
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 17 February 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1048922
This article is part of the Research Topic Targeting Cancer-Associated Fibroblasts: from Bench to Bedside View all 5 articles
Objective: To systematically evaluate the relationship between cancer-associated fibroblasts (CAFs) and clinicopathological characteristics and prognosis of gastric cancer, so as to provide new directions and clinical evidence for the diagnosis and treatment of this disease.
Methods: We searched PubMed, Embase, Web of Science, and The Cochrane Library to identify studies on the correlation between tumor-associated fibroblasts and the diagnosis and prognosis of gastric cancer. Two researchers screened the literature independently to extract data, evaluated the quality of the included studies, and used the Review Manager 5.4 software to perform a meta-analysis.
Results: A total of 14 studies involving a total of 2,703 patients were included. The meta-analysis results showed that high expression of CAFs was associated with stage III–IV gastric cancer (relative risk ratio [RR]=1.59; 95% confidence interval [CI]: [1.24–2.04]; P=0.0003), lymph node metastasis (RR=1.51; 95% CI: [1.23–1.87]; P=0.0001), serosal infiltration (RR=1.56, 95% CI: [1.24–1.95]; P=0.0001), diffuse and mixed types in Lauren classification (RR=1.43; 95% CI: [1.18–1.74]; P=0.0003), vascular invasion (RR=1.99; 95% CI: [1.26–3.14]; P=0.003), and overall survival (hazard ratio [HR]=1.38; 95% CI: [1.22–1.56]; P<0.00001). However, the high expression of CAFs was not significantly correlated with poorly differentiated gastric cancer (RR=1.03; 95% CI: [0.96–1.10]; P=0.45) and gastric cancer with tumor diameter >5 cm (RR=1.34; 95% CI: [0.98–1.83]; P=0.07).
Conclusion: The findings of this meta-analysis demonstrated that high expression of CAFs is closely associated with the traditional pathological indicators related to poor prognosis in gastric cancer, and is a valuable prognostic factor in this setting.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022358165.
Gastric cancer (GC) is a type of malignant tumors with the fifth and fourth highest incidence and mortality rate worldwide, respectively (1). Owing to advances in treatment, the incidence and mortality of GC have decreased in recent years. Nevertheless, this cancer continues to profoundly affect the lives of individuals in East Asia, particularly in China (2). Nowadays, due to extensive screening, as well as the availability of endoscopic or surgical treatment, the overall survival (OS) of patients with GC has been greatly prolonged. However, many patients are diagnosed at a late stage and have poor prognosis. The main treatments for advanced GC include chemotherapy, targeted therapy, and immunotherapy (3). The progress achieved in these treatments is inseparable from the in-depth study of the clinicopathological characteristics of GC. Some biomarkers have been found, such as microsatellite instability, mismatch repair deficient, human epidermal growth factor receptor 2 (HER2), programmed cell death ligand 1 (PD-L1), tumor mutation burden, and Epstein–Barr virus. These biomarkers are linked to the prognosis of GC and play a great role in the molecular typing and treatment of this disease (4).
In addition to targeting the tumor tissue itself, the environment of tumor growth, also termed the tumor microenvironment (TME), has been receiving considerable research attention. Cancer-associated fibroblasts (CAFs) are defined as cells that exist in the stroma of tumors without epithelial, endothelial, or leukocyte markers; they are elongated in shape and do not carry oncogene mutations (5). Studies suggested that CAFs specifically express α-smooth muscle actin (α-SMA) and fibroblast-activation protein (FAP). Hence, CAFs can be identified through their morphology and these cell markers (6). As an important part of the TME, CAFs are directly related to tumor growth, invasion, metastasis, and therapeutic effect (7).
It has been shown that CAF expression is associated with the prognosis of some types of cancer (8, 9). Is high expression of CAFs an efficient biomarker for the differentiation of patients with GC who are at high risk of diagnosis at an advanced stage and poor prognosis? In this meta-analysis, we integrated existing studies to further investigate the relationship between CAFs and the clinicopathological characteristics and prognosis of patients with GC to provide new directions and clinical evidence for molecular typing and targeted therapy of GC.
This study adhered to the PRISMA guidelines (10) for meta-analyses and systematic reviews. This analysis was based on data from previously studies registered on PROSPERO (CRD42022358165).
The inclusion criteria were as follows (1): patients diagnosed with GC based on histopathology (2); expression of CAFs detected by immunohistochemistry, using α-SMA and FAP as markers of CAFs; and (3) studies including available data on one or more of the following clinicopathological characteristics: clinical stage, differentiation, tumor depth, lymph node metastasis, Lauren classification, tumor size, vascular invasion, and OS. The exclusion criteria were (1): reviews, letters, meetings, abstracts, unavailability of full text; and (2) literature written in a language other than English.
PubMed, Embase, Web of Science, and The Cochrane Library were searched for studies on CAFs in GC from the establishment of the database until September 1, 2022. The retrieval was carried out by combining free words with subject terms, and the final retrieval formula was determined by multiple pre-retrieval. The search terms included: CAFs, tumor-associated fibroblasts, stomach neoblasts, GC, etc. The specific search strategy is shown in the Supplementary File. Other eligible studies were retrieved from the references cited in the selected articles and relevant literature.
Two researchers (J.W. and M.W.) independently screened the literature according to the inclusion and exclusion criteria and, subsequently, extracted and cross-checked the data. In case of disagreement, consensus was reached following discussion with a third investigator. The extracted data included publication information (e.g., title, first author, publication time, country), subjects (e.g., sample size, age, markers used for CAF detection, CAF expression determination standard), clinicopathological data (e.g., tumor size, stage, grade, lymph node metastasis, tumor depth, vascular invasion, Lauren classification, follow-up time, follow-up rate, OS, study design scheme, quality). If it was not possible to obtain the original data from the literature, the corresponding author was contacted; in case of no response, data were measured and extracted from the relevant images.
The Newcastle–Ottawa Quality Assessment Scale (NOS) was independently used by two researchers to evaluate the risk of bias of the included studies (11). In case of disagreement, consensus was reached following discussion with a third investigator. The highest NOS score is 9, with scores >6 denoting high-quality research.
The Review Manager 5.4 software was used for the meta-analysis of data included in this study. For continuous and dichotomous data, mean difference and relative risk ratio (RR) were used as the effect size, respectively. Point estimates and 95% confidence intervals (95% CI) were provided for each effect size. The chi-squared test and P-values were used to qualitatively analyze the statistical heterogeneity among the results, and I2 was used to quantitatively analyze the heterogeneity. When I2 ≤ 50% or I2>50%, a fixed effect model or random effect model was used for the meta-analysis, respectively. P-values <0.05 denoted statistically significant differences. The meta-analysis was also analyzed by Stata/SE 16. Publication bias was assessed visually with a funnel plot and the Egger weighted regression statistic, with P<0.05 indicating significant publication bias.
