- 1Department of Orthopedic Oncology, Changzheng Hospital, Second Military Medical University, Shanghai, China
- 2Naval Medical Center, Naval Military Medical University, Shanghai, China
- 3Department of Orthopedic, Changning County People's Hospital, Yunnan, China
Background: Even if COVID-19 vaccine has gradually been adopted in the world, information of side effects and crosstalk in patients with spinal tumors is absent due to the exclusion from clinical research. In this research, we aimed to investigate the efficacy and safety for the patients with spinal tumors treated by denosumab.
Methods: In this retrospective research, 400 patients under treatment of denosumab against spinal tumors in real-clinical experience were grouped into two cohorts according to the treatment of COVID-19 vaccine. And linked hospital data, serum samples and unsolicited related adverse events had been collected from January 22nd 2021 to June 1st 2021 respectively.
Results: 233 patients of all participants who received regular treatment of denosumab were vaccinated by mRNA or inactivated vaccine. Patients of metastatic disease and primary osseous spinal tumor showed similar distribution in both two groups. Over the study period, within 176 patients tested the status of serologic response of vaccine, 88(81.48%) and 41(87.23%) individuals injected one or two inactivated vaccines had effective antibody against SARS-CoV-2 infections. As 21 patients (85.71%) treated by mRNA vaccine did. Considering of the safety of vaccine, most common systemic adverse events were nausea or vomiting (45 events vs 23events). Interestingly, fewer participants in the vaccine group were statistically recorded in local adverse events than in the placebo group (16 events vs 33 events).
Conclusions: Our initial real-clinical experience suggests that COVID-19 vaccines are likely safe and effective in in patients with spinal tumors receiving denosumab treatment.
1 Introduction
The sudden explosion and pandemic caused by coronavirus disease 2019 (COVID-19) (1), which belongs to the same RNA coronavirus family as SARS-CoV and MERS-CoV (2), appears to have been unprecedented. This pandemic has already caused great challenges for the global public health system and has left a heavy burden on the global economy (3). Since the prevalence of cancer seems to be associated with a higher mortality rate due to COVID-19, the development of safe and effective treatments may be crucial for this group of patients (4). Even if several therapies have been tested, including the use of antivirals, corticosteroids, and respiratory support, no specific drug treatments have proven effective against COVID-19 (5). Vaccination is still regarded as the most promising intervention against the spread of the virus. Most patients with spinal tumors are in a generally poor condition or a systemic immunosuppressive state, which can lead to a higher-risk of severe disease and death (6). Furthermore, once these patients have been infected by COVID-19, fatal outcomes may lead to delays in further treatment and a further decline in quality of life (7). However, no studies have focused on the efficacy and safety of COVID-19 vaccines for patients with spinal tumors.
Denosumab, a human monoclonal antibody that targets human RANKL, has been widely used for patients with primary and metastatic spinal tumors. RANKL and RANK are also expressed by cells in the immune system apart from osseous tissue. Meanwhile, RANKL and RANK also played an important role in various immune processes, including lymph node development and activation of dendritic cells, monocytes, and T cells (8–11). Due to the inhibition of the RANK pathway, denosumab may theoretically increase the risk of infections. In the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 months (FREEDOM) clinical trial, data demonstrated that denosumab was associated with a statistically significant increase in serious infection events (SAEs), specifically cellulitis and erysipelas (12) . Therefore, denosumab may be a two-edged sword for cancer patients threatened by COVID-19. Due to the failure of the immune system and weakened autoreactive responses, patients may be more susceptible to COVID-19 infections. In a recent survey, experts even raised concerns that the risk of infection could be worsened if RANKL inhibitors are concomitantly employed with other biological agents of anti-TNF (13). Therefore, clinicians and researchers should especially be concerned about the mutual effects of vaccination and denosumab treatment. However, studies that focus on the efficacy and safety of the vaccine for patients with spinal tumors treated with denosumab have not been conducted as yet.
In our study, we aimed to assess the efficacy and safety of the COVID-19 vaccine for patients with spinal tumors undergoing denosumab treatment. The aim of our study is to provide novel insights for the prevention of COVID-19.
2 Materials and methods
2.1 Study design
We conducted a retrospective study at the Shanghai ChangZheng Hospital on the further investigation of the influence between COVID-19 vaccines and denosumab from January 22nd, 2021 to June 1st, 2021. Our research investigators also shared a questionnaire through social media channels, including WeChat and Weibo, to obtain more information. Unsolicited clinically unidentified signs and symptoms were also recorded when a patient spontaneously volunteered to report symptoms during consultation. Our research was also approved by the local Ethics Committee.
2.2 Patients
All participants enrolled in this study had to meet the following inclusion criteria: (1) the spinal sites were pathologically diagnosed as metastatic malignancy or primary spinal tumor during the period starting January 1st, 2019 to January 22nd, 2021; (2) the patients were treated using 120 mg of denosumab per month (3) over eighteen years of age. (4) participants were able to understand basic details regarding the disease. The exclusion criteria for participation included: (1) a history of COVID-19; (2) treatment using immunosuppressive medication based on programmed cell death 1 (PD-1), hormone therapies such as prednisone, or methylprednisolone concerning brakes in immune tolerance mechanisms, patients with bone marrow suppression caused by targeted therapy and aggressive chemotherapy; and (3) patients who refused to provide consent.
2.3 Vaccination
At present, two types of vaccines, mRNA vaccines, and inactivated vaccines have been widely used in our research. The inactivated vaccine, CoronaVac (Sinovac Life Sciences, Beijing, China), was created using African green monkey kidney cells (Vero cells) that have been inoculated with SARS-CoV-2 (CN02 strain). The other COVID-19 vaccine, which is a replicate of a defective Ad5 vectored vaccine, was also developed by the Beijing Institute of Biotechnology (Beijing, China) and CanSino Biologics (Tianjin, China). It was constructed by cloning an optimized full-length spike gene based on Wuhan-Hu-1 (GenBank accession number YP_009724390) of the tissue plasminogen activator signal peptide gene into an E1 and E3 deleted Ad5 vector (14). As reflected in the survey, all the mRNA vaccine was only injected for a single dose (5ml) (14). All the inactivated vaccines were planned to be injected at 0-14 day, and 0-28 day for 5μg each time (15).
2.4 Efficacy and safety
Data on the basic characteristics of the patients, including demographic characteristics such as age, sex, Body Mass Index (BMI), pathological diagnosis, and comorbidities with rheumatic disease, were recorded. Venous blood was tested regularly on day 20 following denosumab treatment and at 6 months after vaccination. The efficacy of the vaccine was determined by the positive state of anti-SARS-Cov-2 immunoglobulin G (IgG) antibodies (16) (IgG antibodies were tested using the domain of the spike protein of the virus used in a commercial enzyme-linked immunosorbent assay (ELISA) kit as recommended by the manufacturer at local hospitals)
Safety was evaluated using weekly medical consultations to identify treatment-related adverse events resulting from both the vaccine for patients treated by denosumab. Related adverse events were classified into 4 levels according to the National Cancer Institute Common Terminology Criteria for Adverse Events (17): Grade 1 (mild; does not interfere with activity); Grade 2 (moderate; interferes with activity), Grade 3 (severe; prevents daily activity), and Grade 4 (potentially life-threatening; required emergency department visit or admission to hospital).
