
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 25 January 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1062138
This article is part of the Research Topic Case Reports in Thoracic Oncology: 2022 View all 42 articles
Globally, lung cancer is the leading cause of cancer-related mortality. Multiple primary lung cancers (MPLC) account for a very small portion of all primary lung cancer cases. Importantly, a quick and precise differentiation between MPLC and intrapulmonary metastases is directly related to patient prognoses as treatment strategies vary according to pathological type. Synchronous MPLC are most commonly seen in the same lung. Here, we report a rare case of a patient with synchronous MPLC of both lungs. A 67-year-old man, with a 1-month cough and expectoration history, was admitted in our hospital. Computed tomography (CT) chest scan revealed a lower lobe nodule in the left lung and an upper lobe nodule in the right lung. He underwent successive fiberoptic bronchoscopy and CT-guided percutaneous pulmonary aspiration biopsy of both lungs. The pathological diagnosis was squamous cell carcinoma of the left lung and adenocarcinoma of the right lung.
An early diagnosis and the increasing effectiveness of cancer therapies have significantly prolonged overall survival times in cancer patients. However, cancer survivors had a higher risk of developing new malignancies when compared with the general population. This situation poses new problems which are manifested as soaring incidences of multiple primary tumors (1).
Globally, lung cancer is the most common cause of cancer death, with the lungs one of the most common sites in terms of multiple primary malignancy (2). Initial diagnostic criteria for “multiple primary lung cancers” (MPLC) were published in 1975 based on histology and tumor locations (3). However, these criteria could not differentiate MPLC from intrapulmonary metastases (IPM). Furthermore, special cases may be misdiagnosed as MPLC without the pathological confirmation of every lesion, such as lepidic adenocarcinoma, it displays multiple pure ground-glass opacity lesions by computed tomography (CT) (4). In 2015, the World Health Organization Classification of Tumors of the Lung redefined MPLC diagnostic criteria and recommended a multidisciplinary tumor board approach to confirm the diagnosis (5). In addition to histological subtyping, algorithms based on comprehensive clinical and imaging variables and comparative genomic hybridization array information, were also applied to differentiate MPLC from IPM (6, 7).
MPLC is subdivided into two categories depending on the time of diagnosis of each primary site; metachronous MPLC (MMPLC) and synchronous MPLC (SMPLC). MMPLC is common and generally occurs in sequence after more than a 2-year cancer-free interval, while SMPLC is relatively rare and presents simultaneously or within a six months interval (8). In this study, we present a SMPLC case with right upper lobe adenocarcinoma and left lower lobe squamous cell carcinoma.