According to our search strategy, a total of 4,086 studies were identified. After removing duplicate articles, 2,440 studies were selected. By reading the titles and abstracts, we removed 2,391 records because of non-relevance with the theme. After reviewing the full texts of the 46 potentially eligible records in detail, the following studies were excluded: studies with insufficient data (n=26); reviews (n=3); and studies investigating associations between other biomarkers on CAFs (n=6). Eventually, 14 studies (12–25) were included in this meta-analysis. The selection process is illustrated in Figure 1.
These 14 studies, involving a total of 2,703 patients, were included in the pooled analysis. Ten and four studies were conducted in China (12–15, 17–20, 22, 23) and Japan (16, 21, 24, 25), respectively, with publication dates spanning from 2007 to 2020. All studies examined more than one clinicopathological characteristics of GC. OS was recorded in eight studies. Study quality, assessed by the NOS score, ranged from 7 to 8. The characteristics and NOS score of the included studies are shown in Table 1.
A total of 11 studies (13–23) were included, involving 1,951 patients. The meta-analysis results showed that patients with high expression of CAFs were at a significantly increased risk of progressing into stage III–IV GC. The rate of stage III–IV GC in samples with high and low expression of CAFs was 60.7% and 37.8%, respectively. The RR of the study was 1.59 (95% CI: 1.24–2.04; P=0.0003) (Figure 2).
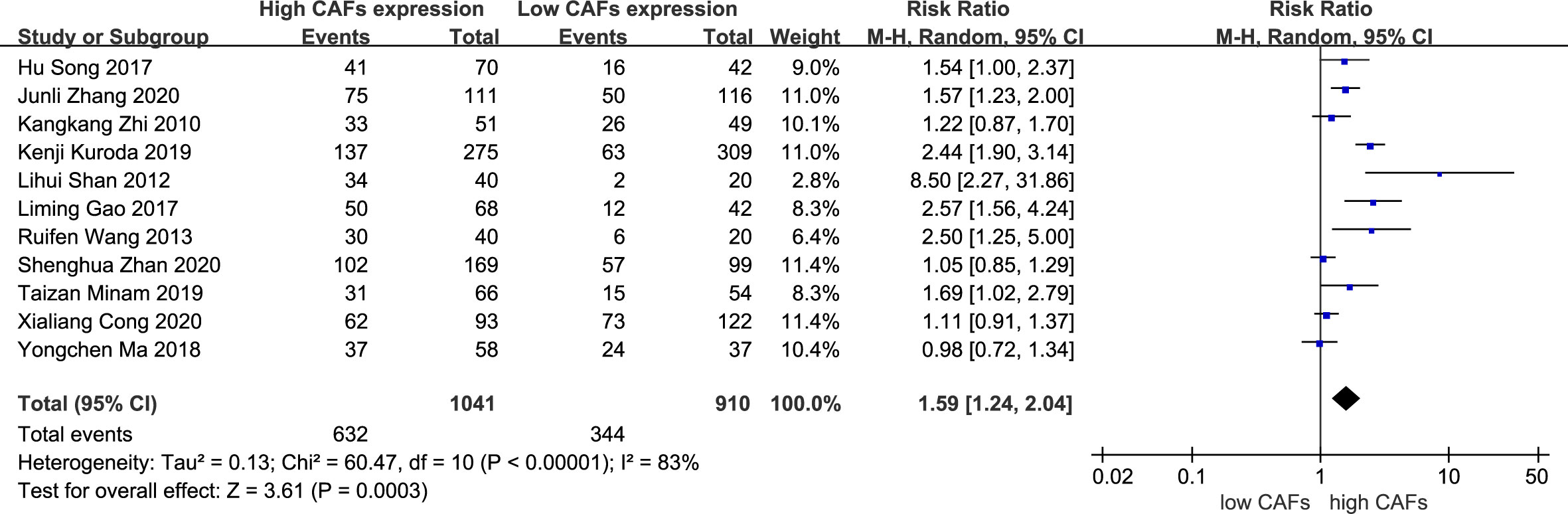
Figure 2 High expression of CAFs is associated with stage III–IV gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
A total of 11 studies (12, 13, 15, 16, 18–21, 23–25) were included, involving 2,201 patients. The meta-analysis results showed that patients with high expression of CAFs were at a significantly increased risk of developing lymph node metastasis. The rate of lymph node metastasis in samples with high and low expression of CAFs was 65.4% and 41.9%, respectively. The RR of the study was 1.51 (95% CI: 1.23–1.87; P=0.0001) (Figure 3).
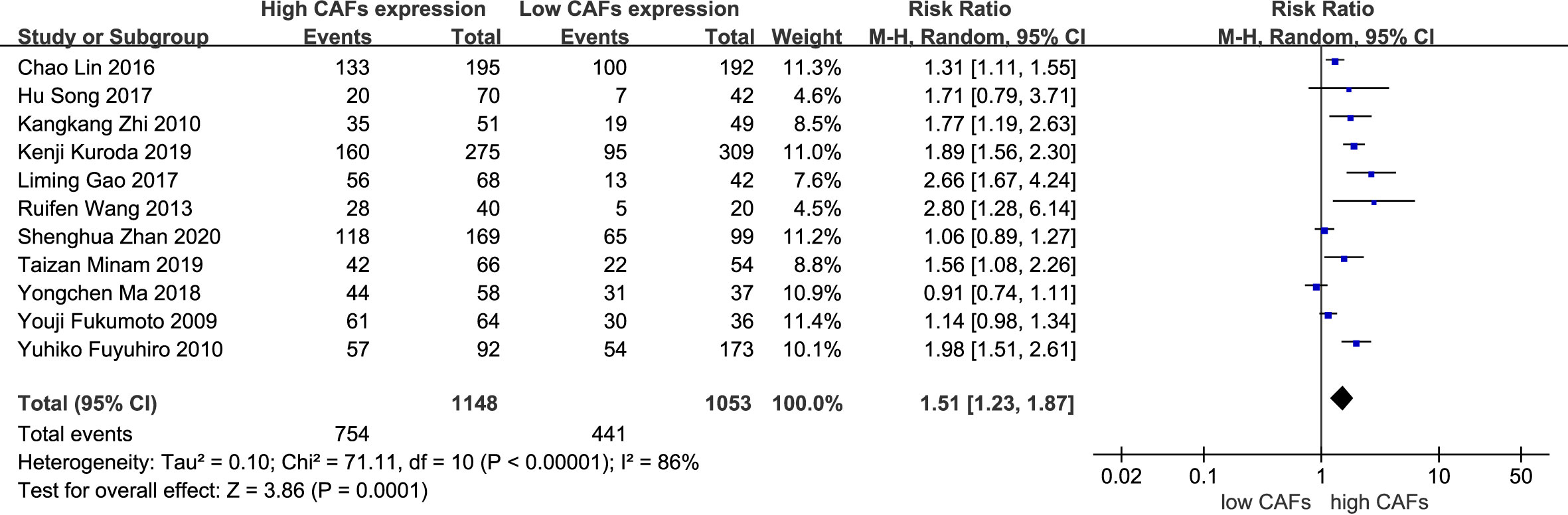
Figure 3 High expression of CAFs is associated with lymph node metastasis of gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
A total of 11 studies (12, 13, 15–21, 23, 25) were included, involving 2,161 patients. The meta-analysis results showed that patients with high expression of CAFs were at a significantly increased risk of serosal infiltration. The rate of serosal infiltration in samples with high and low expression of CAFs was 69.7% and 44.5%, respectively. The RR of the study was 1.56 (95% CI: 1.24–1.95; P=0.0001) (Figure 4).
