
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 02 February 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1037820
This article is part of the Research TopicCase Reports in Gastrointestinal Cancers : 2022View all 33 articles
Background: Primary hepatic angiosarcoma (PHA) is a rare malignant tumor of mesothelial tissue origin in the liver. The diagnosis of PHA relies on pathology, and it is frequently misdiagnosed as multiple hepatic hemangioma. Noncirrhotic portal hypertension is a relatively rare pathological manifestation, and there are few reports of PHA as an uncommon cause of noncirrhotic portal hypertension.
Case summary: A 36-year-old male was admitted with abnormal liver function and suspected drug-induced liver injury (DILI), initially manifesting as multifocal hepatic hemangioma. The liver biopsy revealed features of noncirrhotic portal hypertension (NCPH), and the patient was eventually diagnosed with multifocal hepatic angiosarcoma.
Conclusion: Patients with PHA may present with NCPH in the liver due to injury to hepatic sinusoids; therefore, it is necessary to consider the possibility of unsampled vascular malignancy when hepatic masses are identified, and the histology is consistent with PHA.
Primary hepatic angiosarcoma (PHA), also known as hepatic vascular endothelial sarcoma, hepatic malignant angioendothelioma, or Kupffer cell sarcoma, is a rare malignant tumor of mesothelial tissue origin, accounting for 0.1–2% of primary liver malignancies (1, 2). It is the most common primary malignant mesothelial tumor in the liver and was first reported by Block in 1974 (3). PHA occurs mostly in adults, especially those aged 50 to 70 years old, with a male-to-female ratio of 4:1. Potential pathogenic factors include exposure to chemicals such as arsenic, vinyl chloride monomer, thorium dioxide, and radium (4). The clinical signs and symptoms of primary hepatic angiosarcoma are similar to those of chronic liver diseases, and patients often present with abdominal pain, weight loss, fatigue, and anorexia. Hepatosplenomegaly, abdominal effusion, and jaundice are also common in elderly male patients (5). PHA is fatal, with most patients dying within 6 months of liver failure or bleeding (2).
Non-cirrhotic portal hypertension (NCPH) is a heterogeneous group of liver diseases of vascular origin, usually manifesting as portal hypertension (PHT) but with preserved hepatic synthetic function and near-normal hepatic venous pressure gradient (HVPG) (6). PHA is a rare cause of NCPH; thus, case reports of PHA combined with NCPH are very rare.
Herein, we present the case of a young male patient with an abnormal liver function who was eventually diagnosed with hepatic angiosarcoma. The patient was hospitalized twice, initially misdiagnosed as drug-induced liver injury complicated by NCPH and hepatic hemangioma, and was finally diagnosed as liver angiosarcoma.
A 36-year-old man with a 20-year history of nephrotic syndrome with normal renal function and fluctuating urine protein levels had been treated with glucocorticoids and traditional Chinese medicine for more than 10 years. He was first hospitalized due to abnormal liver function. Routine laboratory tests revealed mild abnormal liver function [total bilirubin (TBIL) 41.9 µmol/L, direct bilirubin (DBIL) 22.7 µmol/L, alanine aminotransferase (ALT) 21.6 U/L, aspartate aminotransferase (AST) 81.1 U/L, gamma-glutamyl transferase (GGT) 517.5 U/L, alkaline phosphatase (ALP) 323 U/L]. Doppler ultrasound, CT scan, and contrast-enhanced MRI scan of the upper abdomen demonstrated multiple small masses in the liver, so hemangioma was considered. Also, the enlarged spleen and collateral vessels indicated the presence of portal hypertension. The patient underwent a percutaneous liver biopsy and soon after felt pain and discomfort in the right upper abdomen with transient blood pressure decline. The ultrasound confirmed bleeding and hemorrhagic shock due to the liver biopsy. The patient recovered after fluid rehydration, blood transfusion, and other supportive treatments. Pathological examination of the liver biopsy (Figure 1A) revealed congested hepatic sinusoids and peri-sinusoid fibrosis, as well as dilation of the interlobular veins and some extended into the surrounding hepatic sinus. These changes in liver histology were consistent with NCPH features that represent venous outflow stenosis with portal hypertension. There was no evidence of extra-hepatic portal vein obstruction, and the patient was diagnosed with a drug-induced liver injury [the Roussel Uclaf Causality Assessment Method (RUCAM) score 5] and secondary NCPH. Ursodeoxycholic acid was given, and regular outpatient follow-up was performed.
