- Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
Purpose: To evaluate the relationship between novel serum lipid index and chemoresistance as well as prognosis of epithelial ovarian cancer (EOC).
Patients and methods: Patients’ serum lipid profiles of 249 cases diagnosed with epithelial ovarian cancer, including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) as well as their ratios, the novel indicators HDL-C/TC and HDL-C/LDL-C, and clinicopathologic characteristics were retrospectively collected and calculated from January 2016 to January 2020 and correlation between serum lipid index and clinicopathological features such as chemoresistance as well as prognosis were evaluated.
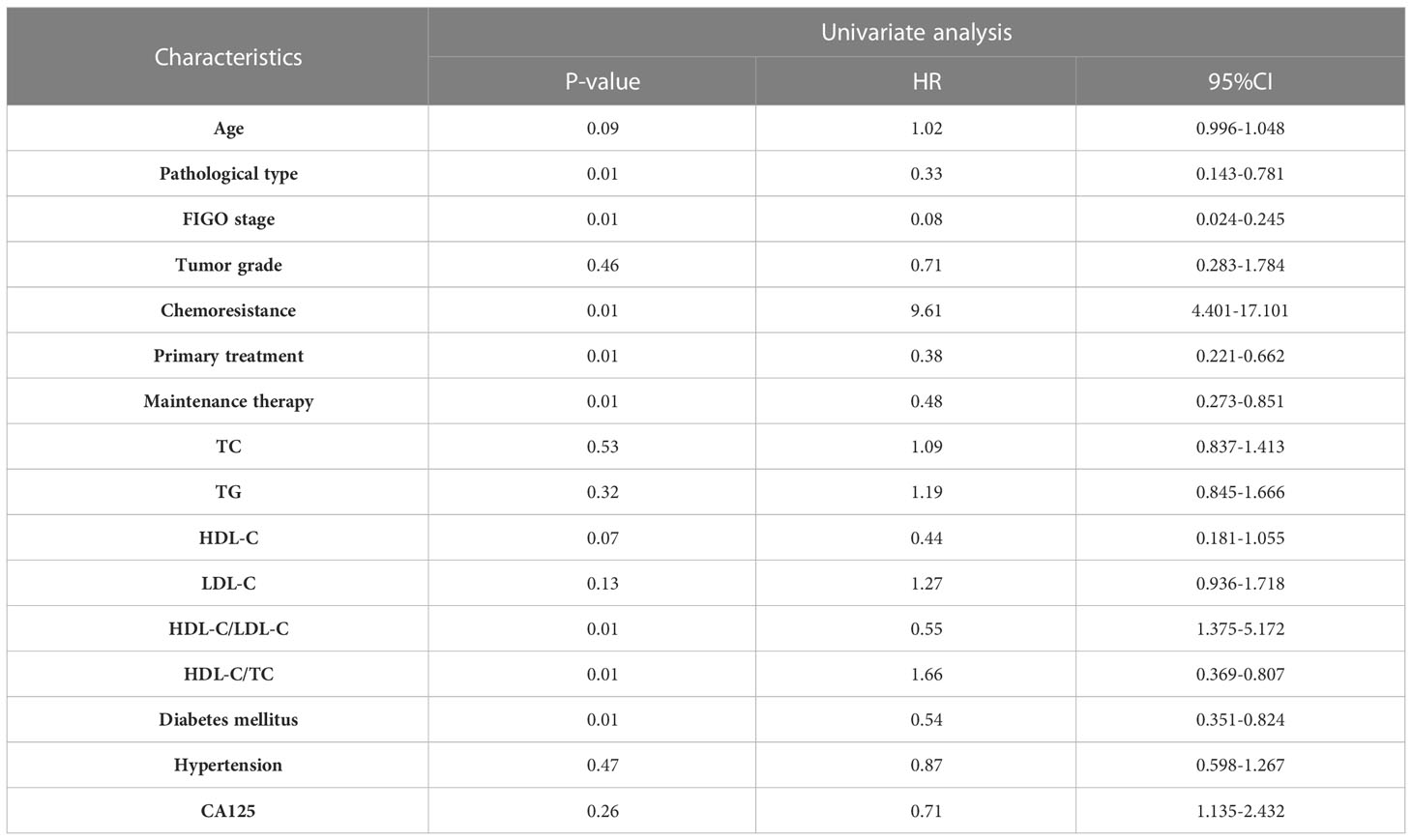
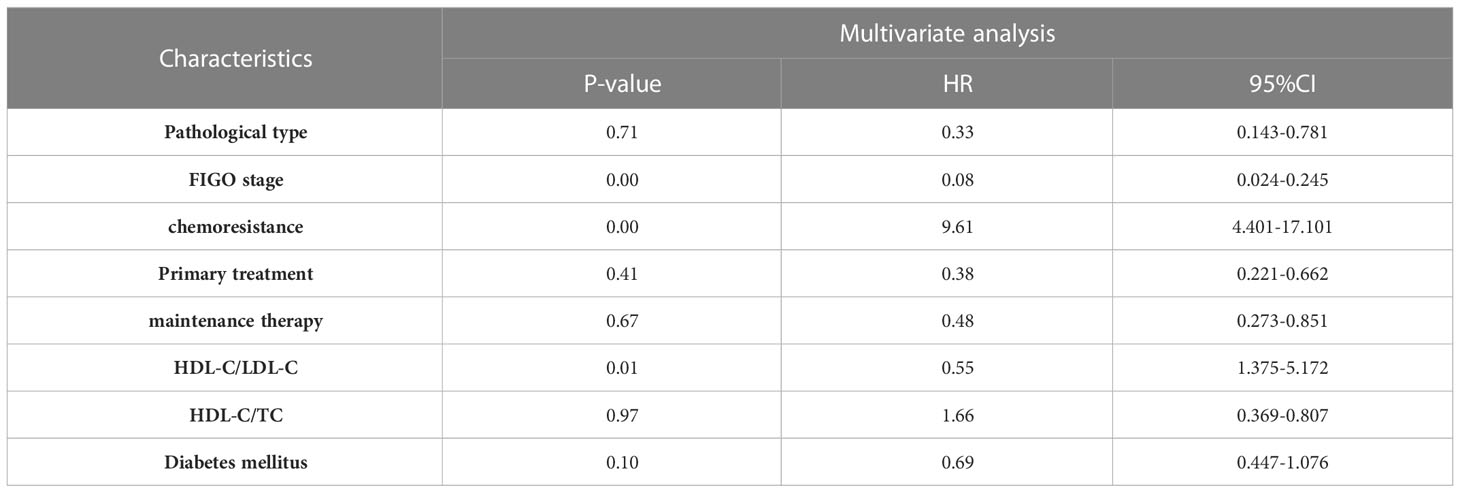
Results: 249 patients pathologically diagnosed EOC who underwent cytoreductive surgery were included in our cohort. The mean age of these patients was 55.20 ± 11.07 years. Binary logistic regression analyses indicated Federation International of Gynecology and Obstetrics (FIGO(stage and HDL-C/TC ratio had significant association with chemoresistance. Univariate analyses demonstrated pathological type, chemoresistance, FIGO stage, neoadjuvant chemotherapy, maintenance treatment, HDL-C/LDL-C ratio, HDL-C/TC ratio were related to Progression-Free Survival (PFS) and Overall Survival (OS) (P<0. 05). Particularly, multivariate analyses indicated that HDL-C/LDL-C ratio was independent protective factors for both PFS and OS.