2.5 Statistical analysis
Categorical variables are presented as frequency and percentage. Continuous variables are presented as a pie chart to determine distribution. χ2 test and Fisher Exact Test were used to compare between the groups. Due to the sample size and concerns regarding false positives in the multivariate analysis, the subgroup analyses were not conducted on different types of cancer (18). All statistical tests were two-tailed. A p< 0.05 was regarded as statistically significant. We also used minimal necessary adjustments on the covariates using directed acyclic graphs (DAG) (Figure S1 in Appendix A).
3 Results
3.1 Patients
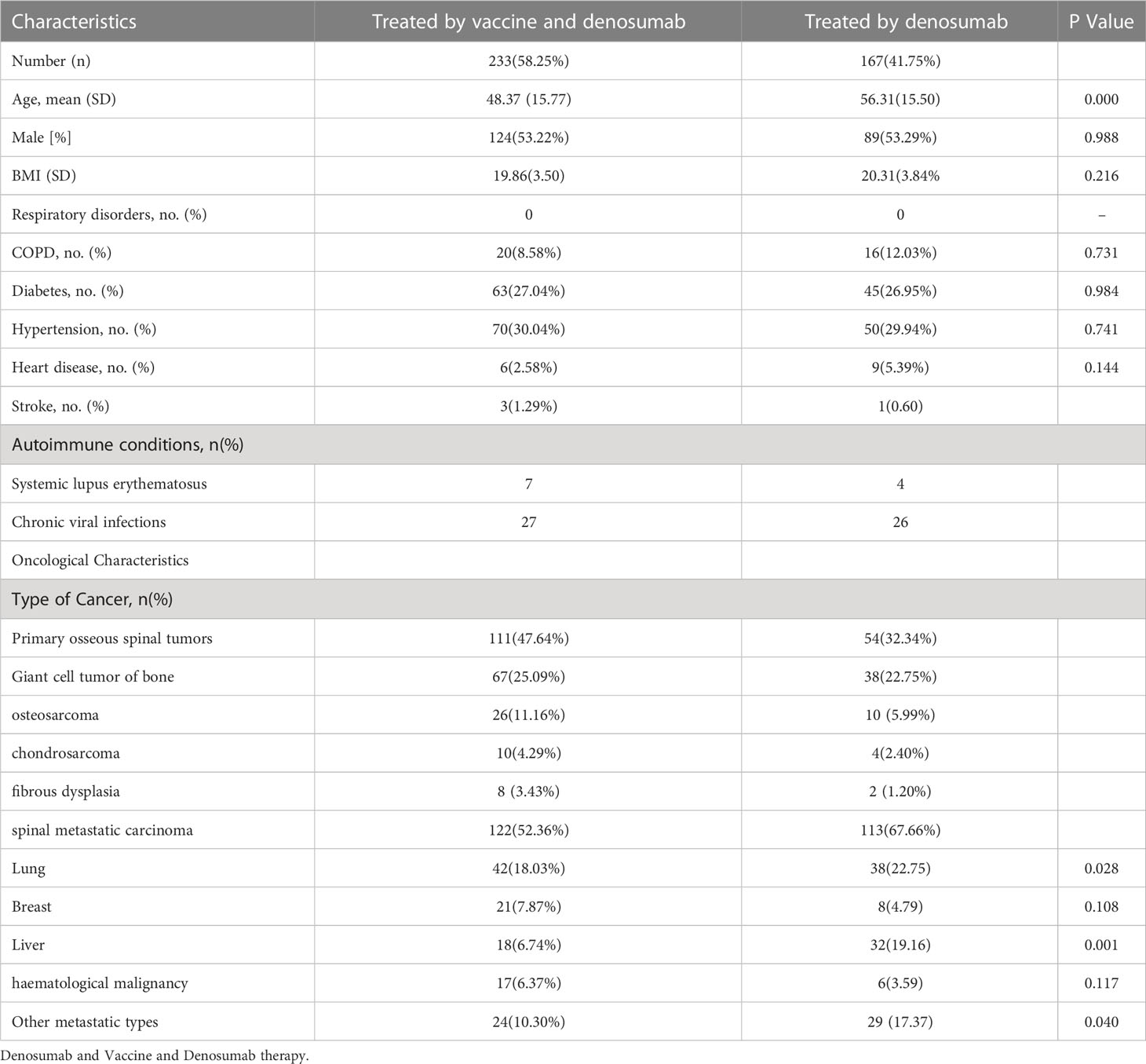
A total of four hundred cancer patients with primary or metastatic spinal tumors undergoing denosumab treatment were included. 233 patients were administered both the COVID-19 vaccine and denosumab, while 167 patients who were treated with denosumab without being administered the vaccine served as the control cohort. The vaccine group was composed of 124 men (53.22%) and the control group comprised 89 men (53.29%).
Among these patients, the most common type of cancer was lung cancer (n=42;18.03%) followed by breast (n=21; 7.87%), liver (n=24; 6.74%), and other metastatic cancers (n=24; 10.30%). Among the 111 (47.64%) patients with primary osseous spinal tumors included in the vaccine group, 67 (25.09%) patients had giant cell tumor of the bone, 26 (11.16%) patients had osteosarcoma, 8 (3.43%) patients had fibrous dysplasia, while 10 (4.29%) patients had chondrosarcoma. A similar oncological distribution was observed in the control group. The types of cancer included in the control cohort were 38 (22.75%) patients with lung cancer, 32 (19.16%) patients with liver cancer, 29 (17.37) patients with metastatic cancers in other tissues, 38 (22.75%) patients with giant cell tumor of the bone, 10 (5.99%) osteosarcoma patients, 2 (1.20%) fibrous dysplasia patients, and 4 (2.40%) patients with chondrosarcoma in the osseous spinal tumors (Table 1 and Figure 1). Furthermore, comorbidities of the patients did also not show any statistical differences, such as hypertension (70, 30.04%; 45, 26.95%), diabetes (63, 27.04%; 45, 26.95%), and other conditions (29, 12.45%; 26, 15.57%).
