
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 April 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1028571
This article is part of the Research Topic Digital Health and Real-World Evidence in Onco-Hematological Patients View all 7 articles
 Zhe Zhuang1†
Zhe Zhuang1† Ying Tian2†
Ying Tian2† Lei Shi3†
Lei Shi3† Dongmei Zou4
Dongmei Zou4 Ru Feng5
Ru Feng5 Wei-wei Tian6
Wei-wei Tian6 Hong Yu7
Hong Yu7 Fei Dong8
Fei Dong8 Aijun Liao9
Aijun Liao9 Yanping Ma10
Yanping Ma10 Qinhua Liu11
Qinhua Liu11 Shuangjiao Liu1
Shuangjiao Liu1 Hongmei Jing8
Hongmei Jing8 Rong Fu7
Rong Fu7 Liang-ming Ma6
Liang-ming Ma6 Hui Liu5
Hui Liu5 Wanling Sun4
Wanling Sun4 Li Bao3*‡
Li Bao3*‡ Yin Wu2*‡
Yin Wu2*‡ Wenming Chen2*‡
Wenming Chen2*‡ Junling Zhuang1*‡
Junling Zhuang1*‡Maintenance treatment is a pivotal part in the whole process management of multiple myeloma (MM), which further deepens response and improves survival. However, evidence of maintenance in non-transplant MM patients is inadequate in real-world practice. Here, we retrospectively analyzed the efficacy and survival of 375 non-transplant MM patients from 11 centers between 2010 and 2021 in north China. After a median of seven cycles of front-line regimens, there were 141, 79, and 155 patients receiving lenalidomide maintenance (L-MT), bortezomib maintenance (B-MT), or thalidomide maintenance (T-MT), respectively. Patients on L-MT and B-MT had significantly greater proportions of high-risk cytogenetic abnormalities (HRCAs) detected by fluorescence in situ hybridization (FISH), which was defined as 1q21 gain, 17p deletion, adverse immunoglobulin heavy chain (IgH) translocations. Although the progression-free survival (PFS) and overall survival (OS) were comparable among the three groups, L-MT and B-MT remedied the negative impact of HRCAs on survival (PFS of patients with HRCAs vs. patients without HRCAs: L-MT, 26.9 vs. 39.2 months, p=0.19; B-MT, 20.0 vs. 29.7 months, p=0.36; OS not reached in all groups). Patients with HRCAs in the T-MT group presented inferior clinical outcomes compared to standard-risk patients (PFS, 12.1 vs. 22.8 months, p=0.02, HR=1.8, 95% CI 1.0–3.4; OS, 54.9 months vs. NR, p<0.001, HR=3.2, 95% CI 1.5–7.0). Achieving complete response (CR) after induction therapy led to superior PFS compared to other degrees of response, regardless of maintenance medication. Furthermore, maintenance duration over 24 months correlated with favorable survival. Due to the large gap of transplant eligibility in China, optimizing maintenance therapy is important for non-transplant MM patients. In this real-world multi-centered study, our findings suggest that clinicians prefer to prescribe lenalidomide or bortezomib as maintenance therapy in high-risk settings, which are superior to thalidomide in non-transplant MM patients. Achievement of CR and maintenance duration over 2 years are positive factors that influence survival.
Multiple myeloma (MM) is the second most prevalent hematological malignancy (1, 2), with an estimated incidence of 0.88–1.17/100,000/year in China (3), and remains incurable despite treatment advances. The incidence of MM has increased, mainly related to aging and the availability of diagnostic approaches. Substantial developments have largely contributed to the improving clinical outcomes of MM patients in the last two decades, such as emerging novel agents, eligibility of autologous stem cell transplantation (ASCT), and strategy of maintenance therapy (4). Even in the era of novel multi-drug induction, maintenance therapy is still one of the essential parts in the whole process management, which sustains and upgrades the response depth and prolongs survival, especially progression-free survival (PFS) (5). Various randomized controlled trials (RCTs) have demonstrated the efficiency and safety of maintenance with a strong immunomodulatory drug (IMiD), lenalidomide; some suggested the benefits of maintenance with bortezomib in patients with high-risk cytogenetics like deletion 17p (6–11). Thalidomide as maintenance has not been recommended in some authoritative guidelines because of the alternative IMiD lenalidomide or its limits of improving PFS and overall survival (OS) in high-risk population (12, 13).
Due to inadequate access to melphalan, ASCT rate is relatively low in Chinese transplant eligible newly diagnosed multiple myeloma (NDMM) patients. Therefore, maintenance after front-line induction seems crucial. However, there has been no adequate data presenting the current situation of maintenance therapy in China, especially in non-transplant patients (14, 15). As economic aspects are considered, thalidomide is still administrated in China. Therefore, we conducted this multi-centered retrospective study on the efficacy and safety of lenalidomide, thalidomide, and bortezomib as maintenance therapy, aiming to delineate the present status of maintenance strategies in real practice in China.
Medical records were extracted to build the Northern China MM Registry database, including those from 11 tertiary hospitals. From the database, non-transplant MM patients diagnosed between 1/1/2010 to 31/7/2021 were screened. Demographic information, international staging system (ISS), revised-ISS (R-ISS), lactic dehydrogenase (LDH), front-line regimens, treatment responses, and adverse events were recorded. CD138-positive marrow cells were sorted for fluorescence in situ hybridization (FISH). Cytogenetic abnormalities (CA) were detected, including amplification of 1q21 (1q21+), deletion 17p (17p−), t(4,14), t(14,16), and t(11,14). High-risk CAs (HRCAs) were defined as 1q21+, 17p−, t(4,14), and t(14,16). Those receiving maintenance therapy by lenalidomide or bortezomib or thalidomide (with or without dexamethasone) after front-line induction therapy and achieving partial response (PR) or better were retrospectively enrolled. The timing and doses of maintenance regimens depended on the respective practice routines. Typically, thalidomide was administrated at 75–150 mg/day. The dose of lenalidomide was 25 mg every other day or 10 mg daily (according to the available dosages, renal function, or adverse reactions), on day 1–21 of 28-day cycle. Bortezomib (1.3 mg/m2 s.c.) was administered every 2 weeks or four doses every 3 months, mainly due to the inconvenience of subcutaneous injection of bortezomib. Dexamethasone was given along in some patients. This study was approved by the Institutional Ethics Committee of Peking Union Medical College Hospital and the Institutional Ethics Committee of the participating centers of the Northern China MM Registry.
The International Myeloma Working Group 2016 efficacy criteria was used to evaluate the response of maintenance therapy (16). The treatment response was evaluated at each follow-up, including stringent complete response (sCR), complete response (CR), very good partial response (VGPR), partial response (PR), minimal response (MR), stable disease (SD), and progression disease (PD).
Electronic medical records were reviewed, and adverse events during maintenance therapy were recorded and graded according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.03, 2010).
