
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol., 21 March 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1012783
This article is part of the Research TopicPrimary and Acquired Resistance in Lung CancerView all 10 articles
 Samuel A. Kareff1
Samuel A. Kareff1 Kunal Gawri2
Kunal Gawri2 Khadeja Khan3
Khadeja Khan3 Deukwoo Kwon4
Deukwoo Kwon4 Estelamari Rodriguez5
Estelamari Rodriguez5 Gilberto de Lima Lopes5
Gilberto de Lima Lopes5 Richa Dawar5*
Richa Dawar5*Current first-line standard therapy for metastatic non-small cell lung cancer without driver mutations involves chemotherapy and immunotherapy combination. Prior to the advent of immune checkpoint inhibition, REVEL, a randomized phase III trial demonstrated improved progression-free and overall survival with ramucirumab and docetaxel (ram+doc) in patients who failed platinum-based first-line therapy. Long-term outcomes related to second-line ramucirumab and docetaxel after first-line immunotherapy exposure remain unknown. We analyzed outcomes for 35 patients from our center whom received ramucirumab and docetaxel following disease progression on chemotherapy and immunotherapy combination. Median progression-free survival among patients who received ram+doc after exposure to immunotherapy was 6.6 months (95% CI = 5.5 to 14.9 months; p<0.0001), and median overall survival was 20.9 months (95% CI = 13.4 months to infinity; p<0.0001). These outcomes suggest that there may a synergistic benefit to combining chemotherapy with anti-angiogenic therapy after immunotherapy exposure. Future analyses should be evaluated prospectively and among a larger patient subset.
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for nearly 80% of all cases, and often presents in the locally advanced or metastatic settings (1). Currently, a combination of chemotherapy and/or immunotherapy (IO) is considered standard first-line treatment for metastatic NSCLC (mNSCLC) without driver mutations, often tailored based on a patient’s programmed death-ligand 1 (PD-L1) status (2). Before the advent of IO as first-line therapy, REVEL, a randomized, multicenter, phase III clinical trial, demonstrated improved progression-free survival (PFS), overall survival (OS), and quality of life (QoL) with ramucirumab and docetaxel (ram+doc) chemotherapy and antiangiogenic combination in patients whose disease progressed after platinum-based doublet first-line chemotherapy compared with docetaxel alone (3, 4). This combination was proposed utilizing ramucirumab as a complete human monoclonal IgG1 antibody with direct vascular endothelial growth factor receptor 2 (VEGFR2) antagonism given already known improved outcomes with docetaxel in platinum-resistant disease (5). Indeed, this biological rationale of overcoming the demonstrated immunosuppressive effect of VEGF has been proven in other lines of therapy and in combination with immunotherapy, such as the IMPOWER150 study which demonstrated improved outcomes for nonsquamous mNSCLC when combining atezolizumab, bevacizumab, and platinum-doublet chemotherapy in the first-line setting (6). There have been studies that have shown promising results in other forms of platinum-resistant tumor histologies, namely, urothelial and gastric cancers (7, 8). There exist data mostly limited to retrospective cohort analyses in East Asia and Europe discussing responses to ram+doc treatment in patients pretreated with IO-based therapy; however, any synergistic benefit has not been proven for patients with mNSCLC (9). Therefore, our study aims to clarify the efficacy and outcomes of this combined therapeutic approach in patients with paclitaxel-resistant mNSCLC.
We performed a retrospective cohort study among all patients with mNSCLC treated at the University of Miami Sylvester Comprehensive Cancer Center. We retrospectively identified all patients with mNSCLC whose disease demonstrated progression after IO-based therapy and then received ram+doc as a subsequent line of therapy between January 1st, 2010 and March 1st, 2020. A total of thirty-nine patients were identified whom met these inclusion criteria. We subsequently excluded 4 patients with EGFR or ALK driver mutations from our analysis. As such, thirty-five (n = 35) patients were included in our final analysis. We assessed patients’ PFS and OS after ram+doc treatment utilizing the Kaplan-Meier method as primary outcomes. We compared our center’s retrospective data with those from REVEL data utilizing a simulation study via Wilcoxon test. Since REVEL data was not available for reproduction, we used median PFS and median OS and corresponding 95% CIs to estimate the distribution of median survival time for our dataset compared to that of REVEL using an approximate Bayesian computation (ABC) approach. We also collected information on adverse events (AEs) during ram+doc treatment as a secondary outcome. This study was approved by the University of Miami Institutional Review Board eProst #20170427.