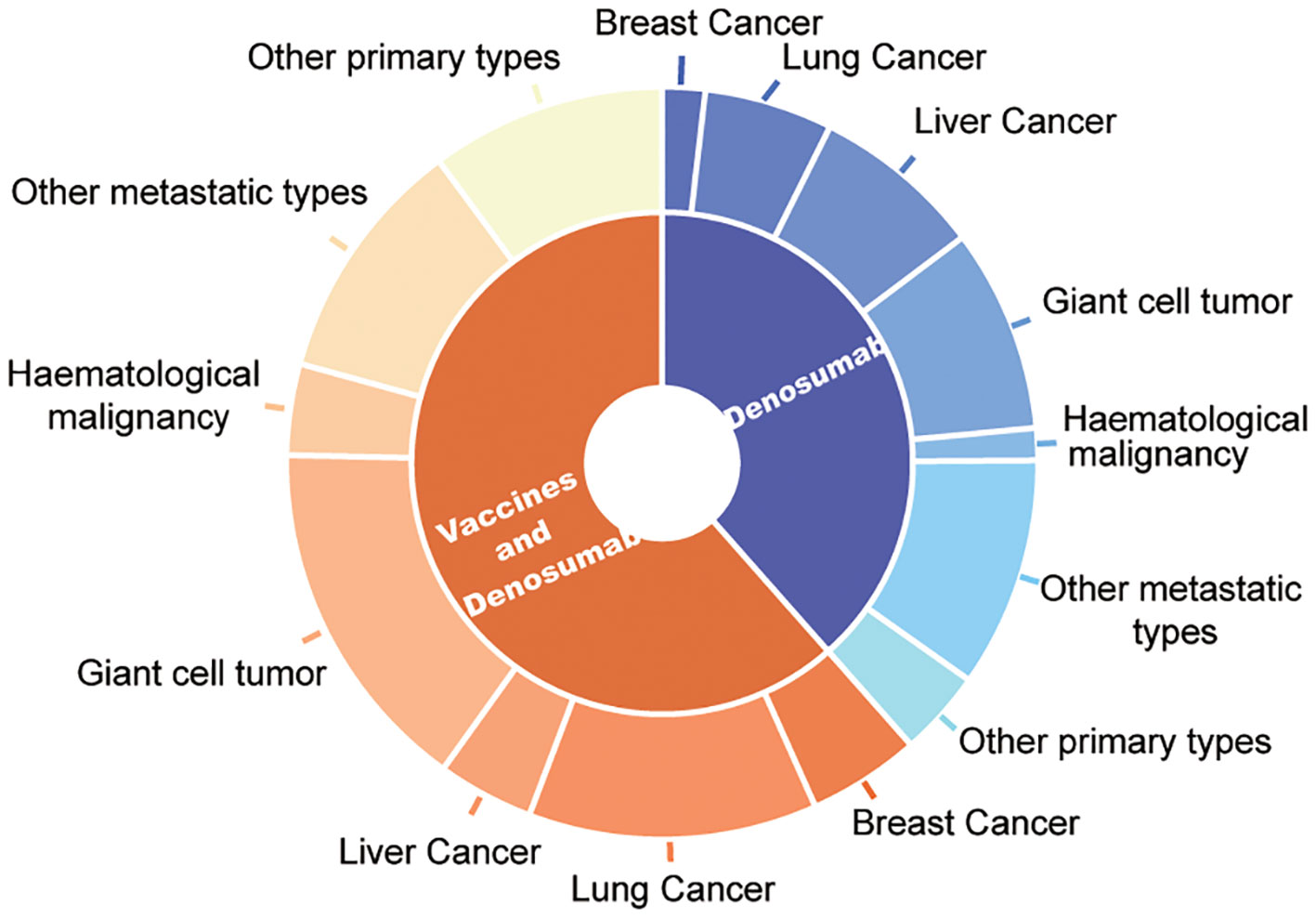
Figure 1 Oncological characteristic distribution of patients treated by denosumab and vaccine and only denosumab therapy.
3.2 Efficacy of COVID-19 vaccines in spinal tumor patients
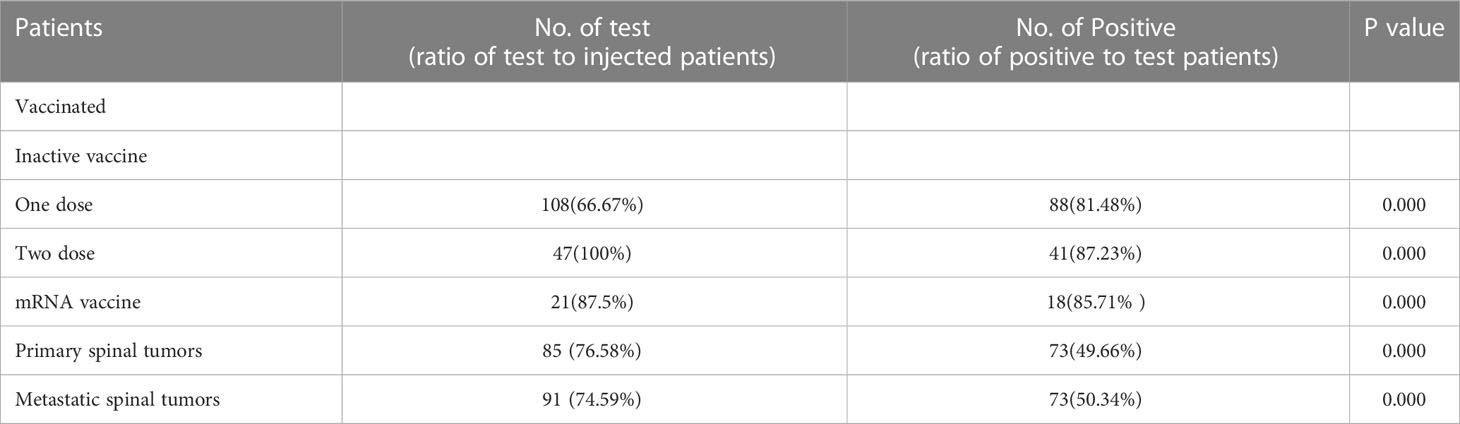
In the vaccinated cohort, 209 89.70% of patients received the inactivated vaccine, while the other 24 patients received the mRNA vaccine. All patients were tested after being vaccinated and the results showed that 147 (83.52%) of these patients showed positive serology after examination for the presence of antibodies. Among the patients who were positive for antibodies, 162 (69.52%) patients had received one dose of the inactivated vaccine, while 47(20.17%) had received two doses of the inactivated vaccine. Over the study period, 88 individuals (81.48%) who were injected with one inactivated vaccine showed effective antibodies against SARS-CoV-2. The results in the 41 patients who had received the second vaccine showed an 87.23% antibody response in the survey. Only one dose of the mRNA vaccine was administered to 24 patients treated with denosumab. Among them, 18 individuals (85.71%) who received the mRNA vaccine also showed a seropositive serologic status (Table 2). In the univariate analysis, all potential risk variables were listed and showed to have a statistically significant influence on the immune response to the vaccine (p<0.005). The risk factors included age, sex, BMI, comorbidities, and the type of vaccine. The results of the multivariable logistic regression (Table 3) did not indicate the causality of these negative seroconversions and potential factors.
