- 1School of Graduate Studies, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 3Department of Oncology, Dongying People’s Hospital, Dongying, China
Background and Purpose: Epidermal growth factor receptor (EGFR)-mutant lung cancers are associated with a high risk of developing brain metastases (BM). Craniocerebral radiotherapy is a cornerstone for the treatment of BM, and EGFR-TKIs act on craniocerebral metastases”. However, whether EGFR-TKIs combined with craniocerebral radiotherapy can further increase the efficacy and improve the prognosis of patients is unclear. This study aimed to evaluate the difference in efficacy between targeted-therapy alone and targeted-therapy combined with radiotherapy in EGFR-mutant lung adenocarcinoma patients with BM.
Materials and Methods: A total of 291 patients with advanced non-small cell lung cancer (NSCLC) and EGFR mutations were enrolled in this retrospective cohort study. Propensity score matching (PSM) was conducted using a nearest-neighbor algorithm (1:1) to adjust for demographic and clinical covariates. Patients were divided into two groups: EGFR-TKIs alone and EGFR-TKIs combined with craniocerebral radiotherapy. Intracranial progression-free survival (iPFS) and overall survival (OS) were calculated. Kaplan–Meier analysis was used to compare iPFS and OS between the two groups. Brain radiotherapy included WBRT, local radiotherapy, and WBRT+Boost.
Results: The median age at diagnosis was 54 years (range: 28–81 years). Most patients were female (55.9%) and non-smokers (75.5%). Fifty-one pairs of patients were matched using PSM. The median iPFS for EGFR-TKIs alone (n=37) and EGFR-TKIs+craniocerebral radiotherapy (n=24) was 8.9 and 14.7 months, respectively. The median OS for EGFR-TKIs alone (n=52) and EGFR-TKIs+craniocerebral radiotherapy (n=52) was 32.1 and 45.3 months, respectively.
Conclusion: In EGFR-mutant lung adenocarcinoma patients with BM, targeted therapy combined with craniocerebral radiotherapy is an optimal treatment.
Introduction
Lung cancer is the leading cause of morbidity and mortality around the globe (1, 2). According to the International Agency for Research on Cancer, 2.2 million new cases of lung cancer were reported worldwide in 2020, accounting for 11.4% of newly reported cancer cases (IARC Biennial Report 2020-2021, World Cancer Report 2020). Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers and most patients are diagnosed at an advanced stage (3). Patients with advanced NSCLC have been treated with individualized molecular-targeted therapy based on the profiles of driver gene alteration, among which mutant epidermal growth factor receptor (EGFR) is an important therapeutic target (4). Brain metastases, the most common site of distant metastasis in NSCLC, is the main cause of poor prognosis (5, 6). Some studies have shown that the incidence of brain metastases in lung adenocarcinoma patients with EGFR mutations is slightly higher than that in patients with wild-type EGFR, with an approximate incidence rate of 60% (7–9), indicating that lung adenocarcinoma patients with EGFR mutations may be more prone to developing brain metastases. With advancements in systemic treatment and radiotherapy techniques, the overall survival (OS) of patients has been further prolonged from 3–6 months to 19–58 months, increasing the risk of developing brain metastases during the entire disease course (10–13). The two aforementioned reasons cause a high incidence of brain metastases in EGFR-mutant patients with lung adenocarcinoma.
In clinical practice, targeted-therapy and radiotherapy are the main treatment options for EGFR-mutant lung adenocarcinoma patients with brain metastases (9, 14). Such a combination has proven to be effective and safe for brain metastases also from other cancers (15).The BRAIN clinical trial showed EGFR-tyrosine kinase inhibitor (TKI) alone was associated with a significantly longer intracranial progression-free survival (iPFS) in patients with craniocerebral metastasis. This clinical trial showed that a median iPFS of 10.0 months (95% confidence interval [CI], 5.6–14.4) in the icotinib group and 4.8 months (95% CI, 2.4–7.2) in the craniocerebral radiotherapy group (14). The results showed that although EGFR-TKIs alone were effective, their therapeutic effect was limited. Craniocerebral radiotherapy is considered a cornerstone of the treatment of brain metastases (16–18). Craniocerebral radiotherapy can destroy the blood–brain barrier, increasing the absorption of chemotherapy or targeted drugs to a great extent, which provides a theoretical basis for the combination of EGFR-TKIs and radiotherapy (19–21). Moreover, some retrospective studies have shown that craniocerebral radiotherapy combined with EGFR-TKIs is more effective than EGFR-TKIs alone (22, 23). However, certain factors that affect the prognosis of patients, such as age, Karnofsky performance status, extracranial metastasis, and number of brain metastases, were not considered in those studies. Studies have shown that these factors are closely related to the prognosis of patients, which may adversely affect the results and conclusions; therefore these studies cannot be used to judge whether EGFR-TKIs combined with craniocerebral radiotherapy indeed increase the efficacy and improve the prognosis of patients (12, 24). The above factors that affect the prognosis of patients with brain metastases should be considered when formulating a treatment plan, especially when radiotherapy is considered.
We performed this retrospective study to eliminate the impact of potential confounding factors on the results to explore the necessity of craniocerebral radiotherapy for EGFR-mutant lung adenocarcinoma patients with brain metastases used propensity score matching (PSM).
Methods and materials
Patient cohort
We screened lung cancer patients diagnosed with EGFR-mutant lung adenocarcinoma and brain metastases at our hospital between September 2008 and September 2020. The inclusion criteria were as follows: pathologically diagnosed with primary lung adenocarcinoma; mutations in EGFR exons 18, 19, or 21; diagnosis of brain metastases using enhanced computed tomography (CT) or magnetic resonance imaging (MRI); detailed clinical information, including treatment options and clinicopathological features; EGFR-TKIs administered (e.g., gefitinib, erlotinib, or icotinib); and no other primary malignancies. Patients with incomplete medical records or those who failed to meet the aforementioned criteria were excluded from the study. The study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute and was conducted in accordance with the Declaration of Helsinki. Patient records were anonymized and identified before data analysis.
The following characteristics were included in the analysis: age, sex, smoking history, EGFR mutation status, tumor-node-metastasis (TNM) stage, treatment scheme that included chemotherapy regimens or EGFR-TKI regimens, and information on craniocerebral radiotherapy. Information on the start date of EGFR-TKIs administration, intracranial progression, most recent follow-up, and death were also recorded. Intracranial progression was defined as radiographic progression of pre-existing brain metastases, development of new brain metastases, or both. iPFS was measured and reviewed at the last follow-up visit. The time to intracranial progression was calculated from the start of EGFR-TKIs or craniocerebral radiotherapy to the date of intracranial progression or death. PFS was defined from the date of the start of EGFR-TKIs or craniocerebral radiotherapy to the date of progression or death. OS was calculated from the date of the pathological diagnosis of lung adenocarcinoma to the date of death or re-examination at the last follow-up.
EGFR genotyping
Genomic DNA was extracted from tumor tissues obtained by fiberoptic bronchoscopy or puncture biopsy. Circulating tumor DNA was isolated from the blood and was purified. EGFR mutations were detected using next-generation sequencing, droplet digital polymerase chain reaction, or amplification refractory mutation system-PCR. However, owing to the lack of clinical data of some patients, the specific mutational site of EGFR could not be identified.
