
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 20 March 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1042548
This article is part of the Research TopicProspective Utilization and Clinical Applications of Artificial Intelligence and Data-driven Automation for RadiotherapyView all 8 articles
Introduction: The goal of this analysis is to validate the 2022 graded prognostic assessment (GPA) for patients with brain metastases from adenocarcinoma of the lung and to discuss its clinical practicability.
Methods/material: 137 patients with adenocarcinoma of the lung were included in this analysis. The disease specific GPA for NSCLC, Lung-molGPA and the GPA for NSCLC adenocarcinoma were calculated. Overall survival was calculated for each GPA group. Additionally, expected and actual OS in the prognostic groups of the GPA available at the time of the patients’ diagnosis was compared.
Results: Median overall survival (OS) from diagnosis of brain metastases was 15 months (95% confidence interval (CI) 9.7–20.3 months). The median OS in the three individual prognostic groups was 7 months for GPA 0-1, 16 months for GPA 1.5-2, 33 months for GPA 2.5-3 and not reached for GPA 3.5-4 (p<0.001). Median survival times for the individual groups were similar to those published in the original GPA publication. Regarding the expected and actual OS when using the available GPA at the time of diagnosis there was an underestimation of survival of more than 3 months for all except the worst prognosis group.
Conclusion: We were able to validate the 2022 GPA for NSCLC adenocarcinoma patients with brain metastases in a similar cohort from a non-academic center. However, the practical applicability regarding the expected median OS might be limited due to the constantly evolving treatment landscape and the consecutive improvement in overall survival.
Brain metastases are quite common in patients with lung cancer with up to 50% of patients either presenting with or developing brain metastases during the course of the disease (1–3). With lung cancer being the second most common cancer and the most common cause of death from cancer worldwide, this results in a substantial amount of patients (4).
The diagnosis of brain metastases leads to complex problems in the management of these patients. On the side of symptom burden, brain metastases may lead to various, possibly severe symptoms, i.e. neurocognitive impairment, personality changes, fluctuating vigilance, epileptic seizures and loss of mobility (5). Regarding therapeutic options, limited penetration of systemic drugs, the loss of targetable mutations and the selection of resistant clones during long disease courses may lead to significant therapeutic challenges (6).
Depending on several factors, i.e. targetable mutations or number of brain metastases, there are multiple treatment options for brain metastases, including radiotherapy (stereotactic or whole brain radiotherapy), surgery and systemic therapy penetrating the blood-brain barrier (chemotherapy, immunotherapy or targeted therapy) (7).
Prognostic scores can be helpful to guide treatment for the primary, for the extracranial metastatic disease, and for the brain metastases. Due to improved therapeutic options, the life expectancy of patients with brain metastases has improved over the last decades (8–10). Consequently, prognostic factors, i.e. the recursive partitioning analysis (RPA) and the graded prognostic assessment (GPA), had to be adapted over time (6, 11–15). Sperduto et al. published the latest version of the GPA for patients with lung cancer in 2022 including the Karnofsky performance status (KPS), age, number of brain metastases, the absence of extracranial metastases, the mutation status (EGFR, ALK) and PD-L1 status (6).
The basis for the development of the GPA scores was an academic cohort. Therefore, the primary aim of this analysis was to validate the new GPA for lung adenocarcinoma with the data from our non-academic tertiary care hospital. Secondarily, we aimed to assess whether the GPA that was available at the time of the patients’ diagnosis would have accurately estimated the overall survival.
A single institutional database from a certified lung cancer unit at a cancer center was searched for patients with adenocarcinoma of the lung with a diagnosis of brain metastases from January 2015 – December 2020. 166 patients were initially identified. Of those, 29 patients had to be excluded from this analysis in accordance with the exclusion criteria stated by Sperduto et al. (6). In ten cases, patient had leptomeningeal disease, 18 patients received best supportive care (BSC) and in one case the amount of available data was insufficient.