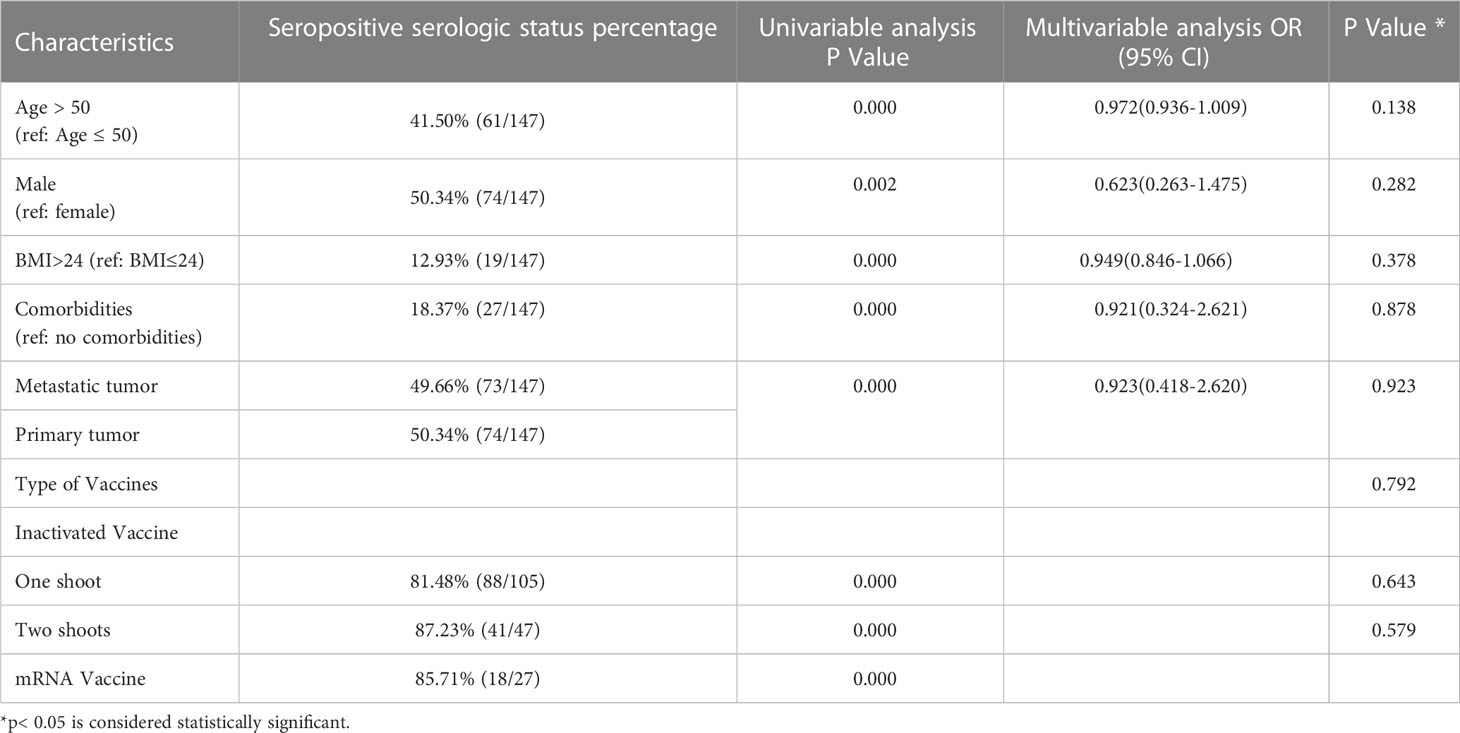
Table 3 Univariable and multivariable analysis of factors potentially associated with serologic response.
3.3 Safety of COVID-19 vaccines for spinal tumor patients
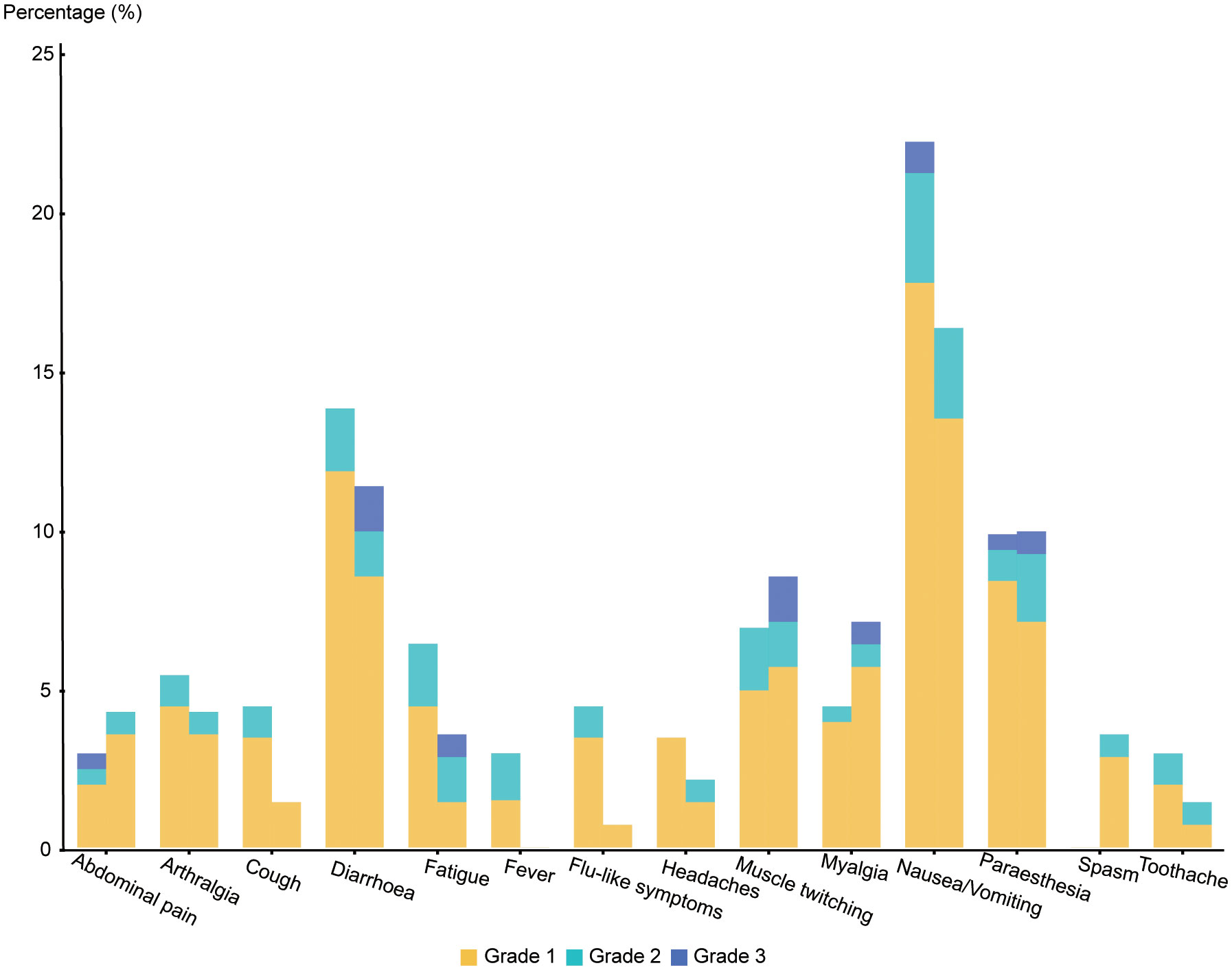
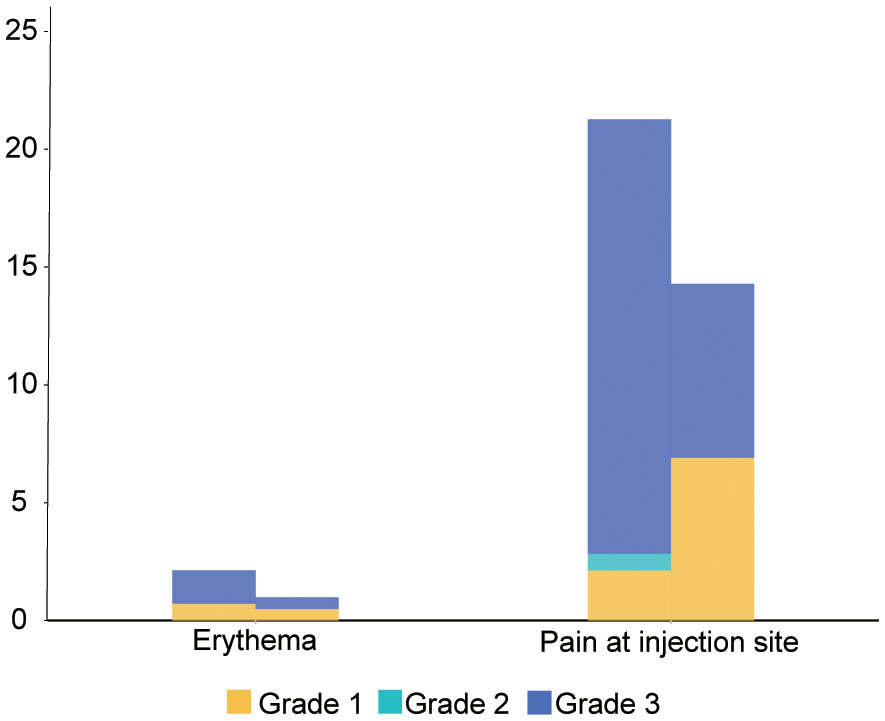
Our research showed that no fatal super sensitivity or life-threatening responses occurred in either group regardless of whether the patients were vaccinated or not. Among the 400 patients, it was observed that systemic adverse events were more frequent in the vaccinated group than in the placebo group (170 events (83.74%) vs. 99 events (70.21%)) (Table 4 and Figure 2). The most common systemic adverse event following vaccination was nausea or vomiting (45 events, 22.17% vs 23events, 16.33%), with most events distributed in grade 1 (36 events, 17.74% vs 19 events, 13.48%) and grade 2 (7 events, 3.45% vs 4 events, 