Radiotherapy
Brain radiotherapy included whole brain radiotherapy (WBRT), local radiotherapy, and WBRT+Boost. The median prescribed dose was 40 Gy (range, total dose: 30–50 Gy, 2-3Gy/per day, 10-25 fractions) and 50 Gy (range, total dose:30–60 Gy, 2-5.5Gy/per day, 10-30 fractions) in patients treated with WBRT and local radiotherapy, respectively. Additionally, in the WBRT+Boost group, the median prescription dose of the whole brain was 37.5 Gy (range, 30–40 Gy, 2-3Gy/per day, 10-20 fractions). Lastly, the median dose of additional radiation boost for local metastases was 12 Gy (range, 7.2–20 Gy, 3 to 10 fractions). The radiation dose was 30 to 60 Gy in 15 to 30 fractions, 2 to 3 Gy per-fraction. In our study, the number of sequential to the principal WBRT course is 46 (52.9%), and the concomitant (integrated) is 41 (47.1%).
Statistical analysis
We used the 1:1 PSM method in SPSS Version26.0 to decrease the effect of potential confounding factors between the two groups to eliminate the influence of these factors on the results. The characteristics of the patients before and after pairing were compared using the Chi-squared test. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used to test the influence of individual variables on survival. Statistical significance was set at P-values<0.05 (two-sided). Statistical analyses were performed using GraphPad Prism version 8.0.1.
Results
Patient characteristics
A total of 291 EGFR-mutant lung adenocarcinoma patients with brain metastases were enrolled in the study, with 61 patients in the EGFR-TKI alone group and 230 in the EGFR-TKI+craniocerebral radiotherapy group. The median age of the patients at diagnosis was 55 years (range, 23–81 years). Most patients were female (62.9%) and non-smokers (77.3%). Moreover, mutations in EGFR exons 19 and 21 were detected in 41.9% (122/291) and 49.1% (143/291) of patients, respectively. Most of the Lung-molGPA scores of the patients were in the 2.5–4 group (167,57.4%). Table 1 shows the clinical features of the two groups.
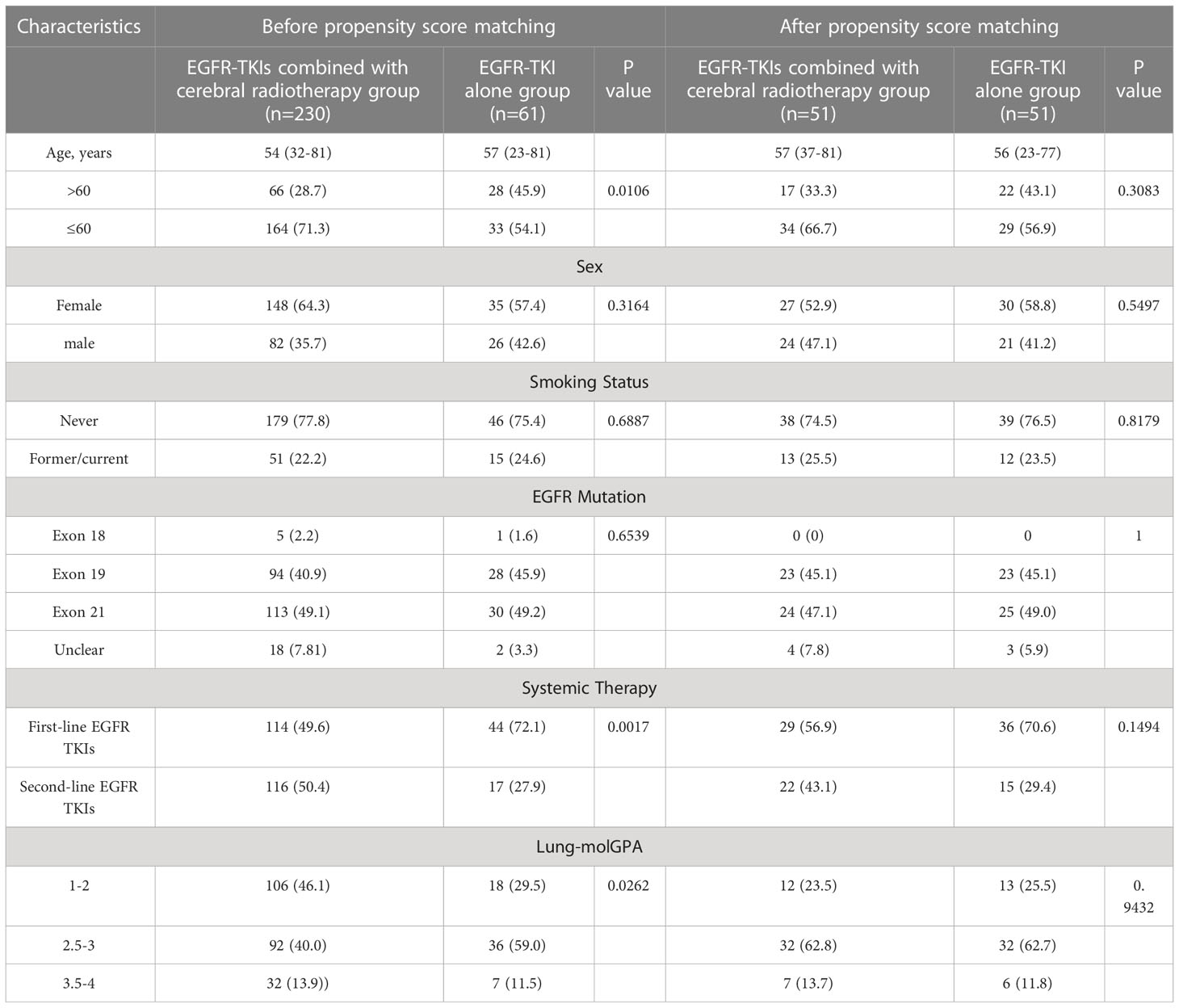
Table 1 Clinical features of patients in the EGFR-TKI alone group and EGFR-TKIs combined with cerebral radiotherapy group with chi-square test for Categorical Variables.
As of March 2022, 195 deaths (67.0%) were recorded. The median follow-up duration was 63.8 months (interquartile range, 40.3–87.5). The median OS was 38.1 months, the median iPFS was 12.8 months, while the median PFS was 12.6 months (Figures 1A–C). There was no difference in OS between the groups with mutations in exons 19 and 21 (41.7 months vs. 36.0 months; log-rank P=0.0624; hazard ratio [HR], 0.7510; 95% CI, 0.5557–1.015; Figure 1D). There was also no difference in OS between the smoker and non-smoker groups (35.8 months vs. 38.3 months; log-rank P=0.1991; HR, 0.7952; 95% CI, 0.5604–1.128; Figure 1E). However, the median OS time of the groups with Lung-molGPA score 1–2 and Lung-molGPA score 2.5–4 was 32.3 months and 48 months, respectively (log-rank P<0.0001; HR, 2.024; 95% CI, 1.502–2.727; Figure 1F).