On December 28th, 2020, a 67-year-old male came to our institute with a 1-month history of fever, cough and expectoration. He had smoked for 20 years. A physical examination revealed moist rales in the left lung base. From routine blood tests, the white blood cell count was 18.68×109/L. Inflammatory indicators, such as C-reactive protein and procalcitonin, were significantly elevated. Tumor marker detection indicated carcinoembryonic antigen serum levels were slightly elevated. Sputum cultures suggested a fungal infection. CT scan identified an infectious lesion in the left lower lobe, accompanied by pulmonary atelectasis and an upper lobe nodule in the right lung (Figure 1A). He then underwent fiberoptic bronchoscopy (FB). The pathological diagnosis from this was squamous cell carcinoma of the left lung. Hematoxylin and eosin (HE) staining showed squamous cell carcinoma (Figure 2A). Immumohistochemical staining (IHC) showed a P40, P63, and CK5/6 positive status (Figures 2B–D). Considering a fungal infection in the left lower lobe and atelectasis, he first received anti-infection therapy for approximately 1 month. The patient was then admitted and reviewed on January 23rd, 2021, a chest CT indicated that the soft tissue mass and obstructive atelectasis in the left lower lobe were absorbed (Figure 1B). Considering bilateral lung metastases before treatment, he received adjuvant chemotherapy as an initial treatment. After four cycles of docetaxel plus nedaplatin (TP) regimen, CT showed a partial response in the left lower lobe; however, the right lung nodule showed no significant change (Figure 1C). To make a definite diagnosis of the right lung lesion and inaugurate timely and responsive clinical treatment, he underwent a percutaneous right lung puncture biopsy guided by CT. Pathological examination showed changes which were completely different from the left lung (Figure 3A). IHC staining showed that CK7 and TTF-1 were strongly positive, while NapsinA was weakly positive (Figures 3B–D), therefore, the pathological diagnosis was adenocarcinoma. Genetic testing indicated a TP53 mutation, EGFR was wild type, and the tumor cell proportion score was 5%. After completion of the 5th TP regimen cycle on May 14th, 2021, CT showed a very good partial response in the left lower lobe, while the right lung nodular was larger than before (Figure 1D). Considering the significant regression of the left lung lesion, the patient commenced adenocarcinoma treatment. He received stereotactic radiotherapy on June 14th, 2021, the prescribed dose was 60.0 Gy in eight fractions. He subsequently received immune checkpoint inhibitor (ICI) therapy in combination with pemetrexed and carboplatin (PP). The right lung lesion had obviously diminished after completion of the first immunochemotherapy cycle (Figure 1E) and he achieved very good remission in both lungs after three cycles of this regimen (Figure 1F). Due to the frequency of grade III-IV myelosuppression and pulmonary infection, he was given another three cycles of pemetrexed plus ICI, followed by intensity modulated radiation therapy for the left lung lesion. The prescribed pulmonary dose was 60.0 Gy, with daily fractions of 2.0 Gy. Finally, paclitaxel plus ICI was administrated every 3 weeks for maintenance treatment. Currently, the patient exhibits no evidence of disease recurrence and progression. Detailed diagnosis information and a treatment flow chart are shown (Figure 4).
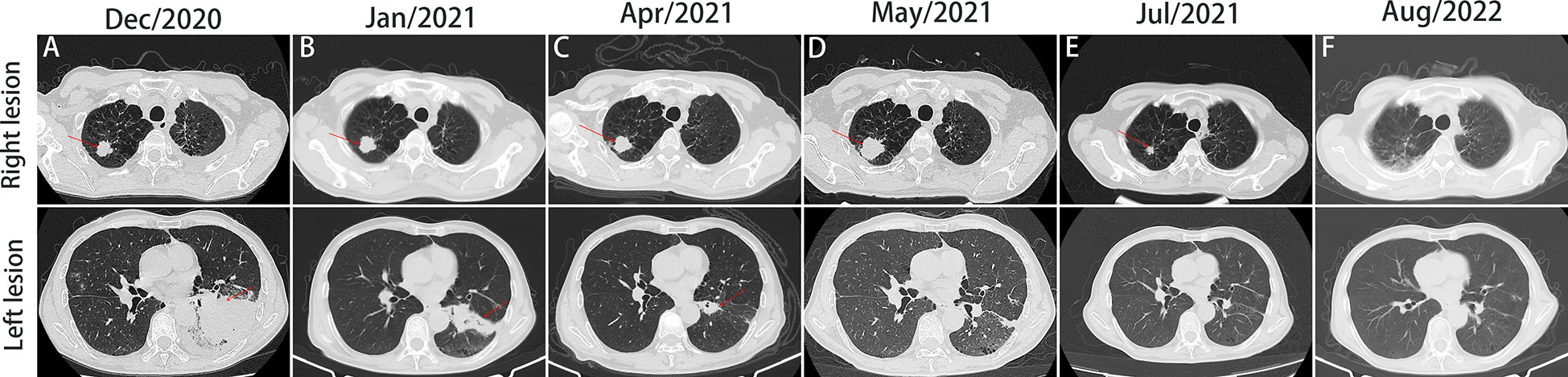
Figure 1 Computed tomography (CT) scan of the patient throughout the whole course of diagnosis and treatment. Figures A-E, imaging changes of chest CT (mediastinal window and pulmonary window) at different times.