Conclusion: The complex serum lipid index HDL-C/TC ratio has a significant correlation with chemoresistance. HDL-C/LDL-C ratio is closely related to the clinicopathological characteristics and prognosis of patients with EOC and is an independent protective factor indicating better outcome.
Introduction
Ovarian cancer (OC) is the most lethal malignant tumor of female reproductive system. The standard treatment for ovarian cancer is cytoreductive surgery combined with platinum-based chemotherapy and maintenance therapy based on tumor heterogeneity (1, 2). Lack of sensitive and effective screening methods, as well as atypical clinical symptoms, leads to initial diagnosis of advanced stage in more than 70% of patients. Although the initial response rate is 70–80% including 40 to 50% complete response, majority of patients relapse within 2 years with subsequent resistance to chemotherapy, leading to a 5-year survival rate of ovarian cancer patients only 45% with a gradually shortened interval between recurrences (3).
Multidisciplinary management of ovarian cancer patients is key to improve outcome. The introduction of imaging biomarkers, such as those obtained with FDG PET/CT, could help improve patient outcome, by precisely staging and restaging, as well as providing a prognostic value (4). More serological markers have also become a hotspot in predicting tumor prognosis.
Disturbance of lipid metabolism in cancer cells can lead to structural changes of tumor cell membrane, turbulence of intracellular energy metabolism, dysregulation of cell signal transduction and gene expression (5). As the source and transforming component of lipid metabolism in tumor microenvironment, serum lipids play an important role in tumorigenesis. A greater synthesis or absorption of lipids has been found in a variety of cancers as the raw materials contributing to the growth of cancer cells and the formation of tumors. Reprogramming of lipid metabolism can also affect the development of tumor cells by changing the fluidity of cell membranes and weakening immune function (6). It is reported that serum lipid levels are closely related to the occurrence and development of malignant tumors such as breast cancer (7), gastric cancer (8) and lung cancer (6). Mesenchyme-derived adipocytes in the tumor microenvironment can promote tumorigenesis of prostate cancer by releasing fatty acids and related factors (9). Adipose stem cells derived from the human omentum can promote proliferation, migration, chemoresistance of ovarian cancer cells and radiation resistance in nude mice model of ovarian cancer (10). There is also a correlation between lipid metabolism in tumor microenvironment and the efficacy of targeted drugs. For example, adipose abundance in breast cancer tumors was significantly correlated with resistance to trastuzumab (11).
The main components of serum lipids are total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). TC is an essential lipid to maintain cell homeostasis and an important part of cell membrane, which is rich in lipid raft and plays a key role in intracellular signal transduction (12). HDL-C maintains normal cellular cholesterol homeostasis by removing excess cholesterol from cells and transporting it to the liver. When the level of HDL-C is low, the accumulated cholesterol is utilized by cancer cells for membrane formation, thereby promoting the development of cancer. On the contrary, LDL-C is a significant carrier of cholesterol from the liver to tissues throughout the body, providing energy and materials for tumor cell proliferation and development (12). It has been reported in literatures, TC、TG、HDL-C and LDL-C are independent factors for survival prediction of various tumors such as gastric cancer (13) and breast cancer (14).
Novel complex indicators of lipid metabolism, such as TG/HDL-C ratio, have been proved superior to that of single TG or HDL-C level in predicting survival of patients with triple negative breast cancer (15) and gastric cancer (7). These results suggest that complex novel indicators might be better predictive index for survival prediction. Correlation between complex lipid indicators and prognosis in patients of ovarian cancer has not been clearly reported. Therefore, this study aimed to explore the correlation between serum lipid levels and chemoresistance as well as prognosis in patients of ovarian cancer.
Material and methods
Patients
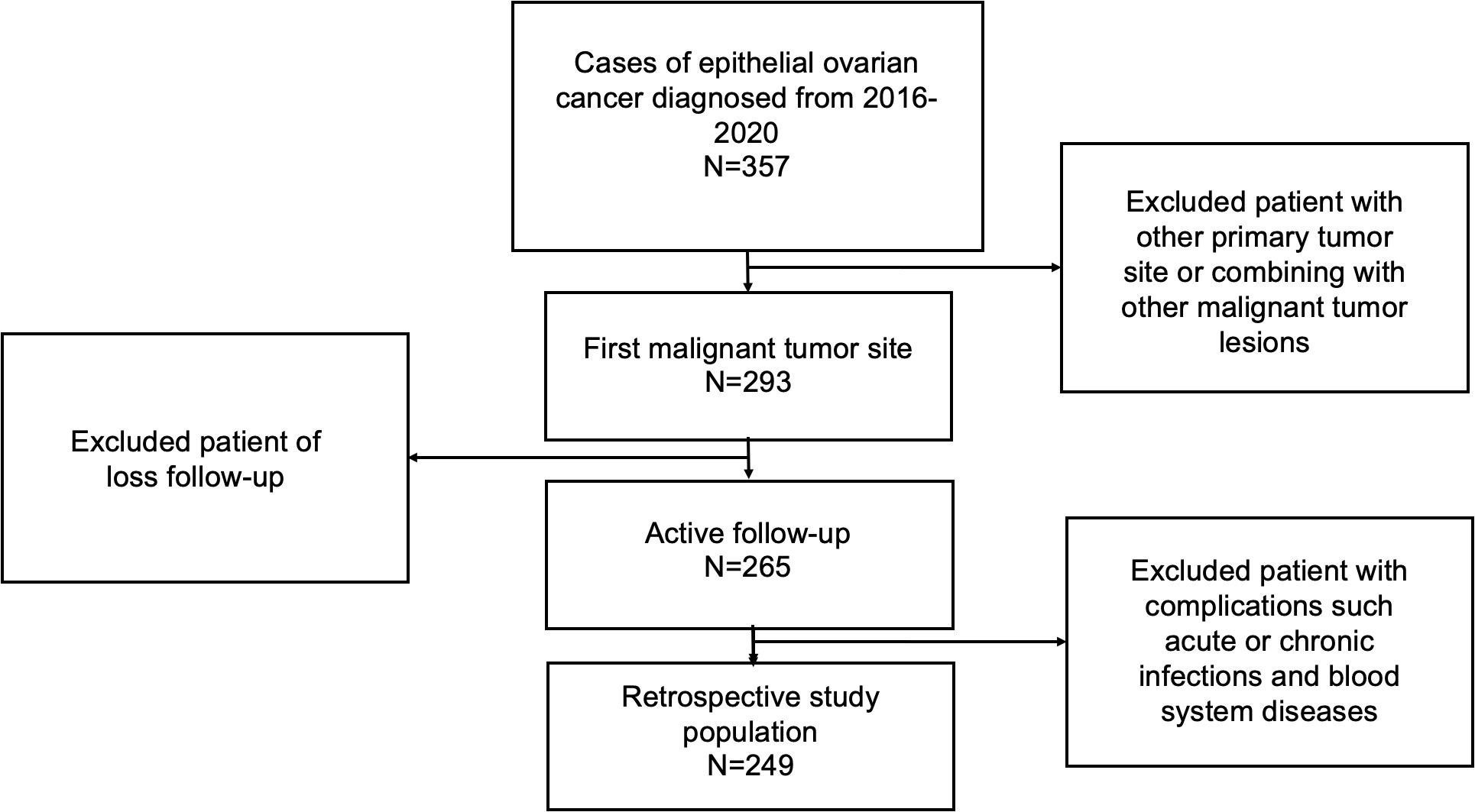