Figure 1 Before PSM: (A) Overall survival (OS) of the entire cohort. (B) Intracranial progression-free survival (iPFS) and (C) progression-free survival (PFS) of the entire cohort. OS of patients stratified according to (D) EGFR mutation status.After PSM: (A) Overall survival (OS) of the entire cohort. (B) Intracranial progression-free survival (iPFS) and (C) progression-free survival (PFS) of the entire cohort. OS of patients stratified according to (D) EGFR mutation status. OS of patients stratified according to (E) Smoking status; OS of patients stratified according to (F) Lung-molGPA.
Survival analysis before PSM
A total of 152 patients had intracranial progression after being administered EGFR-TKIs (EGFR-TKIs+craniocerebral radiotherapy group, 124; EGFR-TKI alone group, 28). The iPFS analysis was compared between the EGFR-TKI+craniocerebral radiotherapy and EGFR-TKI alone groups. The median iPFS in the EGFR-TKIs+craniocerebral radiotherapy group and EGFR-TKI alone group was 12.8 and 11.3 months, respectively (Figure 2A); there were no significant differences in the median iPFS between the two groups (log-rank P=0.2855; HR, 1.376; 95% CI, 0.7661–2.472; Figure 2A).
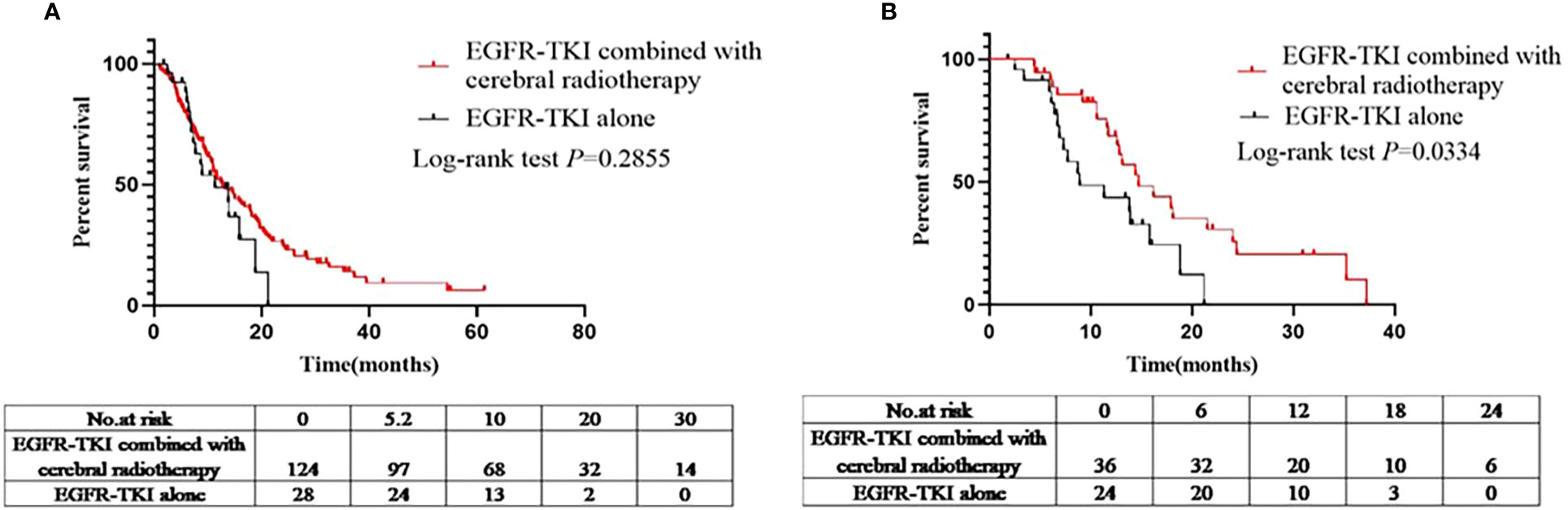
Figure 2 (A) Comparison of iPFS between the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group before PSM. (B) Comparison of iPFS between the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group after PSM.
A total of 252 patients showed disease progression after being administered EGFR-TKIs (EGFR-TKIs+craniocerebral radiotherapy group, 202; and EGFR-TKI alone group, 50). Similarly, PFS analysis was performed in the EGFR-TKI+craniocerebral radiotherapy and EGFR-TKI alone groups; the median PFS in the two groups was 12.6 and 12.4 months, respectively (Figure 3A), with no significant differences (log-rank P=0.9434; HR, 1.014; 95% CI, 0.6871–1.497; Figure 3A).
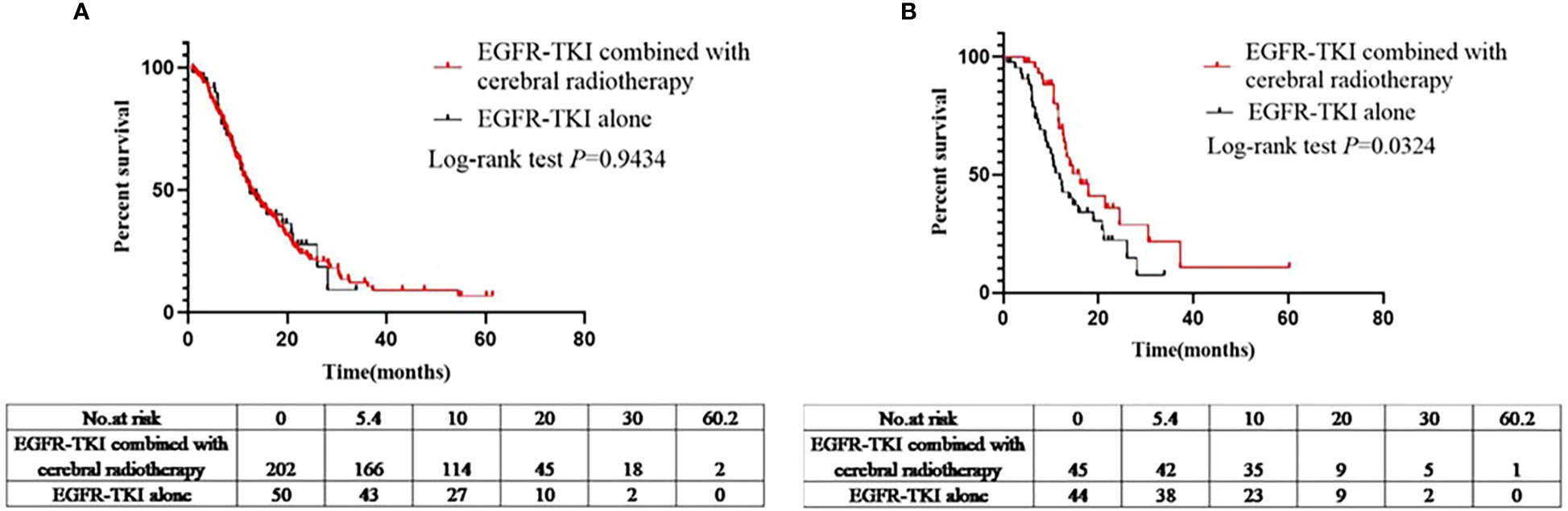
Figure 3 (A) Comparison of PFS between the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group before PSM. (B) Comparison of PFS between the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group after PSM.