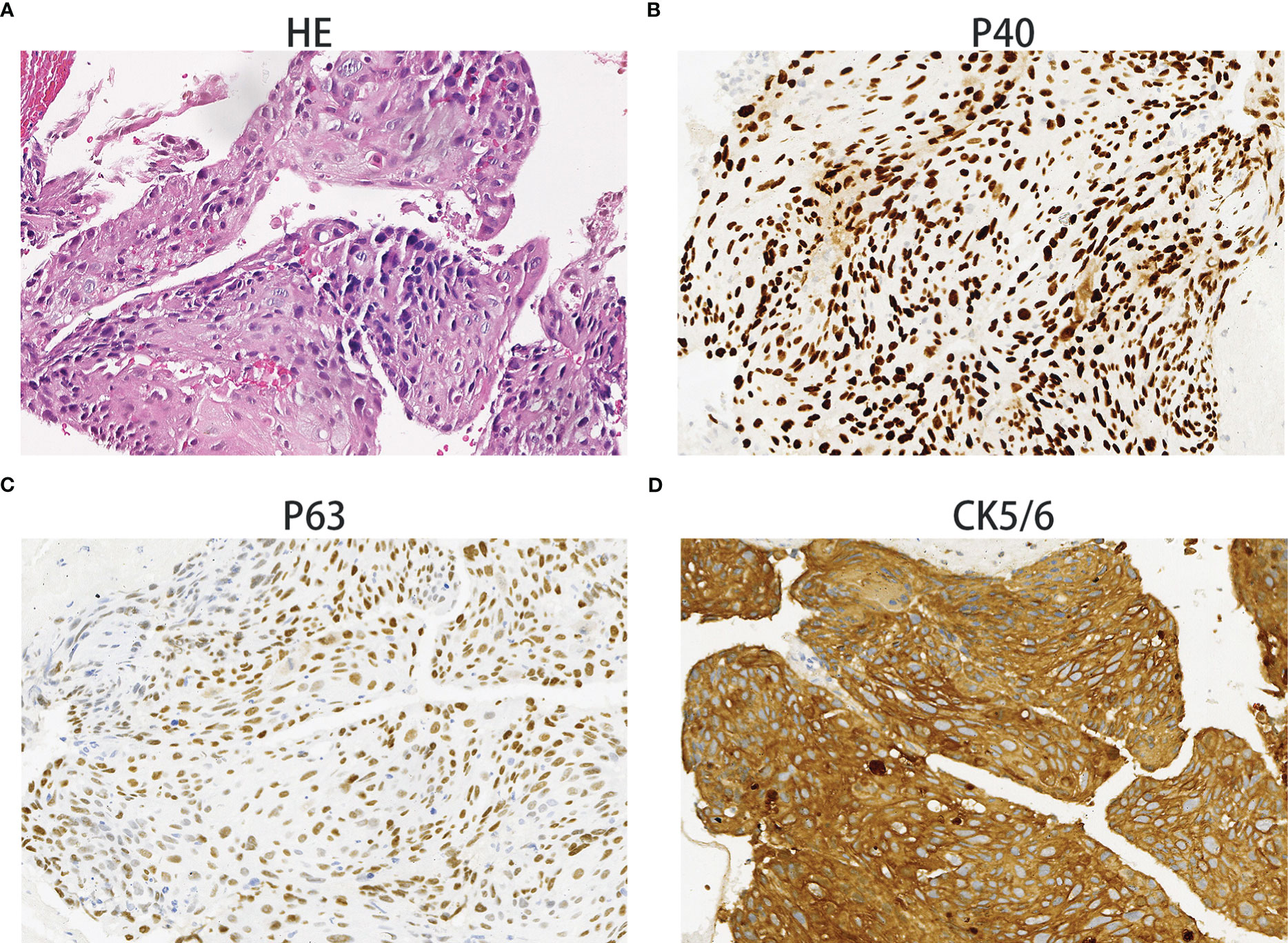
Figure 2 Squamous cell carcinoma. Hematoxylin and eosin staining showing squamous cell carcinoma histology (A). The immunohistochemical examination indicated malignant cells immunoreactive for P40 (B); positive for P63 (C); strongly positive for CK5/6 (D). Magnification 100×.
