stage disease is either combination chemotherapy and involved-field radiation therapy (IFRT) or combination chemotherapy alone. The most widely used first-line chemotherapy regimen for cHL is a combination of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (20). Moreover, Meyer et al. randomized 405 patients with early-stage disease to ABVD alone or subtotal nodal radiation therapy, with or without ABVD therapy (21). Patients who received subtotal nodal radiation therapy had a poorer overall survival (OS) (87% vs 94%), and on long term follow-up, higher rates of death from causes other than HL. A subsequent study randomized 1395 patients with unfavorable disease (large mediastinal masses, extra nodal disease, high erythrocytes sedimentation rate or ≥3 nodal sites) to ABVD for four cycles or standard doses of BEACOPP (bleomycin-etoposide-doxorubicin-cyclophosphamide-vincristine-procarbazine-prednisone) for four cycles, plus either 20 or 30 Gy IFRT (22). Treatment with ABVD plus 30 Gy was superior compared with 20 Gy, however, similar outcomes were seen between 20 and 30 Gy when used with BEACOPP. The German Hodgkin Study Group (GHSG) HD14 trial analyzed 1528 patients to ABVD for four cycles or escalated doses of BEACOPP for two cycles followed by ABVD for two cycles (2 + 2). All patients received 30 Gy IFRT. Freedom from treatment failure was superior with 2 + 2 regimen with a difference of 7.2% at 5 years, however, more acute toxicities were associated with this regimen (23). Based on multiple trials, early-stage favorable disease is generally treated with two cycles of ABVD followed by 20 Gy IFRT (24).
In patients with advanced stage (IIB-IV) disease, the MOPP regimen (nitrogen mustard, vincristine, procarbazine, and prednisone) was initially utilized for patients who progressed after radiation therapy and demonstrated an OS of 48% at 20 years (25). To improve efficacy and minimize toxicity, the ABVD regimen was developed. Multiple randomized studies have compared ABVD to MOPP and ABVD alternating with MOPP, the complete remission rate and freedom from progression was worse for patients receiving MOPP alone (26–29). The ABVD regimen showed superiority with less toxicity and is the most common treatment of choice for patients with advanced HL. The GHSG HD18 trial developed an escalated regimen for advanced-stage disease that consist of dose escalated BEACOPP which has shown higher response rates and progression free survival (PFS) in comparison to ABVD (30). Even though both regimens are associated with late toxic effects, such as secondary malignancies, infertility, cardiovascular disease and lung injury, these sequelae are more common with eBEACOPP (31–33). A randomized comparison of ABVD and BEACOPP of 331 patients with advanced stage HL reported the 7-year rate of freedom from first progression was 85% among patients treated with BEACOPP and 73% among patients treated with ABVD (P=0.004) (34). After completion of planned therapy, the 7-year rate of freedom from a second progression was reported as 88% in the BEACOPP group and 82% in the ABVD group (P=0.12), and the 7-year OS was 89% and 84%, respectively (P=0.39). Notably, severe adverse events were more common in the BEACOPP group than in the ABVD group. Recent trials have used positron emission tomography (PET) scans to identify advanced stage patients who may benefit from intensification or de-escalation of therapy, and the RATHL study is an example of this approach (35). In this study, 1214 patients with advanced HL had an interim PET scan following two cycles of ABVD therapy with those who had a negative scan (Deauville score (DS) 1-3) being randomized to receive ABVD or AVD (without bleomycin) for four more cycles. Patients with a positive PET scan (DS 4 or 5) proceeded to intensification therapy with either four cycles of BEACOPP or three cycles of escalated BEACOPP, followed by a repeat PET scan, if negative, patients received two further cycles of BEACOPP or one cycle of eBEACOPP. In patients with a negative interim PET scan, the 3-year PFS and OS rates in the ABVD group were 85.7% and 97.2% respectively, with similar outcomes in the AVD group with PFS and OS rates of 84.4% and 97.6%, respectively. The AVD group had less pulmonary toxicity. In patients with a positive interim PET scan, intensification of therapy to BEACOPP resulted in 3-year PFS rate of 67.5% and OS rate of 87.8%, suggesting that strategy of response-adapted therapy in HL improves outcomes.
While intensification of therapy to improve efficacy and safety in patients with advanced stage HL has been studied in the past, a more recent approach has been to add novel agents to standard chemotherapy regimens in the frontline setting, include brentuximab vedotin (BV) and PD-1 blocking antibodies. BV is an antibody-drug conjugate targeting CD30 expressed on the cHL cell surface and can be used in combination with standard regimen. The binding of BV to CD30 on the tumor cell membrane triggers a cascade of events which results in apoptotic death of the CD30-expressing cell (36). Checkpoint inhibitors exploit the well-known genetic alterations at 9p24.1 in cHL that leads to the overexpression of the ligands of programmed death-1 (PDL-1), consequently, PD-1 inhibitors have successfully been used to target the PD-1 axis (37–42).
The ECHELON-1 trial assessed the safety and efficacy of A+AVD (brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine) versus ABVD in patients with stage III or IV cHL (43). In this trial, 1334 patients were randomized to receive up to 6 cycles of A+AVD (n=664) or ABVD (n=670). An updated analysis of the trial was presented at the 2022 ASCO annual meeting by the lead author Ansell et al. (44) reported the 6-year PFS of 82.3% with A+AVD and 74.5% with ABVD (hazard ratio [HR] 0.678, 95% CI 0.532-0.863). The 6-year OS rates were 93.9% and 89.4% with A+AVD vs ABVD, respectively, favoring A+AVD over ABVD (HR 0.590, 95% CI 0.396-0.879). Fewer secondary malignancies and more live births were reported with A+AVD. On the basis of these findings, the study recommends A+AVD over ABVD for patients with previously untreated stage III/IV cHL. A relatively recent phase II study of pembrolizumab, a humanized IgG4 monoclonal antibody targeting programmed death-1 (PD-1) protein evaluated outcomes in newly diagnosed cHL when combined with chemotherapy (45). Thirty patients (n=12 with early unfavorable stage and n=18 with advanced stage) were treated with 3 cycles of pembrolizumab monotherapy followed by doxorubicin, vinblastine, and dacarbazine (AVD) for 4 to 6 cycles. After cycle 3 of pembrolizumab monotherapy, 11 patients (37%) showed complete metabolic response (CMR), and 7 of 28 (25%) patients had >90% reduction in metabolic tumor volume on PET scans. All patients achieved CMR after 2 cycles of AVD and at a median follow-up of 22.5 months, there were no changes in terms of therapy, progressions or deaths. The most common adverse events were grade 1 rash and grade 2 infusion reactions. The result of this study suggests that pembrolizumab monotherapy followed by AVD was both effective and safe in patients with newly diagnosed cHL.
Approximately 20-30% of patients with cHL will be refractory to or relapse following frontline treatment (16). Salvage chemotherapy followed by HDC and AHSCT is the standard therapeutic option for patients with r/r cHL that is responsive to chemotherapy. Several studies have proven that HDC followed by AHSCT produce a better long-term disease-free survival and improved outcome than expected with conventional chemotherapy (8, 9, 46, 47). In a randomized trial by Schmitz et al, patients with relapsed Hodgkin’s disease were assigned to two cycles of Dexa-BEAM (dexamethasone and carmustine, etoposide, cytarabine, and melphalan) and either two further courses of Dexa-BEAM or high-dose BEAM and AHSCT in patients with chemosensitive disease. Freedom from treatment failure at 3 years was 55% for BEAM-AHSCT cohort compared with Dexa-BEAM (34%, P=0.019). OS did not differ significantly. Patients with disease progression after AHSCT have a poor prognosis with a median survival of 2.4 years (10, 48, 49). Relapse predictors post-AHSCT include relapse within 12 months of initial treatment, extra nodal disease, bulky disease, active disease at the time of transplant, primary refractory disease, and presence of B symptoms from lymphoma (50, 51).
Given the relatively high rate of post-AHSCT relapse in high risk patients, BV has been approved for maintenance after AHSCT in high risk r/r cHL (52–54). In the AETHERA phase III, double-blinded trial, 329 high risk HL post-AHSCT patients were randomized to receive 1.8 mg/kg of BV or placebo once every 3 weeks for up to 16 cycles starting 30 to 45 days post-transplant (55). Additional inclusion criteria included at least one of the following: primary refractory HL, relapsed HL with initial remission duration of less than 12 months, or extra-nodal involvement at the start of pre-transplantation salvage chemotherapy. Furthermore, patients had to have complete remission, partial remission or stable disease after pre-transplant salvage chemotherapy. Patients who received BV had a longer PFS than those who did not (median 42.9 months vs 24.1 months), and on a 5-year follow-up the PFS difference remained statistically different (59% vs 41%, respectively). While BV has been shown to be an effective maintenance option, it can cause significant peripheral neuropathy, which occurred in 56% of patients on trial in the BV arm in comparison to 16% of patients in the placebo arm. One limitation of this trial is the lack of universal PET scans prior to AHSCT, in fact one-third of patients did not have disease assessment by PET prior to transplant. It is possible that PET scanning done before AHSCT could have more accurately classified patient responses to salvage chemotherapy and the benefit of BV seemed to be diminished in patients who were PET-negative before AHSCT.