The median OS for patients in the EGFR-TKI+craniocerebral radiotherapy and EGFR-TKI alone groups was 37.6 and 38.1 months, respectively (Figure 4A), with no significant differences observed (log-rank P=0.9403; HR, 1.015; 95% CI, 0.6935–1.484; Figure 4A).

Figure 4 (A) Comparison of OS between the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group before PSM. (B) Comparison of OSbetween the EGFR-TKIs combined with cerebral radiotherapy group and EGFR-TKI alone group after PSM.
Univariable and multivariable analyses of covariable with the iPFS, PFS, and OS results
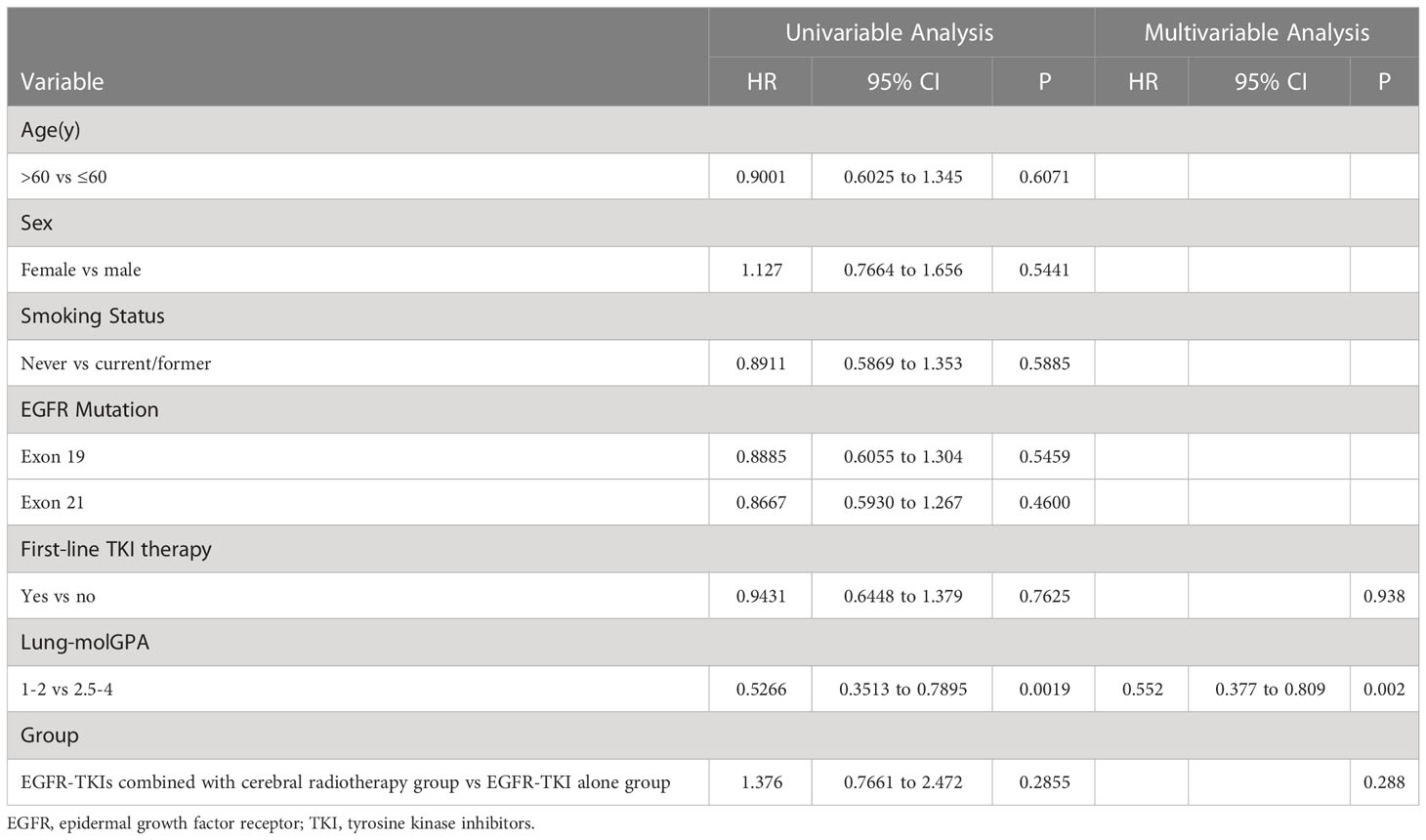
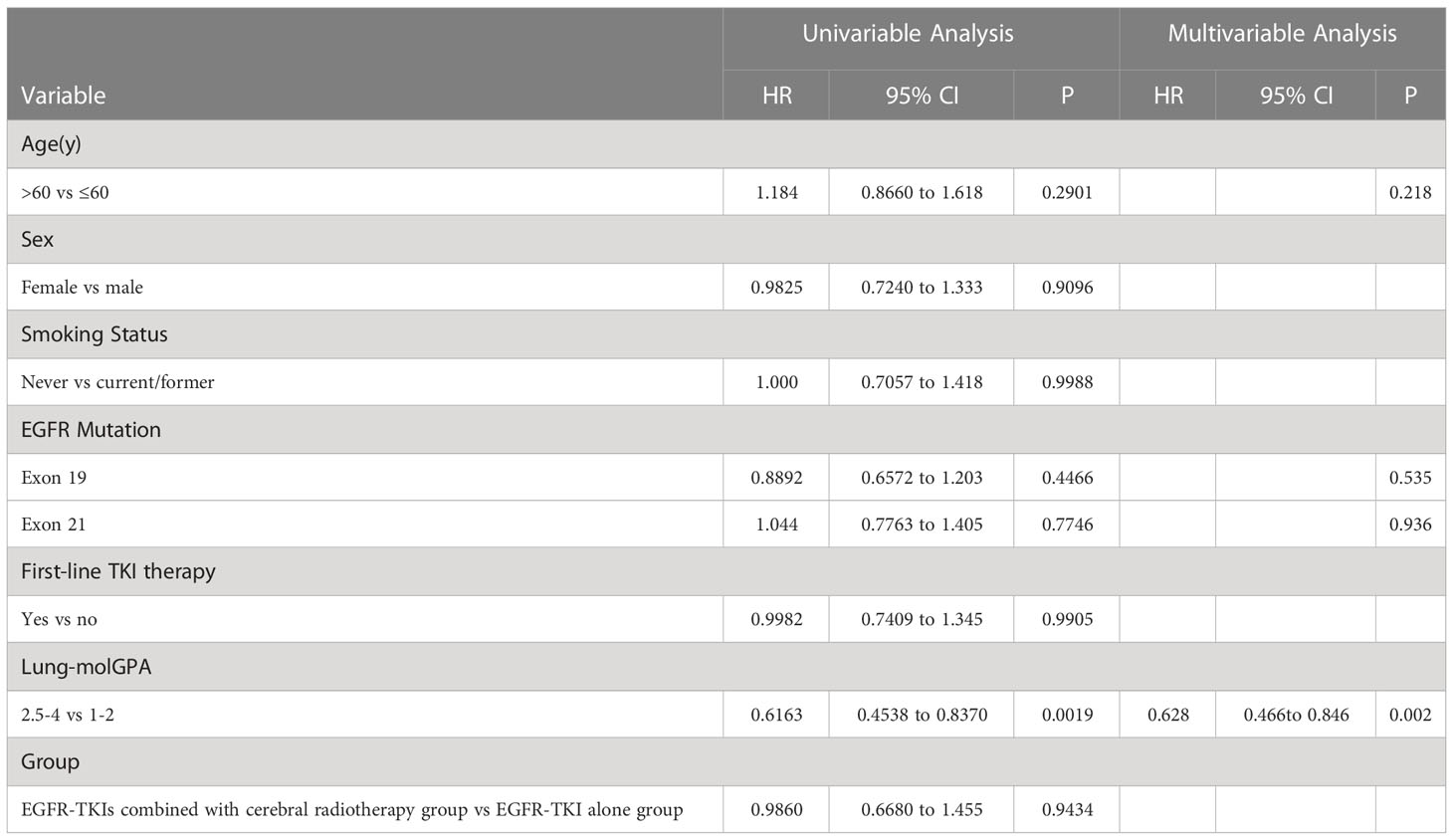
The univariate analysis of covariables with the iPFS (Table 2) and PFS (Table 3) results showed that the effect of sex, age, smoking status, and EGFR mutation was not statistically significant. Multivariate analysis showed that only lung-molGPA was an independent predictive factor (P=0.002 for both).