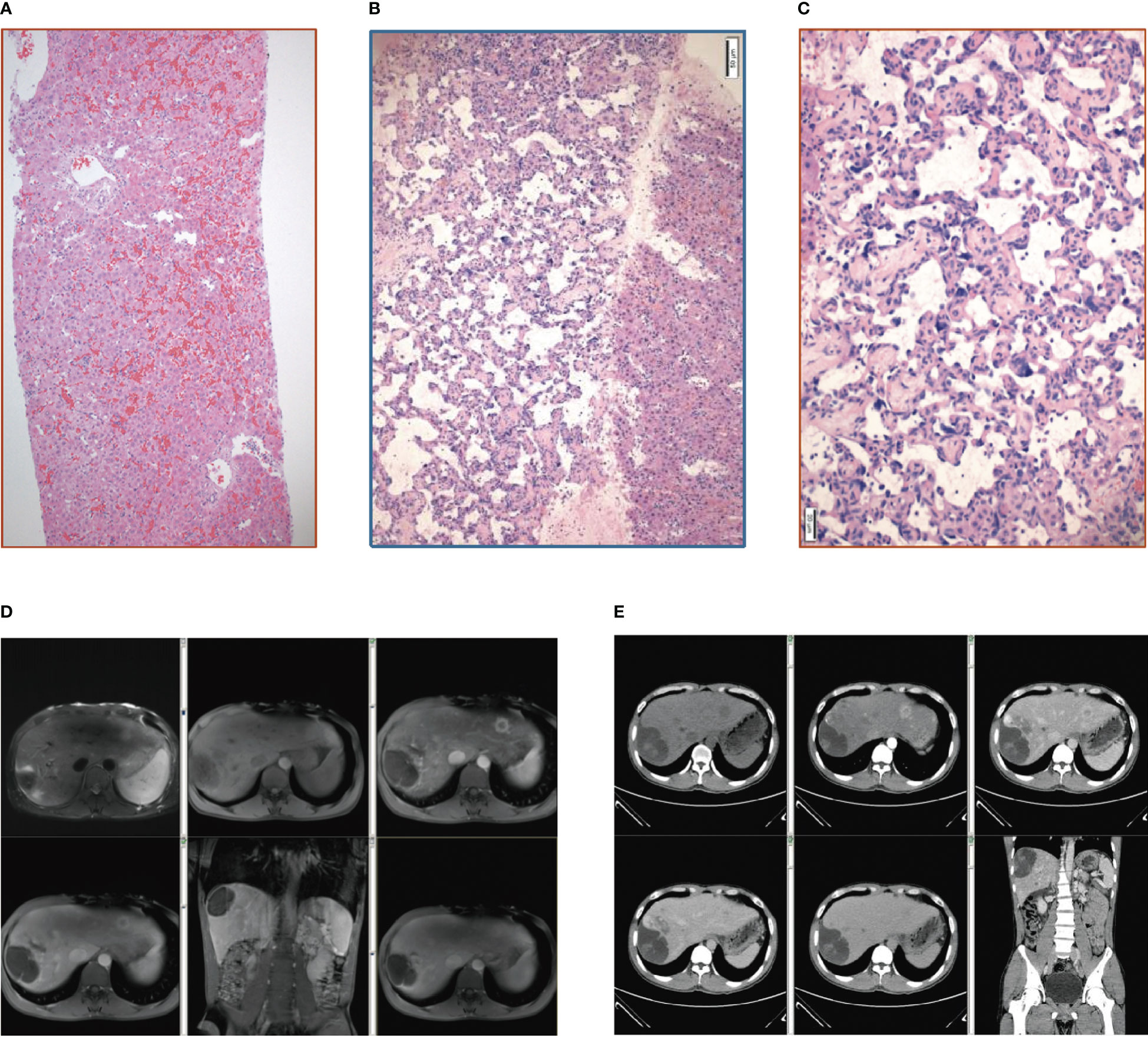
Figure 1 (A) Pathology of the liver tissue: HE staining (X10). (B) Pathology of the liver tissue: HE staining (X100). (C) Pathology of the liver tissue: HE staining (X200). (D) Contrast-enhanced MRI scan of the upper abdomen. (E) Whole abdominal CTV.