Of a total 44 patients treated with ramucirumab and docetaxel at our center, we excluded 6 patients with EGFR mutation, 1 with ALK mutation, and 2 without previous exposure to IO. We report the patient demographics as well as some treatment characteristics for the 35 included patients in Table 1. Patients’ age ranged from 45 to 76 years (median 65 years). There were a total 17 females (48.6%) and 18 males (51.4%) represented in the sample. 19 patients identified as Hispanic/Latinx (54.3%), 12 as white (34.3%), and 1 as African American (2.8%). 28 were tobacco users (80%), while 7 were never-tobacco users (20%). All 35 patients received ICI as first-, second-, or third-line therapy for mNSCLC, which was followed immediately by ram+doc upon disease progression. 33 patients’ tumor histology was adenocarcinoma (94.3%), while 2 patients’ tumor histology was squamous cell carcinoma (5.7%). All patients had disease with at least 3 metastatic sites, listed in the following order of frequency: 1) bone, 2) liver, and 3) brain.
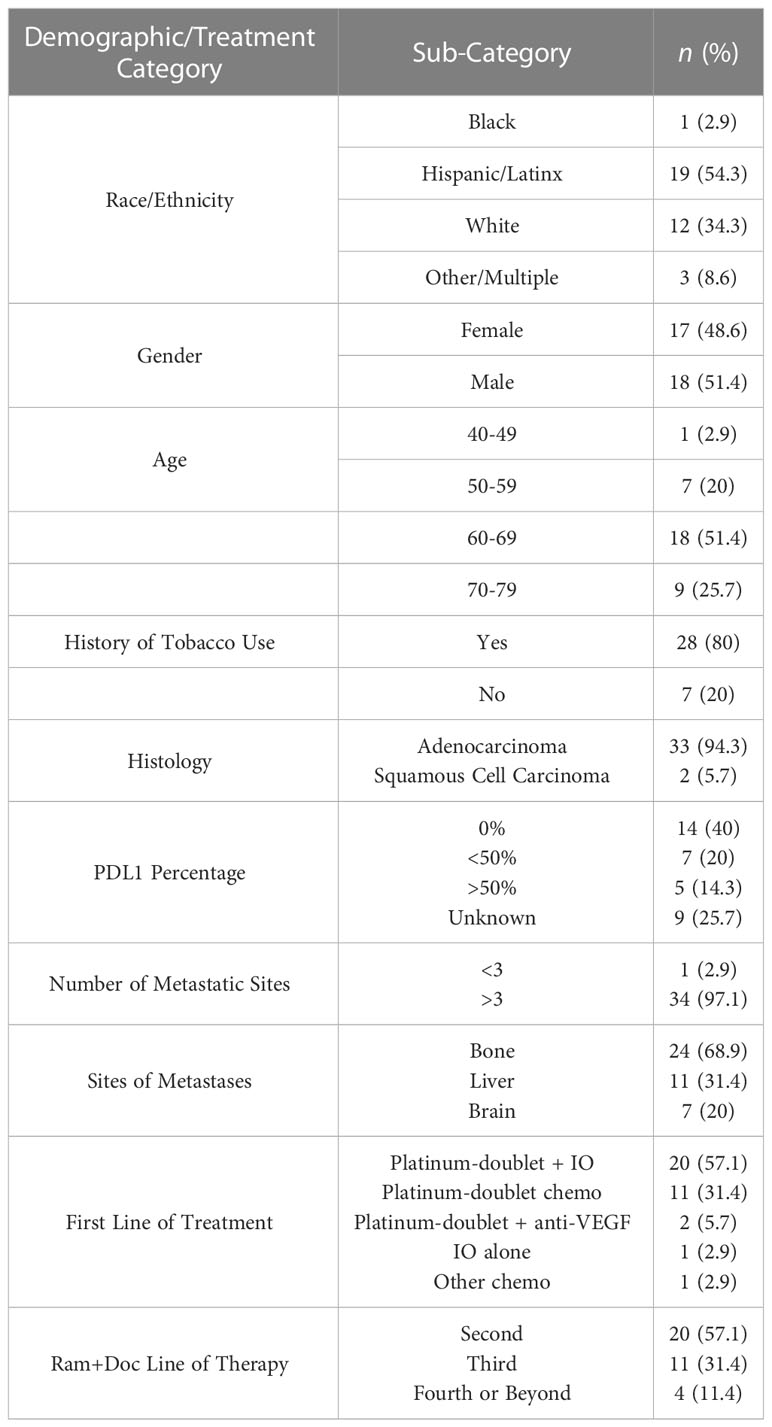
Table 1 Demographics of patients whom received ramucirumab and docetaxel, and characteristics of treatment, at the University of Miami.