249 patients pathologically diagnosed EOC who underwent cytoreductive surgery at the Department of Obstetrics and Gynecology, Beijing University Third Hospital between January 2016 to January 2020 were retrospectively reviewed in our cohort. Patients were included if they: ① pathologically confirmed to be EOC; ② ovary is the primary lesion of tumor; ③ received cytoreductive surgery combined with regular platinum-based chemotherapy in Peking University Third Hospital; ④ had complete preoperative peripheral blood data and complete follow-up; ⑤ There were no acute or chronic infections and blood system diseases, and not complicated with other tumors (Figure 1 shows the workflow for selection procedure of epithelial ovarian cancer patients). This study was ratified by the Ethics Committee of Peking University Third Hospital. Written informed consent was obtained from all the patients in this study.
Follow-up
Patients’ age, Federation of International of Gynecologists and Obstetricians (FIGO) stage, pathological type, tumor grade, therapeutic regimen and complications were collected from clinical attendance records. Follow-up began on the day of surgery and was conducted through direct telecommunications and outpatient visits until October 2021. The median follow-up time was 35 months. Follow-up of patients included blood tests, urine tests, computed tomography, and physical examination. Overall survival (OS) is measured from the date of surgery to the date of death from any cause, or the date of last follow-up visit. Progression-free survival (PFS) is identified from the date of surgery to the date of recurrence which was confirmed by imaging evidence or serum CA125 elevation. Platinum-sensitive is defined when patients have a clear response to the initial platinum-based treatment and achieve clinical remission, and progress or recurrence occurs more than 6 months (including 6 months) after the withdrawal of the previous platinum-containing chemotherapy. Platinum-resistant is defined when patients respond to initial chemotherapy but progresses or relapses occurs within 6 months of completion of chemotherapy.
Statistical analysis
Differences of clinicopathological parameters and relative prognostic parameters between groups were evaluated by independent-samples Student’s t-test or Fisher’s exact test according to the distribution of data, and the normal distribution continuous variables were divided into groups by the median. Chi square test and binary logistic regression analyses were used to analyze the relationship between serum lipid indicators and chemoresistance as well as other clinicopathological characteristics of patients. The survival curves were calculated using the Kaplan–Meier method. Survival analyses were conducted by Cox proportional hazards model. All reported p-values were two-sided. P<0.05 was considered significant, and 95% CIs were calculated. All analyses were performed using the SPSS Statistics version 26.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline clinicopathological characteristics
The mean age of these patients was 55.20 ± 11.07 years. There were 161 patients with FIGO stage III to IV, accounting for 64.66%of the total number. 222 (89.16%) patients were diagnosed of high-grade EOC. Serous carcinoma was found in 191 cases (76.71%), and other pathological types of epithelial ovarian cancer together were 58 cases (23.29%). There were 56 patients (22.49%) who received neoadjuvant chemotherapy. 57 patients (22.89%) received maintenance therapy after surgery. Among 249 patients included in this study, a total of 70 patients (28.11%) had the clinical characteristic of chemoresistance after chemotherapy. Recurrence occurred in 120 patients (48.20%) and 55patients (22.09%) died during the following.
Grouping serum lipid single and complex indicators by median
We divided normal distribution continuous variables TC, HDL-C, LDL-C, HDL-C/LDL-C, HDL-C/TC into low- or high-level groups by median. With TC’s median of 4.65mmol/L as the boundary, 126 patients were in the low TC group and 123 patients in high TC group. Grouped by HDL-C ‘s median of 1.10mmol/L, there were 127 patients in the low HDL-C group and 122 patients in the high HDL-C group. Divided by the LDL-C’s median of 2.90mmol/L, there were 126 patients in the low LDL-C group and 123 patients in the high LDL-C group. The median of normal distribution continuous variable HDL-C/LDL-C was 0.39, 125 patients were in the low HDL-C/LDL-C group and 124 patients in the high HDL-C/LDL-C group. There were 124 patients in the low HDL-C/TC group and 125 patients in the high HDL-C/TC group with the HDL-C/TC’s median of 0.24 as the boundary.
Correlation between clinical features and serum blood lipid levels
Table 1 shows the correlation between clinical features and serum blood lipid levels. Patients of higher TC groups tend to be suffered from serous ovarian cancer. Lower HDL-C/LDL-C groups were associated with advanced FIGO stage and having complication of diabetes mellitus.