SPSS Statistics software (version 20.0; SPSS Inc., Chicago, IL, USA) was used to conduct all statistical analyses. We used the chi-squared test to compare the frequency distributions of categorical variables and one-way ANOVA to compare the numerical variables. Progression-free survival (PFS) time was measured from the initiation of maintenance regimens to the date of disease progression (PD), death, maintenance discontinuation due to toxicity, or the last follow-up. Overall survival (OS) was calculated from the initiation of maintenance regimens to the time of death or the last follow-up. The results reported were as of May 2022. Median PFS and median OS were assessed with the Kaplan–Meier method and compared with the log-rank test. Possible prognostic factors were first screened with univariate analysis; then, a multivariate analysis was conducted with the Cox proportional hazard regression model to ascertain independent prognostic factors. p < 0.05 was considered of statistical significance.
A total of 375 non-transplant patients achieving PR or better after front-line induction were finally recruited in the study, including 141 with lenalidomide (L-MT), 79 with bortezomib (B-MT), and 155 with thalidomide (T-MT). Their baseline characteristics at diagnosis are reported in Table 1. The gender ratio, age, paraprotein type, ISS, and R-ISS were comparable (Table 1). As a distinct bias, the proportion of HRCAs at diagnosis was remarkably lower in the thalidomide group (n=33, 34.0%; p<0.01), compared with 60 (58.8%) patients in lenalidomide-MT and 41 (62.1%) in bortezomib-MT. There were more “double-hit” patients containing any two HRCAs in the bortezomib group (21.2%, p<0.01) than those in the lenalidomide (7.4%) or thalidomide (2.1%) groups. To be noticed, the FISH analyses were performed in each participating center with similar techniques using CD138+ magnetic beads.
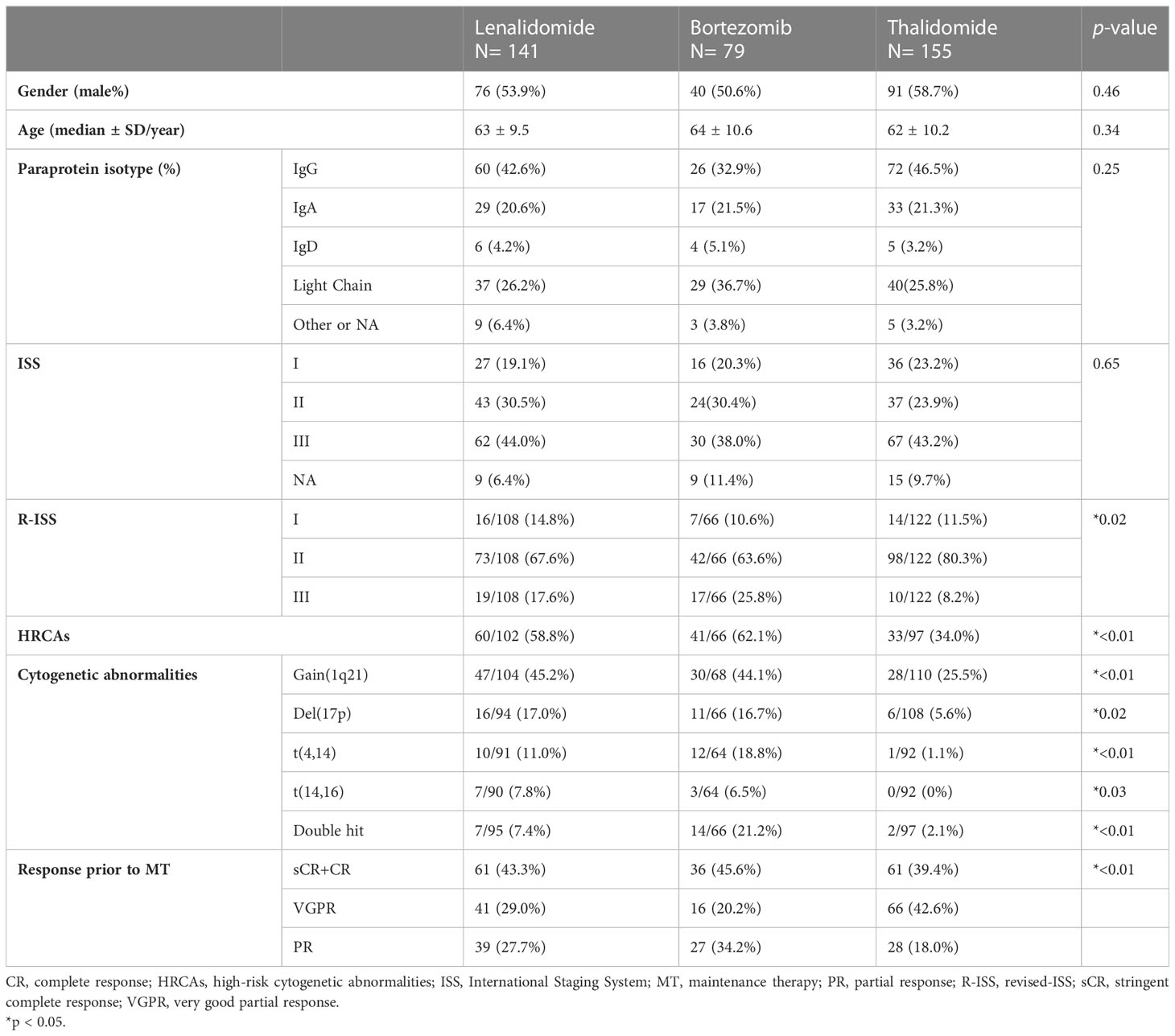
Table 1 Baseline demographic characteristics in non-transplant NDMM patients with lenalidomide, bortezomib, or thalidomide maintenance.
The front-line therapies are listed in Table 2, which were not comparable in the three groups. A total of 125 (88.6%) patients received bortezomib-containing regimens prior to lenalidomide maintenance, 75 (97.4%) prior to bortezomib maintenance, while 96 (61.9%) prior to thalidomide-MT. Thirty-seven (26.2%) patients received lenalidomide-containing regimens prior to lenalidomide-MT, 10 (12.7%) prior to bortezomib-MT, while only 2 (1.3%) prior to thalidomide-MT. The median cycles of induction regimens were seven before maintenance. In the patients receiving lenalidomide-MT, 102 (72.3%) achieved VGPR or better (≥VGPR) response before the initiation of maintenance, with 61 (43.3%) patients achieving CR or sCR. In the bortezomib-MT group, 52 (67.8%) patients achieved ≥VGPR and 36 (45.6%) with CR or sCR. The numbers were 127 (82.0%) and 61 (39.4%) in the thalidomide-MT group, respectively.