2.84%) tumors. The systemic adverse events included nausea or vomiting, fatigue, headaches, muscle or joint pain, fever, gastrointestinal complications, and flu-like symptoms, which showed no significant differences between the two cohorts. Interestingly, statistically fewer participants in the vaccine group recorded local adverse events than in the placebo group (16 events, 7.88% vs 33 events, 23.40%), including pain at the injection site (15 events, 7.39% vs 30 events, 21.28%) and Erythema (1 event, 0.49% vs 3 events, 2.13%) (Figure 3). All adverse events recorded following vaccination were characterized as short-term, mild-to-moderate adverse events. None of the reported complications required admission to the hospital or further special interventions.
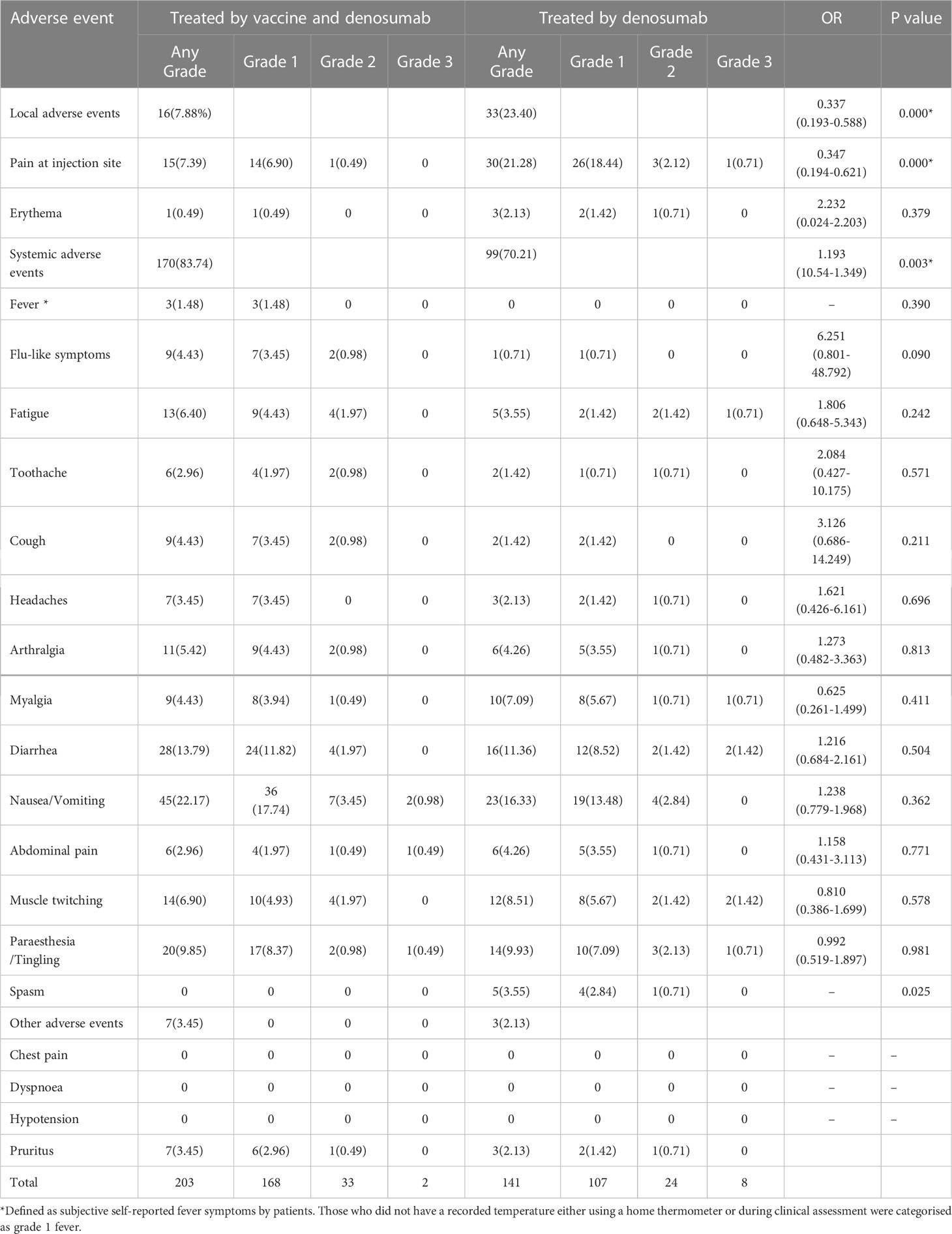
Table 4 Local and systemic adverse events reported after injection of the COVID-19 vaccine and denosumab.