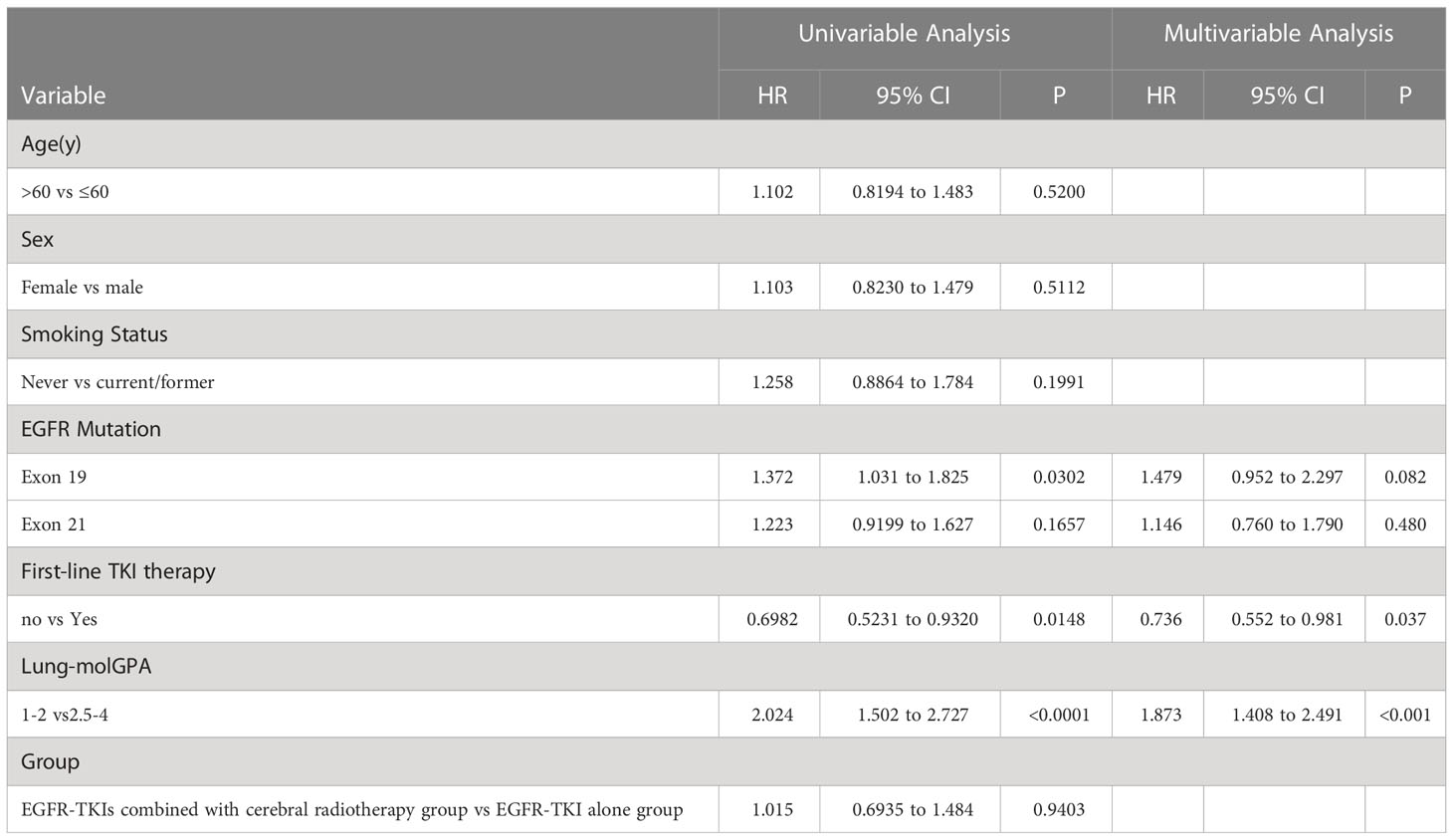
The univariate and multivariate analyses of the covariables are shown in Table 4. Results of the univariate analysis showed that the effects of sex, age, and smoking status were not statistically significant. Multivariate analysis showed that EGFR exon 19 deletion, lung-molGPA, and the start date of EGFR-TKIs were independent predictive factors (P=0.082, HR, 1.479, 95% CI, 0.952–2.297; P ≤ 0.001, HR, 1.873, 95% CI, 1.408–2.491; P=0.9403; P=0.037, HR, 0.736, 95% CI, 0.552–0.981; respectively).
Survival analysis after PSM
According to the results of the Chi-squared test (Table 1), age, time of targeted therapy application, and lung-molGPA score had different constituent ratios (P=0.0106; P=0.0017; P=0.0262, respectively) between the groups. Combined with univariable and multivariable analyses (Tables 2–4), sex, age, TNM stage, lung-molGPA score, and the time of targeted application affected the prognosis of patients. We performed 1:1 PSM to eliminate the influence of these factors on the results. The group indicator was whether it was combined with craniocerebral radiotherapy, and the predictors were sex, age, TNM stage, lung-molGPA score, and the time of targeted application. The predictors were defined as follows: gender classification, i.e., males and females; age, defined as the years at the time of initial diagnosis; TNM staging, classified as III or IV; and the time of targeted application, the time when the patients was first administered targeted drugs. The tendency score was calculated using logistic regression analysis. The nearest neighbor algorithm (caliper: 0.2) was used to match the propensity score.
This study enrolled 102 patients, with 51 patients in each treatment group. The median age at diagnosis was 54 years (range, 28–81 years). Prior to the PSM analysis, women (57, 55.9%) and non-smokers (77, 75.5%) accounted for majority of the patients. Mutations in EGFR exons 19 and 21 were detected in 45.1% (46/102) and 48.0% (49/102) of patients, respectively. The majority of lung-molGPA scores of the patients were in the 2.5–4 group (77,75.5%).