PD-1 inhibitors have also been evaluated as post-AHSCT maintenance therapy with the aim of improving rates of durable remission. Pembrolizumab and nivolumab are two anti-PD-1 antibodies currently approved in cHL patients with r/r disease (56, 57). In a multicohort phase II study of r/r cHL after AHSCT, 30 patients were enrolled to receive 8 cycles of pembrolizumab within 60 days of AHSCT, PFS at 18 months was 82% and OS 100%. Most adverse events (40%) were immune-related, grade 1 or II or higher (58). Similarly, nivolumab was evaluated as maintenance therapy post-AHSCT in high-risk r/r cHL (high risk defined as refractory disease, relapse <12 months, or relapse ≥12 months with extra nodal disease after frontline therapy). In a phase II single arm study, 37 patients were treated with nivolumab for 6 months, PFS was 92.1% at 6 months and the 12-month OS was 100%. The incidence of grade 3 or higher toxicity was 14% (59). Herrera et al. reported in abstract form on 59 patients who received BV and nivolumab maintenance post-AHSCT. Starting between day 30-75 after AHSCT, patients received 1.8 mg/kg of BV and 3 mg/kg nivolumab every 3 weeks for a planned 8 cycles. Forty-nine percent (29) of patients completed all 8 cycles with both drugs. Most common grade 2 or higher immune related adverse events included pneumonitis (12%), AST or ALT elevation (8%), hypothyroidism (5%), and rash (3%). The estimated 18-months PFS and OS for all patients were 95% and 98%, respectively (60). A summary of key studies (61–70) that evaluated the role of immune-checkpoint inhibitors and other novel therapies in cHL is shown in Table 1.
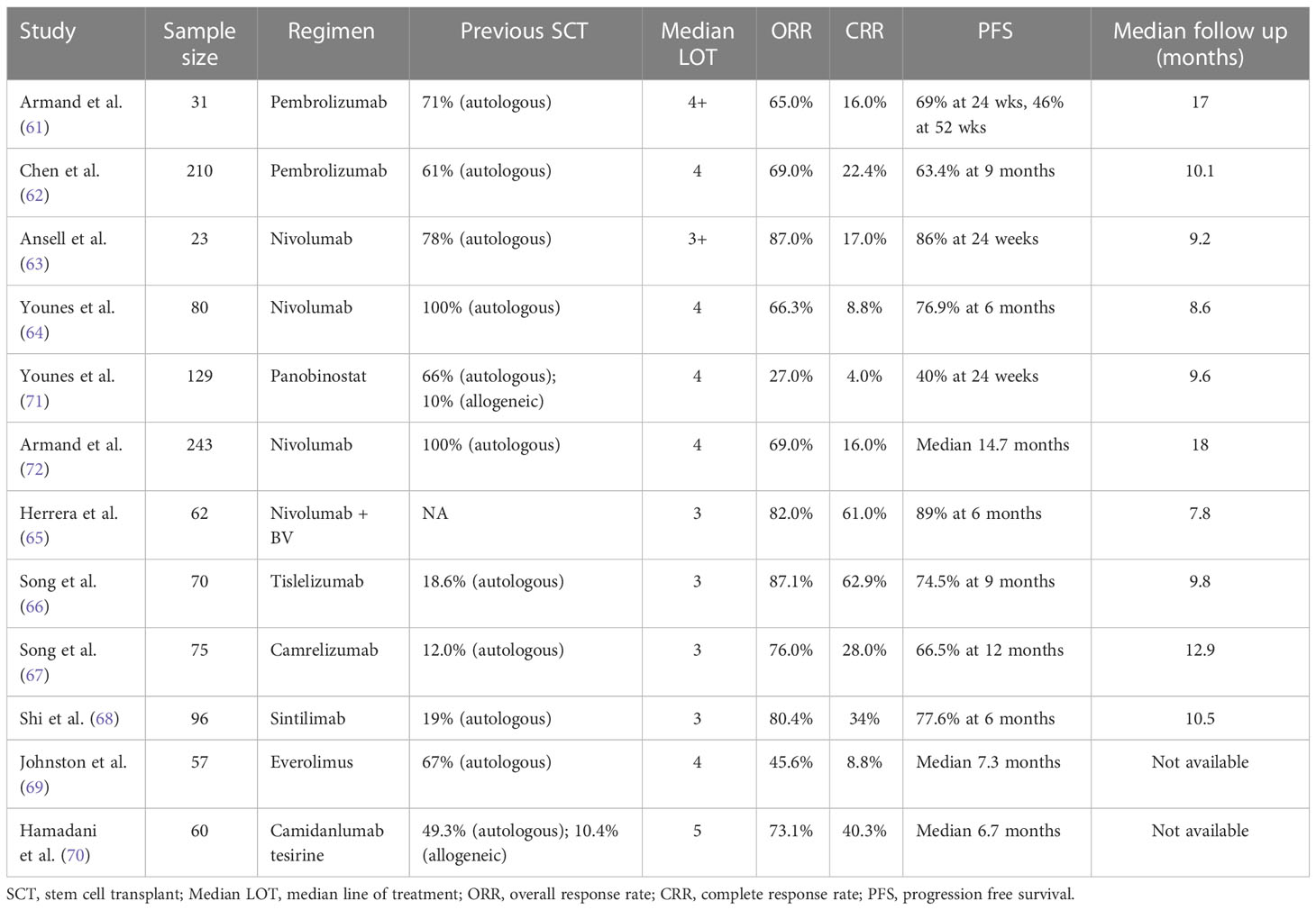
Table 1 Summary of key studies evaluating the role of novel therapies in relapsed and refractory cHL.
Given the success of BV in both advanced stage and r/r cHL, BV has been evaluated as salvage therapy after frontline therapy. Herrera et al. reported on a phase II study with 20 patients enrolled to a BV dose-escalation cohort that consisted of 1.8 mg/kg of BV intravenously every 3 weeks for two cycles (73). Patients with a complete response (CR) after two cycles received two additional cycles of BV at 1.8 mg/kg, while patients with stable disease or partial response (PR) were escalated to 2.4 mg/kg for two cycles. BV escalation was well tolerated however no patient was converted to CR from stable disease or PR. The overall response rate (ORR) was 75% and 43% of patients achieved CR. In total, 50% of patients proceeded directly to AHSCT (without post BV maintenance) with a 2-year PFS of 77%. After AHSCT, the 2-year PFS and OS were 67% and 93%, respectively. The 2-year PFS among patients in CR at the time of AHSCT was 71% compared with 54% in patients not in CR (P=0.12). Similarly, a non-randomized phase II trial evaluated the effect of weekly BV infusion on days 1, 8 and 15 for two 28-day cycles followed by a PET scan (74). Patients who achieved DS of 1 or 2 on PET scan proceeded directly to AHSCT and those with DS 3-5 on PET received two cycles of augmented ICE (isocyanide, carboplatin, etoposide) prior to consolidation with AHSCT. Twenty-seven percent of patients had a CR post-two cycles of BV and 69% of patients receiving augmented ICE subsequently attained a CR. For all patients treated with AHSCT, the 3-year OS and PFS were 95% and 82%, respectively.
The safety and efficacy of BV with bendamustine was evaluated in a phase I/II study of 55 patients with r/r HL (75). Patients received BV on day 1 and bendamustine on days 1 and 2 of a 21-day cycle for up to 6 cycles. Patients could continue on to AHSCT after cycle 2 and after a median of two cycles, the ORR was 93% with 73.6% of patients achieving CR. At a median follow up of 44.5 months, the 3-year OS and PFS were 92% and 60.3%, respectively (3-year PFS was 67.1% in patients who underwent AHSCT and 40.4% who did not undergo AHSCT) (76). A multicenter, phase I/II trial in patients with r/r HL was conducted by the Spanish Lymphoma Group (GELTAMO) (77). In total, 66 patients were assigned to a dose escalation BV with ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin) with ORR of 91%, CR 70% and the 30-month OS and PFS were 91% and 71%, respectively. Another phase I/II study evaluated BV in combination with dexamethasone, cisplatin, and cytarabine (BV-DHAP) for patients with r/r HL (78). Patients were treated with three cycles of therapy over 21 days, and PET was performed after three cycles with those attaining at least PR progressing to AHSCT. A total of 85% ultimately proceeded to AHSCT and the ORR and metabolic CR were 90% and 81%, respectively, with 2-year PFS of 74% and OS of 95%.