The median follow-up durations since maintenance were 24.0, 24.8, and 42.5 months on lenalidomide-MT, bortezomib-MT, and thalidomide-MT, respectively. Further deepening of response (improvement in IMWG response category) was recorded in 15 (10.6%) patients on lenalidomide-MT, 6 (7.6%) patients on bortezomib-MT, and 7 (4.5%) patients on thalidomide-MT (Figure 1A). At last follow-up, 60, 43, and 121 patients had discontinued maintenance therapy. The main reasons for discontinuing maintenance therapy were disease progression (93.3%, 72.1%, and 76.9%), provider/patient preference (1.7%, 18.6%, and 12.4%), and unacceptable toxicity despite dose modification (5.0%, 9.3%, and 10.7%) (Figure 1B).
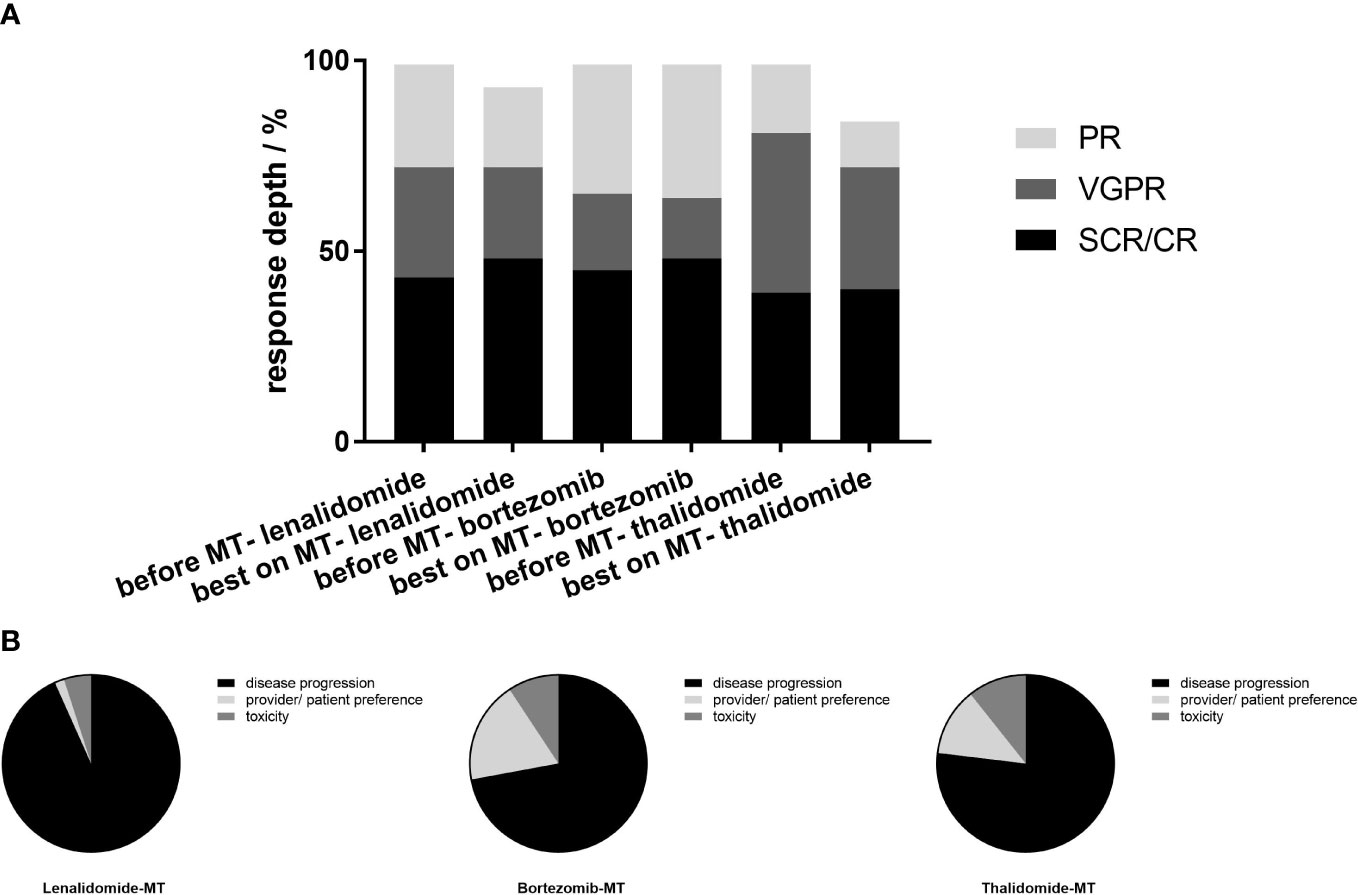
Figure 1 Best response before and during maintenance and reasons for discontinuing maintenance. (A) In the 141 patients on lenalidomide-MT, 61 (43.3%) achieved CR or sCR prior to the initiation of maintenance, 41 (29%) achieved VGPR, while 39(27.7%) achieved PR. In the bortezomib-MT group (n=79), 36(45.6%) achieved CR or sCR prior to the initiation of maintenance, 16 (20.3%) achieved VGPR, and 27 (34.2%) achieved PR. In the thalidomide-MT group (n=155), the ratio was 39.4% (n=61), 42.6% (n=66), and 18.0% (n=28), retrospectively. Best response on maintenance is shown in the adjacent column. On lenalidomide maintenance, 48.2% achieved CR or sCR, 24.8% achieved VGPR, and 21.3% achieved PR. In the bortezomib maintenance group, 48.1%, 16.5%, and 35.4% achieved CR or sCR, VGPR, and PR, respectively, and 40.6%, 32.3%, and 12.3% in patients on thalidomide maintenance. (B) The main reasons for discontinuing maintenance were progression (93.3%, 72.1%, and 76.9% for lenalidomide-MT, bortezomib-MT, and thalidomide-MT, respectively), provider/patient preference (1.7%, 18.6%, and 12.4%), and toxicity (5.0%, 9.3%, and 10.7%).
Until the end of the follow-up, 60 (42.6%) patients on lenalidomide-MT, 31 (39.2%) patients on bortezomib-MT, and 102 (65.8%) patients on thalidomide-MT experienced their first relapse (Figure 2A). The median PFS from maintenance was 27.4, 30.8, and 23.2 months, respectively (p=0.38). The median duration of maintenance treatment was 16.0, 15.6, and 16.0 months, respectively. A total of 23 (16.3%) patients with lenalidomide-MT, 7 (8.9%) patients on bortezomib-MT, and 51 (32.9%) patients with thalidomide-MT died (Figure 2B). The median OS from maintenance was not reached in lenalidomide-MT or bortezomib-MT and 90.7 months in thalidomide-MT (p=0.51).