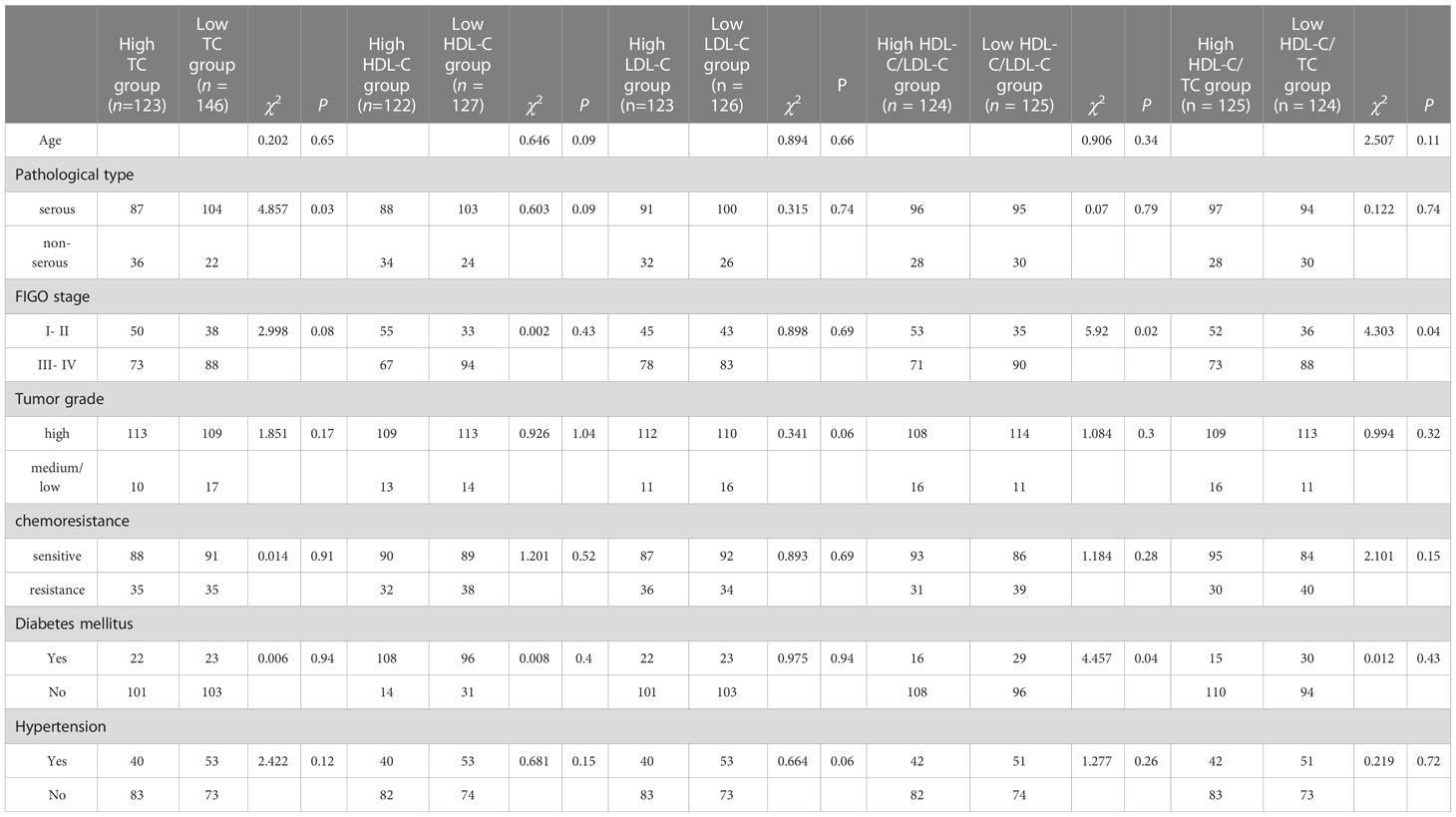
Table 1 Correction between clinicopathological features and different lipid level groups of 249 EOC patients.
Figure 2 shows binary logistic regression analyses indicating FIGO stage and HDL-C/TC ratio had significant correction with chemoresistance. Advanced FIGO stage and lower HDL-C/TC ratio mean a tendency to chemoresistance.
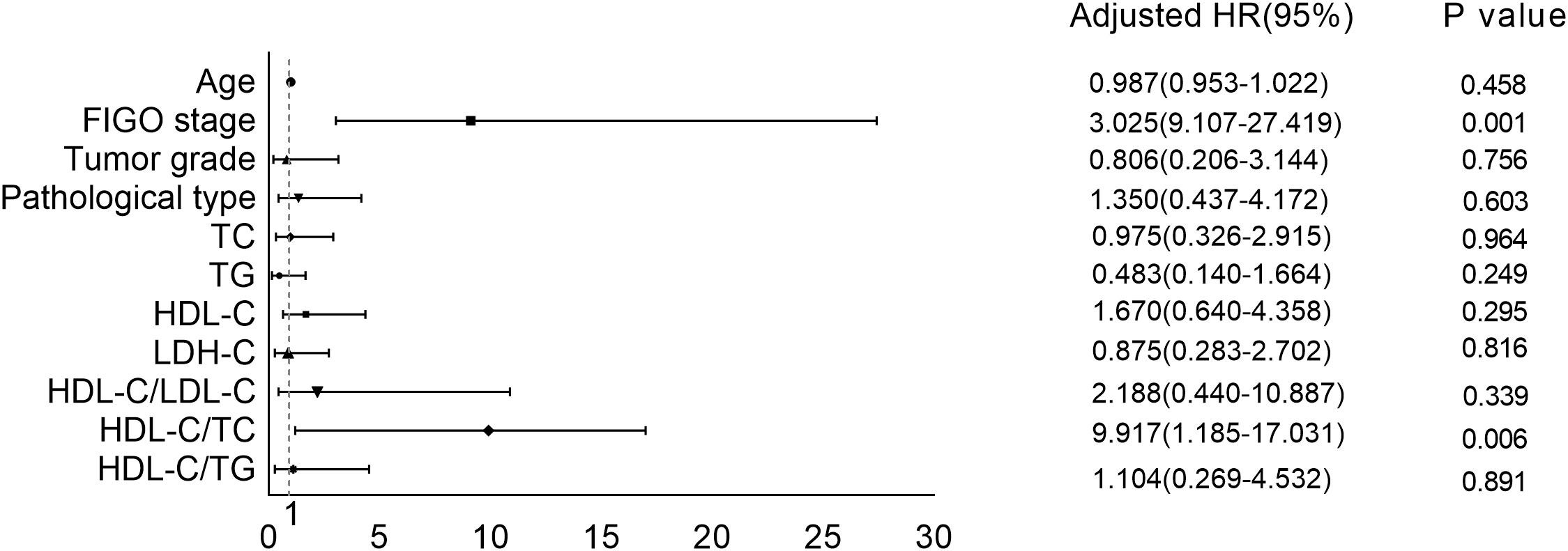
Figure 2 Correction between serum lipid levels as well as other clinical features and chemoresistance.