Median PFS (mPFS) among patients whom received ram+doc therapy after IO exposure was 6.6 months (95% confidence interval [CI] = 5.5 to 14.9 months; p<0.0001] (Figure 1A) and median OS (mOS) was 20.9 months (95% CI = 13.4 months to infinity; p<0.0001) (Figure 1B). There were no statistically significant differences detected among tumor histology. Since the REVEL data were not available for independent reproduction, we utilized the ABC approach to estimate how many p-values are less than 0.0001 in 1,000 tests for simulated two datasets; this approach has been validated elsewhere (10). Given that all 1,000 p-values were less than 0.0001, we found these results to be statistically significant in estimating our CIs. Moreover, since the 95% CIs of our cohort versus that of REVEL did not overlap, we considered these improved mPFS and mOS outcomes to be statistically significant as well.
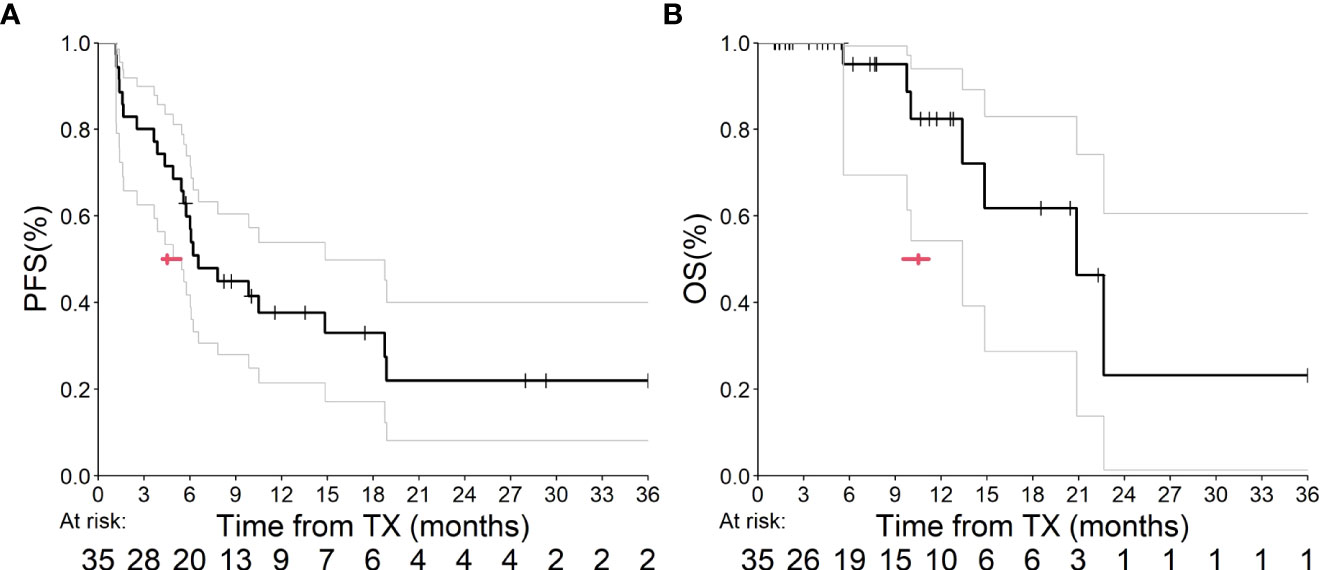
Figure 1 (A, B) This graph depicts PFS (A) and OS (B) from our UM outcomes. The bold line indicates our Kaplan-Meier analysis, and the gray lines represent our 95% CI estimates. The red arrow indicates median estimates within both graphs.