After the PSM analysis, 57 deaths were recorded. The median follow-up duration was 40.3 months (interquartile range, 34.5–63.8) months. The median OS, iPFS, and PFS was 38.1 months, 13.9 months, and 13.8 months, respectively (Figures 1A–C). There was no difference in the OS between groups with mutations in exons 19 and 21 (35.7 months vs. 38.1 months; log-rank P=0.7289; HR, 0.9090; 95% CI, 0.5299–1.559; Figure 1D).
Sixty patients had intracranial progression after treatment with EGFR-TKIs (EGFR-TKIs+craniocerebral radiotherapy group, 36; and EGFR-TKI alone group, 24). Similarly, the median iPFS in the EGFR-TKI+craniocerebral radiotherapy group and EGFR-TKI alone group was 14.7 and 8.9 months, respectively (Figure 2B), with significant differences observed (log-rank P=0.0220; HR, 0.4278; 95% CI, 0.2069–0.8848; Figure 2B).
Ninety-nine patients developed disease progression after using EGFR-TKIs (EGFR-TKIs+craniocerebral radiotherapy group, 45; and EGFR-TKI alone group, 44). The median PFS in the EGFR-TKIs+craniocerebral radiotherapy group and EGFR-TKI alone group was 16.2 and 11.9 months, respectively (Figure 3B); with significant differences observed between the groups (P=0.0324; HR, 0.5524; 95% CI, 0.3208–0.9515; Figure 3B).
Furthermore, after PSM analysis, the median OS of the EGFR-TKI+craniocerebral radiotherapy and EGFR-TKI alone groups were 45.3 and 32.1 months, respectively, with a significant difference observed between the regimens (log-rank, P=0.0106; HR, 0.4924; 95% CI, 0.2861–0.8476; Figure 4B).
Discussion
In our study, iPFS, PFS, and OS in the EGFR-TKI combined with craniocerebral radiotherapy group were not significantly improved as compared to those who were administered TKIs alone prior to PSM. A variety of clinical and pathological factors can affect the prognosis of EGFR-mutant lung adenocarcinoma with brain metastases. Therefore, we speculate that the heterogeneity of these factors between the two groups contributed to the lack of statistical difference in survival analysis (8, 12, 25–27). We initially used a Chi-squared analysis to analyze whether there were differences in the general clinical characteristics between the two groups. The results indicated that differences in age, timing of EGFR-TKI administration, and lung-molGPA existed between the two groups. Univariable and multivariable analyses of covariables associated with iPFS, PFS, and OS also showed that lung-molGPA was independently associated with improved iPFS, PFS, and OS. Therefore, insignificant difference in the survival analysis between the two groups before PSM may be due to uneven clinical features, especially those affected by lung-molGPA. Lung-molGPA reportedly provides a better predictive value, suggesting that lung-molGPA can comprehensively and accurately reflect the prognosis of NSCLC complicated by brain metastases (24, 28–30). These also suggest the importance of individualized therapy for craniocerebral radiotherapy (12, 31–33). Moreover, most previous retrospective studies have shown that EGFR-TKI concurrently administered with radiotherapy was more effective than treatment with EGFR-TKI alone, which was not consistent with the results of our study (10, 19, 34, 35). However, most of these studies did not consider these variables during clinical decision making.
To further elucidate the role and value of craniocerebral radiotherapy in the treatment of patients with lung cancer, we used the PSM method to minimize the impact of these factors, especially the impact of differences in lung-molGPA, which could more accurately compare the effects of EGFR-TKIs combined with craniocerebral radiotherapy and EGFR-TKI alone (36–38). There was no statistical difference in the clinical features between the two groups after matching. After PSM, EGFR-TKIs combined with craniocerebral radiotherapy improved iPFS, OS, and PFS as compared to EGFR-TKIs alone. To the best of our knowledge, our study is the first to use PSM to compare the effects of EGFR-TKIs combined with craniocerebral radiotherapy and EGFR-TKIs alone on the prognosis of patients with EGFR-mutant lung adenocarcinoma with brain metastases. Our results suggest that EGFR-TKIs combined with craniocerebral radiotherapy not only increases the iPFS of the patients but also translates the prolongation of PFS into an improvement in OS. A retrospective study involving a small sample size showed that the combination of craniocerebral radiotherapy and EGFR-TKIs could improve iPFS, while OS and PFS were not significantly prolonged, when compared with EGFR-TKIs alone (19, 22, 23). In another study, the median iPFS and OS of the EGFR-TKIs group combined with the craniocerebral radiotherapy group were significantly longer than those in the EGFR-TKI alone group (39).
Our results emphasize the importance of performing craniocerebral radiotherapy as a treatment for these patients. However, the appropriate timing of craniocerebral radiotherapy requires further investigation. A study showed that in NSCLC patients with EGFR mutations, pre-radiotherapy showed better results, with a median OS of 30 months for patients who received pre-WBRT and 25 months for those who received pre-EGFR-TKIs (23). In patients with EGFR-mutant NSCLC and brain metastases, craniocerebral radiotherapy followed by EGFR-TKIs improved iPFS compared with upfront EGFR-TKI, showing that the delay or lack of craniocerebral radiotherapy was associated with a poor PFS (8). Our study also enrolled patients who received craniocerebral radiotherapy after EGFR-TKI resistance; however, this did not change the results which stated that the iPFS, PFS, and OS of the combined treatment regimen group were longer than those of the EGFR-TKI alone group. Combining the results of previous studies with our results (including the BRAIN clinical trial), we can suggest that patients with better clinical conditions and good prognosis can be treated with EGFR-TKI alone first, which can reduce the long-term adverse events caused by craniocerebral radiotherapy and improve the quality of life of patients (16, 40). For these patients, the progression of brain metastases after EGFR-TKI treatment can be treated with craniocerebral radiotherapy to obtain longer craniocerebral local control and improve the survival prognosis of the patients. However, for patients with worse clinical conditions, especially those with lower lung-molGPA scores, craniocerebral radiotherapy can be combined with EGFR-TKIs concurrently to increase local control. Our results also suggest that prolonged iPFS translates into a better OS, due to which patients can have a better prognosis. Therefore, the combination of craniocerebral radiotherapy and EGFR-TKIs should not be a one-size-fits-all model, and should instead be considered comprehensively to provide patients with the best individualized treatment plan. The formulation of craniocerebral radiotherapy strategies should be based on a comprehensive evaluation of the patient’s lung-molGPA score while making treatment decisions (12, 41, 42).
This study had some limitations. First, it was a single-center, retrospective study with a limited sample size, and thus could have had a selection bias. However, although we used the PSM method to reduce the interference of some potential factors, the individual factors may have still affected our results. Therefore, a multi-institutional, prospective study with a large sample size is required to confirm the findings of this study. Subsequently, the relevant experiments were designed to provide theoretical support that EGFR-TKIs combined with craniocerebral radiotherapy is a superior treatment option, as compared to EGFR-TKIs alone, for future work at the experimental level.