Kaplan–Meier survival curves of patients with ovarian cancer stratified by HDL-C/LDL-C and HDL-C/TC ratios
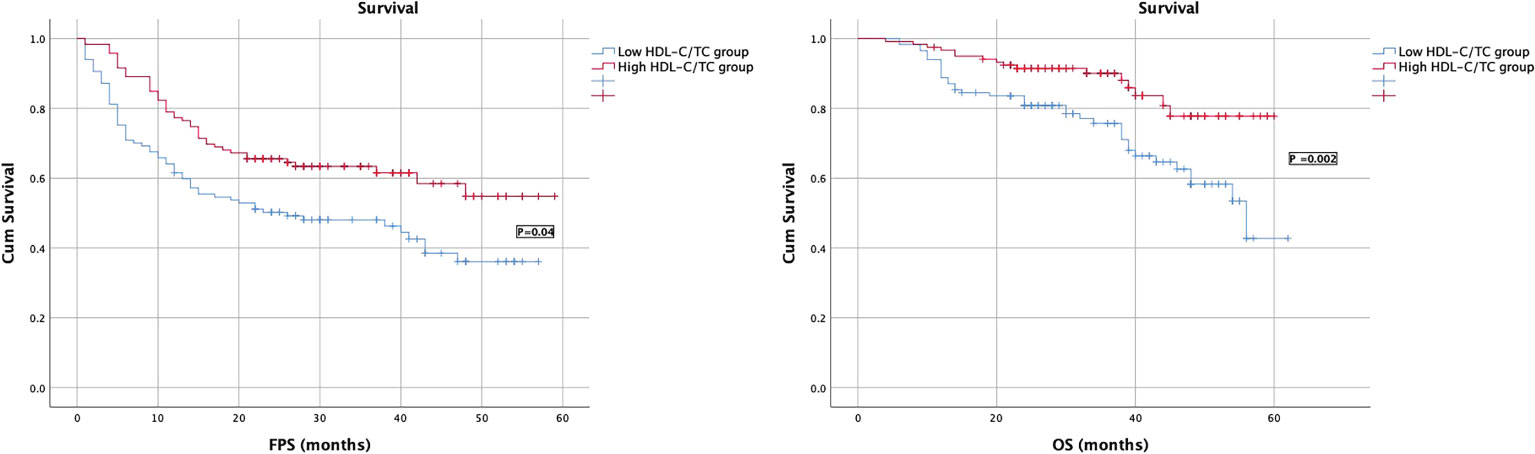
Kaplan–Meier survival analysis showed that patients of higher HDL-C/LDL-C and HDL-C/TC group had longer PFS and OS than those of lower HDL-C/LDL-C group, and the difference was statistically significant (P ≤ 0. 05), as shown in Figures 3, 4.
Univariate and multivariate analysis of FPS and OS
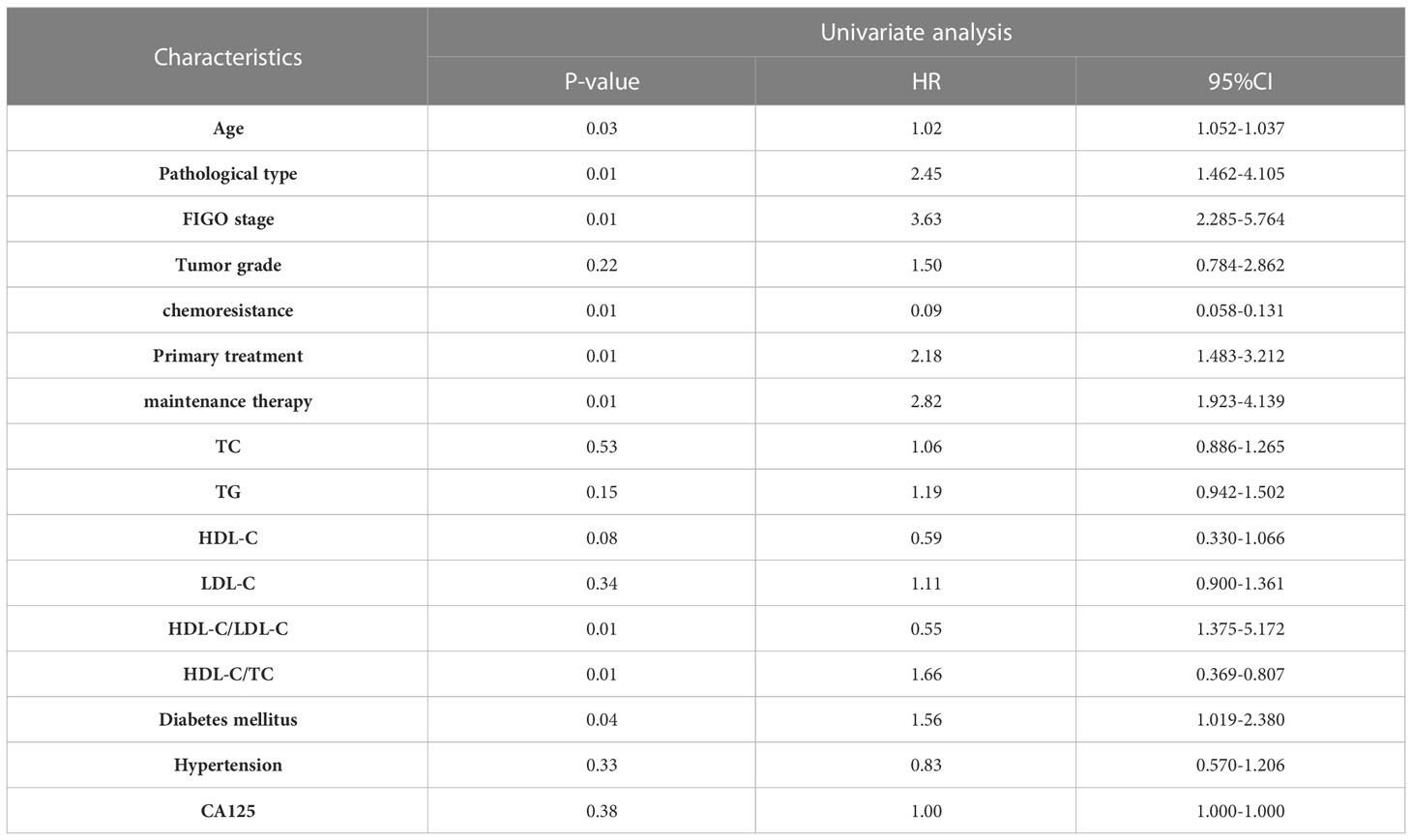
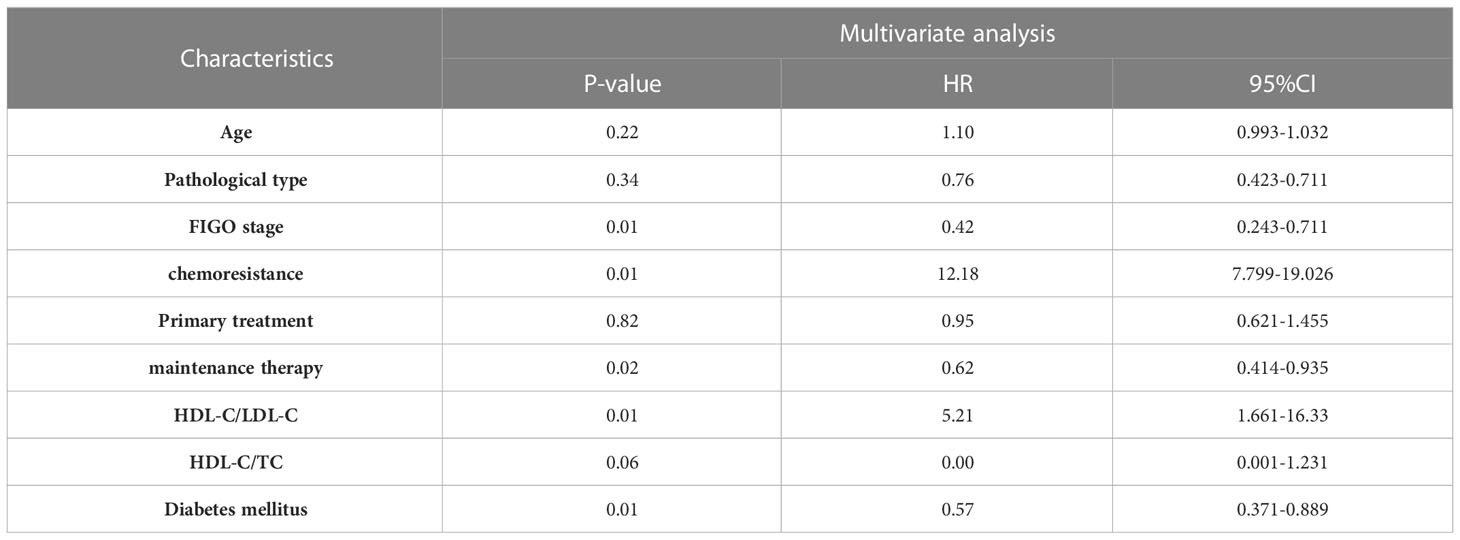
The survival analysis of PFS showed that the patient’s age, FIGO stage, pathological type, chemoresistance, primary treatment, maintenance therapy, HDL-C/LDL-C ratio, HDL-C/TC ratio, and whether having complication of diabetes mellitus were related to PFS (P<0. 05). Cox multivariate analysis showed that chemoresistance, FIGO stage, maintenance therapy, HDL-C/LDL-C ratio, and having complication of diabetes mellitus were independent predictive factors for PFS (P<0. 05), as shown in Tables 2, 3.
The survival analysis of OS showed that FIGO stage, pathological type, chemoresistance, primary treatment, maintenance therapy, HDL-C/LDL-C ratio, HDL-C/TC ratio, and whether having complication of diabetes mellitus were related to OS (P<0. 05). Cox multivariate analysis showed that chemoresistance, FIGO stage, maintenance therapy, and HDL-C/LDL-C ratio were independent predictive factors for PFS (P<0. 05), as shown in Tables 4, 5.
Discussion
In recent years, the relevance of lipid metabolism to the tumor microenvironment and cancer development has become a hot topic of research. To meet the demands of rapid growth, tumor cells try their best to obtain nutrients from outside, one of the main sources of energy is lipids in tumor microenvironment. At the same time, lipids are the main raw material of biofilm, which is important for the stability of cancer cell membrane and the reproduction and metastasis of cancers (16). Serum lipids are the main source of lipids in cancer microenvironment and are therefore closely related to the development of cancer (17). Studies have shown that in some solid tumors such as gastric cancer (18), lung cancer (19) and colon cancer (20), there are correlations between patients’ serum lipid levels and their clinical characteristics and prognosis. In the study of gynecological malignancies and lipid metabolism, researchers have focused on endometrial cancer (21, 22), which has a strong correlation between lipid metabolism and hormones. There has been no in-depth study of association between serum lipids and prognosis of ovarian cancer. Moreover, most of the studies on serum lipids and cancer just focus on single lipid index such as high/low density lipoproteins cholesterol and have not considered the combined effect of these indicators. It is therefore necessary to further explore the association between dyslipidemia and the clinical characteristics of ovarian cancer.