Median age of patients at the diagnosis of brain metastases was 67 years (range, 42 – 87 years) with a female predominance (n = 76; 55.5%). The majority of patients (n = 106; 77.4%) presented with metastatic disease upon first diagnosis. Most patients (n = 76; 55.5%) received systemic therapy as initial treatment for the primary tumor and radiotherapy as primary treatment for the brain metastases (n = 97; 70.8%). The most common type of cranial radiotherapy used in this cohort was whole brain radiotherapy (WBRT, n = 61, 44.5%) followed by stereotactic radiotherapy (SRT, n = 52, 38%) and partial brain radiotherapy (n = 18, 13.1%). In total, 47 patients (34.4%) had an intracranial relapse. The intracranial relapse rates for patients receiving SRT and partial brain radiotherapy were very similar (52% vs. 55%) and lower with WBRT (16.4%). Patients that initially received WBRT had the following treatment in case of cranial relapse: best supportive care (BSC) (n = 2, 20%), surgical resection in (n = 2, 20%), systemic therapy in (n = 3, 30%), re-irradiation in (n = 3, 30% with 2 patients SRT, 1 patient re-WBRT). Patients with initial partial brain RT received surgical resection (n = 1, 10%), systemic therapy (n = 4, 40%) or re-irradiation (n = 5, 50% with 1 patient WBRT, 1 patient partial brain, 3 patients SRT). In patients with initial SRT, the most common therapy in case of recurrence was re-irradiation (n = 16, 59.2% with 8 patients WBRT, 8 patients SRT), followed by systemic therapy (n = 7, 25.9%), surgical resection (n = 3, 11.1%) and BSC (n = 1, 3.7%). Additional patient and treatment related characteristics are shown in Table 1.
The disease specific GPA for NSCLC, Lung-molGPA and the GPA for NSCLC adenocarcinoma were calculated according to the published criteria (6, 14, 15). Survival was calculated from the date of diagnosis of disease as well as the diagnosis of brain metastases until the date of death or last follow-up. Overall survival (OS) was calculated using the Kaplan Meier method. Median OS for the individual GPA prognostic groups was calculated. For group comparison, the log-rank test was used. Due to the very limited number of patients in the prognostic group with GPA 3.5-4.0, this group was excluded from the log-rank test. A p-value ≤ 0.05 was considered statistically significant. For statistical analysis, SPSS version 28 (Statistical Package for Social Sciences, IBM Corp., Armonk, NY, USA) was used. This analysis was approved by the responsible ethics committee.
Median overall survival from first diagnosis of disease was 24 months (95% confidence interval (CI) 16.1 – 32.0 months) and median survival from the diagnosis of brain metastases was 15 months (95% CI 9.7 – 20.3 months). The median OS in the three individual prognostic groups was 7 months (GPA 0-1), 16 months (GPA 1.5-2) and 33 months (GPA 2.5-3), respectively (Chi-Square 34.013, p < 0.001). For the best prognostic group (GPA 3.5 – 4) the median OS was not reached since more than half of the patients were still living. The survival curves are shown in Figure 1. The comparison of the distribution of patients and the median OS in our cohort and the original cohort [from (6)] is shown in Table 2.