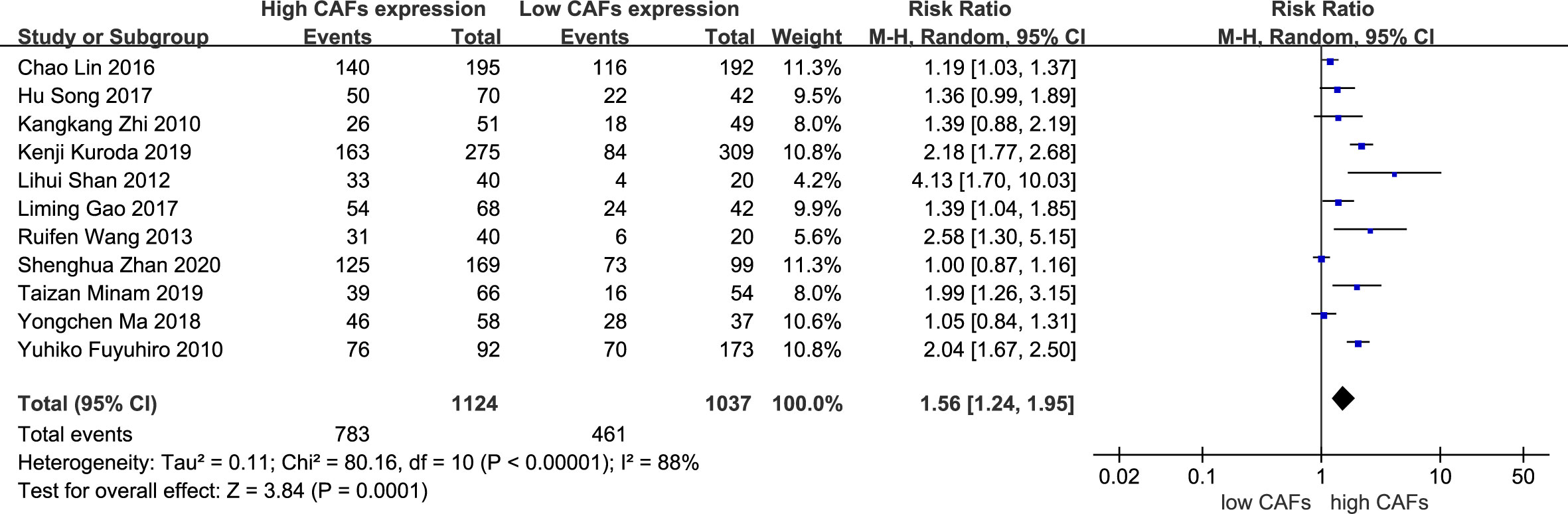
Figure 4 High expression of CAFs is associated with serosal infiltration in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
Four studies (12, 14, 17, 19) were included, involving 734 patients. The meta-analysis results showed that patients with high expression of CAFs were at a significantly increased risk of diffuse and mixed GC in the Lauren classification. The rate of diffuse and mixed GC in the Lauren classification in samples with high and low expression of CAFs was 43.3% and 29.3%, respectively. The RR of the study was 1.43 (95% CI: 1.18–1.74; P=0.0003) (Figure 5).
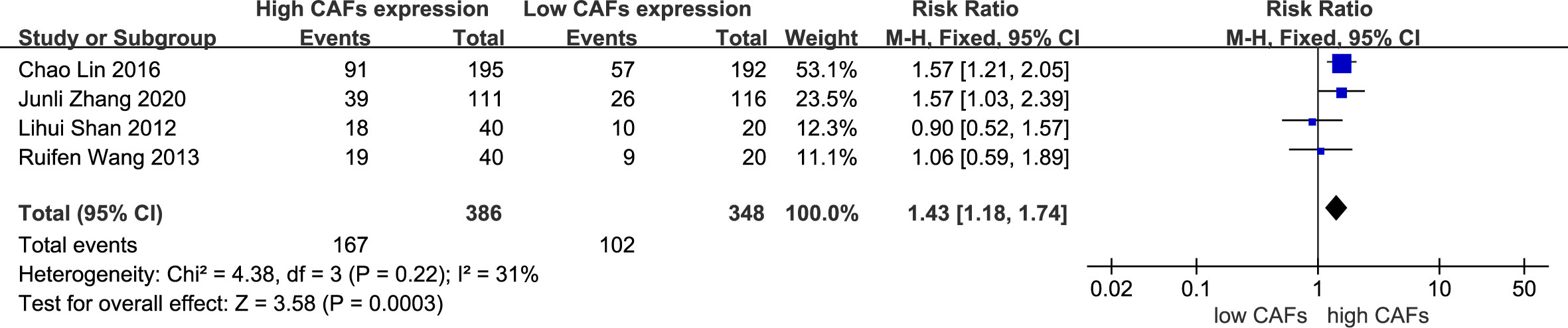
Figure 5 High expression of CAFs is associated with diffuse and mixed gastric cancer in the Lauren classification. CAF, cancer-associated fibroblast; CI, confidence interval.
Four studies (16, 21, 24, 25) were included, involving 1,069 patients. The meta-analysis results showed that patients with high expression of CAFs were at a significantly increased risk of vascular invasion. The rate of vascular invasion in samples with high and low expression of CAFs was 41.0% and 18.5%, respectively. The RR of the study was 1.99 (95% CI: 1.26–3.14; P=0.003) (Figure 6).