The main indicators of lipid metabolism in peripheral blood are total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Cholesterol is an important component of the cell membrane and is essential for maintaining lipid raft stability which promotes cell proliferation (23). In the literature, patients of prostate cancer and cervical cancer with elevated total serum cholesterol levels have a poor prognosis (24, 25). Meanwhile, patients of non-small cell lung cancer, gastric cancer, and primary liver cancer with lower total serum cholesterol levels suggest a poor prognosis (11, 26, 27). A study of 229 patients with ovarian cancer and 233 patients with benign ovarian tumors showed a trend of lower total cholesterol levels in the ovarian cancer group compared to the benign ovarian tumor group, the hypothesis was that the rapid growth of cancer cells requires the involvement of large amounts of total cholesterol, which in turn promotes total cholesterol depletion (14, 28). However, no studies have reported a correlation between serum total cholesterol levels and the prognosis of ovarian cancer patients. In our research, we found that serum cholesterol levels correlated with the type of pathology of the patients, with serous type of ovarian cancer having higher cholesterol levels than other pathological types. High-density lipoprotein cholesterol is known as a vascular scavenger, which reduces cholesterol levels in cancer cells, peripheral tissues and tumor lessens biofilm raw materials by transporting cholesterol from surrounding tissues and converting it into bile acids for excretion from the intestine, which may antagonize the function of intracellular cholesterol in cancer cells to some extent. It has been reported that elevated high density lipoprotein level in peripheral blood is an independent prognostic factor for patients with gastric liver cancers. Zhang D. found that lower levels of HDL were an independent risk factor for the development of ovarian cancer (29). In contrast, LDL is primarily responsible for the distribution of cholesterol between extrahepatic tissues and cells, and elevated serum LDL level can lead to increased levels of cholesterol stored in cancer cells, thereby promoting cell proliferation. Andrew J. retrospectively analyzed serum lipid levels and disease-specific survival in 132 patients with epithelial ovarian cancer and concluded that serum LDL levels were negatively associated with patient disease-specific survival (30). Our study analyzed the relationship between serum HDL, LDL as well as cholesterol levels and the clinical characteristics and prognosis of patients with ovarian cancer and failed to obtain a statistical correlation.