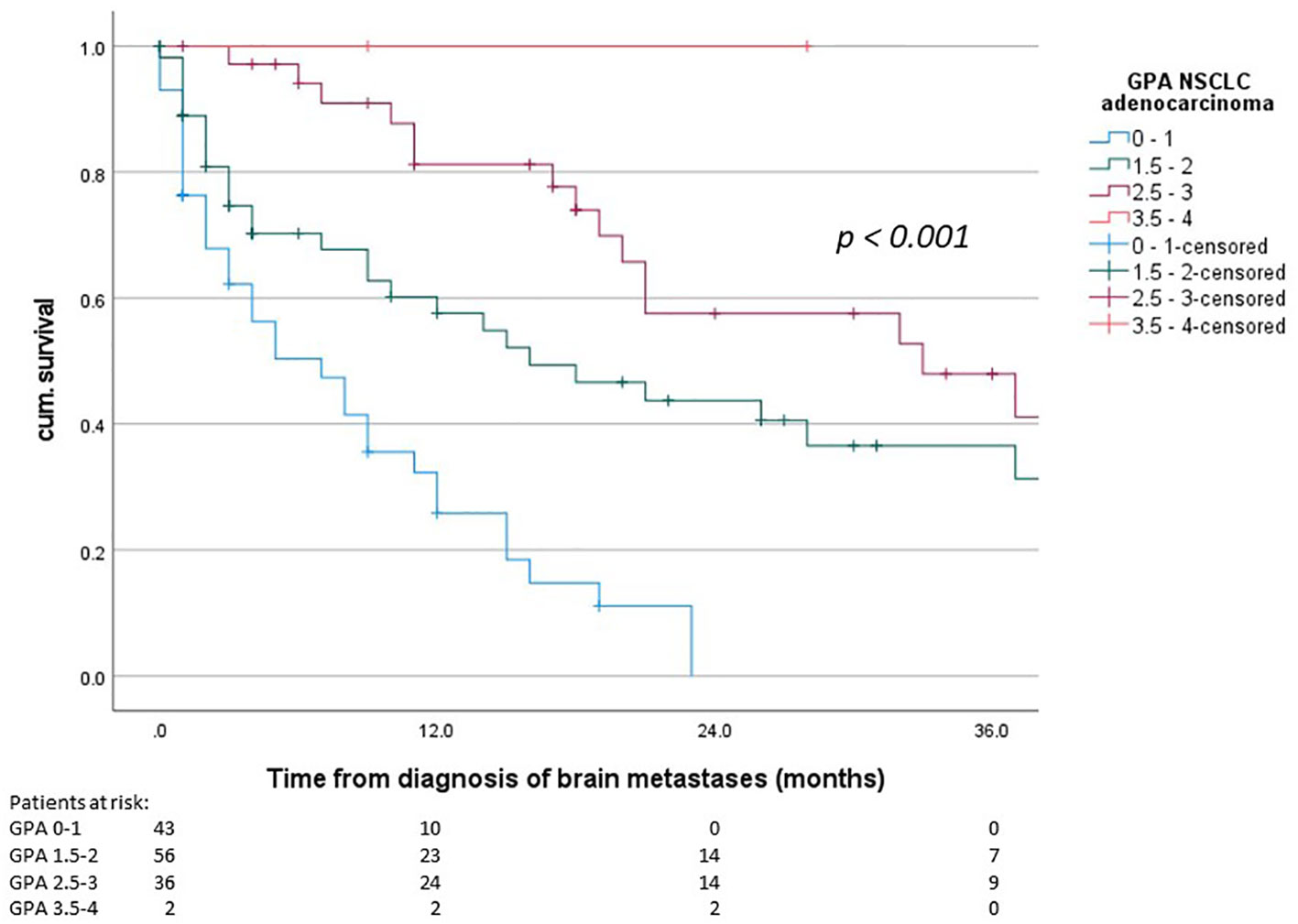
Figure 1 Overall survival from diagnosis of brain metastases, GPA groups according to the 2022 GPA NSCLC adenocarcinoma (p < 0.001).