Brentuximab vedotin combined with nivolumab as a salvage therapy was evaluated in a phase I/II study in patients with r/r cHL (79). In this study, 93 patients were enrolled and of those 91 received the full study treatment with a median follow up of 34.3-months. The ORR for all treated patients was 85%, with 67% achieving CR, and the 3-year PFS was 91% and 3-year OS was 93% for patients who received an AHSCT after study treatment. Even though a high rate of infusion reactions (44%) was observed, the treatment was well tolerated overall. The safety and efficacy of ipilimumab, nivolumab, and BV were evaluated in a phase I study on 61 patients with r/r cHL treated with three different regimens (BV 1.8mg/kg with Ipe 1mg/kg and 3mg/kg; BV 1.2mg/kg and 1.8mg/kg combined with Nivo 3mg/kg; BV 1.2mg/kg and 1.8mg/kg with Nivo 3mg/kg and Ipi 1mg/kg) and ORR was 76%, 89% and 82% with CR rate of 57%, 61% and 73%, respectively (80). The median PFS was 1.2 years for BV-Ipi and not reached for BV-Nivo and BV-Nivo-Ipi. The most common adverse events were grade 3-4, included rash, gastritis, colitis, pancreatitis and arthritis. The phase II randomized portion of the trial is still ongoing (NCT01896999). In the KEYNOTE-204, a randomized multicenter phase III study, 151 patients were randomly assigned to pembrolizumab and 153 to BV (81). After 25 months, median PFS was 13.2 months for pembrolizumab versus 8.3 months for BV (HR=0.65, 95% CI 0.48-0.88). The most common grade 3-5 treatment related toxicity was pneumonitis (4% in the pembrolizumab group and 1% in the BV), neutropenia and peripheral neuropathy were more common in the BV group. The results suggest that pembrolizumab monotherapy is an effective and safe treatment option for patients with r/r cHL who relapse after AHSCT or who are ineligible for AHSCT.
The CHECKMATE-205 trial enrolled patients with disease that relapsed after AHSCT and underwent salvage therapy with nivolumab. ORR was 69% while median PFS and duration of response were 14.7 and 16.6 months, respectively (72). The results of this study led to the addition of checkpoint inhibitors (CPIs) with chemotherapy and subsequently a multicenter prospective trial explored nivolumab in combination with ICE (NICE) as first salvage in 39 patients with r/r HL who were enrolled and treated with nivolumab biweekly for up to 6 cycles (82). PET was performed after cycles 3 and 6, and patients who had not achieved a CR after cycle 6 went to receive two cycles of NICE. After nivolumab alone, the ORR was 81% with a 71% CR rate. Amongst the 9 patients who received NICE, the ORR was 100% with 89% of patients achieving a CR. The 2-year PFS and OS in all treated patients were 72% and 95%, respectively, while the 33 patients who bridged directly to AHSCT had a 2-year PFS of 94%.
Pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin (pembro-GVD) was studied in 39 patients in the second line setting for patients with r/r cHL (83). Those who achieved CR after 2-4 cycles proceeded to AHSCT. The ORR was 100% with 95% of patients achieving CR (92% after 2 cycles) and 95% proceeded to HDC/AHSCT. All patients who proceeded to transplant remained in remission at median post-transplant follow up of 12.5 months. A phase II study evaluated 42 AHSCT eligible patients with r/r cHL treated with pembrolizumab (PEM) added to ICE (84). Of the 42 patients enrolled, 37 patients were evaluable for efficacy and the CMR rate by PET following two cycles of PEM-ICE was 86.5% and the 24-month PFS was 88.2%. However, one patient died of cardiac arrest during stem cell collection, while another patient died due to acute respiratory failure secondary to engraftment syndrome early post-AHSCT.
Some studies have suggested that treatment with CPIs may re-sensitize patients to chemotherapy (85–87). Moreover, a multicenter study evaluated 81 heavily pretreated patients who progressed on CPIs were rechallenged, and sensitized patients to their subsequent treatment with ORR to post-CPI therapy of 62%, median PFS of 6.3 months and median OS of 21 months (87). There was no significant difference in OS. Subsequently, Calabretta et al. reported on 28 patients with r/r cHL, and of those, 26 (92%) were refractory to the last chemotherapy prior to CPIs (88). Following rechallenge with chemotherapy, 23 (82%) experienced a CR and 3 (11%) PR. Twenty-five patients proceeded to alloHSCT, and at a median follow-up of 21 months, median PFS and OS were not reached. A summary of key studies (89–92) that evaluated the role of immunotherapy and chemotherapy in the salvage setting in cHL is shown in Table 2.
Anti-PD-1 agents have been used extensively to treat cHL. Camrelizumab, a novel anti-PD-1 immune checkpoint inhibitor has been evaluated in a multicenter, phase II study in 75 patients with r/r cHL with median follow-up of 36.2 months (93). Median PFS was 22.5 months and 36-month OS rate was 82.7% (95% CI 72.0-89.5). The most common toxicity was grade 1 or 2 reactive capillary endothelial proliferation with spontaneous regression. A separate multicenter phase II study evaluated the efficacy and safety of GLS-010 or Fiberesima, an anti-PD-1, in Chinese patients with r/r cHL (94). The 12-month PFS and OS rate were 78% (95% CI 67.5-85.6) and 99% (95% CI 91.9-99.8), respectively. Treatment-related adverse events occurred in 92.9% of participants with grade III in 28.2% and most common were abnormal hepatic function, hyperuricemia and neutropenia. However, there is limited data on the long-term survival outcome of those patients after remission with immune-checkpoint inhibitors.
Treating r/r cHL remains challenging and multiple agents have been explored in this space, and one such agent are histone deacetylation (HDACs) inhibitors which function by blocking post-transcriptional histone modification in regulating gene transcription (95). Vorinostat (SAHA) inhibits STAT6 phosphorylation and transcription in HL cell lines which leads to decreased expression and secretion of Th2-type cytokines and chemokines (TARC and IL-5) and converse increase in Th1-type cytokines/chemokines (IP-10) (96). The Southwest Oncology Group (SWOG) reported on a phase II study of 25 patients with relapsed HL treated with single agent Vorinostat (97). While it was well tolerated, it only produced modest clinical activity, 1 patient (4%) achieving a PR and median PFS was 4.8 months. Mocetinostat (MGCD-0103) is a selective inhibitor of HDAC 1, 2, 3 (class I) and 11 (class IV) isoforms, which causes hyperacetylation of histones, and selectively induce apoptosis and cell cycle blockade in various cancer cell lines in a dose-dependent manner (98). The safety and efficacy of Mocetinostat was evaluated in a phase II study with an overall disease control rate of 34.8% and 25% for the 110-mg and 85-mg cohorts, respectively (99). After at least 2 cycles of therapy, 81% had a decrease in tumor measurements, however, 47% discontinued therapy due to disease progression (57% in the 85-mg cohort and 34% in 110-mg cohort). Panobinostat (LBH 589) is a pan-deacetylase inhibitor targeting epigenetic and non-epigenetic oncogenic pathways (100). Panobinostat was evaluated in a phase II study of 61 patients with r/r HL, and 53 patients completed two or more cycles of therapy and had at least one post-baseline imaging studies. Responses include one CR and 10 PR, with up to 92% decrease in tumor burden. The most common grade 3 and 4 adverse events were thrombocytopenia (77%), anemia (20%) and neutropenia (16%). In a similar study, 129 heavily pretreated patients were treated with Panobinostat with median time to response (TTR) of 2.3 months (71). Tumor reduction occurred in 74%, including 23% PR and 4% CR. Median PFS was 6.1 months and 1-year OS rate was 78%. Other HDAC inhibitors include Entinostat (SNDX-275) and ITF 2357 have shown encouraging clinical activity in r/r HL (101, 102).
Lymphocyte-activation gene 3 (LAG-3) is nearly always expressed in the tumor microenvironment of cHL and inhibitors of LAG-3 are now in clinical trials. In vitro studies have shown that anti-PD1 immunotherapy-resistant HL has CD8 lymphocytes depleted in microenvironment and overexpress the LAG-3 on CD4+ helper T lymphocytes (103). Timmerman et al. evaluated the safety and efficacy of favezelimab (MK-4280), a humanized IgG4 LAG-3 inhibitor, given with pembrolizumab every 3 weeks to 33 patients with r/r cHL refractory to anti-PD-1 therapy (104). At a median follow-up of 16.5 months, ORR for patients receiving favezelimab 800 mg was 31%, CR 7%, PR 24% and 66% of the responders had an anti-PD1-based regimen as most recent line of therapy. For all patients, median PFS and OS were 9-mo and 26-mo respectively, while 12-mo PFS and OS rates were 39% and 91% respectively. The most common adverse events were non-hematologic (hypothyroid, nausea, fatigue, etc). Another interesting target is the T-cell immunoglobulin and ITIM domains (TIGIT), which is an immune checkpoint receptor known to negatively regulate T cell functions and highly co-expressed with PD-1 on both CD4 and CD8 T cells in cancers (105). A study conducted by Annibali et al, 19/34 (56%) HL patients were TIGIT positive and of those, 16 (84%) were also PD-1 positive. Out of 15 TIGIT negative, only 4 (27%) were PD-1 positive, but (100%) were PD-L1 positive. Blockade of TIGIT with vibostolimab (MK-7684) has demonstrated antitumor activity in multiple pre-clinical tumor models. A multicohort, phase II study (106) is currently enrolling patients to evaluate the safety and efficacy of vibostolimab with pembrolizumab in patients with r/r cHL (NCT05005442). Camidanlumab tesirine is an anti-CD25 antibody drug conjugate that has been evaluated in r/r cHL. A recent phase I study explored camidanlumab tesirine in a highly pretreated patient population administered once every 3 weeks (107). Overall response rate was 71.4% and CR 48.6% in cHL cohort at 45 µg/kg camidanlumab tesirine with a median follow-up of 9.2 months. Toxicities were dose limiting with most common being rash, anemia, pyrexia and increased γ-glutamyl transferase. Five of 133 patients (3.8%) developed serious neurologic events of Guillain-Barré syndrome (GBS)/polyradiculopathy considered likely immune-related and at least possibly study-drug related but without correlation to dose. Treatment of relapse and refractory cHL remains an unmet need and further studies are needed to evaluate novel agents. A summary of recently completed or ongoing clinical trials of novel agents is shown in Table 3.