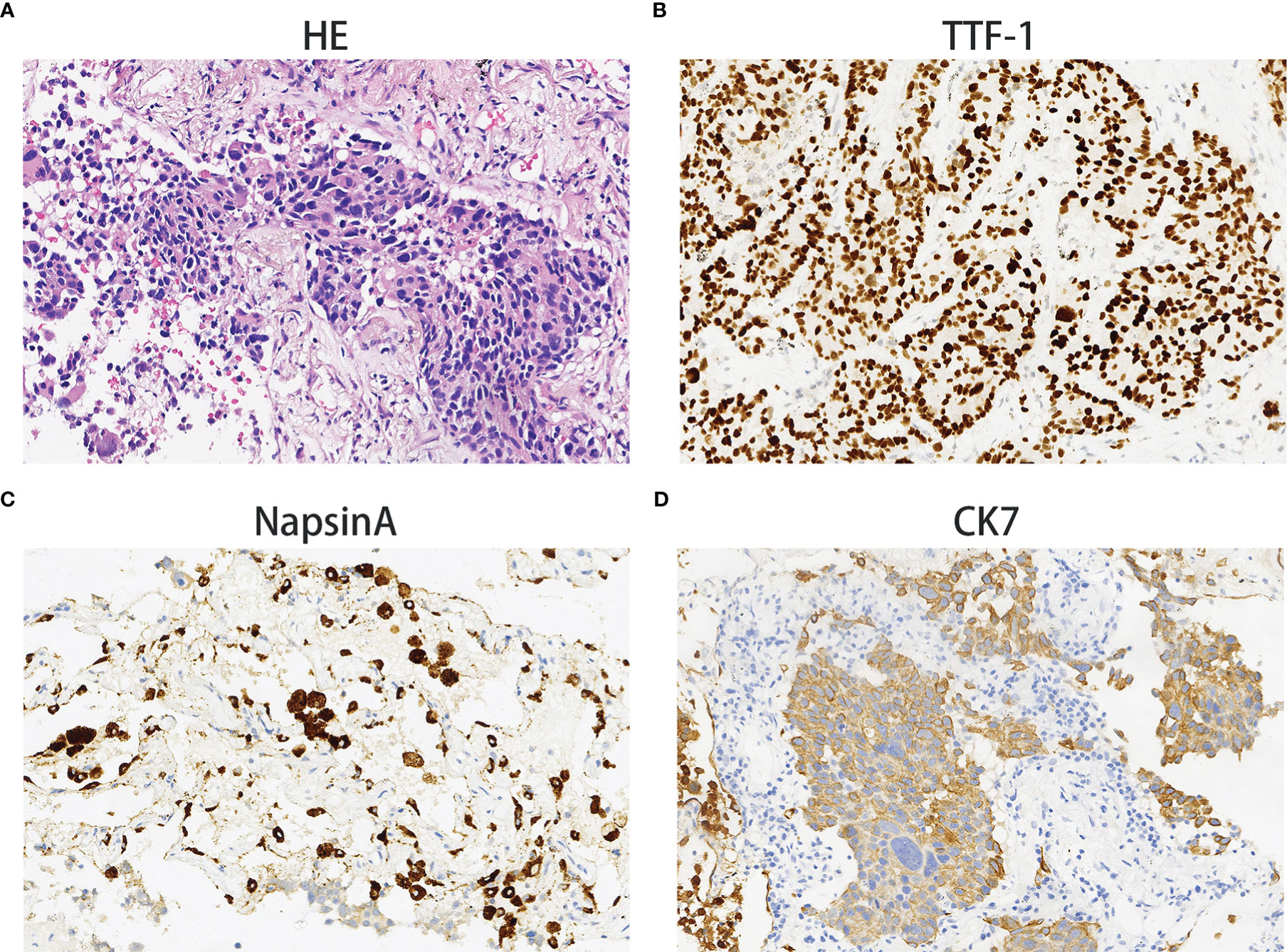
Figure 3 Adenocarcinoma. Hematoxylin and eosin staining showing adenocarcinoma histology (A). The immunohistochemical examination indicated malignant cells immunoreactive for TTF-1 (B); weakly positive for NapsinA (C); strongly positive for CK7 (D). Magnification 100×.