Three months after discharge, an outpatient contrast-enhanced MRI scan of the upper abdomen depicted enlarged hepatic masses. Considering the possibility of malignancy (Figure 1D), the patient was readmitted to the hospital. Routine laboratory tests revealed abnormal liver function [albumin (ALB) 21 g/L, TBIL 35.7 µmol/L, DBIL 14.0 µmol/L, ALT 67 U/L, AST 70 U/L, GGT 147 U/L, ALP 240 U/L]. Liver contrast-enhanced ultrasound and positron emission tomography-computed tomography (PET-CT) indicated the possibility of primary liver malignancy with intratumoral hemorrhage. Whole abdominal CTV demonstrated multiple masses in the liver and an enlarged spleen with a spleen-renal venous shunt (Figure 1E). The patient was transferred to the hepatobiliary surgery department and underwent laparoscopic resection of the liver lesions. Liver lesion biopsy pathology (Figures 1B, C) demonstrated that the tumor cells were slit and cable-like with a vascular network and growth along the liver sinusoids. Immunohistochemistry revealed high expression of CD34, CD31, and ERG (ETS transcription factor).
The patient was diagnosed with primary hepatic angiosarcoma and was treated with lenvatinib. In May 2020, he stopped taking lenvatinib due to progressively exacerbating liver function and died of severe intra-abdominal infection and acute kidney failure.
PHA is a rare malignant tumor originating from the liver sinusoidal endothelial cells. Herein, we reported the case of a male patient who was admitted to hospital due to abnormal liver function. CT and MRI play an important role in diagnosing hepatic angiosarcoma, and there are four main types of PHA radiological presentation: multiple nodules, massive masses, massive masses with multiple nodules, and diffuse invasive micronodular tumors. Most lesions present hypodense on CT scans, but some are hyperdense due to spontaneous intraperitoneal or intratumoral hemorrhage (7). On contrast injection, most nodular lesions depict low-density enhancement, and some show irregular or annular enhancement. The MRI reflects the hemorrhagic, heterogeneous, and multivascular nature of the PHA lesions, typically areas of high signal intensity on T1-weighted images and distinct heterostructures on T2-weighted images, suggesting intratumoral hemorrhage and fibrous septa (8). Hepatic angiosarcoma can be distinguished from hepatic hemangiomas or other liver tumors by CT and MRI. Previous studies have reported that using non-enhanced, multiphase-enhanced, and late-delayed CT and MR imaging is an optimal imaging technique for accurately assessing PHA (7, 9). However, due to its rarity, PHA is easily misdiagnosed as multiple hepatic hemangioma.