We observed six patients with treatment-related adverse events related to ramucirumab: two patients with Grade 1 hypertension; two patients with proteinuria (one Grade 1 and one Grade 2); one patient with Grade 3 hemoptysis, and one with Grade 2 fatigue. Three of these patients experiencing adverse events (n = 3/7; 42.8%) required dose reduction. Docetaxel use led to 31 adverse events: 15 patients with fatigue, with at least one Grade 3, three patients with skin/nail changes, one patient with Grade 3 myalgias, 2 patients with neutropenia (1 Grade 3), 4 patients with anemia, 4 patients with neuropathy (1 Grade 3), and 2 patients with cough/COPD exacerbation. Three of the patients with Grade 3 adverse events (n = 3/4; 75%) required dose reduction of docetaxel. An additional patient demonstrated Grade 2 arthritis that was attributed to previous IO exposure, and it is unclear how ram+doc subsequent therapy mediated this toxicity. In total, seven patients (n = 7/35; 20%) required treatment discontinuation during the course of therapy. Further detailed results are available in Table 2.
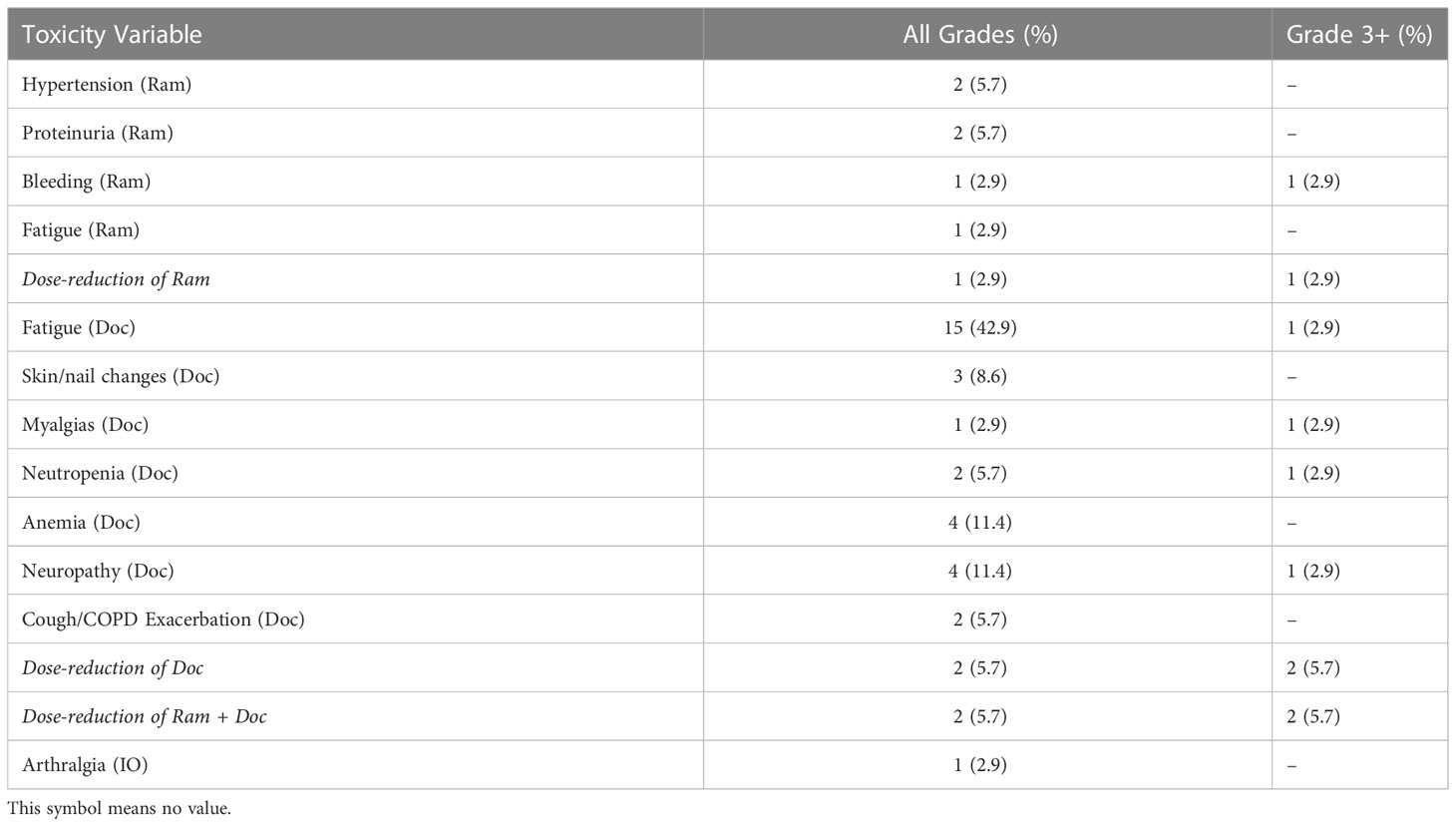
Table 2 Treatment-related adverse events related to ramucirumab and docetaxel among patients in the UM Cohort.
In our retrospective analysis, we found a statistically longer mPFS and mOS for patients with mNSCLC treated with ram+doc after progression on IO at our center compared to the original cohort reported in the REVEL study, which first analyzed this combination chemotherapy and antiangiogenic-therapy regimen in 2014 (Figures 1A, B). Our results did not show a difference in these primary outcomes based on tumor histology.