Conclusion
Our study showed that in patients with EGFR-mutant lung adenocarcinoma and brain metastases, craniocerebral radiotherapy plays an important role in improving the survival and prognosis, indicating that EGFR-TKIs combined with craniocerebral radiotherapy might be a better therapeutic option for this patient population. Furthermore, a combination strategy should be developed for individualized treatment plans based on various prognostic parameters.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong Cancer Hospital and Institute. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, GD, and XT. Methodology, GD. Software, GD. Validation, GD and XT. Formal analysis, GD and ZL. Resources, GD. Data curation, GD, XT, YL and YZ. Writing—original draft preparation, GD. Writing—review and editing, GD and ZL. Supervision, JL and ZL. All authors contributed to the article and approved the submitted version.
Funding
This study was supported jointly by Taishan Scholars Program of Shandong Province (No. ts20190982), the National Natural Science Foundation of China (Grant No. 82272752). And there are no competing interests.
Acknowledgments
This study would not have been possible without the consistent and valuable reference materials that I received from my supervisor, whose insightful guidance and enthusiastic encouragement in the course of my shaping this thesis definitely gain my deepest gratitude.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1049855/full#supplementary-material
References
1. Tungsukruthai S, Reamtong O, Roytrakul S, Sukrong S, Vinayanwattikun C, Chanvorachote P. Targeting AKT/mTOR and bcl-2 for autophagic and apoptosis cell death in lung cancer: Novel activity of a polyphenol compound. Antioxid (Basel) (2021) 10:534. doi: 10.3390/antiox10040534
2. Han L, Huang Z, Liu Y, Ye L, Li D, Yao Z, et al. MicroRNA-106a regulates autophagy-related cell death and EMT by targeting TP53INP1 in lung cancer with bone metastasis. Cell Death Dis (2021) 12:1037. doi: 10.1038/s41419-021-04324-0
3. Kauffmann-Guerrero D, Kahnert K, Kiefl R, Sellmer L, Walter J, Behr J, et al. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: A prospective study. Sci Rep (2021) 11:10919. doi: 10.1038/s41598-021-90397-y
4. Hung MS, Lung JH, Lin YC, Fang YH, Huang SY, Jiang YY, et al. Comparative analysis of two methods for the detection of EGFR mutations in plasma circulating tumor DNA from lung adenocarcinoma patients. Cancers (Basel) (2019) 11:803. doi: 10.3390/cancers11060803
5. Li J, Jing W, Jia W, Zhai X, Zhu H, Yu J. Downregulation of lncRNA XR_429159.1 linked to brain metastasis in patients with limited-stage small-cell lung cancer. Front Oncol (2021) 11:603271. doi: 10.3389/fonc.2021.603271
6. Rounis K, Skribek M, Makrakis D, Petris LD, Agelaki S, Ekman S, et al. Correlation of clinical parameters with intracranial outcome in non-small cell lung cancer patients with brain metastases treated with pd-1/Pd-L1 inhibitors as monotherapy. Cancers (Basel) (2021) 13:1562. doi: 10.3390/cancers13071562
7. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer (2015) 88:108–11. doi: 10.1016/j.lungcan.2015.01.020
8. Bhatt VR, Kedia S, Kessinger A, Ganti AK. Brain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol (2013) 31:3162–4. doi: 10.1200/JCO.2013.49.8915
9. Yu HA, Sima C, Feldman D, Liu LL, Vaitheesvaran B, Cross J, et al. Phase 1 study of twice weekly pulse dose and daily low-dose erlotinib as initial treatment for patients with EGFR-mutant lung cancers. Ann Oncol (2017) 28:278–84. doi: 10.1093/annonc/mdw556
10. Dong K, Liang W, Zhao S, Guo M, He Q, Li C, et al. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl Lung Cancer Res (2019) 8:268–79. doi: 10.21037/tlcr.2019.06.12
11. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys (2016) 95:673–9. doi: 10.1016/j.ijrobp.2016.01.037
12. Deng G, Zhang Y, Ke J, Wang Q, Qin H, Li J, et al. Effect of brain radiotherapy strategies on prognosis of patients with EGFR-mutant lung adenocarcinoma with brain metastasis. J Transl Med (2021) 7:503. doi: 10.1186/s12967-021-03161-1
13. Vadalà RE, Santacaterina A, Sindoni A, Platania A, Arcudi A, Ferini G, et al. Stereotactic body radiotherapy in non-operable lung cancer patients. Clin Transl Oncol (2016) 18(11):1158–9. doi: 10.1007/s12094-016-1552-7
14. Foggetti G, Li C, Cai H, Hellyer JA, Lin WY, Ayeni D, et al. Genetic determinants of EGFR-driven lung cancer growth and therapeutic response in vivo. Cancer Discovery (2021) 11:1736–53. doi: 10.1158/2159-8290.CD-20-1385
15. Gagliano A, Prestifilippo A, Cantale O, Ferini G, Fisichella G, Fontana P, et al. Role of the combination of cyclin-dependent kinase inhibitors (CDKI) and radiotherapy (RT) in the treatment of metastatic breast cancer (MBC): Advantages and risks in clinical practice. Front Oncol (2021) 11:643155. doi: 10.3389/fonc.2021.643155
16. Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): A multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med (2017) 5:707–16. doi: 10.1016/S2213-2600(17)30262-X
17. Petoukhova A, Snijder R, Wiggenraad R, Wit LDB, der Wouden IM, Florijn M, et al. Quality of automated stereotactic radiosurgery plans in patients with 4 to 10 brain metastases. Cancers (Basel) (2021) 13:3458. doi: 10.3390/cancers13143458
18. Khalifa J, Mazieres J, Gomez-Roca C, Ayyoub M, Moyal EC. Radiotherapy in the era of immunotherapy with a focus on non-Small-Cell lung cancer: Time to revisit ancient dogmas? Front Oncol (2021) 11:662236. doi: 10.3389/fonc.2021.662236
19. Zheng H, Liu QX, Hou B, Zhou D, Li JM, Lu X, et al. Clinical outcomes of WBRT plus EGFR-TKIs versus WBRT or TKIs alone for the treatment of cerebral metastatic NSCLC patients: a meta-analysis. Oncotarget (2017) 8:57356–64. doi: 10.18632/oncotarget.19054
20. Brown PD, Ahluwalia MS, Khan OH, Asher AL, Wefel JS, Gondi V. Whole-brain radiotherapy for brain metastases: Evolution or revolution. J Clin Oncol (2018) 36:483–91. doi: 10.1200/JCO.2017.75.9589
21. Chen CH, Lee HH, Chuang HY, Hung JY, Huang MY, Chong IW. Combination of whole-brain radiotherapy with epidermal growth factor receptor tyrosine kinase inhibitors improves overall survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Cancers (2019) 11:1092. doi: 10.3390/cancers11081092