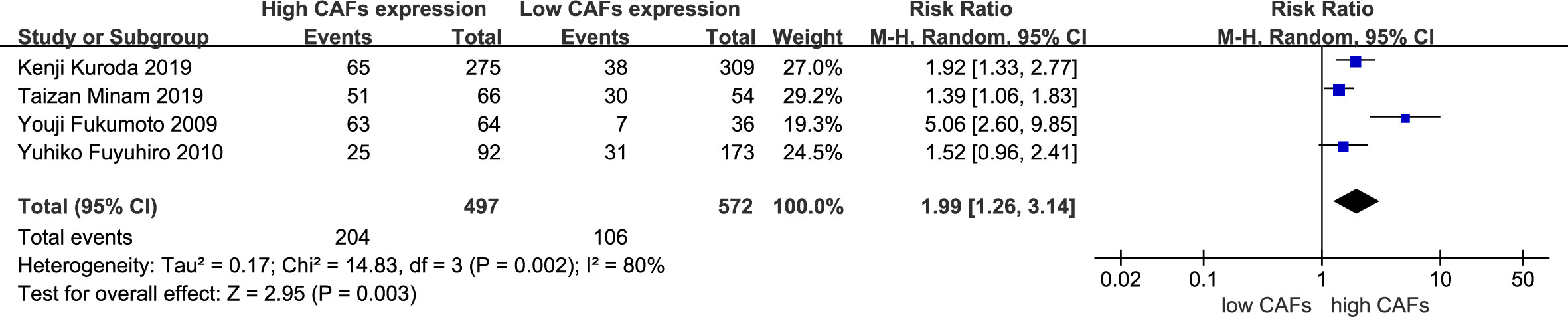
Figure 6 High expression of CAFs is associated with vascular invasion in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
There was no significant correlation between CAF expression and poorly differentiated GC. Thirteen studies (12–20, 22–25) were included, involving 2,583 patients. The RR of the study was 1.03 (95% CI: 0.96–1.10; P=0.45) (Figure 7). In addition, CAF expression was not significantly correlated with GC with a tumor diameter >5 cm. Six studies were included (14–16, 18, 20, 22), involving 1,504 patients. The RR of the study was 1.34 (95% CI: 0.98–1.83; P=0.07) (Figure 8).
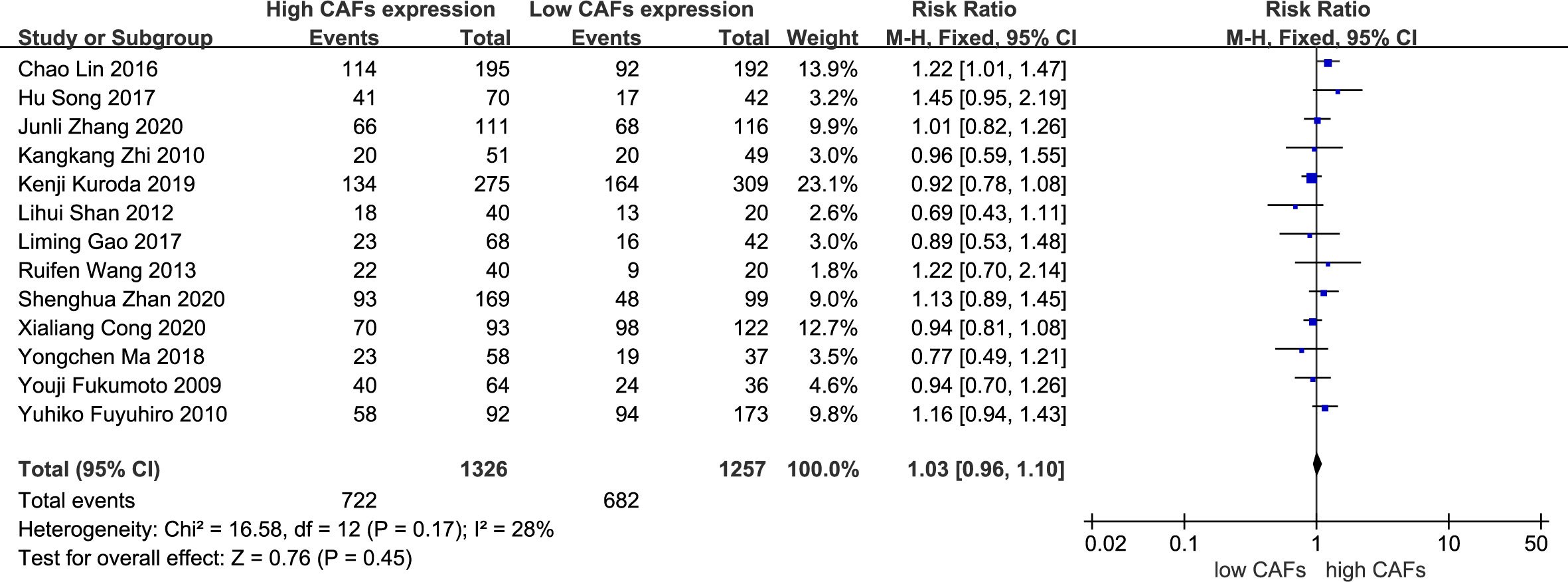
Figure 7 Correlation between CAFs and differentiation in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
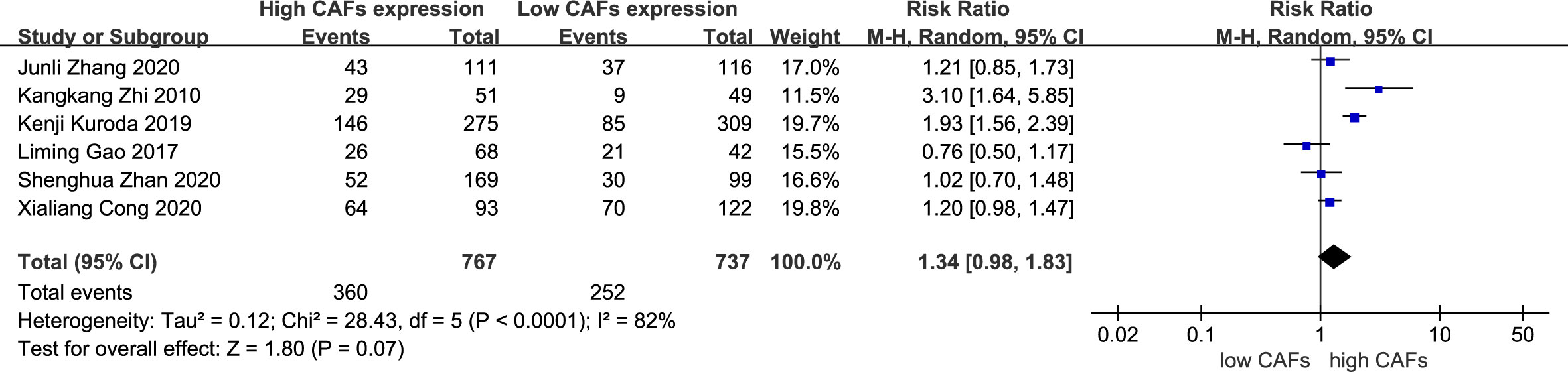
Figure 8 Correlation between CAFs and tumor size in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
Eight studies (12, 13, 16, 20, 21, 23–25) were included. The meta-analysis results showed that high expression of CAFs was significantly associated with poor OS in patients with GC. The hazard ratio (HR) was 1.38 (95% CI: 1.22–1.56; P<0.00001) (Figure 9).