All the participants were analyzed for their risk of developing related adverse events, serious adverse events (>2 grades), and any grade of systemic adverse events. Cancer patients with fewer comorbidities and metastatic sites were less likely to report adverse events of any grade compared with those with more comorbidities and metastatic sites (OR 2.520 [95%CI 1.430–4.442]; p = 0.001 vs. OR 1.839 [95%CI 1.128–2.996]; p = 0.015). However, age, BMI, comorbidities, metastatic cancer, or receiving the vaccines, did not show any statistical difference in developing grade ≥2 adverse events (Tables 4, 5 and Supplementary Data).
4 Discussion
The spine is one of the most common sites of metastatic and primary osseous tumors (19, 20). All active untreated spinal lesions can lead to local irreversible events that are associated with spinal cord compression and pathological fractures (21). Once these events occur, they have a devastating destructive impact on the quality of life and require further multidisciplinary synthetic therapy. The nuclear kappa B ligand (RANKL) signaling pathway has been proven to be highly expressed in osteoclasts and performs an important role in the regulation of bone resorption–production. Due to the inhibition of osteoclast-mediated bone destruction, denosumab has been approved by the FDA for the treatment of unresectable giant cell tumor of bone and metastatic spinal cancers (22). However, a study on RANKL expression in primary osseous spinal tumors showed that RANKL is also expressed at high levels in fibrous dysplasia, osteosarcoma, chondrosarcoma, and enchondroma (23). Animal experiments and drug sensitivity tests have also shown that denosumab can inhibit the growth of primary tumors (24). Based on the results of preclinical research studies, denosumab has been gradually applied as a novel method of treatment for primary spinal tumors (23, 25, 26). Therefore, patients with metastatic malignancies and primary spinal tumors being treated with denosumab were included and analyzed in our research study. Nonetheless, the global outbreak caused by COVID-19 has become an unparalleled challenge, especially for the comprehensive treatment of patients with spinal tumors since December 2019 (1, 27). It has been reported that severe complications were more likely to occur in elderly and fatal patients who have been diagnosed with malignant tumors (28). Therefore, vaccination against severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2) may represent an unprecedented method of treatment in the fight against the pandemic. However, only a few clinical studies have focused on the unique responses of patients with spinal tumors to COVID-19 vaccines. According to our knowledge, this is the first research study to determine the efficacy of the inactivated and mRNA COVID-19 vaccines for patients being treated using denosumab. Therefore, our study aimed to demonstrate whether COVID-19 vaccination has an influence on the rate of adverse events and the efficacy of treatment in this group of patients.
A positive result for antibodies and significant IgG levels are potential predictive factors of vaccine resistibility and efficacy (29). Our results indicated that 81.48% and 85.71% of patients who were administered one dose of the inactivated vaccine and the mRNA vaccine, respectively, showed a positive serological response against COVID-19. After the second inactivated vaccine was injected, the rate of seroconversion increased from 81.48% to 87.23%. The interim analysis of the results of 2 randomized clinical trials confirmed 95.8% efficacy of the inactivated vaccine against SARS-CoV-2 in healthy adults (15). Another research study conducted on 364 cancer patients who received the inactivated vaccine showed that the rate of seroconversion in cancer patients with breast cancer may even reach 93.3%, and may be as high as 94.7% in patients with digestive cancers (30). Our investigation showed that vaccinated patients treated with denosumab seroconverted antibodies were at levels less than that of normal individuals and cancer patients. Furthermore, early clinical data appeared to show a higher tendency of COVID-19 infection among patients being treated with denosumab (31). Clinical trials have also suggested that denosumab therapy may increase the risk of infection in cancer patients. Severe infections were reported at a rate of 2.3% vs 0.8% in denosumab-treated patients compared with placebo-treated patients with early-stage breast cancer (32). Similar results were also obtained in androgen-dependent prostate cancer patients (33). Compared with the results of clinical trials and studies on the molecular mechanisms involved (34–36), a lower vaccination efficacy and higher risk of infection may be associated with immunity and denosumab treatment. The potential influence of immunosuppression on RANKL inhibitors may be because RANKL expression and the immune system share multiple pathways involved in B-cell differentiation and T-cell survival (37). Our results showed that although a lower rate of seroconversion was observed in the vaccine group, a sufficient antibody response to SARS-CoV-2 is acceptable for patients with spinal tumors. We also evaluated whether denosumab treatment has an influence on vaccination efficacy. In the multivariate analysis, the type of vaccine, age, sex, BMI, and the metastatic site did not lead to a significant difference while the potential risk factors in the univariate analysis were proven to be correlated. Therefore, the relationship between vaccine immunogenicity and denosumab treatment may need to be urgently further investigated.
Comparison with the placebo group showed a higher tendency of abdominal adverse events in the cohort of patients with cancer who were vaccinated but the result was not statistically significant. The rate of nausea or vomiting and diarrhea reached 22.17% and 13.79%, respectively, and were the most common systemic adverse events reported in our cohort. However, in post-COVID-19 vaccine trial conducted on cancer patients vaccinated (38, 39) with the inactivated vaccine, fatigue and headaches were the most frequently reported systemic adverse events (8.17% and 8.79%, respectively) but only reached a rate of 6.40% and 3.45%, respectively, in our research study. Interestingly, our results suggest that patients who have been vaccinated may have a lower rate of local adverse events than the control group. Moreover, in our research study, the rate of local adverse events, including pain and erythema, was much lower than that which was previously reported in studies conducted on elderly patients (pain 42.4% and erythema 1.7%) (40) and cancer patients (pain 31.52% and erythema 33.46%) (39). Due to the method of vaccine administration, patients being treated with denosumab experience more pain as a result of both subcutaneous injections of the vaccine and disease treatment. Additionally, it can be observed that local adverse events are more subjective and are usually neglected compared to systemic adverse events.