The standard of care at first relapse after frontline therapy is HDC followed by AHSCT as part of salvage therapy. Patients who are eligible and achieve a complete metabolic response should proceed to AHSCT in second remission as the treatment related mortality is significantly lower when compared to alloHSCT. More recently with the availability of newer agents, the timing of alloHSCT is less clear. The decision considering alloHSCT should be individualized given the risk and potential benefit, however, in a selected group of patients it is potentially curative. We recommend alloHSCT be considered in all patients who have relapse of disease post AHSCT or are ineligible for AHSCT due to insufficient disease response with the caveat that patients must have responding lymphoma prior to proceeding to alloHSCT. The role of alloHSCT in patients with r/r cHL has been controversial due to high transplant-related mortality (TRM) as well as transplant related complications associated with acute and chronic graft-versus host disease (GVHD) (108). However, reduced-intensity conditioning (RIC) alloHSCT has shown a promising result with reduced TRM in patients with r/r cHL. In a clinical trial by Alvarez et al, 40 patients were treated with RIC alloHSCT demonstrated 2-year OS and PFS 48% ± 10% and 32% ± 10%, respectively (109). In chemo sensitive disease, the results were even better, 63% ± 12% and 55% ± 16%, respectively. In a phase II study (HDR-ALLO study), 92 patients with r/r cHL were treated with salvage chemotherapy followed by RIC alloHSCT, PFS rate was 48% at 1 year and 24% at 4 years (110). Patients who were allografted in complete remission, OS rate was 71% at 1 year and 43% at 4 years. The incidence of relapse rate was lower in patients with chronic GVHD. The addition of cyclophosphamide post-transplant showed better outcomes with 3-year OS, PFS, relapse rate and 1-year non-relapse mortality (NRM) rates of 63%, 59%, 21% and 20%, respectively (111). Post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis was associated with significant improvement in PFS and GRFS.
Patients with cHL with r/r disease may benefit from alloHSCT, but many lack a matched sibling donor (MSD). A study conducted by Ahmed et al. included 596 adult patients who received a first RIC alloHSCT using either a haplo-PTCy (n=139) or MSD/calcineurin inhibitor (CNI)-based (n=457) approach (112). On multivariate analysis, no significant difference was seen between haplo/PTCy and MSD/CNI-based approaches in terms of OS (HR 1.07, P=0.66) or PFS (HR 0.86, P=0.22). The haplo/PTCy platform was associated with higher risk of grades II to IV acute GVHD (OR 1.73, P=0.007) however, the risk of grades III to IV acute GVHD was not different between the 2 cohorts. The haplo/PTCy cohort had a significant reduction in chronic GVHD risk (HR 0.45, P < 0.001) and a significant reduction in relapse risk (HR 0.74, P= 0.03). A European study retrospectively compared the outcome of patients with HL who received PTCy-based haplo alloHSCT (n=98) vs HLA MSD (n=338) vs HLA-matched unrelated donor (MUD) (n=273) transplantation (113). The median follow-up of survivors was 29 months. Haplo alloHSCT was associated with a lower risk of chronic GVHD (26%) compared with MUD (41%, P=0.04). The 2-year cumulative incidence of relapse progression was 39%, 49%, and 32% in haplo, MSD and MUD, respectively. The two-year OS, PFS were 67% and 43% for haplo, 71% and 38% for MSD, and 62% and 45% for MUD, respectively. The rate of extensive chronic GVHD and relapse-free survival was significantly better for haplo (40%) compared with MSD (28%, P=0.049) and similar to MUD (38%, P=0.59). Based on the results of these studies there is support that haplo/PTCy alloHSCT in cHL results in survival comparable to traditional alloHSCT approaches with potentially better outcomes in terms of GVHD and relapse.
AHSCT is considered the standard treatment for patients with r/r HL (9). Risk factors that have been repeatedly found to be strong predictors of failure after AHSCT include HL refractory to frontline therapy and <12 months to first relapse (53, 55). For patients with high-risk of relapse after AHSCT, an alternative consolidation strategy with alloHSCT could be a potential option to improve their outcome (49, 50). The use of RIC has resulted in a significant reduction of the NRM but this strategy requires several months for the allogeneic effect to develop, thus in patients with an aggressive HL the disease might progress. In this setting, a tandem auto-RIC-SCT approach has the potential of providing effective cytoreduction to keep the lymphoma under control. A retrospective study conducted by Bento et al. analyzed 126 patients treated with tandem AHSCT- RIC-alloHSCT (114). The median number of lines prior to AHSCT was two (33% of the patients received >3 lines) and 41% were transplanted with active disease. The median follow-up was 44 months and 3-year PFS, OS, incidence of relapse, and NRM after the tandem were 53%, 73%, 34%, and 13%, respectively. A similar result was reported by Mariotti et al. in another study (115). The results suggest that this might be an effective treatment for a high-risk population.
It is important to note that several studies have suggested PD-1 blockade prior to alloHSCT is associated with higher-than-normal rates of early transplant-related complications. Merryman et al. reported on a retrospective cohort of 209 cHL patients who received alloHSCT after PD-1 blockade with a median follow-up of 24 months (116). The 2-year GVHD and relapse-free survival (GRFS), PFS and OS were 47%, 69%, and 82%, respectively. The 180-day cumulative incidence (CI) of grade 3-4 acute GVHD was 15%, while the 2-year CI of chronic GVHD was 34%. Post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis was associated with significant improvements in PFS and GRFS. An international retrospective study reported on the outcomes of 39 patients with advanced lymphoma who received treatment with a PD-1 inhibitor at a median time of 62 days before alloHSCT (117). One-year OS and PFS rates were 89% and 76%, respectively, while the 1-year cumulative incidences of grade 2-4, grade 3-4, and grade 4 acute GVHD were 44%, 23%, and 13%, respectively. The incidence of grade 4 GVHD was higher than prior studies (13% vs 3% to 4%). In another multicenter retrospective analysis, 31 patients with relapse post-alloHSCT received anti-PD-1 with ORR of 77% (118). At last follow-up, there were 8 (26%) deaths related to new-onset GVHD after anti-PD1, 17 (55%) patients developed treatment-emergent GVHD after initiation of anti-PD1 and grade III-IV or severe chronic GVHD occurred in 9 patients. The results indicate that PD-1 blockade is highly efficacious but frequently complicated by rapid onset of severe and treatment-refractory GVHD. A similar finding was reported by Herbaux et al. analyzed 20 patients with HL relapsing after alloHSCT received anti-PD-1 (119). GVHD occurred in 6 patients (30%) and two patients died as a result of GVHD. The 1-year PFS was 58.2% and OS 78.7%. These results prompted a warning from the United States Food and Drug Administration and recommend caution about using PD-1 blockade in close proximity prior to alloHSCT.