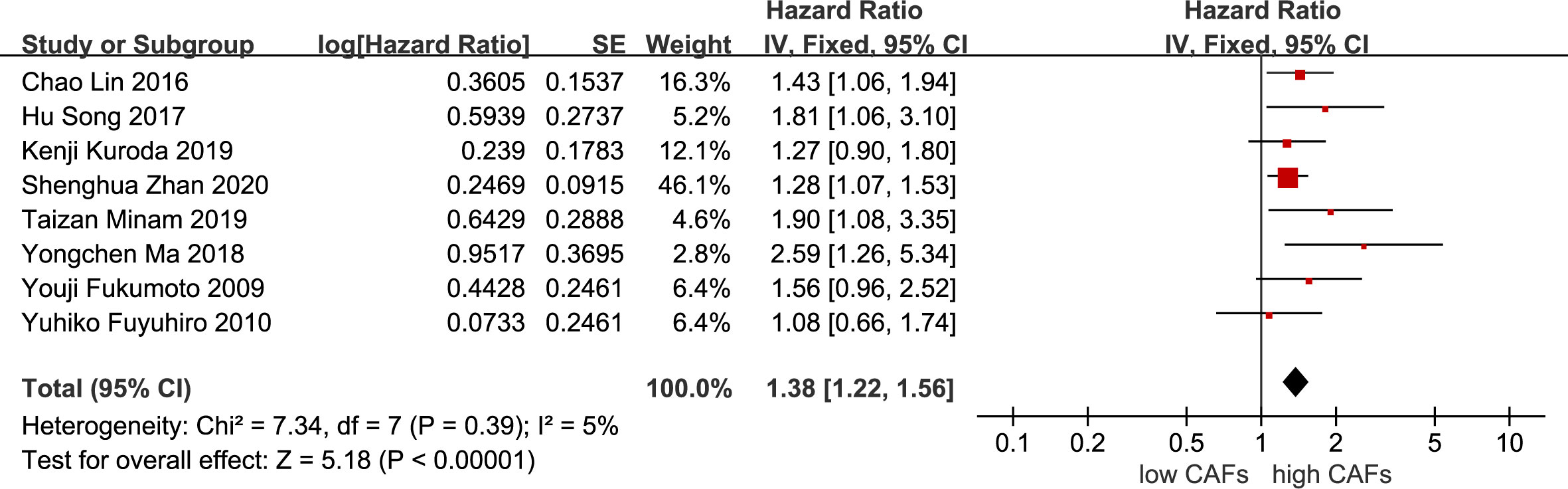
Figure 9 Association between CAFs and OS in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval; OS overall survival; SE, standard error.
Among the included studies, eight studies (12, 14–16, 20–22, 25) used α-SMA as a marker for CAF detection, while the remaining six studies (13, 17–19, 23, 24) used FAP. The specific role of CAFs identified by different markers in the development of GC is also controversial. Therefore, we performed a subgroup analysis based on these two different markers for the results of more than five included studies.
Subgroup analysis of high expression of CAFs and stage III–IV gastric cancer showed that FAP (RR, 2.06; 95% CI: 1.14–3.74; P=0.02)was more closely related to stage III-IV gastric cancer than α-SMA (RR, 1.43; 95% CI: 1.07–1.92; P=0.01) (Figure 10). Unexpectedly, although the result were not statistically significant in the meta-analysis of high expression of CAFs and tumor size in gastric cancer (P=0.07), in the subgroup analysis, we found a significant association between α-SMA and tumor diameter >5 cm (RR, 1.47; 95% CI: 1.07–2.02; P=0.02) (Figure 11). In addition, the FAP subgroup was also more strongly associated with poor OS (RR, 1.82; 95% CI: 1.32–2.51; P=0.0003) (Figure 12).

Figure 10 Subgroup analysis of high expression of CAFs and stage III–IV gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
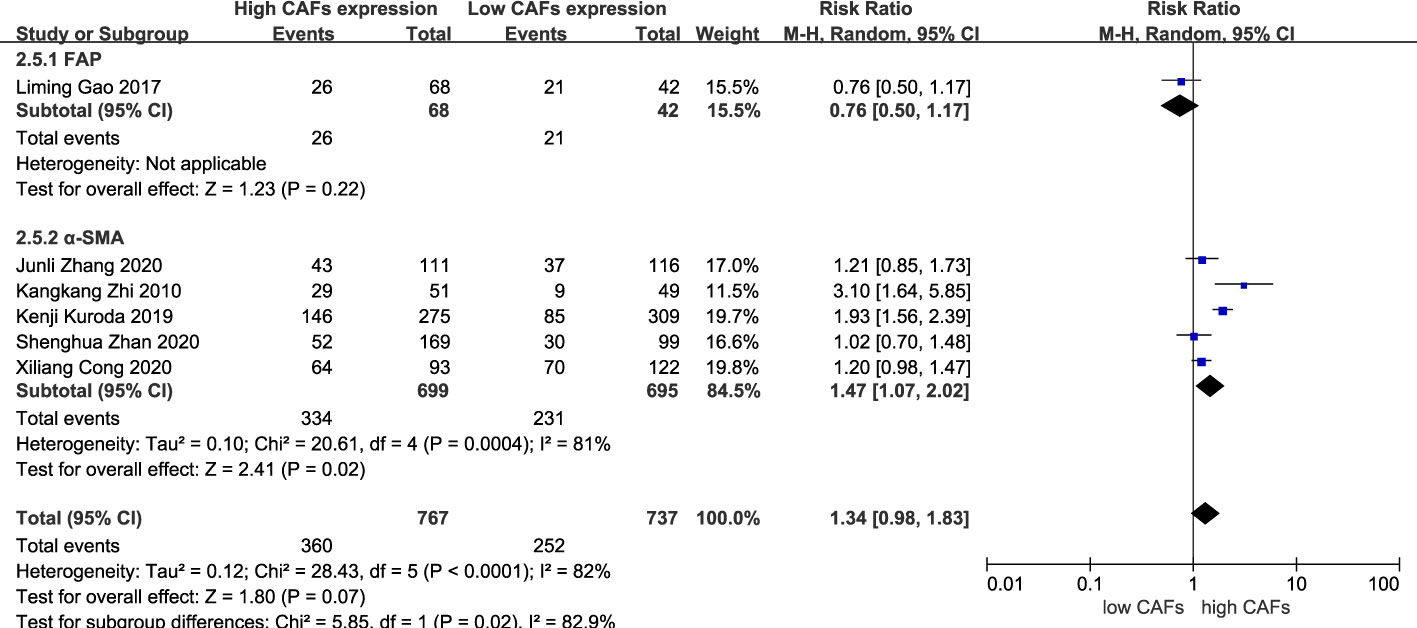
Figure 11 Subgroup analysis of high expression of CAFs and tumor size in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval.