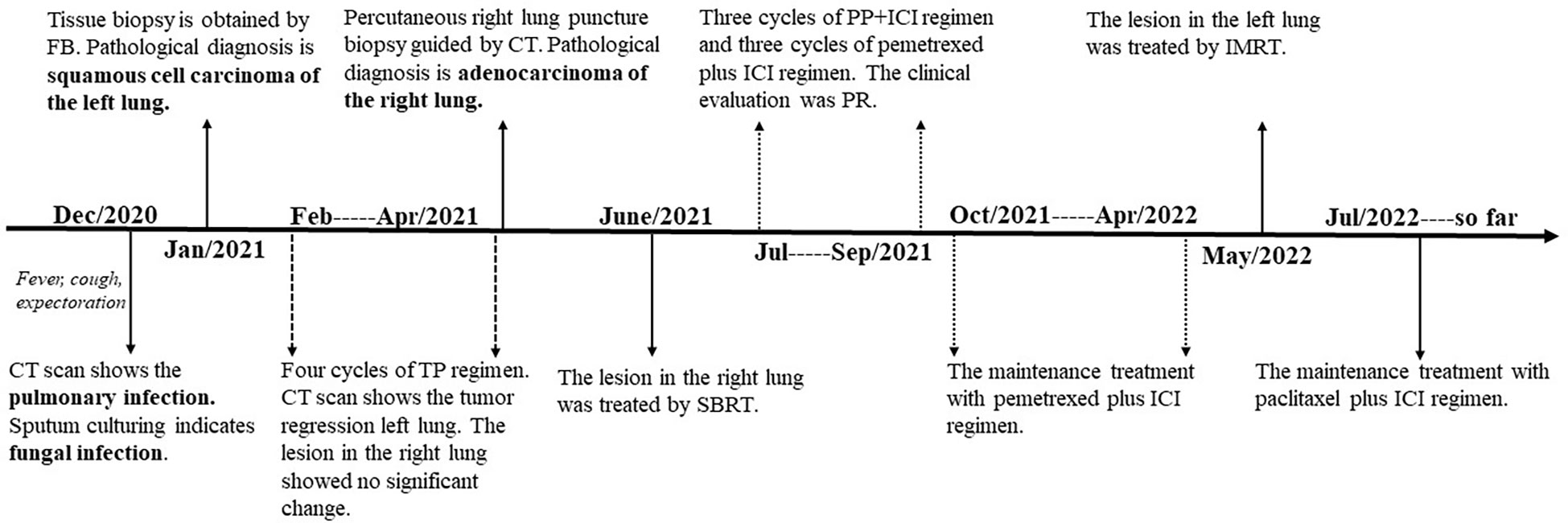
Figure 4 The timeline of the patient’s events from diagnosis to treatment. CT, computed tomography; FB, fiberoptic bronchoscopy; TP, docetaxel plus nedaplatin; SBRT, stereotactic body radiation therapy; ICI, immune checkpoint inhibitor; PP, pemetrexed plus carboplatin; PR, partial remission; IMRT, intensity-modulated radiation therapy.
We report a patient with SMPLC who was successively diagnosed with left lung squamous cell carcinoma and right lung adenocarcinoma. SMPLC were identified in 1924 and then were widely recognized. The estimated SMPLC incidence accounts for 0.2%–8% of all lung cancers and has steadily increased over the past three decades (9).
MPLC may be caused by intrinsic and non-intrinsic cancer risk factor; intrinsic risk factors are defined as the genetic mutations caused by DNA replication errors, including EGFR, KRAS, TP53, or PARP1 mutations (10). Non-intrinsic risk factors refer to modified endogenous factors, including lifestyle, radiation, and any other endogenous factors. It is accepted that smoking and the widespread use of high-resolution CT and positron emission tomography-CT contribute to MPLC occurrence (9).
Currently, no golden diagnostic criteria exist for MPLC due to the tumor heterogeneity and a poor understanding of associated clinicopathological characteristics. Therefore, a diagnosis should be considered by a multidisciplinary tumor board based on clinical manifestations, imaging features, pathological characteristics, and molecular genetic characteristics (11). MPLC stage classification is critical for patients, because staging affects initial treatment choices. For MMPLC, the second tumor should be staged as the primary according to the 7th Tumor-Node-Metastasis (TNM) classification guidelines. However, SMPLC staging is ambiguous, each tumor should be staged separately and only one TNM stage should be provided based on all combined tumors (8). In some situations, SMPLC is defined as the highest pathological stage (12).
Surgery is the cornerstone treatment for early MPLC without lymph node involvement. For surgery, the indications and contraindications must be strictly understood. Tumor’s size and location, Eastern Cooperative Oncology Group performance status, and cardiopulmonary function must be considered before surgery. Operational styles are also closely related to patient quality of life and prognosis. Pneumonectomy has a high risk of postoperative respiratory failure and may herald a poor prognosis. Though segmentectomy or wedge resection has a risk of local recurrence (up to 15%), it is tolerable for patients with compromised pulmonary function who are unfit for more extensive resection (13). The Lung Study Group recommends that pneumonectomy should be avoided whenever possible, thus limited resection remains the mainstay treatment for MPLC.