Chimeric antigen receptor (CAR) T-cell against CD-30 is a directed novel cellular therapy where patient’s own immune cells are engineered ex vivo to recognize target cancer antigens. A phase I trial evaluated 18 heavily pretreated patients who received anti-CD30 CAR T-cells (120). This study used multiple combinations of drugs for conditioning. Patients received a conditioning regimen that included 3 forms: (I) FC (fludarabine, 3 daily doses of 25 mg/m2 + cyclophosphamide, at a total dose of 30 mg/kg) to deplete endogenous leukocytes; (II) GMC-like chemotherapy (gemcitabine 1 g + mustargen 10 mg + cyclophosphamide, at a total dose of 30 mg/kg) to inhibit the disease progression in a short time and eliminate endogenous leukocytes; and (III) PC (nab-paclitaxel 150 mg/m2 + cyclophosphamide 30 mg/kg) to deplete the stromal compartment and eliminate endogenous leukocytes. The individual absolute dose was administrated at the discretion of a physician according to treatment history and bone marrow tolerance. The ORR was 39% (all partial responders) with 28% of patients showing stable disease at two months and a median PFS of 6 months. All patients experienced Grade 1 or 2 febrile syndrome at the time of infusion that resolved without intervention. In another phase I/II trial of anti-CD30 CAR T-cells in r/r cHL, 41 heavily pretreated patients underwent lymphodepletion with varying regimens of bendamustine alone, bendamustine-fludarabine or cyclophosphamide-fludarabine (121). The ORR was 72% with 60% of patients attaining a CR. The one-year PFS and OS rates were 41% and 94%, respectively. Five patients had a CR more than a year out from infusion and more than a third showed durable response. The most common adverse event was cytopenia related to lymphodepletion with a favorable profile in terms of CART related toxicity. Subsequently, a phase II, multi-national trial (CHARIOT NCT04268706) which is ongoing, reported in abstract form on 15 patients with r/r cHL treated with CD30 CART (122). This was a heavily pre-treated patient population with 6 median prior therapies, with a range of 4 to 19. At the time of reporting, 12 patients had received CD30 CART infusion and were evaluable, the ORR at Day 42 was 100% (5/5), CR and PR rates were 80% (4) and 20% (1), respectively. The most common adverse events were hematologic and without significant CART associated toxicity such as cytokine release syndrome or neurotoxicity.
Voorhees and colleagues evaluated factors that were associated with PFS after anti-CD30 CAR T-cells therapy in r/r cHL (123). The tumor burden measured by using Flourine-18 fluorodeoxyglucose (F-FDG) PET imaging or metabolic tumor volume (MTV) showed a direct correlation between MTV and PFS. Patients with higher MTV (>60 ml) before CAR T-cells therapy and lymphodepletion had a lower 1-year PFS (14%) compared to those with a low MTV (58%) and interestingly, patients who responded to bridging therapy with a decrease in MTV had an improved 1-year PFS (40%) compared to those who showed no reduction in tumor burden post bridging therapy (1-year PFS 0%). However, bridging therapy was not associated with a significant difference in PFS neither the persistence of anti-CD30 CAR T-cells. In another study by Voorhees and colleagues, they evaluated the role of checkpoint inhibitors in patients with disease progression following CAR T-cell treatment and found a clinical benefit in all patients including those that had not responded to checkpoint inhibition prior to CAR T-cell therapy, presumably due to reprogramming and reactivating CAR T-cells that persisted after the initial infusion (124).
Other types of cellular therapy have also been explored with encouraging results of an ongoing trial with natural killer (NK) cells derived from umbilical cord blood (CB) and activated with a novel bispecific antibody known as AFM13 (targeting CD16A and CD30) demonstrated safety and efficacy in r/r CD30+ lymphoma (NCT04074746) (125). Patients received two cycles of fludarabine/cyclophosphamide followed by AFM13-precomplexed CB-NK cells (day 0) and 3 weekly intravenous infusion of AFM13. Eighteen patients completed both planned cycles and therapy was well tolerated. The ORR was 89% and at median follow-up of 6 months, PFS/OS across all dose levels were 52% and 81%, respectively. All patients treated at the recommended dose for phase II responded, for an overall response rate of 100% with 62% in complete remission. Expansion of CB-NK cells occurred as early as 3 days post infusion. The preliminary results of this study indicate high tolerability and activity. In summary, randomized clinical trials are needed to assess the long-term safety and efficacy of these novel cellular therapies.
In the frontline setting, patients are treated according to age. For early-stage HL, we generally recommend a combined modality approach with chemotherapy (ABVD and/or eBEACOPP) and radiation, or risk-adapted PET-guided approach. Patients aged <60 years with advanced stage disease and no significant comorbidities, we recommend AAVD. Young women with disease in the thorax who may receive radiation exposure to breast tissue, we recommend chemotherapy alone approaches. In patients aged >60 years, the intensity of therapies is based on comorbidities. In the relapsed setting, chemoimmunotherapeutic or chemotherapy-free approaches can be used. AHSCT is considered the standard treatment for patients with r/r cHL and among those who experience r/r cHL after HDC/AHSCT, single agent and combinations with novel agents including BV and/or PD1 has demonstrated substantial efficacy and favorable toxicity. For a select population of patients, alloHSCT should be considered. Ongoing trials are evaluating the efficacy of CAR T-cells therapy in r/r cHL.
Classical Hodgkin lymphoma is a rare lymphoma, and while the majority of patients will respond to frontline therapy, up to 30% of patients may experience relapsed or refractory disease. The therapeutic approach to each patient depends on clinical prognostic factors, comorbidities and toxicity profile. Undeniably high dose chemotherapy and AHSCT remains the standard consolidation for patients whose disease responds to salvage systemic therapy, however, treatment options have dramatically changed over the years. The integration of novel therapies with standard regimen has achieved higher response rates and durable benefits with tolerable toxicity. Brentuximab vedotin and checkpoint inhibitors have shown survival benefits in patients whose HL has relapsed after AHSCT. More recently, CAR T-cell therapy has demonstrated exceptional response rates and safety, however longer follow up is required to confirm durability of responses. The new era of immune-chemotherapy and targeted agents continues to provide significant clinical benefits, although there is still a subset of patients with refractory disease who do not experience durable long-term responses with current treatment paradigms, and this remains a significant unmet need.
The authors confirm contribution to the paper as follows: study conception and design: SA and FU; data collection: FU, DD, NO, and OO; draft manuscript preparation: all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Flora DR, Parsons SK, Liu N, Yu KS, Holmes K, Flores C, et al. Classical Hodgkin lymphoma; real-world observations from physicians, patients, and caregivers on the disease and its treatment (CONNECT)-a cross-sectional survey of patients with stage III or IV classical Hodgkin lymphoma compared by age. Blood (2021) 138(Supplement 1):1966–6. doi: 10.1182/blood-2021-147354
2. Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J haematology (2019) 184(1):45–59. doi: 10.1111/bjh.15614
3. Greaves P, Clear A, Coutinho R, Wilson A, Matthews J, Owen A, et al. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J Clin Oncol (2013) 31(2):256–62. doi: 10.1200/JCO.2011.39.9881
4. Liu Y, Abdul Razak FR, Terpstra M, Chan FC, Saber A, Nijland M, et al. The mutational landscape of Hodgkin lymphoma cell lines determined by whole-exome sequencing. Leukemia (2014) 28(11):2248–51. doi: 10.1038/leu.2014.201
5. Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet (British edition) (2021) 398(10310):1518–27. doi: 10.1016/S0140-6736(20)32207-8
6. Yung L, Linch D. Hodgkin's lymphoma. Lancet (2003) 361(9361):943–51. doi: 10.1016/S0140-6736(03)12777-8
7. Ansell SM. Hodgkin Lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J hematology (2018) 93(5):704–15. doi: 10.1002/ajh.25071
8. Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant hodgkin's disease: Results of a BNLI randomised trial. Lancet (1993) 341(8852):1051–4. doi: 10.1016/0140-6736(93)92411-L
9. Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive hodgkin's disease: A randomised trial. Lancet (British edition) (2002) 359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9
10. Ferme C, Mounier N, Berger F, Salles G, Divine M, Brice P, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced hodgkin’s disease in relapse or failure after initial chemotherapy: Results of the groupe d’Études des lymphomes de l’Adulte H89 trial. J Clin Oncol (2002) 20(2):467–75. doi: 10.1200/JCO.2002.20.2.467
11. Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N, Rancea M. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database systematic Rev (2013) 6:CD009411. doi: 10.1002/14651858.CD009411.pub2
12. Singh AK, McGuirk JP. Allogeneic stem cell transplantation: A historical and scientific overview. Cancer Res (Chicago Ill) (2016) 76(22):6445–51. doi: 10.1158/0008-5472.CAN-16-1311
13. Butcher BW, Collins RH. The graft-versus-lymphoma effect: clinical review and future opportunities. Bone marrow Transplant (Basingstoke) (2005) 36(1):1–17. doi: 10.1038/sj.bmt.1705008
14. Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood (2018) 131(15):1689–97. doi: 10.1182/blood-2017-09-772673
15. Brierley CK, Jones FM, Hanlon K, Peniket AJ, Hatton C, Collins GP, et al. Impact of graft-versus-lymphoma effect on outcomes after reduced intensity conditioned-alemtuzumab allogeneic haematopoietic stem cell transplantation for patients with mature lymphoid malignancies. Br J haematology (2019) 184(4):547–57. doi: 10.1111/bjh.15685
16. Perales M-A, Ceberio I, Armand P, Burns LJ, Chen R, Cole PD, et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: Guidelines from the American society for blood and marrow transplantation. Biol Blood marrow transplantation (2015) 21(6):971–83. doi: 10.1016/j.bbmt.2015.02.022
17. Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner H-H, et al. Hodgkin Lymphoma. Nat Rev Dis primers (2020) 6(1):61–1. doi: 10.1038/s41572-020-0189-6
19. Brusamolino E, Lazzarino M, Orlandi E, Canevari A, Morra E, Castelli G, et al. Early-stage hodgkin's disease: Long-term results with radiotherapy alone or combined radiotherapy and chemotherapy. Ann Oncol (1994) 5:S101–6. doi: 10.1093/annonc/5.suppl_2.S101
20. Evens AM, Yu KS, Liu N, Surinach A, Holmes K, Flores C, et al. Classical Hodgkin lymphoma; real-world observations from physicians, patients, and caregivers on the disease and its treatment (CONNECT): Physician first-line treatment preferences for stage III or IV classical Hodgkin lymphoma. Blood (2021) 138(Supplement 1):2467–7. doi: 10.1182/blood-2021-152497
21. Meyer R, Gospodarowicz M, Connors J, Pearcey R, Wells W, Winter J, et al. ABVD alone versus radiation-based therapy in limited-stage hodgkin's lymphoma. New Engl J Med (2012) 366(5):399–408. doi: 10.1056/NEJMoa1111961
22. Eich H, Diehl V, Görgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable hodgkin's lymphoma: Final analysis of the German Hodgkin study group HD11 trial. J Clin Oncol (2010) 28(27):4199–206. doi: 10.1200/JCO.2010.29.8018
23. Von Tresckow B, Plütschow A, Fuchs M, Klimm B, Markova J, Lohri A, et al. Dose-intensification in early unfavorable hodgkin's lymphoma: Final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol (2012) 30(9):907–13. doi: 10.1200/JCO.2011.38.5807
24. Engert A, Plütschow A, Eich H, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage hodgkin's lymphoma. New Engl J Med (2010) 363(7):640–52. doi: 10.1056/NEJMoa1000067
25. Longo D, Young R, Wesley M, Hubbard S, Duffey P, Jaffe E, et al. Twenty years of MOPP therapy for hodgkin's disease. J Clin Oncol (1986) 4(9):1295–306. doi: 10.1200/JCO.1986.4.9.1295
26. Bonadonna G, Valagussa P, Santoro A. Alternating non-cross-resistant combination chemotherapy or MOPP in stage IV hodgkin's disease. A report of 8-year results. Ann Internal Med (1986) 104(6):739–46. doi: 10.7326/0003-4819-104-6-739
27. Canellos G, Anderson J, Propert K, Nissen N, Cooper M, Henderson E, et al. Chemotherapy of advanced hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. New Engl J Med (1992) 327(21):1478–84. doi: 10.1056/NEJM199211193272102
28. Viviani S, Bonadonna G, Santoro A, Bonfante V, Zanini M, Devizzi L, et al. Alternating versus hybrid MOPP and ABVD combinations in advanced hodgkin's disease: ten-year results. J Clin Oncol (1996) 14(5):1421–30. doi: 10.1200/JCO.1996.14.5.1421
29. Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced hodgkin's disease: Report of an intergroup trial. J Clin Oncol (2003) 21(4):607–14. doi: 10.1200/JCO.2003.12.086
30. Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET-guided treatment in patients with advanced-stage hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin study group. Lancet (2017) 390(10114):2790–802. doi: 10.1016/S0140-6736(17)32134-7
31. Eichenauer DA, Thielen I, Haverkamp H, Franklin J, Behringer K, Halbsguth T, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: A report from the German Hodgkin study group. Blood (2014) 123(11):1658–64. doi: 10.1182/blood-2013-07-512657
32. Franklin J, Eichenauer DA, Becker I, Monsef I, Engert A. Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis. Cochrane Database Syst Rev (2017) 9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2
33. André MPE, Carde P, Viviani S, Bellei M, Fortpied C, Hutchings M, et al. Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials. Cancer Med (2020) 9(18):6565–75. doi: 10.1002/cam4.3298
34. Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. ABVD versus BEACOPP for hodgkin's lymphoma when high-dose salvage is planned. New Engl J Med (2011) 365(3):203–12. doi: 10.1056/NEJMoa1100340
35. Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Treatment guided by interim PET-CT scan in advanced hodgkin's lymphoma. N Engl J Med (2016) 374(25):2419–29. doi: 10.1056/NEJMoa1510093
36. Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood (2016) 128(12):1562–6. doi: 10.1182/blood-2016-02-699850
37. Weber J. Immune checkpoint proteins: A new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol (2010) 37(5):430–9. doi: 10.1053/j.seminoncol.2010.09.005
38. Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nat (London) (2011) 471(7338):377–81. doi: 10.1038/nature09754
39. Green MR, Rodig S, Juszczynski P, Jing O, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin Cancer Res (2012) 18(6):1611–8. doi: 10.1158/1078-0432.CCR-11-1942
40. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large b-cell lymphoma. Blood (2010) 116(17):3268–77. doi: 10.1182/blood-2010-05-282780
41. Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol (2016) 34(23):2690–7. doi: 10.1200/JCO.2016.66.4482
42. Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for reed-sternberg cells in Hodgkin lymphoma. Blood (2017) 130(22):2420–30. doi: 10.1182/blood-2017-03-770719
43. Straus DJ, Długosz-Danecka M, Connors JM, Alekseev S, Illés Á, Picardi M, et al. Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematology (2021) 8(6):e410–e21. doi: 10.1016/S2352-3026(21)00102-2
44. Ansell SM, Connors JM, Radford JA, Kim WS, Gallamini A, Ramchandren R, et al. First-line brentuximab vedotin plus chemotherapy to improve overall survival in patients with stage III/IV classical Hodgkin lymphoma: An updated analysis of ECHELON-1. J Clin Oncol (2022) 40(16_suppl):7503–3. doi: 10.1200/JCO.2022.40.16_suppl.7503
45. Allen PB, Savas H, Evens AM, Advani RH, Palmer B, Pro B, et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood (2021) 137(10):1318–26. doi: 10.1182/blood.2020007400
46. Josting A, Katay I, Rueffer U, Winter S, Tesch H, Engert A, et al. Favorable outcome of patients with relapsed or refractory hodgkin's disease treated with high-dose chemotherapy and stem cell rescue at the time of maximal response to conventional salvage therapy (Dex-BEAM). Ann Oncol (1998) 9(3):289–95. doi: 10.1023/A:1008283909959
47. Moskowitz C, Kewalramani T, Nimer S, Gonzalez M, Zelenetz A, Yahalom J. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory hodgkin's disease. Br J Haematology (2004) 124(5):645–52. doi: 10.1111/j.1365-2141.2003.04828.x
48. Goodman K, Riedel E, Serrano V, Gulati S, Moskowitz C, Yahalom J. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory hodgkin's lymphoma. J Clin Oncol (2008) 26(32):5240–7. doi: 10.1200/JCO.2007.15.5507
49. Arai S, Fanale M, deVos S, Engert A, Illidge T, Borchmann P, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leukemia lymphoma (2013) 54(11):2531–3. doi: 10.3109/10428194.2013.798868
50. Lazarus H, Loberiza F, Zhang M, Armitage J, Ballen K, Bashey A, et al. Autotransplants for hodgkin's disease in first relapse or second remission: A report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplantation (2001) 27(4):387–96. doi: 10.1038/sj.bmt.1702796