The typical histology of hepatic angiosarcoma is tumor vascular-like cavities lined by spindle cells that project into the lumen to form papillary structures. The degree of differentiation of tumor cells varies greatly, with well-differentiated tumor cells resembling hemangioma and poorly differentiated tumor cells having obvious atypia. Tumor cells can look spongy with no obvious vascular space, giant cells, and pathological mitoses. They often spread along the sinus, terminal liver vein, and portal vein branches, and multilayer or prominent vascular lumen growth on the liver plate causes liver plate dissociation. Hepatocytes atrophy or disappear, the vascular cavity is enlarged, and the rapidly growing tumor tissue has visible residual hepatocytes, vascular lumen visible blood clots, and tumor cell debris (10). Immunohistochemically, liver sections are positive for CD31, CD34, D2-40, and factor VIII-associated antigens, with CD31 and factor VIII-associated antigens being the most specific markers (11).
Interestingly, this patient also presented with NCPH features. NCPH is a vascular liver disease of unknown etiology that presents clinically as portal hypertension (examples include thrombocytopenia secondary to hypersplenism and bleeding from esophagogastric varices or hypertensive gastropathy) with preserved hepatic synthetic function and near-normal hepatic venous pressure gradient (HVPG). It is characterized by increased pressure in the portal vein and its branches due to fibrosis of the intima and the absence of cirrhosis. The diagnosis of NCPH is mainly based on the following signs: a) hepatic hemodynamics and portal venography showing a high gradient of the portal sinus with fewer branches and increased portal caliber in the absence of thrombosis; b) liver biopsy showing thickening of portal vein fibers and no evidence of cirrhosis, necrosis, or inflammation (12). Although there is evidence that certain toxic substances (e.g., arsenic salts, thorium sulfate, vinyl chloride) may cause NCPH, the cause is often not identified. It has been suggested that some histological lesions of NCPH, such as hepatic sinusoidal cell hyperplasia, may lead to the development of angiosarcoma (4, 13, 14); however, there is no clear association between NCPH and hepatic angiosarcoma. In addition, a series of specific risk factors, such as arsenic, vinyl chloride, and androgenic steroids, which have a latency period of up to 20 years for their carcinogenic effects, have been reported in 40% of patients with hepatic angiosarcoma (15, 16). As previously mentioned, these risk factors are also associated with NCPH development; therefore, NCPH and hepatic angiosarcoma may share the same triggering environmental factors. To our knowledge, there is a lack of literature describing the association between hepatic angiosarcoma and NCPH, with only eight published reports of NCPH and hepatic angiosarcoma-related cases, of which two patients had prior exposure to arsenic (17, 18), four patients with prior exposure to polyvinyl chloride (19), and two patients with unknown prior exposure history (19, 20). Furthermore, there have been reported cases of liver failure (21), which may be caused by portal fibrosis, destruction of the terminal portal nerve root in the liver, and liver parenchyma atrophy secondary to poor portal perfusion. In addition, PHA liver biopsies are extremely prone to bleeding, and as in this case, the liver biopsy caused transient hemorrhagic shock. Therefore, in patients with suspected PHA, non-surgical liver biopsy should be performed with extreme caution and careful evaluation before surgery is required.
Due to the rare incidence of PHA, there are no established treatment guidelines. Hepatectomy may be considered when the lesion is localized to one liver lobe. Patient survival may be prolonged with improved diagnostic techniques and liver transplantation, but the recurrence rate remains very high, and the prognosis is very poor (10). Also, PHA is considered an absolute contraindication to liver transplantation due to its dismal results. There are a few reports that chemotherapy is effective for PHA, and palliative care can be considered in cases of inoperable surgery. Local chemotherapy, such as transcatheter arterial chemoembolization (TACE) or systemic chemotherapy, can effectively prolong the life of patients, even equal to surgical outcomes (22, 23). PHA is a vascular-derived malignancy, so anti-angiogenic therapy may be a new potential strategy. There is a report of thalidomide combined with radiotherapy in a PHA patient with neck metastasis achieving a tumor-free state (23). Targeted therapies such as sorafenib and bevacizumab have limited efficacy in treating primary angiosarcoma of other organs; however, only one case of PHA was included in these studies (24, 25). An attempt to combine pazopanib, a PD-1 inhibitor, and RAK cells yielded effective results in an elderly PHA patient (26). These new approaches, alone or in combination with other therapeutic modalities, such as surgery and chemotherapy, need further investigation to assess their role in prolonging patient survival. Personalized therapeutic algorithms according to the genetics, molecular biology, histopathological features, and behavior of the tumors should be elaborated for the management of PHA patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions: XW, XY, QG, BW, ZL collected and analyzed the data. XW, XY wrote the paper. YS and ZH critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Chinese National Natural Science Foundation (nos. 81870425), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No.2022490480), and the Fundamental Research Funds for the Central Universities and Scientific Research Fund of Zhejiang University(XY2021030).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PHA, primary hepatic angiosarcoma; DILI, drug-induced liver injury; NCPH, noncirrhotic portal hypertension; TBIL, total bilirubin; DBIL, direct bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, glutamyl transferase; ALP, alkaline phosphatase; PET-CT, positron emission tomography – computed tomography; HVPG, hepatic venous pressure gradient; RUCAM, Roussel Uclaf Causality Assessment Method.