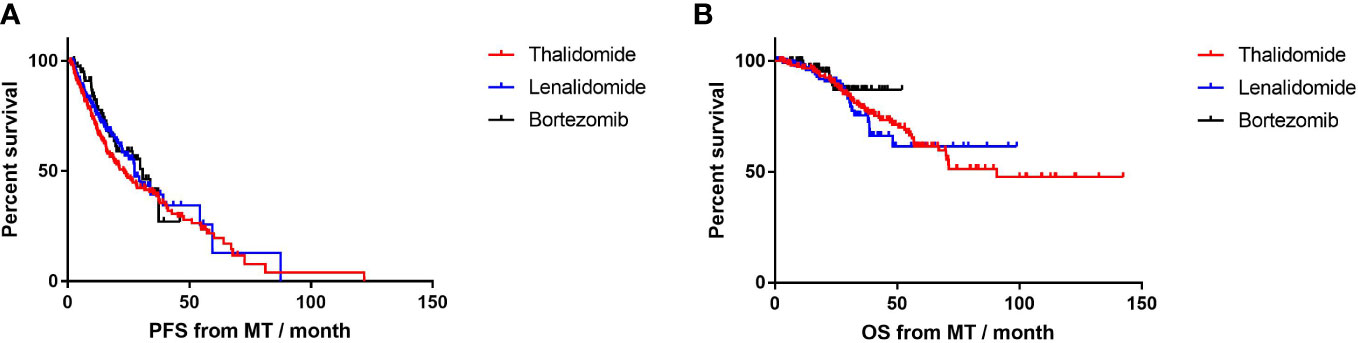
Figure 2 Progression-free survival (PFS) and overall survival (OS) in patients receiving lenalidomide, bortezomib, and thalidomide as maintenance. (A) The median PFS of lenalidomide, bortezomib, and thalidomide maintenance was 27.4, 30.8, and 23.2 months, respectively. (B) The median OS of lenalidomide, bortezomib, and thalidomide maintenance was not reached, not reached, and 90.7months, respectively.
In patients on thalidomide maintenance, those who achieved CR or sCR before MT had prolonged PFS (median, 40.1 m, n=61) compared to those with VGPR or worse (median, 17.2 m, n=94; p=0.003; HR=0.54, 95% CI 0.37–0.81; Figure 3), while PFS was comparable for those with response of CR/sCR and ≤VGPR in lenalidomide-MT (27.4 vs. 25.0 months, p=0.10) and bortezomib-MT (NR vs 29.7m, p=0.16). Patients in each group had similar OS despite different response depths before MT. In the meantime, PFS and OS were not affected by induction regimens in all maintenance groups (Supplementary Figure S1).
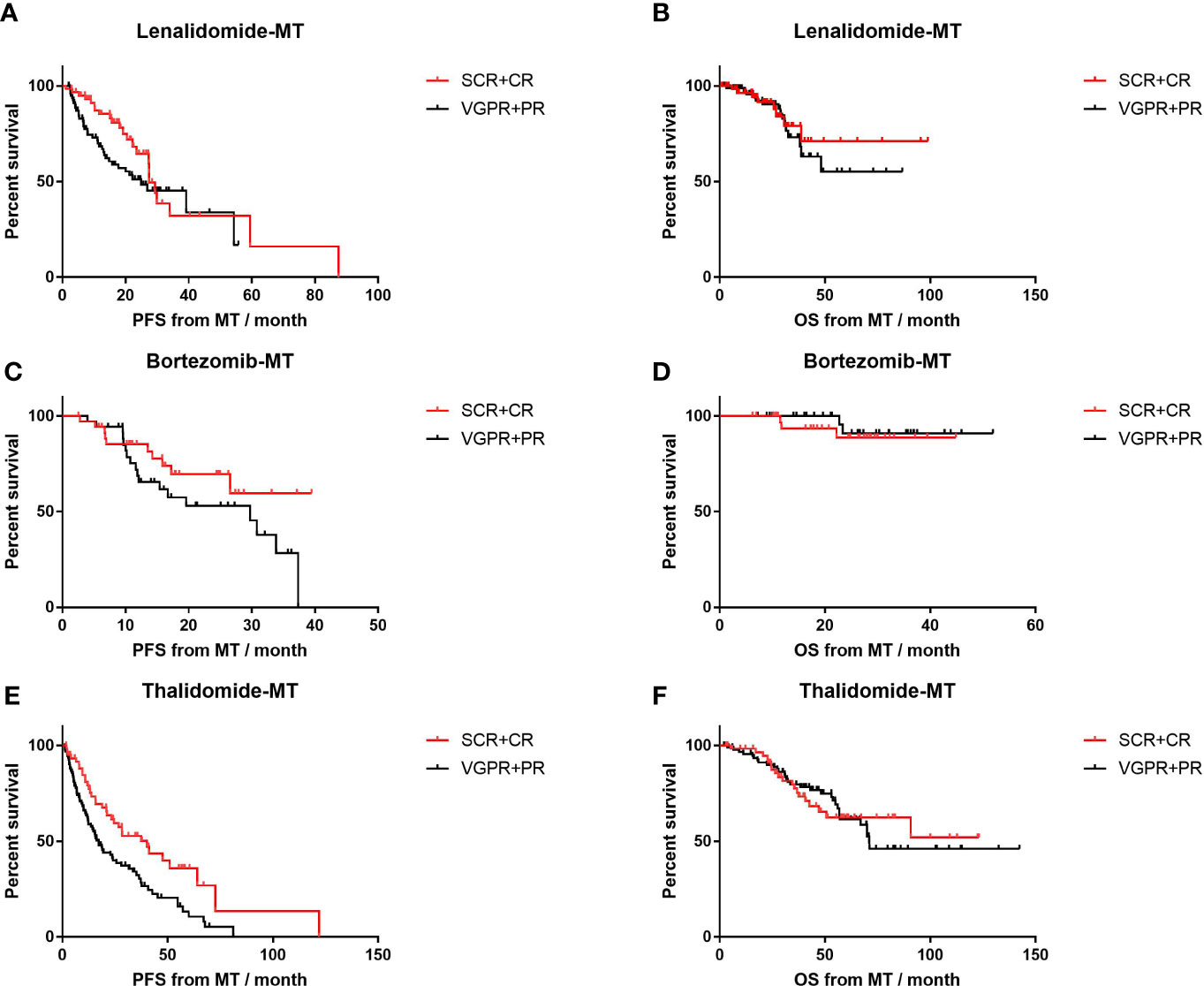
Figure 3 Impact of front-line response depth on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib, and thalidomide maintenance. (A, B) The median PFS and median OS of patients with front-line response of sCR or CR (n=61) versus front-line response of PR or VGPR (n=80) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with front-line response of sCR or CR (n=36) versus front-line response of PR or VGPR (n=43) in patients on bortezomib maintenance. (E, F) The median PFS and median OS of patients with front-line response of sCR or CR (n=61) versus front-line response of PR or VGPR (n=94) in patients on thalidomide maintenance.