51. Sureda A, Constans M, Iriondo A, Arranz R, Caballero M, Vidal M, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in hodgkin's lymphoma autografted after a first relapse. Ann Oncol (2005) 16(4):625–33. doi: 10.1093/annonc/mdi119
52. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory hodgkin's lymphoma. J Clin Oncol (2012) 30(18):2183–9. doi: 10.1200/JCO.2011.38.0410
53. Moskowitz C, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood (2018) 132(25):2639–42. doi: 10.1182/blood-2018-07-861641
54. Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab vedotin with chemotherapy for stage III or IV hodgkin’s lymphoma. New Engl J Med (2018) 378(4):331–44. doi: 10.1056/NEJMoa1708984
55. Moskowitz C, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi M, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with hodgkin's lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2015) 385(9980):1853–62. doi: 10.1016/S0140-6736(15)60165-9
56. Zinzani PL, Lee HJ, Armand P, Johnson N, Brice P, Radford J, et al. Three-year follow-up of keynote-087: Pembrolizumab monotherapy in Relapsed/Refractory classic Hodgkin lymphoma. Blood (2019) 134(Supplement_1):240–0. doi: 10.1182/blood-2019-127280
57. Armand P, Engert A, Younes A, Lee H, Santoro A, Zinzani P, et al. Nivolumab for relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic cell transplantation (auto-HCT): Extended follow-up of the phase 2 single-arm CheckMate 205 study. Blood (2018) 132(Supplement 1):2897–7. doi: 10.1182/blood-2018-99-112067
58. Armand P, Chen Y, Redd R, Joyce R, Bsat J, Jeter E, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood (2019) 134(1):22–9. doi: 10.1182/blood.2019000215
59. Bachier C, Schade H, Zoghi B, Ramakrishnan A, Shah N. A phase II single arm study of nivolumab as maintenance therapy after autologous stem cell transplantation in patients with Hodgkin lymphoma at risk of relapse or progression. Blood (2021) 138(Supplement 1):2455–5. doi: 10.1182/blood-2021-148139
60. Herrera A, Chen L, Nieto Y, Holmberg L, Johnston P, Mei M, et al. Consolidation with nivolumab and brentuximab vedotin after autologous hematopoietic cell transplantation in patients with high-risk Hodgkin lymphoma. Blood (2020) 136(Supplement 1):19–20. doi: 10.1182/blood-2020-136384
61. Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol (2016) 34(31):3733–9. doi: 10.1200/JCO.2016.67.3467
62. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson N, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for Relapsed/Refractory classic Hodgkin lymphoma. J Clin Oncol (2017) 35(19):2125–32. doi: 10.1200/JCO.2016.72.1316
63. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott E, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin's lymphoma. New Engl J Med (2015) 372(4):311–9. doi: 10.1056/NEJMoa1411087
64. Younes A, Santoro A, Shipp M, Zinzani P, Timmerman J, Ansell S, et al. Nivolumab for classical hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol (2016) 17(9):1283–94. doi: 10.1016/S1470-2045(16)30167-X
65. Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood (2018) 131(11):1183–94. doi: 10.1182/blood-2017-10-811224
66. Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou D, et al. Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia (2020) 34(2):533–42. doi: 10.1038/s41375-019-0545-2
67. Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Cancer Res (2019) 25(24):7363–9. doi: 10.1158/1078-0432.CCR-19-1680
68. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2 trial. Lancet Haematology (2019) 6(1):e12–9. doi: 10.1016/S2352-3026(18)30192-3
69. Johnston PB, Pinter-Brown LC, Warsi G, White K, Ramchandren R. Phase 2 study of everolimus for relapsed or refractory classical Hodgkin lymphoma. Exp Hematol Oncol (2018) 7:12. doi: 10.1186/s40164-018-0103-z
70. Hamadani M, Collins GP, Samaniego F, Spira AI, Davies A, Radford J, et al. Phase 1 study of ADCT-301 (camidanlumab tesirine), a novel pyrrolobenzodiazepine-based antibody drug conjugate, in relapsed/refractory classical Hodgkin lymphoma. Blood (2018) 132(Supplement 1):928–8. doi: 10.1182/blood-2018-99-118198
71. Younes A, Sureda A, Ben-Yehuda D, Zinzani P, Ong T, Prince H, et al. Panobinostat in patients with Relapsed/Refractory hodgkin's lymphoma after autologous stem-cell transplantation: Results of a phase II study. J Clin Oncol (2012) 30(18):2197–203. doi: 10.1200/JCO.2011.38.1350
72. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/JCO.2017.76.0793
73. Herrera A, Palmer J, Martin P, Armenian S, Tsai N, Kennedy N, et al. Autologous stem-cell transplantation after second-line brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Ann Oncol (2018) 29(3):724–30. doi: 10.1093/annonc/mdx791
74. Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory hodgkin's lymphoma: A non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol (2015) 16(3):284–92. doi: 10.1016/S1470-2045(15)70013-6
75. LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood (2018) 132(1):40–8. doi: 10.1182/blood-2017-11-815183
76. LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Three-year outcomes with brentuximab vedotin plus bendamustine as first salvage therapy in relapsed or refractory Hodgkin lymphoma. Br J Haematology (2020) 189(3):e86–90. doi: 10.1111/bjh.16499
77. Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez A, Pinana J, et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO group). Ann Oncol (2019) 30(4):612–20. doi: 10.1093/annonc/mdz009
78. Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: The phase II HOVON/LLPC transplant BRaVE study. Haematologica (2021) 106(4):1129–37. doi: 10.3324/haematol.2019.243238
79. Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood (2021) 138(6):427–38. doi: 10.1182/blood.2020009178
80. Diefenbach C, Hong F, Ambinder R, Cohen J, Robertson M, David K, et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: Phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematology (2020) 7(9):e660–70. doi: 10.1016/S2352-3026(20)30221-0
81. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2021) 22(4):512–24. doi: 10.1016/S1470-2045(21)00005-X
82. Mei M, Lee H, Palmer J, Chen R, Tsai N, Chen L, et al. Response-adapted anti-PD-1–based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood (2022) 139(25):3605–16. doi: 10.1182/blood.2022015423
83. Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol (2021) 39(28):3109–17. doi: 10.1200/JCO.21.01056
84. Bryan LJ, Casulo C, Allen P, Smith SE, Savas H, Karmali R, et al. Pembrolizumab (PEM) added to ICE chemotherapy results in high complete metabolic response rates in Relapsed/Refractory classic Hodgkin lymphoma (cHL): A multi-institutional phase II trial. Blood (2021) 138(Supplement 1):229–9. doi: 10.1182/blood-2021-145111
85. Rossi C, Gilhodes J, Maerevoet M, Herbaux C, Morschhauser F, Brice P, et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: A series from lysa centers. Am J Hematol (2018) 93:1042–9. doi: 10.1002/ajh.25154
86. Casadei B, Argnani L, Morigi A, Lolli G, Broccoli A, Pellegrini C, et al. Effectiveness of chemotherapy after anti-PD-1 blockade failure for relapsed and refractory Hodgkin lymphoma. Cancer Med (2020) 9(21):7830–6. doi: 10.1002/cam4.3262
87. Carreau NA, Pail O, Armand P, Merryman R, Advani RH, Spinner MA, et al. Checkpoint blockade treatment may sensitize Hodgkin lymphoma to subsequent therapy. Oncologist (2020) 25:878–85. doi: 10.1634/theoncologist.2020-0167
88. Calabretta E, Guidetti A, Ricci F, Di Trani M, Monfrini C, Magagnoli M, et al. Chemotherapy after PD-1 inhibitors in relapsed/refractory Hodgkin lymphoma: Outcomes and clonal evolution dynamics. Br J Haematol (2022) 198(1):82–92. doi: 10.1111/bjh.18183
89. Santoro A, Mazza R, Pulsoni A, Re A, Bonfichi M, Zilioli V, et al. Five-year results of the BEGEV salvage regimen in relapsed/refractory classical Hodgkin lymphoma. Blood Advances (2020) 4(1):136–40. doi: 10.1182/bloodadvances.2019000984
90. Lynch R, Cassaday R, Smith S, Fromm J, Cowan A, Warren E, et al. Dose-dense brentuximab vedotin plus ifosfamide, carboplatin, and etoposide for second-line treatment of relapsed or refractory classical Hodgkin lymphoma: A single centre, phase 1/2 study. Lancet Haematology (2021) 8(8):e562–71. doi: 10.1016/S2352-3026(21)00170-8
91. Stamatoullas A, Ghesquieres H, Clement filliatre L, Quittet P, Morschhauser F, Ribrag V, et al. Brentuximab vedotin in first Refractory/Relapsed classical Hodgkin lymphoma patients treated by chemotherapy (ICE) before autologous transplantation. final analysis of phase II study. Blood (2019) 134(Supplement_1):132–2.