1. Mani H, Van Thiel DH. Mesenchymal tumors of the liver. Clinics liver Dis (2001) 5:219–57, viii. doi: 10.1016/S1089-3261(05)70162-8
2. Molina E, Hernandez A. Clinical manifestations of primary hepatic angiosarcoma. Digestive Dis Sci (2003) 48:677–82. doi: 10.1023/A:1022868221670
3. Block JB. Angiosarcoma of the liver following vinyl chloride exposure. JAMA (1974) 229:53–4. doi: 10.1001/jama.1974.03230390029019
4. Falk H, Thomas LB, Popper H, Ishak KG. Hepatic angiosarcoma associated with androgenic-anabolic steroids. Lancet (London England) (1979) 2:1120–3. doi: 10.1016/S0140-6736(79)92515-7
5. Kielhorn J, Melber C, Wahnschaffe U, Aitio A, Mangelsdorf I. Vinyl chloride: Still a cause for concern. Environ Health Perspect (2000) 108:579–88. doi: 10.1289/ehp.00108579
6. Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol (2014) 60:421–41. doi: 10.1016/j.jhep.2013.08.013
7. Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary hepatic angiosarcoma: Findings at CT and MR imaging. Radiology (2002) 222:667–73. doi: 10.1148/radiol.2223010877
8. Nelson V, Fernandes NF, Woolf GM, Geller SA, Petrovic LM. Primary liposarcoma of the liver: A case report and review of literature. Arch Pathol Lab Med (2001) 125:410–2. doi: 10.5858/2001-125-0410-PLOTL
9. Peterson MS, Baron RL, Rankin SC. Hepatic angiosarcoma: findings on multiphasic contrast-enhanced helical CT do not mimic hepatic hemangioma. AJR. Am J roentgenology (2000) 175:165–70. doi: 10.2214/ajr.175.1.1750165
10. Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2015) 41:1137–43. doi: 10.1016/j.ejso.2015.04.022
11. Rao P, Lahat G, Arnold C, Gavino AC, Lahat S, Hornick JL, et al. Angiosarcoma: A tissue microarray study with diagnostic implications. Am J dermatopathology (2013) 35:432–7. doi: 10.1097/DAD.0b013e318271295a
12. Alonso S, Bañares R, Barrio J, Rincón D, Nuñez O, Alvárez E, et al. [Diagnostic utility of hepatic hemodynamics study in hepatoportal sclerosis]. Gastroenterologia y hepatologia (2001) 24:473–7. doi: 10.1016/S0210-5705(01)70217-8
13. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison cases unknown etiology Am J Pathol (1978) 92:349–76. doi: 10.1111/j.1365-2990.1977.tb00605.x
14. Thomas LB, Popper H, Berk PD, Selikoff I, Falk H. Vinyl-chloride-induced liver disease. from idiopathic portal hypertension (Banti's syndrome) to angiosarcomas. New Engl J Med (1975) 292:17–22. doi: 10.1056/NEJM197501022920104
15. Salgado M, Sans M, Forns X, Bruguera M, Castells A, Navasa M, et al. [Hepatic angiosarcoma: A report of a case associated with treatment with arsenic salts and a review of the literature]. Gastroenterologia y hepatologia (1995) 18:132–5. doi: 10.1002/ajh.23367