22. Miyawaki E, Kenmotsu H, Mori K, Harada H, Mitsuya K, Mamesaya N, et al. Optimal sequence of local and EGFR-TKI therapy for EGFR-mutant non-small cell lung cancer with brain metastases stratified by number of brain metastases. Int J Radiat Oncol Biol Phys (2019) 104:604–13. doi: 10.1016/j.ijrobp.2019.02.051
23. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-Small-Cell lung cancer: A retrospective multi-institutional analysis. J Clin Oncol (2017) 35:1070–77. doi: 10.1200/JCO.2016.69.7144
24. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol (2017) 3:827–31. doi: 10.1001/jamaoncol.2016.3834
25. Shi L, Tang J, Tao H, Guo L, Wu H, Liu Z, et al. Detection of EGFR mutations in cerebrospinal fluid of EGFR-mutant lung adenocarcinoma with brain metastases. Front Oncol (2021) 11:622142. doi: 10.3389/fonc.2021.622142
26. Li C, Zhang B, Guo J, Hu F, Nie W, Zheng Q, et al. Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) combined with chemotherapy delay brain metastasis in patients with EGFR-mutant lung adenocarcinoma. Target Oncol (2019) 14:423–31. doi: 10.1007/s11523-019-00649-1
27. Shen CI, Huang HC, Chiang CL, Luo YH, Shiao TH, Chiu CH. Effects of different brain surveillance strategies on outcomes for patients with EGFR-mutant metastatic lung adenocarcinoma under targeted therapy. Lung Cancer (2019) 138:52–7. doi: 10.1016/j.lungcan.2019.10.001
28. Li J, Jing W, Zhai X, Jia W, Zhu H, Yu J. Estimating survival in patients with non-Small-Cell lung cancer and brain metastases: A verification of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). Onco Targets Ther (2021) 14:1623–31. doi: 10.2147/OTT.S288928
29. Chen K, Yu X, Zhang F, Xu Y, Zhang P, Huang Z, et al. Applicability of the lung-molGPA index in non-small cell lung cancer patients with different gene alterations and brain metastases. Lung Cancer (2018) 125:8–13. doi: 10.1016/j.lungcan.2018.08.023
30. Wang Q, Tan X, Deng G, Fu S, Li J, Li Z. Dynamic changes in the systemic immune-inflammation index predict the prognosis of EGFR-mutant lung adenocarcinoma patients receiving brain metastasis radiotherapy. BMC Pulm Med (2022) 22:75. doi: 10.1186/s12890-022-01866-7
31. Afshar P, Mohammadi A, Tyrrell PN, Cheung P, Sigiuk A, Plataniotis KN, et al. [Formula: see text]: Deep learning-based radiomics for the time-to-event outcome prediction in lung cancer. Sci Rep (2020) 10:12366. doi: 10.1038/s41598-020-69106-8
32. Fan C, Zhao C, Wang F, Li S, Wang J. Significance of PTEN mutation in cellular process, prognosis, and drug selection in clear cell renal cell carcinoma. Front Oncol (2019) 9:357. doi: 10.3389/fonc.2019.00357
33. Palmisciano P, Ferini G, Khan R, Bin-Alamer O, Umana GE, Yu K, et al. Neoadjuvant stereotactic radiotherapy for brain metastases: Systematic review and meta-analysis of the literature and ongoing clinical trials. Cancers (Basel). (2022) 14(17):4328. doi: 10.3390/cancers14174328
34. Chen Y, Wei J, Cai J, Liu A. Combination therapy of brain radiotherapy and EGFR-TKIs is more effective than TKIs alone for EGFR-mutant lung adenocarcinoma patients with asymptomatic brain metastasis. BMC Cancer (2019) 19:793. doi: 10.1186/s12885-019-6005-6
35. He ZY, Li MF, Lin JH, Lin D, Lin RJ. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: a retrospective cohort study. Cancer Manag Res (2019) 11:2129–38. doi: 10.2147/CMAR.S184922
36. Sun H, Liu C, Zhang J, Yang G, Han D, Liu T, et al. Twice-daily thoracic radiotherapy by intensity-modulated radiation therapy (IMRT) compared with simultaneous integrated boost IMRT (SIB-IMRT) with concurrent chemotherapy for patients with limited-stage small cell lung cancer. a propensity-score matched analysis. Radiother Oncol (2022) 172:140–6. doi: 10.1016/j.radonc.2022.01.022
37. Wang ZN, Jiang XB, Lu J, Guo XY, He ZQ, Duan H, et al. Survival benefit from surgical resection in lung cancer patients with brain metastases: A single-center, propensity-matched analysis cohort study. Ann Surg Oncol (2022) 29(6):3684–93. doi: 10.1245/s10434-022-11365-y
38. Torasawa M, Yoshida T, Yagishita S, Shimoda Y, Shirasawa M, Matsumoto Y, et al. Nivolumab versus pembrolizumab in previously-treated advanced non-small cell lung cancer patients: A propensity-matched real-world analysis [published online ahead of print, 2022 Apr 4]. Lung Cancer (2022) 167:49–57. doi: 10.1016/j.lungcan.2022.03.020
39. Liu Y, Wang J, Wu J, Yang Q, Zeng Y, Wu D, et al. The efficacy of first-generation EGFR-TKI combined with brain radiotherapy as the first-line treatment for lung adenocarcinoma patients with brain metastases and EGFR sensitive mutations: A retrospective study. Technol Cancer Res Treat (2021) 20:1533033821997819. doi: 10.1177/1533033821997819
40. Ferini G, Viola A, Valenti V, Tripoli A, Molino L, Marchese V, et al. Whole brain irradiation or stereotactic RadioSurgery for five or more brain metastases (WHOBI-STER): A prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin Transl Radiat Oncol (2021) 32:52–8. doi: 10.1016/j.ctro.2021.11.008
41. Karlsson AT, Hjermstad MJ, Omdahl T, Aass N, Skovlund E, Hellebust TP, et al. Overall survival after initial radiotherapy for brain metastases; a population based study of 2140 patients with non-small cell lung cancer. Acta Oncol (2021) 60(8):1054–60. doi: 10.1080/0284186X.2021.1924399
42. Zhang T, Zhang Y, Zhou L, Deng S, Huang M, Liu Y, et al. Applicability of the adjusted graded prognostic assessment for lung cancer with brain metastases using molecular markers (Lung-molGPA) in a Chinese cohort: A retrospective study of multiple institutions. Cancer Med (2020) 9:8772–81. doi: 10.1002/cam4.3485
Keywords: Adenocarcinoma of lung, brain neoplasms, carcinoma, non-small-cell lung, progression-free survival (PFS)
Citation: Deng G, Tan X, Li Y, Zhang Y, Wang Q, Li J and Li Z (2023) Effect of EGFR-TKIs combined with craniocerebral radiotherapy on the prognosis of EGFR-mutant lung adenocarcinoma patients with brain metastasis: A propensity-score matched analysis. Front. Oncol. 13:1049855. doi: 10.3389/fonc.2023.1049855
Received: 21 September 2022; Accepted: 30 January 2023;
Published: 09 February 2023.
Edited by:
Rongrong Zhou, Xiangya Hospital, Central South University, ChinaReviewed by:
Gianluca Ferini, Rem Radiotherapy, ItalyJianchun Duan, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Deng, Tan, Li, Zhang, Wang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbin Li, bGlqaWFuYmluQG1zbi5jb20=; Zhenxiang Li, bGl6eDAxMDhAMTYzLmNvbQ==
 Guangchuan Deng1,2
Guangchuan Deng1,2 Xiaojing Tan
Xiaojing Tan Jianbin Li
Jianbin Li Zhenxiang Li
Zhenxiang Li