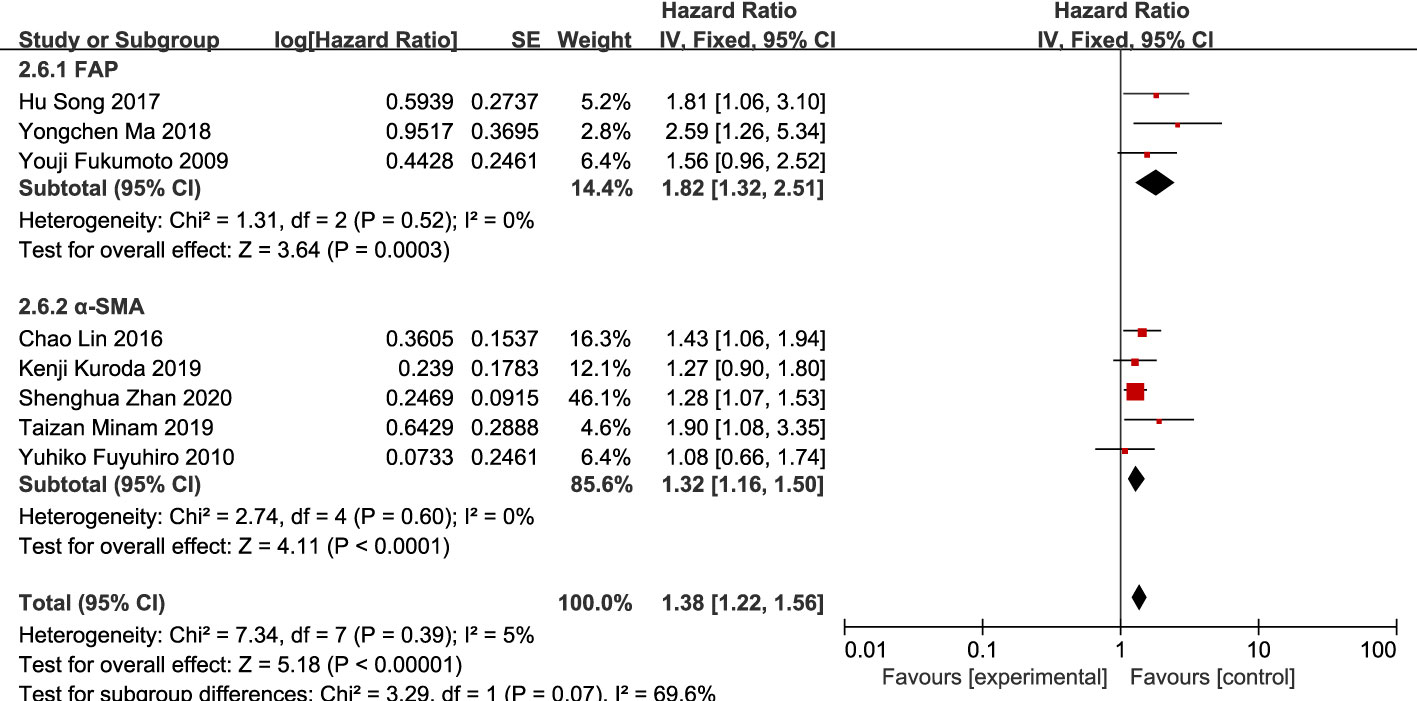
Figure 12 Subgroup analysis of high expression of CAFs and OS in gastric cancer. CAF, cancer-associated fibroblast; CI, confidence interval; OS overall survival; SE, standard error.
We conducted this meta-analysis after eliminating studies one by one, and the results showed no significant changes, indicating that the stability of the results was good.
The publication bias of the outcomes with more than 10 studies was assessed using visual examination of funnel plots and the Egger weighted regression statistic. The graph is shown in the Supplementary File. No significant publication bias was indicated.
Cancer is not limited to the presence of malignant tumor cells. It is characterized by a fundamental imbalance of the entire cell environment, termed TME, which is a complex dynamic system composed of cellular and non-cellular components (26). CAFs are one of the most important components in the TME and play an essential role in the occurrence and development of tumors. In recent years, an increasing number of studies have focused on tumor-associated fibroblasts and reported their roles in tumor proliferation, invasion, metastasis, drug resistance, etc. (27). Numerous studies investigated targeted therapies for CAFs (28). A meta-analysis of the association of CAFs with the prognostic characteristics of oral squamous cell carcinoma and head and neck squamous cell carcinoma was previously conducted, revealing significant correlations (29, 30). The correlation between CAFs and the prognosis of gastrointestinal tumors was also analyzed. However, due to the small number of GC studies included in this meta-analysis, the correlations between individual GC pathological features and CAFs were not analyzed (31).
This study systematically analyzed the relationship between CAFs and clinicopathological characteristics and prognosis of GC. A total of 14 studies were included, with sample sizes ranging from 60 to 594 patients. The present study confirmed that high expression of CAFs was closely associated with pathological indicators related to advanced GC (e.g., stage, lymph node metastasis, and vascular metastasis), suggesting that CAFs play a key role in GC invasion and metastasis. Several basic research studies confirmed that CAFs promote GC invasion and metastasis by inducing epithelial–mesenchymal transition (EMT), extracellular matrix (ECM) remodeling, and tumor angiogenesis. EMT alters the morphology of tumor cells from tightly arranged epithelial cells to loosely structured mesenchymal cells. This effect weakens the adhesion between tumor cells and enhances their motility, thus facilitating detachment from the primary site and transfer to other sites. Studies have confirmed that inflammatory cytokines, such as interleukin 6 (IL-6), IL-11, and IL-33, secreted by CAFs promote EMT through downstream signaling pathways, such as Janus kinase and signal transducer and activator of transcription and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways. Moreover, they promote the migration and invasion of GC cells, resulting in peritoneal dissemination of GC (32–35). Downregulation of mirNA-214 in CAFs induces EMT and promotes the migration and invasion of GC cells (36). In ECM remodeling, CAFs can degrade the ECM by expressing matrix metalloproteinases and collagenase, and change its structure and hardness through the release of transforming growth factor-β (TGF-β) (37). CAFs can also increase the hardness of the ECM by regulating factors related to cytoskeleton formation, thereby promoting tumor invasion and metastasis. Recent studies have found that hyaluronan and proteoglycan link protein 1, the most significantly upregulated gene in CAFs of GC, promotes invasion and metastasis through TGF-β-mediated ECM remodeling (38). Regarding tumor angiogenesis, blood vessels are the main channel of tumor cell metastasis and the main source of nutrients for tumor cells. The formation of tumor blood vessels is a sign of malignancy. CAFs promote tumor angiogenesis by secreting cytokines, such as vascular endothelial growth factor, platelet-derived growth factor, and TGF-β (39). Studies have confirmed that CAF-derived hepatocyte growth factor promotes angiogenesis, vascular mimicry, and mosaic vascular formation through the phosphatidylinositol 3 kinase/protein kinase B and extracellular regulated kinase 1/2 signaling pathways (40).