92. Moskowitz A, Advani R, Bartlett N, Vose J, Ramchandren R, Feldman T, et al. Brentuximab vedotin and nivolumab for relapsed or refractory classic Hodgkin lymphoma: Long-term follow-up results from the single-arm phase 1/2 study. Blood (2019) 134(Supplement_1):238–8. doi: 10.1182/blood-2019-122576
93. Wu J, Song Y, Chen X, Lin T, Cao J, Liu Y, et al. Camrelizumab for relapsed or refractory classical Hodgkin lymphoma: Extended follow-up of the multicenter, single-arm, phase 2 study. Int J Cancer (2022) 150(6):984–92. doi: 10.1002/ijc.33852
94. Lin N, Zhang M, Bai H, Liu H, Cui J, Ke X, et al. Efficacy and safety of GLS-010 (zimberelimab) in patients with relapsed or refractory classical Hodgkin lymphoma: A multicenter, single-arm, phase II study. Eur J Cancer (2022) 164:117–26. doi: 10.1016/j.ejca.2021.07.021
95. Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene (2007) 26(37):5420–32. doi: 10.1038/sj.onc.1210610
96. Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood (2008) 112(4):1424–33. doi: 10.1182/blood-2008-01-133769
97. Kirschbaum M, Goldman B, Zain J, Cook J, Rimsza L, Forman S, et al. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest oncology group study S0517. Leukemia Lymphoma (2012) 53(2):259–62. doi: 10.3109/10428194.2011.608448
98. Fournel M, Bonfils C, Hou Y, Yan P, Trachy-Bourget MC, Kalita A, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther (2008) 7(4):759–68. doi: 10.1158/1535-7163.MCT-07-2026
99. Younes A, Oki Y, Bociek R, Kuruvilla J, Fanale M, Neelapu S, et al. Mocetinostat for relapsed classical hodgkin's lymphoma: An open-label, single-arm, phase 2 trial. Lancet Oncol (2011) 12(13):1222–8. doi: 10.1016/S1470-2045(11)70265-0
100. Younes A, Ong TC, Ribrag V, Engert A, Ben-Yehuda D, McCabe R, et al. Efficacy of panobinostat in phase II study in patients with relapsed/refractory Hodgkin lymphoma (HL) after high-dose chemotherapy with autologous stem cell transplant. Blood (2009) 114(22):923–3. doi: 10.1182/blood.V114.22.923.923
101. Khaskhely NM, Buglio D, Shafer J, Bollard CM, Younes A. The histone deacetylase (HDAC) inhibitor entinostat (SNDX-275) targets Hodgkin lymphoma through a dual mechanism of immune modulation and apoptosis induction. Blood (2009) 114(22):1562–2. doi: 10.1182/blood.V114.22.1562.1562
102. Carlo-Stella C, Guidetti A, Viviani S, Bonfante V, Valagussa P, Marchiano` A, et al. Phase II trial of combination of the histone deacetylase inhibitor ITF2357 and meclorethamine demonstrates clinical activity and safety in heavily pretreated patients with relapsed/refractory Hodgkin lymphoma (HL). Blood (2008) 112(11):2586–6. doi: 10.1182/blood.V112.11.2586.2586
103. Michot J, Mouraud S, Adam J, Lazarovici J, Bigenwald C, Rigaud C, et al. CD8+ T lymphocytes immune depletion and LAG-3 overexpression in Hodgkin lymphoma tumor microenvironment exposed to anti-PD-1 immunotherapy. Cancers (2021) 13(21):5487. doi: 10.3390/cancers13215487
104. Timmerman J, Lavie D, Johnson N, Avigdor A, Borchmann P, Andreadis C, et al. Favezelimab (anti–LAG-3) plus pembrolizumab in patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) after anti–PD-1 treatment: An open-label phase 1/2 study. J Clin Oncol (2022) 40(16_suppl):7545–5. doi: 10.1200/JCO.2022.40.16_suppl.7545
105. Annibali O, Bianchi A, Grifoni A, Tomarchio V, Tafuri M, Verri M, et al. May tigit (T cell ig and ITIM domain) expression be a new target for Hodgkin lymphoma? Blood (2019) 134(Supplement_1):5235–5. doi: 10.1182/blood-2019-130794
106. Yusuf R, Jemielita T, Marinello P. Safety and efficacy of vibostolimab and pembrolizumab in patients with relapsed or refractory hematologic malignancies: A multicohort, open-label, phase 2 study. Blood (2021) 138(Supplement 1):2484–4. doi: 10.1182/blood-2021-151728
107. Hamadani M, Collins GP, Caimi PF, Samaniego F, Spira A, Davies A, et al. Camidanlumab tesirine in patients with relapsed or refractory lymphoma: A phase 1, open-label, multicentre, dose-escalation, dose-expansion study. Lancet Haematology (2021) 8(6):e433–45. doi: 10.1016/S2352-3026(21)00103-4
108. Milpied N, Fielding A, Pearce R, Ernst P, Goldstone A. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed hodgkin's disease. European group for blood and bone marrow transplantation. J Clin Oncol (1996) 14(4):1291–6. doi: 10.1200/JCO.1996.14.4.1291
109. Alvarez I, Sureda A, Caballero M, Urbano-Ispizua A, Ribera J, Canales M, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed Hodgkin lymphoma: Results of a Spanish prospective cooperative protocol. Biol Blood Marrow Transplantation (2006) 12(2):172–83. doi: 10.1016/j.bbmt.2005.09.009
110. Sureda A, Canals C, Arranz R, Caballero D, Ribera J, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory hodgkin's lymphoma. results of the HDR-ALLO study - a prospective clinical trial by the grupo espanol de Linfomas/Trasplante de medula osea (GEL/TAMO) and the lymphoma working party of the European group for blood and marrow transplantation. Haematologica (2012) 97(2):310–7. doi: 10.3324/haematol.2011.045757
111. Castagna L, Bramanti S, Devillier R, Sarina B, Crocchiolo R, Furst S, et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transplantation (2017) 52(5):683–8. doi: 10.1038/bmt.2016.348
112. Ahmed S, Kanakry J, Ahn K, Litovich C, Abdel-Azim H, Aljurf M, et al. Lower graft-versus-Host disease and relapse risk in post-transplant cyclophosphamide–based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood Marrow Transplantation (2019) 25(9):1859–68. doi: 10.1016/j.bbmt.2019.05.025
113. Martínez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: A registry study of the lymphoma working party of the European society for blood and marrow transplantation. J Clin Oncol (2017) 35(30):3425–32. doi: 10.1200/JCO.2017.72.6869
114. Bento L, Boumendil A, Finel H, Khvedelidze I, Blaise D, Fegueux N, et al. Tandem autologous-reduced intensity allogeneic stem cell transplantation in high-risk relapsed Hodgkin lymphoma: A retrospective study of the lymphoma working party-EBMT. Bone Marrow Transplant (2021) 56(3):655–63. doi: 10.1038/s41409-020-01075-y
115. Mariotti J, Bramanti S, Devillier R, Furst S, El Cheikh J, Sarina B, et al. Tandem autologous-haploidentical transplantation is a feasible and effective program for refractory Hodgkin lymphoma. Bone Marrow Transplant (2018) 53(3):366–70. doi: 10.1038/s41409-017-0032-1
116. Merryman R, Castagna L, Giordano L, Ho V, Corradini P, Guidetti A, et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia (2021) 35(9):2672–83. doi: 10.1038/s41375-021-01193-6
117. Merryman R, Kim H, Zinzani P, Carlo-Stella C, Ansell S, Perales M, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood (2017) 129(10):1380–8. doi: 10.1182/blood-2016-09-738385
118. Haverkos B, Abbott D, Hamadani M, Armand P, Flowers M, Merryman R, et al. PD-1 blockade for relapsed lymphoma post–allogeneic hematopoietic cell transplant: High response rate but frequent GVHD. Blood (2017) 130(2):221–8. doi: 10.1182/blood-2017-01-761346
119. Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood (2017) 129(18):2471–8. doi: 10.1182/blood-2016-11-749556
120. Wang C, Wu Z, Wang Y, Guo Y, Dai H, Wang X, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: An open-label phase I trial. Clin Cancer Res (2017) 23(5):1156–66. doi: 10.1158/1078-0432.CCR-16-1365
121. Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J Clin Oncol (2020) 38(32):3794–804. doi: 10.1200/JCO.20.01342
122. Ahmed S, Flinn I, Mei M, Riedell P, Armand P, Grover N, et al. Safety and efficacy profile of autologous CD30.CAR-T-Cell therapy in patients with relapsed or refractory classical Hodgkin lymphoma (CHARIOT trial). Blood (2021) 138(Supplement 1):3847–7.
123. Voorhees T, Zhao B, Oldan J, Hucks G, Khandani A, Dittus C, et al. Pretherapy metabolic tumor volume is associated with response to CD30 CAR T cells in Hodgkin lymphoma. Blood Advances (2022) 6(4):1255–63. doi: 10.1182/bloodadvances.2021005385
124. Voorhees T, Grover N, Beaven A, Park S, Morrison J, Cheng C, et al. Retrospective cohort study analyzing the safety and efficacy of anti-PD-1 therapy following CD30 CAR-T cell therapy in Relapsed/Refractory Hodgkin lymphoma. Blood (2019) 134(Supplement_1):3233–3. doi: 10.1182/blood-2019-122846
125. Nieto Y, Banerjee P, Kaur I, Bassett R, Kerbauy L, Basar R, et al. Abstract CT003: Innate cell engager (ICE®) AFM13 combined with preactivated and expanded cord blood (CB)-derived NK cells for patients with refractory/relapsed CD30+ lymphoma. Cancer Res (2022) 82(12_Supplement):CT003–3. doi: 10.1158/1538-7445.AM2022-CT003
Keywords: Classical Hodgkin lymphoma (cHL), relapsed and refractory Hodgkin's lymphoma, chemoimmunotherapy, hematopoietic stem cell transplant, chimeric antigen receptor (CAR) T-cell therapy
Citation: Ullah F, Dima D, Omar N, Ogbue O and Ahmed S (2023) Advances in the treatment of Hodgkin lymphoma: Current and future approaches. Front. Oncol. 13:1067289. doi: 10.3389/fonc.2023.1067289
Received: 11 October 2022; Accepted: 07 February 2023;
Published: 03 March 2023.
Edited by:
Matthew Mei, City of Hope National Medical Center, United StatesReviewed by:
Saurabh Chhabra, Mayo Clinic Arizona, United StatesCopyright © 2023 Ullah, Dima, Omar, Ogbue and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sairah Ahmed, c2FobWVkM0BtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.