Previous literatures as well as the results of our study reveal that there are few studies focusing on the prognostic evaluation of serum lipid levels in ovarian cancer and some conclusions are controversial. Therefore, we firstly introduced the previously reported high-density lipoprotein cholesterol/total cholesterol (HDL-C/TC) and high-density lipoprotein cholesterol/low-density lipoprotein cholesterol (HDL-C/LDL-C) as two composite lipid markers to better investigate association between serum lipid levels and clinical characteristics and prognosis of ovarian cancer. Previous studies have shown that in gastric cancer, HDL-C/TC ratio has better prognostic evaluation than a single HDL-C level (6). A similar study has demonstrated that TG/HDL was positively correlated with clinical features such as FIGO stage and pathological type of endometrial cancer (15). Our results show that HDL-C/LDL-C and HDL - C/TC ratios are negatively associated with patients’ FIGO stage. One of the mechanisms may be that a higher HDL-C/TC ratio increases the amount of cholesterol brought into the blood vessels by HDL thus reducing the amount of free cholesterol in cancer cells and weakening the ability of cancer cells to proliferate and metastasize. Using multiple regression analysis, we concluded that the HDL-C/TC ratio was negatively associated with chemoresistance in patients. It has been documented that cholesterol levels are elevated in the tumor microenvironment of ovarian cancer patients which can upregulate the expression of the drug efflux pump proteins ABCG2 and MDR1, as well as cholesterol receptor LXRα/β to reduce the sensitivity of ovarian cancer cells to chemotherapeutic agents (31).
In terms of prognosis, univariate analysis showed that the type of tumor pathology, FIGO stage, chemoresistance, HDL-C/LDL-C, HDL-C/TC, the presence of neoadjuvant chemotherapy and maintenance therapy were associated with progression free survival (PFS) and overall survival (OS) in patients with ovarian cancer. FIGO stage and chemoresistance were independent risk factors for FPS and OS. HDL-C/LDL-C ratio and post-operative maintenance therapy were independent protective factors for FPS. HDL-C/LDL-C ratio was also an independent protective factor for patients’ OS. Patients with higher HDL-C/LDL-C ratio have longer FPS and OS (p<0. 05). The mechanism may be that the conversion and metabolism of cholesterol within ovarian cancer cells affects cell proliferation, chemoresistance and metastasis, which in turn affects patient prognosis.
Our study shows that the HDL-C/TC and HDL-C/LDL-C ratios are closely related to the FIGO stage, chemoresistance and prognosis of ovarian cancer patients. Previous studies have suggested that statins such as lovastatin and Fluvastatin may play a potential role in cancer therapy strategy by modulating patients’ serum lipid levels. For example, statin can significantly reduce the risk of breast, colorectal, ovarian, and pancreatic cancer (32–35). In non-small cell lung cancer, lovastatin combined with gefitinib produces a synergistic anti-tumor effect, reducing cancer cell proliferation by inhibiting the epidermal growth factor receptor (36). Studies on ovarian cancer have found that fluvastatin and cisplatin can synergistically induce apoptosis of ovarian cancer cells by modulating Ras pathway (37). Based on our findings and relevant literature data, statins are expected to play an important role in the treatment of ovarian cancer in the future (38, 39).
This research is a single-center study with a relatively small sample size, so further multi-center, large-sample studies are needed to verify the relationship between serum lipids and clinical features and prognosis of ovarian cancer. We will conduct further molecular research on the relationship between lipid metabolism and biological behaviors of ovarian cancer cells and closely link them to patient’s serum lipid levels and prognosis, bringing new thinking to the treatment of ovarian cancer. Statins’ role in modulating lipid metabolism of tumor microenvironment therefore changing characteristics of cancer cells requires further investigation.
Conclusion
In conclusion, serum lipid profiles are associated with the chemoresistance and prognosis of epithelial ovarian cancer. Higher HDL-C/LDL-C ratio and higher HDL-C/TC levels were protective factors for epithelial ovarian cancer, indicating novel index for screening and follow-up of ovarian cancer. At present, statins have been used in anti-tumor therapy. With the progress of research, lipid-lowering drugs are expected to play a more important role in improving the prognosis of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: YL and HG; methodology: YL; software: YL; formal analysis: YL; writing - original draft: YL; all authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Department of Obstetrics and Gynecology, Peking University Third Hospital for precious clinical statistics related to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Piver MS. Treatment of ovarian cancer at the crossroads: 50 years after single-agent melphalan chemotherapy. Oncol (Williston Park) (2006) 20(10):1156, 1158.
2. Zhang Y, Luo G, Li M, Guo P, Xiao Y, Ji H, et al. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer (2019) 19(1):984. doi: 10.1186/s12885-019-6139-6
3. Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer (2009) 9(9):665–74. doi: 10.1038/nrc2714
4. Delgado Bolton RC, Aide N, Colletti PM, Ferrero A, Paez D, Skanjeti A, et al. EANM guideline on the role of 2-[(18)F]FDG PET/CT in diagnosis, staging, prognostic value, therapy assessment and restaging of ovarian cancer, endorsed by the American college of nuclear medicine (ACNM), the society of nuclear medicine and molecular imaging (SNMMI) and the international atomic energy agency (IAEA). Eur J Nucl Med Mol Imaging (2021) 48(10):3286–302. doi: 10.1007/s00259-021-05450-9
5. Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med (2021) 218(1):e20201606. doi: 10.1084/jem.20201606
6. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature (2009) 457(7229):557–61. doi: 10.1038/nature07665
7. Kosalka P, Johnson C, Turek M, Sulpher J, Law A, Botros J, et al. Effect of obesity, dyslipidemia, and diabetes on trastuzumab-related cardiotoxicity in breast cancer. Curr Oncol (2019) 26(3):e314–21. doi: 10.3747/co.26.4823
8. Sun H, Huang X, Wang Z, Zhang G, Mei Y, Wang Y, et al. Triglyceride-to-high density lipoprotein cholesterol ratio predicts clinical outcomes in patients with gastric cancer. J Cancer (2019) 10(27):6829–36. doi: 10.7150/jca.35939