For patients with adverse cytogenetic abnormalities (Figure 4), thalidomide-MT resulted in significantly impaired PFS (12.1 vs. 22.8 months, p=0.02; HR=1.8, 95% CI 1.0–3.4) and OS (54.9 months vs. NR, p<0.001; HR=3.2, 95% CI 1.5–7.0). In contrast, PFS was comparable in patients with HRCA or not on lenalidomide-MT (26.9 vs. 39.2 months, p=0.19) or bortezomib-MT (20.0 vs. 29.7 months, p=0.36), respectively. OS was not reached in either subgroup. The 4-year survival rate was 83.3% versus 88.1% in the lenalidomide-MT group, and 90.2% versus 88.0% in the bortezomib-MT group, respectively.
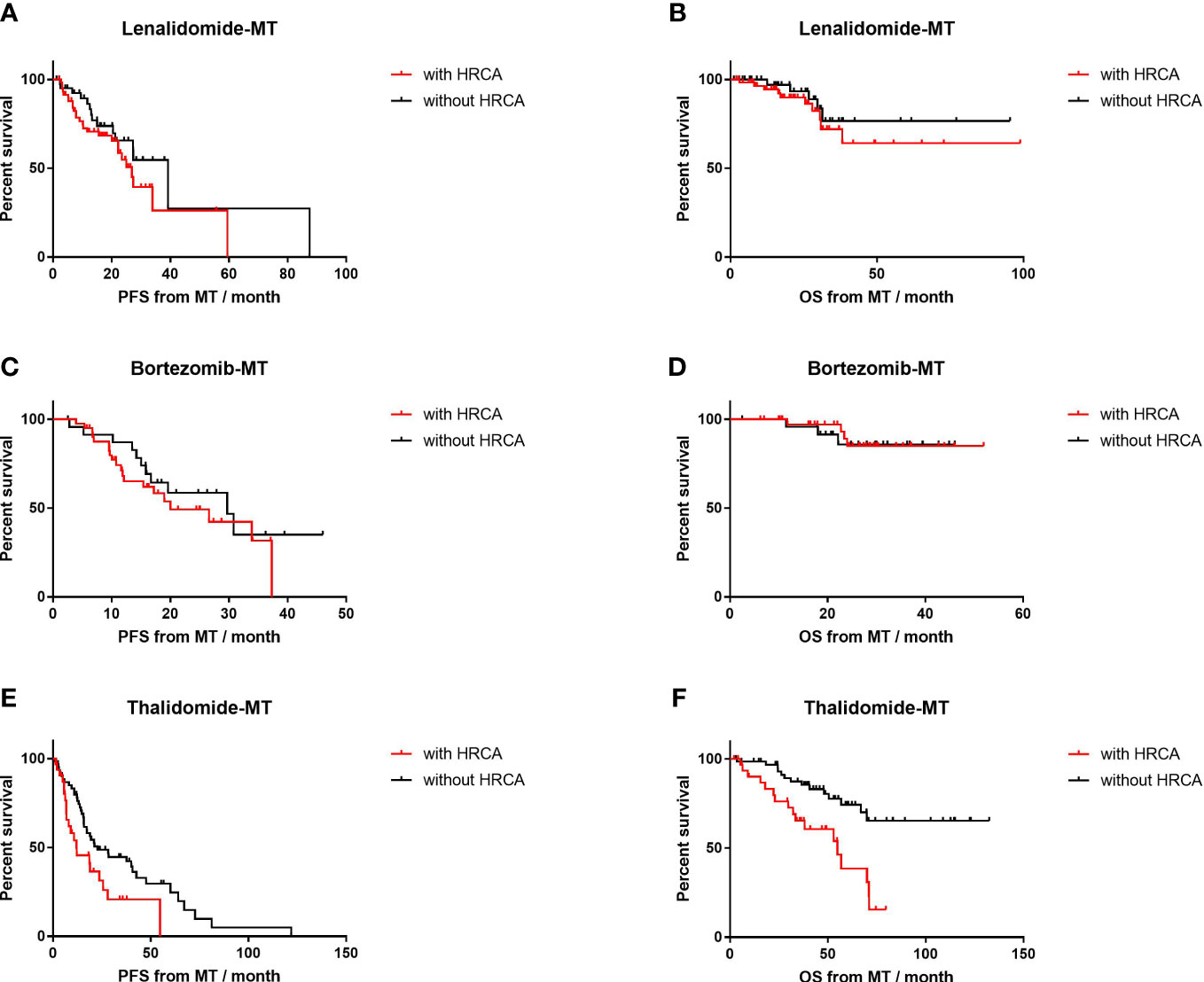
Figure 4 Impact of high-risk cytogenetics on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib, and thalidomide maintenance. (A, B) The median PFS and median OS of patients with high-risk cytogenetics (n=60) versus those without (n=42) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with high-risk cytogenetics (n=41) versus those without (n=25) in patients on bortezomib maintenance. (E, F) The median PFS and median OS of patients with high-risk cytogenetics (n=35) versus those without (n=62) in patients on thalidomide maintenance.
To be more specific, 1q21+ on thalidomide-MT was an inferior predictor of shortened median PFS (12.2 vs. 22.8 months, p=0.07) and impaired median OS (54.9 months vs. NR, p<0.01; HR=2.4, 95% CI 1.1–5.5), as shown in Supplementary Figure S2. However, in the lenalidomide group, the median PFS was similar for those with 1q21+ or not (26.9 vs. 27.4 months, p=0.84); 4-year OS was 89.4% and 82.5% (p=0.37), respectively. In the bortezomib group with 1q21+ or not, the median PFS was 26.6 vs. 29.7 months (p=0.99); 4-year OS was 96.7% and 84.2% (p=0.14).
In the circumstance of 17p−, thalidomide-MT also resulted in impaired PFS (6.8 vs. 22.8 months, p=0.04; HR=3.0, 95% CI 0.6–16.0) and impaired OS (32.3 vs. 71.1 months, p=0.005; HR=4.0, 95% CI 0.6–26.5). As for lenalidomide-MT, deletion 17p or not did not significantly affect PFS (22.2 vs. 27.4 months, respectively; p=0.11); OS was NR (p=0.73). The median PFS for patients on bortezomib-MT with 17p deletion was shorter, yet comparable to those without 17p− (19.5 vs. 29.7 months, p=0.52), and OS was comparable (NR vs. NR, p=0.12; Supplementary Figure S3).
Only one patient presented with high-risk IgH translocation in the thalidomide-MT group, while patients with t(4,14) or t(14,16)) on lenalidomide-MT or bortezomib-MT had similar median PFS and OS as those without (Supplementary Figure S4).
In the meantime, PFS and OS were not affected by baseline ISS stage in all maintenance groups.