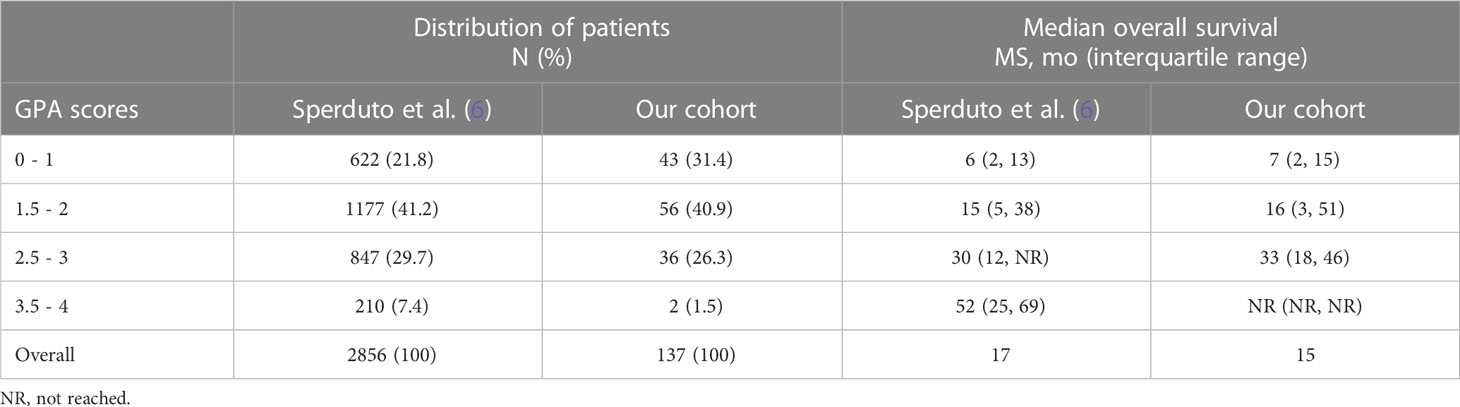
Table 2 Comparison of the distribution of patients and median OS of the original cohort and our cohort.
In this cohort, 45 patients had the diagnosis of brain metastases before the publication of the Lung-mol GPA in 06/2017. For these patients (diagnosed 01/2015 – 05/2017) the disease specific GPA from 2012 would have been applied (14) in clinical practice. For the patients diagnosed from 06/2017 – 12/2020 the Lung-molGPA would have been applied (15).
The median overall survival of the patients diagnosed until the end of 05/2017 was 18 months and 14 months for the patients diagnosed thereafter (06/2017 – 12/2020). The comparison of the expected median OS according to the respective GPA in the different prognostic groups and the actual median OS in this cohort is shown in Table 3. Notably, the separation between survival curves is still statistically significant for both groups (until end of 05/2017 p = 0.035, from 06/2017 p < 0.001).
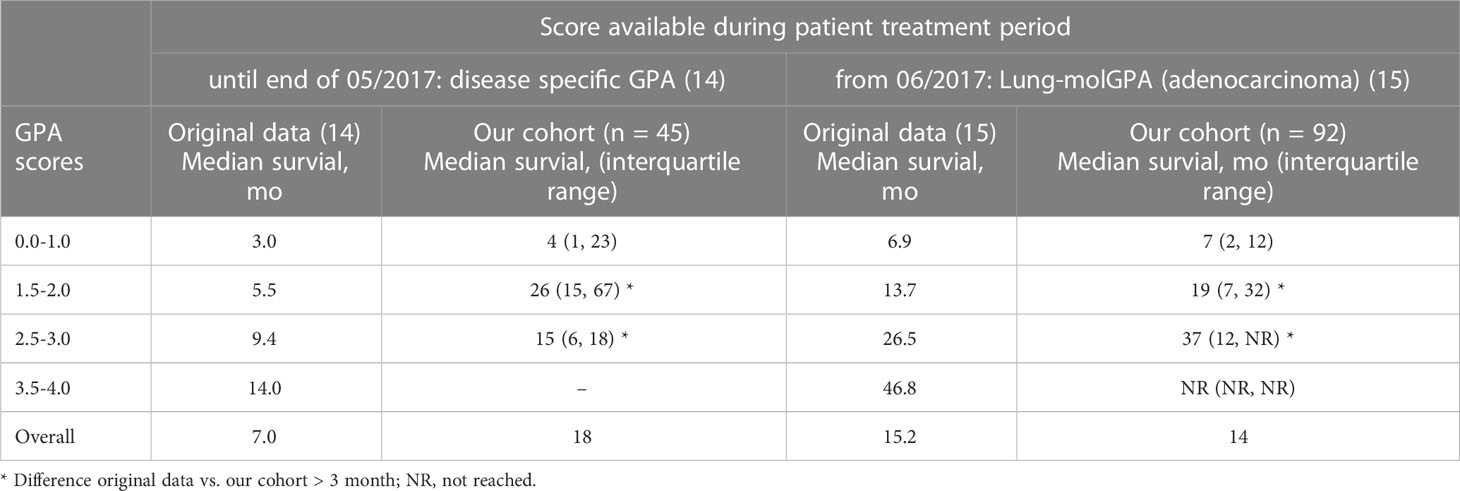
Table 3 Comparison of the expected median OS according to the available prognostic score and the actual median OS of patients treated in the time period.