Denosumab treatment may also lead to complications, such as fatigue, nausea, dyspnea, and hypocalcemia (41). Therefore, we aimed to determine whether denosumab treatment could influence the rate of any grade of adverse events caused by crosstalk with the vaccine. Fever and flu-like symptoms are also frequently reported complications of denosumab, but the rate of these symptoms did not increase in the cohort that was vaccinated and treated with denosumab compared with vaccinated cancer patients who were not treated with denosumab (39, 42, 43). Osteonecrosis of the Jaw (ONJ) is a previously reported complication of denosumab that affects 8% of therapeutic patients. Nevertheless, we did not observe the occurrence of ONJ in our research study. The incidence of toothache in cancer patients who were vaccinated and treated with denosumab was similar to that of patients who were being treated with denosumab but not vaccinated (2.96% vs 1.42%). As Raje et al (44) have indicated, the higher incidence may be related to prolonged exposure to therapy during the trial, which might account for the absence of ONJ incidence in our study.
We also analyzed the risk of the occurrence of adverse events in patients being treated with denosumab who were vaccinated. Analyses based on age, sex, and the metastatic site did not show significant differences in the patient cohorts, while comorbidities represented a risk factor for developing a mild or severe grade of adverse events and systemic adverse events. The application of vaccination in the current study suggests the relative safety of the vaccine, as vaccination was not a risk factor for adverse events.
A total of 122 (52.36%) patients were diagnosed with metastatic cancer in the vaccine group and some of those patients had been treated using other types of chemotherapy or molecular targeted therapies for cancer in situ and had other potential metastatic sites. Up till now, the specific relationship between chemotherapy and virus infection has not been verified (45, 46). Even if immunotherapy was excluded, we cannot demonstrate that chemoradiotherapy or multiple metastatic sites will not obstruct immunological functions or the seroconversion of antibodies. However, the multivariate analysis showed that there were no statically significant differences observed between primary tumors and metastatic cancer. This finding may indicate that chemoradiotherapy or multiple metastatic sites are not major factors that affect the efficacy and safety of the vaccine. However, further research should also be conducted to obtain more persuasive evidence on the effect of vaccination and other anticancer treatments.
Our study also has serval limitations. The clinical information was retrospectively collected. The choice of type and dose of COVID-19 vaccine depended more on limited supply, vaccine acceptancy, culture, and sociodemographic factors instead of clinical intervention. A study conducted on a larger cohort along with a longer follow-up period should be conducted to fully assess the effects of vaccination and denosumab treatment. Our results confirmed a satisfactory rate of seroconversion after vaccination in patients with spinal tumors being treated with denosumab. Serve adverse events were not observed in our study. Therefore, at this historically challenging moment, our results indicate that COVID-19 vaccines are likely to be safe and effective for patients with spinal tumors being treated using denosumab. Based on the results of this study we recommend that spinal tumor patients being treated using denosumab should get vaccinated in a timely manner to get through current pandemic conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethics Committee of Chang Zheng hospital and granted a waiver of informed consent from study participants.
Author contributions
PW, BL, and SZ contributed to conception and design of the study. YX organized and collected the database. ZZ performed the statistical analysis. PW wrote the first draft of the manuscript. SD, DB, and HY wrote sections of the manuscript. WX and JX supervised the whole project. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Project of China (2016YFC0902100), the National Natural Science Foundation of China (81702888), and Shanghai Youth doctor assistance program(QNYS09). All patients and their families who were invited in the study and all health-care workers who are fighting against the COVID-19 pandemic have rendered help to those patients in need for further research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1034466/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med (2020) 382(8):727–33. doi: 10.1056/NEJMoa2001017
2. Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, et al. Novel coronavirus 2019-nCoV: Prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci (2020) 24(4):2012–9. doi: 10.26355/eurrev_202002_20379
3. Meo S, Al-Khlaiwi T, Usmani A, Meo A, Klonoff D and Hoang T. Biological and epidemiological trends in the prevalence and mortality due to outbreaks of novel coronavirus COVID-19. J King Saud University. Sci (2020) 32(4):2495–9. doi: 10.1016/j.jksus.2020.04.004
4. Kuderer N, Choueiri T, Shah D, Shyr Y, Rubinstein S, Rivera D, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet (London England) (2020) 395(10241):1907–18. doi: 10.1016/S0140-6736(20)31187-9
5. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci (2020) 250:117583. doi: 10.1016/j.lfs.2020.117583
6. Au L, Boos LA, Swerdlow A, Byrne F, Shepherd STC, Fendler A, et al. Cancer, COVID-19, and antiviral immunity: The CAPTURE study. Cell (2020) 183(1):4–10. doi: 10.1016/j.cell.2020.09.005
7. Wilke LG, Nguyen TT, Yang Q, Hanlon BM, Wagner KA, Strickland P, et al. Analysis of the impact of the COVID-19 pandemic on the multidisciplinary management of breast cancer: Review from the American society of breast surgeons COVID-19 and mastery registries. Ann Surg Oncol (2021) 28(10):5535–43. doi: 10.1245/s10434-021-10639-1
8. Ahern E, Smyth MJ, Dougall WC and Teng MWL. Roles of the RANKL-RANK axis in antitumour immunity - implications for therapy. Nat Rev Clin Oncol (2018) 15(11):676–93. doi: 10.1038/s41571-018-0095-y
9. González-Suárez E, Sanz-Moreno A. RANK as a therapeutic target in cancer. FEBS J (2016) 283(11):2018–33. doi: 10.1111/febs.13645
10. Li B, Wang P, Jiao J, Wei H, Xu W and Zhou P. Roles of the RANKL-RANK axis in immunity-implications for pathogenesis and treatment of bone metastasis. Front Immunol (2022) 13:824117. doi: 10.3389/fimmu.2022.824117
11. Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regener (2020) 40:2. doi: 10.1186/s41232-019-0111-3
12. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med (2009) 361(8):756–65. doi: 10.1056/NEJMoa0809493