The duration of maintenance <2 years or longer had a distinct influence on PFS (Figure 5). In the lenalidomide-MT group (18.1 vs. 54.3 months, p<0.001; HR=4.9, 95% CI 3.0–8.2), bortezomib-MT group (16.7 vs. 37.3 months, p<0.001; HR=6.3, 95% CI 3.1–12.9), and thalidomide-MT group (12.1 vs. 57.2 months, p=0.001; HR=4.9, 95% CI 3.3–7.4). The results of OS were similar according to maintenance duration.
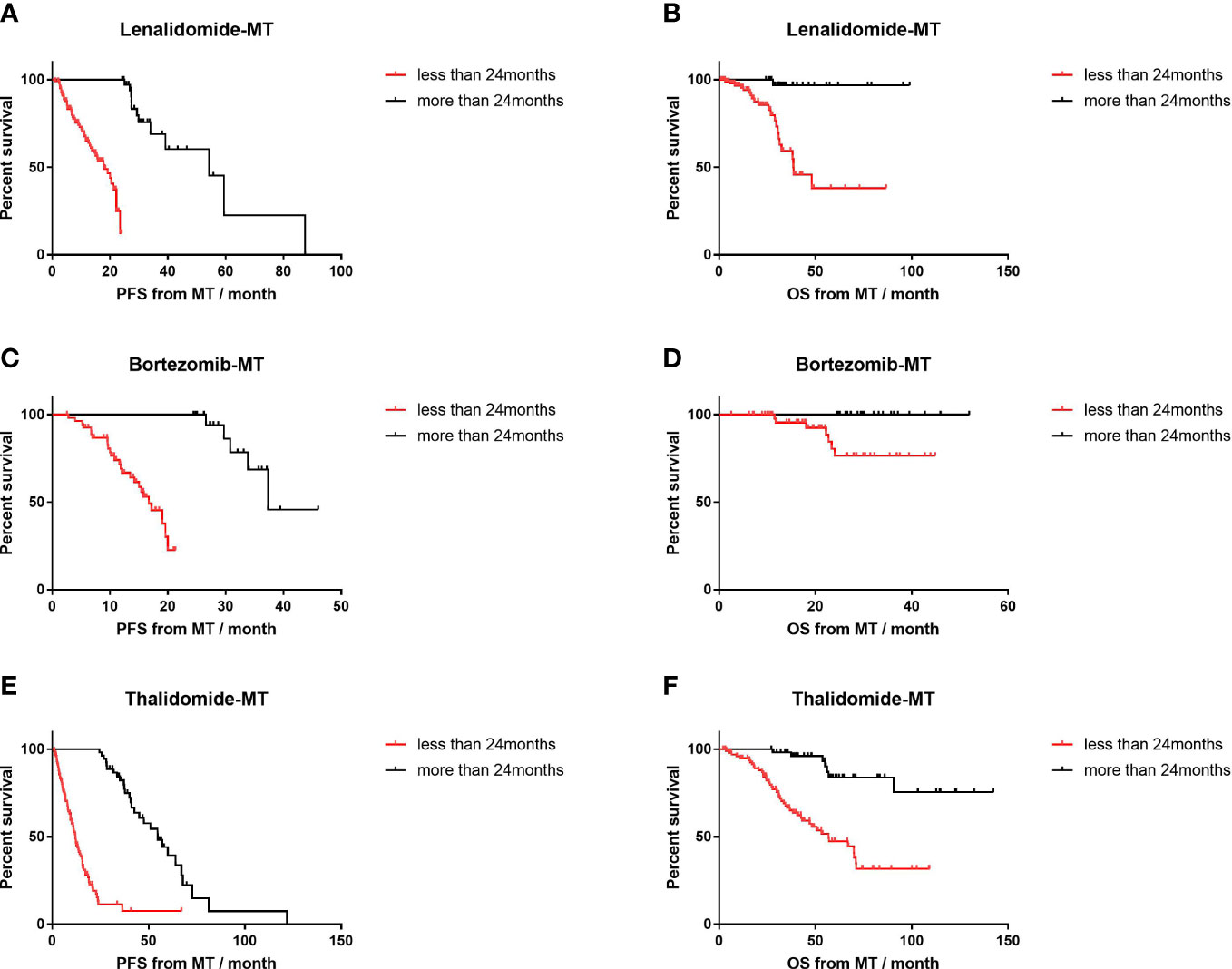
Figure 5 Impact of maintenance duration on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib, and thalidomide maintenance. (A, B) The median PFS and median OS of patients with maintenance duration <24 months (n=103) versus those with longer maintenance (n=38) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with maintenance duration <24 months (n=56) versus those with longer maintenance (n=23) in patients on bortezomib maintenance. (E, F) The median PFS and median OS of patients with maintenance duration <24 months (n=101) versus those with longer maintenance (n=54) in patients on thalidomide maintenance.
Lenalidomide or bortezomib maintenance had a non-superior impact on survival compared to thalidomide maintenance in the multivariate analysis after adjusting for high-risk cytogenetics by FISH, response depth prior to maintenance, and maintenance duration (Table 3). Meanwhile, multivariate analysis by Cox model confirmed that deepened front-line response (CR or sCR) had an independent protective impact on PFS and prolonged maintenance duration on PFS and OS.
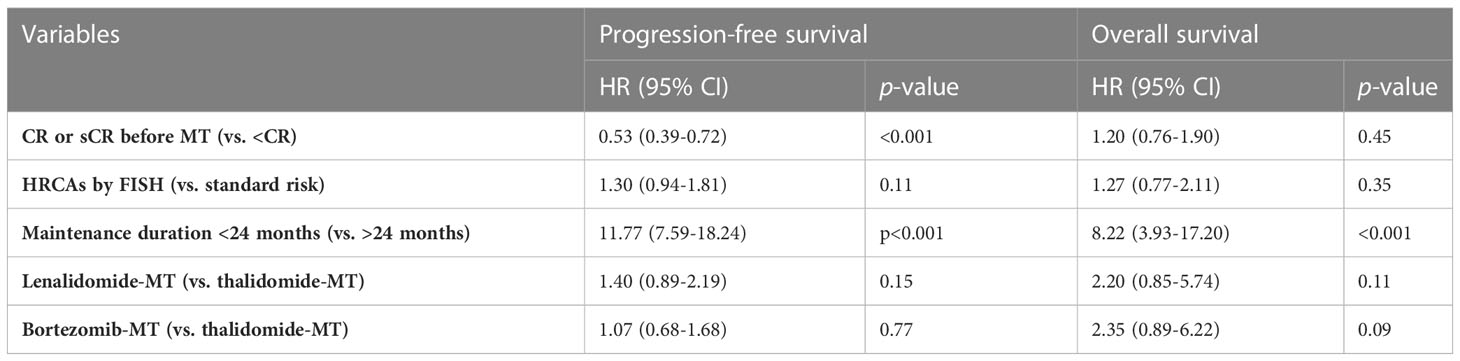
Table 3 Multivariate analysis by Cox model for lenalidomide or bortezomib vs. thalidomide maintenance.