Systemic chemotherapy is crucial for patients with mediastinal lymph node and distant metastasis. For patients with MMPLC, the second tumor should be treated as a primary tumor. However, the SMPLC treatment strategy is that each tumor should be staged and treated separately (14). Due to differences in chemotherapy regimens between adenocarcinoma and squamous cell carcinoma, it is often difficult for clinicians to select which regimen should be first perform for SMPLC patients with distinct tumor histology. It remains to be determined whether both effective regimen or sequential treatment for each tumor should be the first choice. In our study, the patient benefited from sequential treatments. After five TP regimen cycles, the lesion shrank and obstructive pneumonia improved significantly in this lung. Then, the right lung nodule significantly was reduced after the replacement of the PP regimen. Our strategy indicated that sequential treatment was effective for patients with SMPLC in both lungs with different pathological types, especially when the problem was more severe in one lung, such as severe pulmonary infection, obstructive pneumonia or pulmonary atelectasis.
Previous studies reported that ICI plus chemotherapy generated higher objective remission rates and superior overall survival and progression-free survival rates in patients with previously untreated, metastatic, non-small cell lung cancer (NSCLC) when compared with single chemotherapy (15, 16). Our patient benefited from combined ICI and chemotherapy in adjuvant and maintenance treatment phases. Thus, ICI may be recommended for patients with advanced MPLC, but this hypothesis requires further research.
Radiotherapy improves local control rates in cancer. Intensity- modulated radiation therapy or three-dimensional conformal radiation is still an important treatment strategy for stage I–IIIB inoperable patients with lung cancers (17). Several studies reported that stereotactic body radiation therapy (SBRT) generated similar clinical outcomes in early NSCLC when compared with surgical treatment (18). Furthermore, SBRT may be a potential cure approach for early stage MPLC as it achieves promising long-term tumor control and survival (19). Given these factors, radiation is indispensable for MPLC treatment.
The 5-year overall survival for MPLC ranges from 20% to 70% (12). The prognosis is related to several clinical factors. Patients with same tumor histology are relatively favorable when compared with those with different histology (20). Patients with SMPLC or early MMPLC have lower survival rates when compared with patients with late MMPLC (21). Pneumonectomy has a higher early postoperative mortality rate and a shorter overall survival rate when compared with limited resection (21). Multivariable analyses have indicated that pathological stage and lymph node metastases are associated with prognosis (21). Therefore, the MPLC diagnosis and treatment should be determined by a multi-disciplinary team.
In conclusion, we reported the rare but interesting case of a patient with SMPLC in both lungs. The patient was first misdiagnosed with squamous cell carcinoma of the left lung, accompanied by right IPM and mediastinal lymph node metastasis. He received sequential chemotherapy plus ICI and radiotherapy without surgical resection, and is doing well under ICI maintenance. We believe this interesting case may provoke debate in the literature about the precise diagnosis and effective treatment for MPLC cases. Our result also indicated that the proper tumor subclassification (with IHC and molecular methods) was important for patients with SMPLC to receive individualize treatment and achieve better outcome.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics and Scientific Committee of Hubei University of Medicine with approval number XYY2021002. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Conceptualization, DZ; data curation and writing, writing—review and editing, YL, YD, and HY; funding acquisition, DZ. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation of China (Grants number: 82200214), Key Research and Development Project of Hubei (Grants number: 2022BCE028), Innovative Research Program of Xiangyang No.1 People’s Hospital (Grants number: XYY2021Q02), Platform Special Fund for Scientific Research of Xiangyang No.1 People’s Hospital (Grants number: XYY2022P05) and Key projects of Xiangyang Science and Technology Bureau (2021YL26).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Spratt JS Jr., Hoag MG. Incidence of multiple primary cancers per man-year of follow up: 20-year review from the Ellis fischel state cancer hospital. Ann surgery. (1966) 164(5):775–84. doi: 10.1097/00000658-196611000-00001