9. Liotti A, La Civita E, Cennamo M, Crocetto F, Ferro M, Guadagno E, et al. Periprostatic adipose tissue promotes prostate cancer resistance to docetaxel by paracrine IGF-1 upregulation of TUBB2B beta-tubulin isoform. Prostate (2021) 81(7):407–17. doi: 10.1002/pros.24117
10. Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PloS One (2013) 8(12):e81859. doi: 10.1371/journal.pone.0081859
11. Duong MN, Cleret A, Matera EL, Chettab K, Mathe D, Valsesia-Wittmann S, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res (2015) 17(1):57. doi: 10.1186/s13058-015-0569-0
12. Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech (2013) 6(6):1353–63. doi: 10.1242/dmm.011338
13. Pih GY, Gong EJ, Choi JY, Kim MJ, Ahn JY, Choe J, et al. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Res Treat (2021) 53(2):445–56. doi: 10.4143/crt.2020.599
14. Taroeno-Hariadi KW, Hardianti MS, Sinorita H, Aryandono T. Obesity, leptin, and deregulation of microRNA in lipid metabolisms: Their contribution to breast cancer prognosis. Diabetol Metab Syndr (2021) 13(1):10. doi: 10.1186/s13098-020-00621-4
15. Dai D, Chen B, Wang B, Tang H, Li X, Zhao Z, et al. Pretreatment TG/HDL-c ratio is superior to triacylglycerol level as an independent prognostic factor for the survival of triple negative breast cancer patients. J Cancer (2016) 7(12):1747–54. doi: 10.7150/jca.15776
16. Wang B, Rong X, Palladino END, Wang J, Fogelman AM, Martín MG, et al. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell (2018) 22(2):206–220.e4. doi: 10.1016/j.stem.2017.12.017
17. Radisauskas R, Kuzmickienė I, Milinavičienė E, Everatt R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas) (2016) 52(2):89–98. doi: 10.1016/j.medici.2016.03.002
18. Zou Y, Wu L, Yang Y, Ding Z, Huang J, Li P, et al. Serum lipid levels correlate to the progression of gastric cancer with neuroendocrine immunophenotypes: A multicenter retrospective study. Transl Oncol (2021) 14(1):100925. doi: 10.1016/j.tranon.2020.100925
19. Lin X, Lu L, Liu L, Wei S, He Y, Chang J, et al. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J Clin Lipidol (2017) 11(4):1073–81. doi: 10.1016/j.jacl.2017.05.004
20. Broitman SA. Dietary cholesterol, serum cholesterol, and colon cancer: A review. Adv Exp Med Biol (1986) 206:137–52. doi: 10.1007/978-1-4613-1835-4_13
21. Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. Int J Cancer (2009) 124(12):2938–41. doi: 10.1002/ijc.24285
22. Swanson CA, Potischman N, Barrett RJ, Berman ML, Mortel R, Twiggs LB, et al. Endometrial cancer risk in relation to serum lipids and lipoprotein levels. Cancer Epidemiol Biomarkers Prev (1994) 3(7):575–81.
23. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer (2020) 1874(1):188394. doi: 10.1016/j.bbcan.2020.188394
24. YuPeng L, YuXue Z, PengFei L, Cheng C, YaShuang Z, DaPeng L, et al. Cholesterol levels in blood and the risk of prostate cancer: A meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev (2015) 24(7):1086–93. doi: 10.1158/1055-9965.EPI-14-1329
25. Lin F, Zheng R, Yu C, Su Y, Yan X, Qu F, et al. Predictive role of serum cholesterol and triglycerides in cervical cancer survival. Int J Gynecol Cancer (2021) 31(2):171–6. doi: 10.1136/ijgc-2020-001333
26. Ma C, Wang X, Guo J, Liu P. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: a retrospective study. Lipids Health Dis (2021) 20(1):69. doi: 10.1186/s12944-021-01492-y
27. Wen Y, Wang G, Chen HD, Li X, Lyu ZY, Feng XS, et al. [Total cholesterol and the risk of primary liver cancer in Chinese males: A prospective cohort study]. Zhonghua Yu Fang Yi Xue Za Zhi (2020) 54(7):753–9.
28. Kitson SJ, Lindsay J, Sivalingam VN, Lunt M, Ryan NAJ, Edmondson RJ, et al. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: A prospective case control study. Gynecol Oncol (2018) 148(1):154–60. doi: 10.1016/j.ygyno.2017.11.019
29. Zhang D, Xi Y, Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: A systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev (2021) 30(2):161–70. doi: 10.1097/CEJ.0000000000000597
30. Li AJ, Elmore RG, Chen IY, Karlan BY. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol Oncol (2010) 116(1):78–81. doi: 10.1016/j.ygyno.2009.09.027
31. Kim S, Lee M, Dhanasekaran DN, Song YS. Activation of LXRa/beta by cholesterol in malignant ascites promotes chemoresistance in ovarian cancer. BMC Cancer (2018) 18(1):1232.
32. Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res (2018) 20(1):144. doi: 10.1186/s13058-018-1066-z
33. Ling Y, Yang L, Huang H, Hu X, Zhao C, Huang H, et al. Prognostic significance of statin use in colorectal cancer: A systematic review and meta-analysis. Med (Baltimore) (2015) 94(25):e908. doi: 10.1097/MD.0000000000000908
34. Majidi A, Na R, Jordan SJ, De Fazio A, Webb PM. Statin use and survival following a diagnosis of ovarian cancer: A prospective observational study. Int J Cancer (2021) 148(7):1608–15. doi: 10.1002/ijc.33333
35. Zhang Y, Liang M, Sun C, Qu G, Shi T, Min M, et al. Statin use and risk of pancreatic cancer: An updated meta-analysis of 26 studies. Pancreas (2019) 48(2):142–50. doi: 10.1097/MPA.0000000000001226
36. Park IH, Kim JY, Jung JI, Han JY. Lovastatin overcomes gefitinib resistance in human non-small cell lung cancer cells with K-ras mutations. Invest New Drugs (2010) 28(6):791–9. doi: 10.1007/s10637-009-9319-4
37. Taylor-Harding B, Orsulic S, Karlan BY, Li AJ. Fluvastatin and cisplatin demonstrate synergistic cytotoxicity in epithelial ovarian cancer cells. Gynecol Oncol (2010) 119(3):549–56. doi: 10.1016/j.ygyno.2010.08.017
38. Zaleska M, Mozenska O, Bil J. Statins use and cancer: An update. Future Oncol (2018) 14(15):1497–509. doi: 10.2217/fon-2017-0543
Keywords: serum lipids, epithelial ovarian cancer, chemoresistance, prognosis, novel serum lipid index
Citation: Li Y, Shang C, Liang H, Zhang K, Wu Y and Guo H (2023) Associations of novel serum lipid index with epithelial ovarian cancer chemoresistance and prognosis. Front. Oncol. 13:1052760. doi: 10.3389/fonc.2023.1052760
Received: 24 September 2022; Accepted: 24 January 2023;
Published: 13 February 2023.
Edited by:
Connie Irene Diakos, Royal North Shore Hospital, AustraliaReviewed by:
Felice Crocetto, Federico II University Hospital, ItalyRoberto C. Delgado Bolton, Hospital San Pedro, Spain
Copyright © 2023 Li, Shang, Liang, Zhang, Wu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Guo, YnlzeWdoeUAxNjMuY29t
 Yuan Li
Yuan Li Chunliang Shang
Chunliang Shang Hongyan Guo
Hongyan Guo