Maintenance therapy has been considered as an important part of the whole process management of treatment strategies in multiple myeloma (MM). The depth of response is further improved during maintenance, even switching to MRD negativity. The schema of maintenance for post-transplant patients is relatively perspicuous. However, key questions related to maintenance in non-transplant patients are ambiguous, such as timing to start, approaches, and duration. Oral agents like lenalidomide or ixazomib have been approved for maintenance in transplant-ineligible NDMM patients based on randomized controlled trial (RCT) data, while evidence from real-world study (RWS) is quite inadequate in such population. To address the unmet need of insufficient data, we conducted this multi-centered study to summarize the real-world patient characteristics, maintenance patterns and clinical outcomes in non-transplant NDMM patients in China. Our findings demonstrated that lenalidomide and bortezomib were superior to thalidomide for further improvement of response and achieved benefit of survival regardless of cytogenetic risk.
The special situation in China was that melphalan was not available in China’s mainland until the end of 2018. Even in China’s tertiary hospitals, the ASCT rate was only 15%–30%. Therefore, the majority of patients in our study were younger than 65 years. After being reimbursed in 2017, branded or generic bortezomib and lenalidomide were comprehensively administrated in MM patients. Compared to a further 4.5% improvement of response by thalidomide during maintenance, the boosting of response in bortezomib maintenance was greater, and lenalidomide achieved an extra 10% deepening. In the community-based UPFRONT study, single-agent bortezomib maintenance for only five cycles following bortezomib-based induction therapies improved response depth in approximately 16% of patients (17).The baseline data were of distinct bias in three groups. Only approximately one-third of the patients in thalidomide group presented high-risk characteristics, which was over 50% in the other two approaches. The percentages of any single high-risk cytogenetic abnormality including 1q21+, 17p−, and adverse IGH rearrangements were lower in thalidomide-treated patients so was that of R-ISS 3. Therefore, PFS or OS in lenalidomide or bortezomib groups could be compromised by the selection bias of more high-risk patients. Even so, our data clearly demonstrated that both lenalidomide and bortezomib could reverse the negative impact of high-risk cytogenetics on PFS and OS (Figure 4) compared to those with standard-risk. Although the efficacy of lenalidomide as maintenance in high-risk patients was controversial, recent Myeloma XI trial has suggested that high-risk sub-population in lenalidomide maintenance group had significant longer PFS compared to that of placebo (10). While bortezomib is listed as the “other recommended regimen” in most guidelines (18), many trials have proved its advantage in high-risk disease. Mayo Clinic consensus recommended bortezomib for patients with high-risk cytogenetics (6, 11, 19), both in front-line and maintenance settings. By contrast, this study confirmed that thalidomide maintenance could not overcome the inferior impacts of high-risk disease in real-world practice.
An important issue regarding maintenance in non-transplant scenario was patients’ status, mainly front-line regimens and response status. Although maintenance therapy started after 4–12 cycles of induction regimens or if stable disease was obtained in some RCT studies, all patients in our study initiated their maintenance with a median seven cycles of front-line therapy and achieved PR or better. We found that compared to response of PR or VGPR, achieving CR or sCR as before maintenance was an independent protecting factor for PFS (Tables 3). Meanwhile, survival outcome in maintenance with novel drugs like lenalidomide or bortezomib was not affected by front-line response (Figure 3). In addition, deep response rates during maintenance with lenalidomide or bortezomib were further enhanced yet fell in the thalidomide group. These trends coincided with the results in some RCT studies (7, 20).
The maintenance duration in transplant-ineligible patients is still indefinite. Previous studies demonstrated that lengthy maintenance could result in better survival and depth of response (21, 22). In spite of continuous treatment, such as the FIRST trial, PFS in patients with continuous lenalidomide and dexamethasone (Rd) was 26 months (23), which was similar in our L-MT group (27.4 months). Our data also confirmed that maintenance duration had an independent favorable impact on survival (Figure 5). Disease progression was still the main reason for drug withdrawal. Only approximately one-third of patients on thalidomide or bortezomib and one-fourth on lenalidomide could persist to keep medication for more than 2 years. The failure from late progression was mainly due to non-optimal response, which is a problem with compromised induction treatment. Thus, the question in non-transplant patients was not “how long the maintenance will be” but “how long the maintenance could be.” More potent regimens are approved for transplant-ineligible patients such as anti-CD38 antibody, daratumumab, lenalidomide, and dexamethasone (DRd) for continuous administration. Therefore, deeper response even MRD negativity could be achieved and translate into longer PFS.
As a retrospective study, there were limitations to be considered. One was the missing data in cytogenetics, which lost a substantial number of patients for high-risk cytogenetics subgroup analysis. However, only incorporating patients with integrative information also caused bias. We recruited all patients who met the inclusion criteria in this study. A full-time research assistant was responsible for all patients’ follow-up. The missing rate of the whole cohort was <5%. Another limitation was the relatively limited follow-up duration for lenalidomide group and bortezomib group. Lenalidomide and bortezomib were first reimbursed by China’s National Healthcare in September 2017. Therefore, these novel drugs were affordable in most myeloma patients since then.
In this multi-centered real-world study, lenalidomide, bortezomib, or thalidomide after front-line therapy in non-transplant NDMM patients produced similar PFS and OS. However, patients with lenalidomide or bortezomib comprised a greater proportion of high-risk cytogenetic abnormalities. These HRCAs drag down survival in patients with thalidomide, while lenalidomide and bortezomib remedy the negative effect during maintenance. Clinicians in real practice prefer to recommend lenalidomide or bortezomib as maintenance therapy for patients with HRCAs, while thalidomide is still an option for patients with standard risk. Schema of prolonged maintenance duration improved survival despite maintenance regimens. Furthermore, in clinical settings with limited resources to ASCT, maintenance therapy should be highlighted to delay progression.
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Peking Union Medical College Hospital, as well as the Institutional Ethics Committee of the participated centers from the Northern China MM Registry. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JZ designed the experiment. All authors contributed to clinical data collection. ZZ analyzed the data and prepared the tables and figures. ZZ wrote the first draft and JZ revised the draft. All authors reviewed and approved the revised manuscript.
This study was funded by the Capital Health Development Scientific Research Fund (Grant No. 2022-2-4013) and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-048).
The authors thank the medical staff and physicians who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1028571/full#supplementary-material
Supplementary Figure 1 | Impact of different induction regimens on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib and thalidomide maintenance. (A, B) The median PFS and median OS of patients who received VRD (n=27), B-based (without IMiDs, n=98), L-based (without PIs, n=10) and other regimens (n=6) as induction therapy, on lenalidomide maintenance. (C, D) The median PFS and median OS of patients who received VRD (n=8), B-based (without IMiDs, n=69), L-based (without PIs, n=2) and other regimens (n=0) as induction therapy, on bortezomib maintenance. (E, F) The median PFS and median OS of patients who received VRD (n=0), B-based (without IMiDs, n=96), L-based (without PIs, n=2) and other regimens (n=57) as induction therapy, on thalidomide maintenance.