2. Shoji F, Yamazaki K, Miura N, Katsura M, Oku Y, Takeo S, et al. Postoperative management of multiple primary cancers associated with non-small cell lung cancer. Anticancer Res (2018) 38(6):3773–8. doi: 10.21873/anticanres.12660
3. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc surgery. (1975) 70(4):606–12. doi: 10.1016/S0022-5223(19)40289-4
4. Kim HK, Choi YS, Kim J, Shim YM, Lee KS, Kim K. Management of multiple pure ground-glass opacity lesions in patients with bronchioloalveolar carcinoma. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2010) 5(2):206–10. doi: 10.1097/JTO.0b013e3181c422be
5. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630
6. Suh YJ, Lee HJ, Sung P, Yoen H, Kim S, Han S, et al. A novel algorithm to differentiate between multiple primary lung cancers and intrapulmonary metastasis in multiple lung cancers with multiple pulmonary sites of involvement. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15(2):203–15. doi: 10.1016/j.jtho.2019.09.221
7. Ostrovnaya I, Olshen AB, Seshan VE, Orlow I, Albertson DG, Begg CB. A metastasis or a second independent cancer? Evaluating the clonal origin of tumors using array copy number data. Stat Med (2010) 29(15):1608–21. doi: 10.1002/sim.3866
8. Jiang L, He J, Shi X, Shen J, Liang W, Yang C, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: Systematic review and meta-analysis. Lung Cancer (Amsterdam Netherlands). (2015) 87(3):303–10. doi: 10.1016/j.lungcan.2014.12.013
9. Warth A, Macher-Goeppinger S, Muley T, Thomas M, Hoffmann H, Schnabel PA, et al. Clonality of multifocal nonsmall cell lung cancer: Implications for staging and therapy. Eur Respir J (2012) 39(6):1437–42. doi: 10.1183/09031936.00105911
10. Izumi M, Oyanagi J, Sawa K, Fukui M, Ogawa K, Matsumoto Y, et al. Mutational landscape of multiple primary lung cancers and its correlation with non-intrinsic risk factors. Sci Rep (2021) 11(1):5680. doi: 10.1038/s41598-021-83609-y
11. Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest (2013) 143(5 Suppl):e191S–210S. doi: 10.1378/chest.12-2354
12. Liu M, He W, Yang J, Jiang G. Surgical treatment of synchronous multiple primary lung cancers: a retrospective analysis of 122 patients. J Thorac disease. (2016) 8(6):1197–204. doi: 10.21037/jtd.2016.04.46
13. Kocaturk CI, Gunluoglu MZ, Cansever L, Demir A, Cinar U, Dincer SI, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surgery. (2011) 39(2):160–6. doi: 10.1016/j.ejcts.2010.05.037
14. Jiang L, He J, Shi X, Shen J, Liang W, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: Systematic review and meta-analysis. lung cancer. J Int Assoc Study Lung Cancer (2015) 87(3):303-10. doi: 10.1016/j.lungcan.2014.12.013
15. Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(3):387–97. doi: 10.1016/S1470-2045(19)30801-0
16. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. New Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
17. Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat oncology biology physics. (2003) 57(3):875–90. doi: 10.1016/S0360-3016(03)00743-0
18. Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat oncology biology physics. (2012) 84(5):1060–70. doi: 10.1016/j.ijrobp.2012.07.2354
19. Chang JY, Liu YH, Zhu Z, Welsh JW, Gomez DR, Komaki R, et al. Stereotactic ablative radiotherapy: A potentially curable approach to early stage multiple primary lung cancer. Cancer (2013) 119(18):3402–10. doi: 10.1002/cncr.28217
20. Jung EJ, Lee JH, Jeon K, Koh WJ, Suh GY, Chung MP, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer (Amsterdam Netherlands). (2011) 73(2):237–42. doi: 10.1016/j.lungcan.2010.11.008
Keywords: multiple primary lung cancers, intrapulmonary metastases, squamous cell carcinoma, adenocarcinoma, synchronous MPLC
Citation: Liu Y, Yu H, Dong Y and Zhang D (2023) Case Report: A case of synchronous right upper lobe adenocarcinoma and left lower lobe squamous cell carcinoma treated with immune checkpoint inhibitor plus chemotherapy. Front. Oncol. 13:1062138. doi: 10.3389/fonc.2023.1062138
Received: 05 October 2022; Accepted: 13 January 2023;
Published: 25 January 2023.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanCopyright © 2023 Liu, Yu, Dong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongdong Zhang, emhhbmdkb25nZG9uZ0B3aHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.