With the constant development of new therapies for non-small cell lung cancer (NSCLC) and as a consequence, the improved prognosis of patients, there also has been an adaptation of the proposed prognostic scores. The Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) for all patients with brain metastases was introduced in 1997 (11, 12). It included age, KPS score, controlled primary tumor and the absence of extracranial metastases. In 2008, a graded prognostic assessment (GPA) for patients with brain metastases was introduced by Sperduto et al. including similar factors like the RPA (13). The same group proposed a disease specific GPA (with NSCLC and small cell lung cancer (SCLC) having a similar scoring system but a different prognosis) 4 years later in 2012; with again similar factors in the lung cancer group (age, KPS score, absence of extracranial metastases, number of brain metastases) (14). With further improvement of therapies and subsequently survival, Sperduto et al. further adapted their prognostic score and introduced the Lung-molGPA in 2017, including molecular markers, i.e. EGFR and ALK, for the first time (15). This score was recently further updated with a newly introduced separation of NSCLC adenocarcinoma and NSCLC non-adenocarcinoma, not only regarding the prognosis as in the the Lung-molGPA but also for the score itself (6). Additionally, the presence of a PD-L1 status was included. Furthermore, during the adaption of the scores the cutoff for age was raised from 60 to 70 years (from 2017) and the grading for the number of brain metastases was changed (also from 2017) (14, 15).
Regarding survival, the median overall survival more than doubled from the publication of the RPA (7 months) to the latest 2022 GPA (17 months) (6, 11, 12). In our cohort, the median overall survival was inferior with 15 months, which rather resembles the 2017 Lung-molGPA adenocarcinoma cohort (15). However, the difference was very small with 2 months and likely attributable to the distribution of included patients in the prognostic groups. In our cohort, 72.3% of patients were in the worst two prognostic groups (GPA 0-1, GPA 1.5-2) as opposed to 63.0% in the 2022 cohort of Sperduto et al. (6). Generally, the median OS in the individual subgroups of the original paper and this cohort were very similar with a maximum difference of three months in the GPA 2.5 – 3 group. For the best prognostic group (GPA 3.5 – 4.0), no conclusion is possible due to the very limited amount of patients in cohort analyzed in this work. The median overall survival in the individual prognostic group was very similar with the 2022 GPA being highly significant for overall survival (p < 0.001). This prognostic group was also rather underrepresented in the original cohort (7.4%) and even lower in this cohort (1.5%). This might be attributable to this being a non-academic cohort.