Supplementary Figure 2 | Impact of 1q21 amplification on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib and thalidomide maintenance. (A, B) The median PFS and median OS of patients with 1q21 amplification (n=47) versus those without (n=57) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with 1q21 amplification (n=30) versus those without (n=38) in patients on bortezomib maintenance. (E, F) The median PFS and median OS of patients with 1q21 amplification (n=30) versus those without (n=78) in patients on thalidomide maintenance.
Supplementary Figure 3 | Impact of 17p deletion on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib and thalidomide maintenance. (A, B) The median PFS and median OS of patients with 17p deletion (n=16) versus those without (n=78) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with 17p deletion (n=11) versus those without (n=55) in patients on bortezomib maintenance. E, F The median PFS and median OS of patients with 17p deletion (n=6) versus those without (n=102) in patients on thalidomide maintenance.
Supplementary Figure 4 | Impact of high-risk IgH translocation on progression-free survival (PFS) and overall survival (OS) of lenalidomide, bortezomib and thalidomide maintenance. (A, B) The median PFS and median OS of patients with t(4,14) or t(14,16) (n=15) versus those without (n=76) in patients on lenalidomide maintenance. (C, D) The median PFS and median OS of patients with t(4,14) or t(14,16) (n=15) versus those without (n=49) in patients on bortezomib maintenance.
1. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up(†). Ann Oncol Off J Eur Soc Med Oncol (2021) 32:309–22. doi: 10.1016/j.annonc.2020.11.014
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
3. Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, et al. Incidence and mortality of multiple myeloma in China, 2006-2016: an analysis of the global burden of disease study 2016. J Hematol Oncol (2019) 12:136. doi: 10.1186/s13045-019-0807-5
4. Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the united states. Blood adv (2017) 1:282–87. doi: 10.1182/bloodadvances.2016002493
5. Dimopoulos MA, Jakubowiak AJ, McCarthy PL, Orlowski RZ, Attal M, Bladé J, et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J (2020) 10:17. doi: 10.1038/s41408-020-0273-x
6. Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30:2946–55. doi: 10.1200/JCO.2011.39.6820
7. Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. New Engl J Med (2012) 366:1782–91. doi: 10.1056/NEJMoa1114138
8. McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. New Engl J Med (2012) 366:1770–81. doi: 10.1056/NEJMoa1114083
9. Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New Engl J Med (2012) 366:1759–69. doi: 10.1056/NEJMoa1112704
10. Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2019) 20:57–73. doi: 10.1016/S1470-2045(18)30687-9
11. Goldschmidt H, Lokhorst HM, Mai EK, van der Holt B, Blau IW, Zweegman S, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia (2018) 32:383–90. doi: 10.1038/leu.2017.211
12. Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, et al. Long-term follow-up of MRC myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res an Off J Am Assoc Cancer Res (2013) 19:6030–8. doi: 10.1158/1078-0432.CCR-12-3211
13. Morgan GJ, Gregory WM, Davies FE, Bell SE, Szubert AJ, Brown JM, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC myeloma IX results and meta-analysis. Blood (2012) 119:7–15. doi: 10.1182/blood-2011-06-357038
14. Shen M, Huang ZX, Zhang JJ, Li X. [Clinical analysis of 25 non-transplanted multiple myeloma patients treated with bortezomib maintenance]. Zhongguo shi yan xue ye xue za zhi. (2021) 29:131–36. doi: 10.19746/j.cnki.issn.1009-2137.2021.01.021
15. Han X, Jin C, Zheng G, He D, Zhao Y, Li Y, et al. Different patient subgroup different maintenance, proteasome inhibitors or immunomodulators maintenance for newly diagnosed multiple myeloma: A 7-year single-center date in China. Front Oncol (2021) 11:665217. doi: 10.3389/fonc.2021.665217
16. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol (2016) 17:e328–e46. doi: 10.1016/S1470-2045(16)30206-6
17. Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol Off J Am Soc Clin Oncol (2015) 33:3921–9. doi: 10.1200/JCO.2014.58.7618
18. Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18:1685–717. doi: 10.6004/jnccn.2020.0057
19. Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli D, et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement. Bone Marrow Transplant. (2019) 54:353–67. doi: 10.1038/s41409-018-0264-8
20. Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, López de la Guía A, et al. Maintenance therapy with bortezomib plus thalidomide or bortezomib plus prednisone in elderly multiple myeloma patients included in the GEM2005MAS65 trial. Blood (2012) 120:2581–8. doi: 10.1182/blood-2012-05-427815
21. Alonso R, Cedena MT, Wong S, Shah N, Ríos-Tamayo R, Moraleda JM, et al. Prolonged lenalidomide maintenance therapy improves the depth of response in multiple myeloma. Blood Adv (2020) 4:2163–71. doi: 10.1182/bloodadvances.2020001508
22. Ho M, Zanwar S, Kapoor P, Gertz M, Lacy M, Dispenzieri A, et al. The effect of duration of lenalidomide maintenance and outcomes of different salvage regimens in patients with multiple myeloma (MM). Blood Cancer J (2021) 11:158. doi: 10.1038/s41408-021-00548-7
Keywords: maintenance therapy, high-risk disease, real-world study, multicenter, multiple myeloma
Citation: Zhuang Z, Tian Y, Shi L, Zou D, Feng R, Tian W-w, Yu H, Dong F, Liao A, Ma Y, Liu Q, Liu S, Jing H, Fu R, Ma L-m, Liu H, Sun W, Bao L, Wu Y, Chen W and Zhuang J (2023) Lenalidomide or bortezomib as maintenance treatment remedy the inferior impact of high-risk cytogenetic abnormalities in non-transplant patients with newly diagnosed multiple myeloma: a real-world multi-centered study in China. Front. Oncol. 13:1028571. doi: 10.3389/fonc.2023.1028571
Received: 26 August 2022; Accepted: 29 March 2023;
Published: 20 April 2023.
Edited by:
Laura Lopez-Perez, Universidad Politécnica de Madrid, SpainReviewed by:
Catarina Geraldes, Coimbra Hospital and University Center, PortugalCopyright © 2023 Zhuang, Tian, Shi, Zou, Feng, Tian, Yu, Dong, Liao, Ma, Liu, Liu, Jing, Fu, Ma, Liu, Sun, Bao, Wu, Chen and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Bao, YmFvbGlscTkwOUBzaW5hLmNvbQ==; Yin Wu, d3VkeHVhbkAxMjYuY29t; Wenming Chen, d2VubWluZ19jaGVuQHlhaG9vLmNvbQ==; Junling Zhuang, emh1YW5nanVubGluZ0BwdW1jaC5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.