The subanalysis performed to determine the degree of differentiation in GC included the largest number of studies and cases in this investigation. There were no significant differences or heterogeneity among the studies. Unlike in head and neck squamous cell carcinoma, CAFs were not significantly correlated with the degree of differentiation in GC. Further basic research is warranted to confirm the relationship between differentiation and CAFs in GC. In addition, some indicators (e.g., microsatellite instability, HER2, PD-L1, Epstein–Barr virus, KI-67, etc.) cannot be comprehensively analyzed because the number of studies focusing on these indicators is currently insufficient; hence, it is important to further investigate the correlation between indicators in the future.
Although the present study demonstrated that high expression of CAFs is closely associated with the traditional pathological indicators related to poor prognosis in GC, CAFs did not show significant advantages over those characteristics and prognostic markers. Traditionally, CAFs have been broadly differentiated based on their morphology and specific markers. However, in recent years, the progress of single-cell omics technology has enabled researchers to further distinguish various types of CAFs and conduct more detailed studies on their specific roles (41–44). Through single-cell sequencing of GC tissues and adjacent mucosal samples, Li et al. (45) identified four subsets of CAFs with different properties, namely myofibroblastic CAFs, pericytic CAFs, inflammatory CAFs, and ECM CAFs. Inflammatory CAFs and ECM CAFs show enhanced invasive activity and mobilize surrounding immune cells to construct a tumor-friendly microenvironment, which is associated with poor prognosis of GC. Recently, a pan-cancer single-cell analysis reveal the heterogeneity of CAF and revealed their different activation pathways and roles (46). The article proposed that a high proportion of FAP+ CAFs was significantly associated with poor OS, which was similar to the conclusion obtained in our study. The article also proposed that α-SMA+ CAFs are closely related to angiogenesis, but there were not enough samples in the studies we included. So, additional studies are needed to examine the relationship between CAF heterogeneity and the pathological features and prognosis of GC.
This study had some limitations. Firstly, all studies included in this meta-analysis were conducted in China (n=10) and Japan (n=4). Hence, there was a lack of studies from other countries. This may be related to the global distribution of GC. Although GC is the fifth most malignant type of cancer worldwide, its incidence is low in European and American countries. Secondly, most studies did not adopt uniform standards for the determination of CAF expression, and the distinction between high and low CAF expression was not completely consistent. Thus, the results of this meta-analysis may be biased to some extent.
This study analyzed the relationship between CAF expression and clinicopathological indicators and prognosis of GC. The results showed that high expression of CAFs was closely associated with the traditional pathological indicators related to poor prognosis of GC, and may be valuable predictor of poor prognosis in this setting. This may provide new directions for research on the related mechanism and targeted therapy of GC. However, more high-quality studies are warranted to verify the above conclusions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Concept and design: JW. Acquisition of data: JW and MW. Statistical analysis: JW and MW. Interpretation of data: all authors. Writing of the original draft of the manuscript: JW and MW. Review and editing of the manuscript: all authors. All authors contributed to the article and approved the submitted version.
We thank all the authors of the enrolled published papers for their valued contributions to the field.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1048922/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) . 68(6):394–424. doi: 10.3322/caac.21492
2. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent (2022) 2(1):1–9. doi: 10.1016/j.jncc.2022.02.002
3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
4. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
5. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1
6. Ma Z, Chen M, Yang X, Xu B, Song Z, Zhou B, et al. The role of cancer-associated fibroblasts in tumorigenesis of gastric cancer. Curr Pharm Des (2018) 24(28):3297–302. doi: 10.2174/1381612824666180601094056
7. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol (2021) 18(12):792–804. doi: 10.1038/s41571-021-00546-5
8. Galbo PM Jr., Zang X, Zheng D. Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin Cancer Res (2021) 27(9):2636–47. doi: 10.1158/1078-0432.CCR-20-4226
9. Kobayashi H, Gieniec KA, Lannagan TRM, Wang T, Asai N, Mizutani Y, et al. The origin and contribution of cancer-associated fibroblasts in colorectal carcinogenesis. Gastroenterology (2022) 162(3):890–906. doi: 10.1053/j.gastro.2021.11.037
10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
11. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
12. Lin C, Liu H, Shen ZB, Zhang H, He HY, Li H, et al. Increased expression of alpha SMA is associated with poor prognosis in patients with gastric cancer after surgical resection. Int J Clin Exp Med (2016) 9(6):11157–65. Available at: https://e-century.us/files/ijcem/9/6/ijcem0024111.pdf.
13. Song H, Liu QY, Huang ZW. High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer. Biomed Res (2017) 28(18):7779–83.Available at: https://www.biomedres.info/biomedical-research/high-expression-of-fibroblast-activation-protein-is-an-adverse-prognosticator-in-gastric-cancer.pdf.