Although prognostic scores can be a helpful tool in clinical practice to estimate prognosis and guide treatment decisions, there are some critical aspects to consider. In case of the 2022 GPA, this is for once the more detailed separation of the KPS (e.g. ≤ 70%, 80%, 90-100% for adenocarcinoma and ≤ 60%, 70%, 80%, 90%, 100% for SCLC) which might be challenging to obtain correctly in clinical practice. There are known biases for the estimation of the performance status due to the subjective nature and KPS may vary in these patients on a daily basis (16–20). Another factor to consider is the reason for the constant updates of the GPA as a consequence of the constant improvement of therapeutic options and hence, the overall survival of these patients. This might be less important for some tumor entities but especially in lung cancer there is rapid development, e.g. regarding CNS penetrating systemic therapies (21–26). In this scenario, a score that is based on an older cohort of patients might not be entirely accurate even at the time of publication. This cohort for example was treated between January 2015 and December 2020. The 2017 Lung-mol GPA [cohort 2006 – 2014 (15)] was published in June 2017 and would have been used for most patients in this cohort (92 patients (67%) were diagnosed from June 2017 on). Although the separation of the survival curves with the 2017 Lung-molGPA was still very good in the cohort analyzed in this work, the median survival times differed significantly for some groups. For the prognostic group with a GPA 0-1, the median OS was quite similar (6.9 months in the work of Sperduto et al. (15) vs. 5 months in this cohort), but for the GPA scores 1.5-2 (13.7 months in (15) vs. 21 months in this cohort) and 2.5-3 (26.5 months in (15) vs. 37 months in this cohort) the median OS differed by up to 10 months. Therefore, prognostic scores should be used with caution, especially in rapidly evolving fields.
The limitations of this analysis lie in the limited amount of patients, specifically across subgroups, and the retrospective nature of the analysis.
In conclusion, the 2022 GPA for NSCLC adenocarcinoma patients with brain metastases was validated in a similar cohort from a non-academic center. However, the expected median OS might likely change dynamically in this patient group due to the rapidly evolving therapeutic options and the subsequently constantly improving OS.
The data analyzed in this study is subject to the following licenses/restrictions: Hospital internal database. Requests to access these datasets should be directed to Y2hyaXN0aW5hLnNjaHJvZWRlckBrc3cuY2g=.
The studies involving human participants were reviewed and approved by Cantonal Ethics Committee Zurich (BASEC-Nr. 2020-02112, 2020-02124). The patients/participants provided their written informed consent to participate in this study. Consent for patients who were deceased was covered BASEC Nr. 2020-02124, which is an Ethics approval according to article 34 HFG.
All authors contributed to the article and have read and agreed to the published version of the manuscript.
This work was supported by a personal grant of the corresponding author (C.Schröder) from the “Filling the gap” program of the University of Zurich, Switzerland.
The authors thank all participating patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol (2017) 19(11):1511–21. doi: 10.1093/neuonc/nox077
2. Quan AL, Videtic GM, Suh JH. Brain metastases in small cell lung cancer. Oncol (Williston Park) (2004) 18(8):961–72. discussion 74, 79-80, 87.
3. Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer (2018) 19(4):e373–e9. doi: 10.1016/j.cllc.2018.01.007
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Maqbool T, Agarwal A, Sium A, Trang A, Chung C, Papadakos J. Informational and supportive care needs of brain metastases patients and caregivers: A systematic review. J Cancer Educ (2017) 32(4):914–23. doi: 10.1007/s13187-016-1030-5
6. Sperduto PW, De B, Li J, Carpenter D, Kirkpatrick J, Milligan M, et al. Graded prognostic assessment (GPA) for patients with lung cancer and brain metastases: Initial report of the small cell lung cancer GPA and update of the non-small cell lung cancer GPA including the effect of programmed death ligand 1 and other prognostic factors. Int J Radiat Oncol Biol Phys (2022) 114(1):60–74. doi: 10.1016/j.ijrobp.2022.03.020
7. Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol (2022) 40(5):492–516. doi: 10.1200/JCO.21.02314
8. Liu Q, Tong X, Wang J. Management of brain metastases: History and the present. Chin Neurosurg J (2019) 5:1. doi: 10.1186/s41016-018-0149-0
9. Steindl A, Brunner TJ, Heimbach K, Schweighart K, Moser GM, Niziolek HM, et al. Changing characteristics, treatment approaches and survival of patients with brain metastasis: Data from six thousand and thirty-one individuals over an observation period of 30 years. Eur J Cancer (2022) 162:170–81. doi: 10.1016/j.ejca.2021.12.005
10. Tsao MN. Brain metastases: Advances over the decades. Ann Palliat Med (2015) 4(4):225–32.doi: 10.3978/j.issn.2224-5820.2015.09.01
11. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys (1997) 37(4):745–51. doi: 10.1016/S0360-3016(96)00619-0
12. Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys (2000) 47(4):1001–6. doi: 10.1016/S0360-3016(00)00547-2
13. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys (2008) 70(2):510–4. doi: 10.1016/j.ijrobp.2007.06.074
14. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol (2012) 30(4):419–25. doi: 10.1200/JCO.2011.38.0527
15. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol (2017) 3(6):827–31. doi: 10.1001/jamaoncol.2016.3834
16. Agarwal JP, Chakraborty S, Laskar SG, Mummudi N, Patil VM, Prabhash K, et al. Prognostic value of a patient-reported functional score versus physician-reported karnofsky performance status score in brain metastases. Ecancermedicalscience (2017) 11:779. doi: 10.3332/ecancer.2017.779
17. Broderick JM, Hussey J, Kennedy MJ, DM OD. Patients over 65 years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. J Geriatr Oncol (2014) 5(1):49–56. doi: 10.1016/j.jgo.2013.07.010
18. Roila F, Lupattelli M, Sassi M, Basurto C, Bracarda S, Picciafuoco M, et al. Intra and interobserver variability in cancer patients' performance status assessed according to karnofsky and ECOG scales. Ann Oncol (1991) 2(6):437–9. doi: 10.1093/oxfordjournals.annonc.a057981
19. Sorensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. inter-observer variability study Br J Cancer (1993) 67(4):773–5.doi: 10.1038/bjc.1993.140
20. Taylor AE, Olver IN, Sivanthan T, Chi M, Purnell C. Observer error in grading performance status in cancer patients. Support Care Cancer (1999) 7(5):332–5. doi: 10.1007/s005200050271
21. Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-Small-Cell lung cancer. J Clin Oncol (2020) 38(14):1505–17. doi: 10.1200/JCO.19.03136
22. Gadgeel SM, Shaw AT, Govindan R, Gandhi L, Socinski MA, Camidge DR, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-Small-Cell lung cancer. J Clin Oncol (2016) 34(34):4079–85. doi: 10.1200/JCO.2016.68.4639
23. Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21(5):655–63. doi: 10.1016/S1470-2045(20)30111-X
24. Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol (2016) 17(4):452–63. doi: 10.1016/S1470-2045(15)00614-2
25. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-Small-Cell lung cancer. J Clin Oncol (2018):JCO2018783118.doi: 10.1200/JCO.2018.78.3118
26. Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir Med (2019) 7(5):437–46. doi: 10.1016/S2213-2600(19)30053-0
Keywords: adenocarcinoma, brain metastases, overall survival, prognosis, lung
Citation: Schröder C, Windisch P, Lütscher J, Zwahlen DR and Förster R (2023) Validation and discussion of clinical practicability of the 2022 graded prognostic assessment for NSCLC adenocarcinoma patients with brain metastases in a routine clinical cohort. Front. Oncol. 13:1042548. doi: 10.3389/fonc.2023.1042548
Received: 12 September 2022; Accepted: 16 February 2023;
Published: 20 March 2023.
Edited by:
Susanne Rogers, Aarau Cantonal Hospital, SwitzerlandReviewed by:
Luis Schiappacasse, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2023 Schröder, Windisch, Lütscher, Zwahlen and Förster. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Schröder, Y2hyaXN0aW5hLnNjaHJvZWRlckBrc3cuY2g=
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.