16. Neshiwat LF, Friedland ML, Schorr-Lesnick B, Feld1man S, Glucksman WJ, Russo RD Jr. Hepatic angiosarcoma. Am J Med (1992) 93:219–22. doi: 10.1016/0002-9343(92)90054-F
17. Dueñas C, Pérez-Alvarez JC, Busteros JI, Sáez-Royuela F, Martin-Lorente JL, Yuguero L, et al. Idiopathic portal hypertension and angiosarcoma associated with arsenical salts therapy. J Clin Gastroenterol (1998) 26:303–5. doi: 10.1097/00004836-199806000-00019
18. Oshimoto H, Saitoh S, Makita F, Matsumoto T, Konaka K, Takehara K, et al. [A case of hepatic angiosarcoma associated with idiopathic portal hypertension]. Nihon Shokakibyo Gakkai zasshi = Japanese J gastro-enterology (1996) 93:573–7.
19. Lee FI, Smith PM, Bennett B, Williams DM. Occupationally related angiosarcoma of the liver in the united kingdom 1972-1994. Gut (1996) 39:312–8. doi: 10.1136/gut.39.2.312
20. Sanz-García C, Matilla-Peña A, Nogales-Rincón O, Núñez-Martínez O, Clemente-Ricote G. [Hepatoportal sclerosis and liver angiosarcoma: An infrequent association with a possible common etiology]. Gastroenterologia y hepatologia (2005) 28:555–7. doi: 10.1157/13080609
21. Okudaira M, Ohbu M, Okuda K. Idiopathic portal hypertension and its pathology. Semin liver Dis (2002) 22:59–72. doi: 10.1055/s-2002-23207
22. Ozden I, Bilge O, Erkan M, Cevikbaş U, Acarli K. Five years and 4 months of recurrence-free survival in hepatic angiosarcoma. J hepato-biliary-pancreatic Surg (2003) 10:250–2. doi: 10.1007/s00534-003-0849-4
23. Huang NC, Kuo YC, Chiang JC, Hung SY, Wang HM, Hung YM, et al. Hepatic angiosarcoma may have fair survival nowadays. Medicine (2015) 94:e816. doi: 10.1097/MD.0000000000000816
24. Ray-Coquard I, Italiano A, Bompas E, Le Cesne A, Robin YM, Chevreau C, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French sarcoma group (GSF/GETO). oncologist (2012) 17:260–6. doi: 10.1634/theoncologist.2011-0237
25. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol Off J Eur Soc Med Oncol (2013) 24:257–63. doi: 10.1093/annonc/mds237
Keywords: primary hepatic angiosarcoma, noncirrhotic portal hypertension, diagnosis, treatment, pathology
Citation: Wu X, Yu X, Gan Q, Wang B, Lin Z, Shi Y and Huang Z (2023) Primary hepatic angiosarcoma with noncirrhotic portal hypertension: A case report. Front. Oncol. 13:1037820. doi: 10.3389/fonc.2023.1037820
Received: 06 September 2022; Accepted: 23 January 2023;
Published: 02 February 2023.
Edited by:
Yu-Chen Fan, Qilu Hospital, Shandong University, ChinaReviewed by:
Shang Wan, Sichuan University, ChinaCopyright © 2023 Wu, Yu, Gan, Wang, Lin, Shi and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuxiong Huang, aHVhbmd6dXhpb25nMjE1QGhvdG1haWwuY29t; Yu Shi, emp1c2hpeXVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.