14. Zhang J, Li S, Zhao Y, Ma P, Cao Y, Liu C, et al. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann Transl Med (2020) 8(14):877. doi: 10.21037/atm-20-4843
15. Zhi K, Shen X, Zhang H, Bi J. Cancer-associated fibroblasts are positively correlated with metastatic potential of human gastric cancers. J Exp Clin Cancer Res (2010) 29(1):66. doi: 10.1186/1756-9966-29-66
16. Kuroda K, Yashiro M, Sera T, Yamamoto Y, Kushitani Y, Sugimoto A, et al. The clinicopathological significance of thrombospondin-4 expression in the tumor microenvironment of gastric cancer. PLoS One (2019) 14(11):e0224727. doi: 10.1371/journal.pone.0224727
17. Shan LH, Sun WG, Han W, Qi L, Yang C, Chai CC, et al. Roles of fibroblasts from the interface zone in invasion, migration, proliferation and apoptosis of gastric adenocarcinoma. J Clin Pathol (2012) 65(10):888–95. doi: 10.1136/jclinpath-2012-200909
18. Gao LM, Wang F, Zheng Y, Fu ZZ, Zheng L, Chen LL. Roles of fibroblast activation protein and hepatocyte growth factor expressions in angiogenesis and metastasis of gastric cancer. Pathol Oncol Res (2019) 25(1):369–76. doi: 10.1007/s12253-017-0359-3
19. Wang RF, Zhang LH, Shan LH, Sun WG, Chai CC, Wu HM, et al. Effects of the fibroblast activation protein on the invasion and migration of gastric cancer. Exp Mol Pathol (2013) 95(3):350–56. doi: 10.1016/j.yexmp.2013.10.008
20. Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, et al. Overexpression of B7-H3 in α-SMA-Positive fibroblasts is associated with cancer progression and survival in gastric adenocarcinomas. Front Oncol (2019) 9:1466. doi: 10.3389/fonc.2019.01466
21. Minami T, Aoyagi K, Kawahara A, Murakami N, Isobe T, Tanaka Y, et al. Evaluation of the expression of bone marrow-derived mesenchymal stem cells and cancer-associated fibroblasts in the stroma of gastric cancer tissue. Ann Gastroenterol Surg (2020) 4(4):464–74. doi: 10.1002/ags3.12347
22. Cong X, Zhang Y, Zhu Z, Li S, Yin X, Zhai Z, et al. CD66b+ neutrophils and α-SMA+ fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med (2020) 9(8):2761–73. doi: 10.1002/cam4.2939
23. Ma Y, Zhu J, Chen S, Li T, Ma J, Guo S, et al. Activated gastric cancer-associated fibroblasts contribute to the malignant phenotype and 5-FU resistance via paracrine action in gastric cancer. Cancer Cell Int (2018) 18:104. doi: 10.1186/s12935-018-0599-7
24. Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S, Tsujitani S, et al. Clinical findings on fibroblast activation protein in patients with gastric cancer. Yonago Acta Med (2009) 52(1):21–5. Available at: http://www.lib.tottori-u.ac.jp/yam/yam/yam52-1/52_021-025.pdf.
25. Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Doi Y, et al. Myofibroblasts are associated with the progression of scirrhous gastric carcinoma. Exp Ther Med (2010) 1(4):547–51. doi: 10.3892/etm_00000086
26. Rojas A, Araya P, Gonzalez I, Morales E. Gastric tumor microenvironment. Adv Exp Med Biol (2020) 1226:23–35. doi: 10.1007/978-3-030-36214-0_2
27. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol (2019) 12(1):86. doi: 10.1186/s13045-019-0770-1
28. Jung JG, Le A. Targeting metabolic cross talk between cancer cells and cancer-associated fibroblasts. Adv Exp Med Biol (2018) 1063:167–78. doi: 10.1007/978-3-319-77736-8_12
29. Dourado MR, Guerra ENS, Salo T, Lambert DW, Coletta RD. Prognostic value of the immunohistochemical detection of cancer-associated fibroblasts in oral cancer: a systematic review and meta-analysis. J Oral Pathol Med (2018) 47(5):443–53. doi: 10.1111/jop.12623
30. Knops AM, South A, Rodeck U, Martinez-Outschoorn U, Curry JM. Cancer-associated fibroblast density, prognostic characteristics, and recurrence in head and neck squamous cell carcinoma: a meta-analysis. Front Oncol (2020) 10:565306. doi: 10.3389/fonc.2020.565306
31. Gu JJ, Wang CY, Xu XX, Zhao L, Zhou JF, Bai CM, et al. Immunohistochemical detection of cancer-associated fibroblasts in gastrointestinal cancer as a potential prognostic biomarker of survival: Meta-analysis. Transl Cancer Res (2020) 9(11):6629–38. doi: 10.21037/tcr-20-2365
32. Yasuda T, Koiwa M, Yonemura A, Miyake K, Kariya R, Kubota S, et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep (2021) 34(8):108779. doi: 10.1016/j.celrep.2021.108779
33. Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial–mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget (2017) 8(13):20741–50. doi: 10.18632/oncotarget.15119
34. Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene (2020) 39(7):1414–28. doi: 10.1038/s41388-019-1078-x
35. Wang XX, Che XF, Bai M, Fan YB, Liu YP, Qu XJ. Cancer-associated fibroblasts-stimulated interleukin-11 promotes metastasis of gastric cancer cells mediated by upregulation of MUC1. Exp Cell Res (2018) 368(2):184–93. doi: 10.1016/j.yexcr.2018.04.028
36. Wang R, Sun Y, Yu W, Yan Y, Qiao M, Jiang R, et al. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J Exp Clin Cancer Res (2019) 38(1):20. doi: 10.1186/s13046-018-0995-9
37. Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer (2020) 1873(2):188356. doi: 10.1016/j.bbcan.2020.188356
38. Zhang T, Li X, He Y, Wang Y, Shen J, Wang S, et al. Cancer-associated fibroblasts-derived HAPLN1 promotes tumour invasion through extracellular matrix remodeling in gastric cancer. Gastric Cancer (2022) 25(2):346–59. doi: 10.1007/s10120-021-01259-5
39. Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol (2019) 16(5):282–95. doi: 10.1007/s10120-021-01259-5
40. Ding XS, Xi WQ, Ji J, Cai Q, Jiang JL, Shi M, et al. HGF derived from cancer-associated fibroblasts promotes vascularization in gastric cancer via PI3K/AKT and ERK1/2 signaling. Oncol Rep (2018) 40(2):1185–95. doi: 10.3892/or.2018.6500
41. Kumar V, Ramnarayanan K, Sundar R, Padmanabhan N, Srivastava S, Koiwa M, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discovery (2022) 12(3):670–91. doi: 10.1158/2159-8290.CD-21-0683
42. Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell (2021) 39(6):866–82.e11. doi: 10.1016/j.ccell.2021.03.012
43. Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun (2018) 9(1):5150. doi: 10.1038/s41467-018-07582-3
44. Chen Z, Zhou L, Liu L, Hou Y, Xiong M, Yang Y, et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun (2020) 11(1):5077. doi: 10.1038/s41467-020-18916-5
45. Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics (2022) 12(2):620–38. doi: 10.7150/thno.60540
Keywords: cancer-associated fibroblasts, gastric cancer, clinicopathological characteristics, prognosis, meta-analysis
Citation: Wei J, Wang M and Li G (2023) Cancer-associated fibroblasts, and clinicopathological characteristics and prognosis of gastric cancer: A systematic review and meta-analysis. Front. Oncol. 13:1048922. doi: 10.3389/fonc.2023.1048922
Received: 20 September 2022; Accepted: 06 February 2023;
Published: 17 February 2023.
Edited by:
Jinqiu Jacky Yuan, Seventh Affiliated Hospital, Sun Yat-sen University, ChinaReviewed by:
Xiaowen Liu, Fudan University, ChinaCopyright © 2023 Wei, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guixiang Li, bGd4LmRvY3Rvci4wMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.