13. Andrews NA. Denosumab and the treatment of rheumatoid arthritis: In an occupied field, where will a RANKL inhibitor fit in? ibms bonekey (2008) 5(10):351–6. doi: 10.1138/20080340
14. Zhu F, Li Y, Guan X, Hou L, Wang W, Li J, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet (London England) (2020) 395(10240):1845–54. doi: 10.1016/S0140-6736(20)31208-3
15. Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. Jama (2020) 324(10):951–60. doi: 10.1001/jama.2020.15543
16. He Z, Ren L, Yang J, Guo L, Feng L, Ma C, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet (2021) 397(10279):1075–84. doi: 10.1016/S0140-6736(21)00238-5
17. Carhill AA, Cabanillas ME, Jimenez C, Waguespack SG, Habra MA, Hu M, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab (2013) 98(1):31–42. doi: 10.1210/jc.2012-2909
18. Chiu CY, Jung J, Chen W, Weeks DE, Ren H, Boehnke M, et al. Meta-analysis of quantitative pleiotropic traits for next-generation sequencing with multivariate functional linear models. Eur J Hum Genet (2017) 25(3):350–9. doi: 10.1038/ejhg.2016.170
19. Liede A, Hernandez RK, Tang ET, Li C, Bennett B, Wong SS and Jandial D. Epidemiology of benign giant cell tumor of bone in the Chinese population. J Bone Oncol (2018) 12:96–100. doi: 10.1016/j.jbo.2018.07.003
20. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop (1995) 19(5):309–11. doi: 10.1007/BF00181116
21. Costa L, Badia X, Chow E, Lipton A and Wardley A. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Supportive Care cancer: Off J Multinat. Assoc Supportive Care Cancer (2008) 16(8):879–89. doi: 10.1007/s00520-008-0418-0
23. Yamagishi T, Kawashima H, Ogose A, Ariizumi T, Sasaki T, Hatano H, et al. Receptor-activator of nuclear KappaB ligand expression as a new therapeutic target in primary bone tumors. PloS One (2016) 11(5):e0154680. doi: 10.1371/journal.pone.0154680
24. Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther (2007) 9 Suppl 1(Suppl 1):S7. doi: 10.1186/ar2171
25. Anderson P, Kopp L, Anderson N, Cornelius K, Herzog C, Hughes D, et al. Novel bone cancer drugs: investigational agents and control paradigms for primary bone sarcomas (Ewing's sarcoma and osteosarcoma). Expert Opin Investig Drugs (2008) 17(11):1703–15. doi: 10.1517/13543784.17.11.1703
26. Savvidou OD, Bolia IK, Chloros GD, Papanastasiou J, Koutsouradis P, Papagelopoulos PJ. Denosumab: Current use in the treatment of primary bone tumors. Orthopedics (2017) 40(4):204–10. doi: 10.3928/01477447-20170627-04
27. Meng Y, Lu W, Guo E, Liu J, Yang B, Wu P, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol (2020) 13(1):75. doi: 10.1186/s13045-020-00907-0
28. Zhang J, Zhong W and Wu Y. Cancer treatment in the coronavirus disease pandemic. Lung Cancer (Amsterdam Netherlands) (2021) 152:98–103. doi: 10.1016/j.lungcan.2020.12.012
29. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA (2021) 325(21):2204–6. doi: 10.1001/jama.2021.7489
30. Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Dehghani M, Nabavifard M, et al. Immunogenicity and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest (2022) 40(1):26–34. doi: 10.1080/07357907.2021.1992420
31. Blanch-Rubió J, Soldevila-Domenech N, Tío L, Llorente-Onaindia J, Ciria-Recasens M, Polino L, et al. Influence of anti-osteoporosis treatments on the incidence of COVID-19 in patients with non-inflammatory rheumatic conditions. Aging (2020) 12(20):19923–37. doi: 10.18632/aging.104117
32. Ellis G, Bone H, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin oncology: Off J Am Soc Clin Oncol (2008) 26(30):4875–82. doi: 10.1200/JCO.2008.16.3832
33. Smith M, Egerdie B, Hernández Toriz N, Feldman R, Tammela T, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. New Engl J Med (2009) 361(8):745–55. doi: 10.1056/NEJMoa0809003
34. Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy, and safety. Nat Rev Clin Oncol (2022) 19:385–401. doi: 10.1038/s41571-022-00610-8
35. Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, Mesa-Chavez F, Barrientos-Gutiérrez T, Tagliamento M, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer (2022) 160:243–60. doi: 10.1016/j.ejca.2021.10.014
36. Renema N, Navet B, Heymann M-F, Lezot F, Heymann D. RANK–RANKL signalling in cancer. Biosci. Rep (2016) 36(4):e00366. doi: 10.1042/BSR20160150
37. Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int (2011) 22(2):435–46. doi: 10.1007/s00198-010-1326-y
38. So ACP, McGrath H, Ting J, Srikandarajah K, Germanou S, Moss C, et al. COVID-19 vaccine safety in cancer patients: A single centre experience. Cancers (2021) 13(14):3573. doi: 10.3390/cancers13143573
39. Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. a prospective observational study in Italy. Eur J Cancer (Oxford England: 1990) (2021) 157:441–9. doi: 10.1016/j.ejca.2021.08.035
40. Saeed B, Al-Shahrabi R, Alhaj S, Alkokhardi Z and Adrees A. Side effects and perceptions following sinopharm COVID-19 vaccination. Int J Infect dis.: IJID Off Publ Int Soc Infect Dis (2021) 111:219–26. doi: 10.1016/j.ijid.2021.08.013
41. Lipton A, Stopeck A, Rv M, Henry DH, Richardson GE, Rodriguez GI, et al. A meta-analysis of results from two randomized, double-blind studies of denosumab versus zoledronic acid (ZA) for treatment of bone metastases. J Clin Oncol (2010) 28(15_suppl):9015–5. doi: 10.1200/jco.2010.28.15_suppl.9015
42. Waissengrin B, Agbarya A, Safadi E, Padova H and Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol (2021) 22(5):581–3. doi: 10.1016/S1470-2045(21)00155-8
43. van der Veldt A, Oosting S, Dingemans A, Fehrmann R, GeurtsvanKessel C, Jalving M, et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med (2021) 27(4):568–9. doi: 10.1038/s41591-021-01240-w
44. Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol (2018) 19(3):370–81. doi: 10.1016/S1470-2045(18)30072-X
45. Vento S, Cainelli F and Temesgen Z. Lung infections after cancer chemotherapy. Lancet Oncol (2008) 9(10):982–92. doi: 10.1016/S1470-2045(08)70255-9
Keywords: COVID-19, vaccine, spinal tumor, denosumab, immunology
Citation: Wang P, Li B, Zhou S, Xin Y, Zhu Z, Duan S, Bai D, Yuan H, Xu W and Xiao J (2023) Efficacy and safety of COVID-19 vaccines for patients with spinal tumors receiving denosumab treatment: An initial real−clinical experience study. Front. Oncol. 13:1034466. doi: 10.3389/fonc.2023.1034466
Received: 16 September 2022; Accepted: 28 February 2023;
Published: 22 March 2023.
Edited by:
Yong Zhou, West China Hospital, Sichuan University, ChinaReviewed by:
Riccardo Ghermandi, Rizzoli Orthopedic Institute (IRCCS), ItalyAnnan Hu, Zhongshan Hospital, Fudan University, China
Copyright © 2023 Wang, Li, Zhou, Xin, Zhu, Duan, Bai, Yuan, Xu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianru Xiao, amlhbnJ1eGlhbzgzQDE2My5jb20=; Wei Xu, eHV3ZWljaGFuZ3poZW5nQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Pengru Wang
Pengru Wang Bo Li1†
Bo Li1† Wei Xu
Wei Xu Jianru Xiao
Jianru Xiao