These findings are striking in that they demonstrate a possible synergy between IO pre-treatment and exposure to second-line (or beyond) antiangiogenic-therapy, namely, ramucirumab, in combination with docetaxel. These findings have been echoed in other retrospective cohorts in East Asia. For example, a retrospective review of 99 patients in multiple Japanese centers found a statistically significant mPFS response of 5.9 months in those pre-treated with IO compared to those who did not have IO exposure (2.6 months) (11). These findings were further bolstered in a post-hoc sub-group analysis of the original REVEL study in which East Asian patients demonstrated a mPFS of 4.88 months and mOS of 15.4 months (12).
Furthermore, our cohort did not demonstrate unexpected and/or significant adverse events, thereby supporting the safety of ram+doc as combination therapy. This observation also mirrors outcomes in other retrospective cohorts, such as a review of 77 patients in Germany that did not demonstrate unexpected toxicities, i.e., no more than 9.09% febrile neutropenia, fatigue, mucositis, stomatitis, or ileus (13). Similarly, another retrospective analysis in Japan estimated up to 16.7% total (and 11.1% Grade 3 or more) pneumonitis, which is more frequent than the 2.9% of pneumonitis as well as COPD exacerbation observed in our cohort (14).
Our findings also echo activity among a similar combination of docetaxel with nintedanib, a tyrosine kinase inhibitor that has activity against multiple kinases including VEGF. This combination was originally approved based on the LUME-Lung1 study which demonstrated improved PFS and OS compared to docetaxel alone, particularly in adenocarcinoma histology, compared to docetaxel alone in the second-line setting (15). Real-world outcomes mirror those reported at our institution after treatment with chemo- and immunotherapy. Specifically, the VARGADO cohort demonstrated a mPFS of 6.4 months, with a 1-year OS rate of 52% in the third-line setting (16). Furthermore, another German cohort reported a mOS of 8.4 months in adenocarcinoma histology specifically (17).
Two strengths of our study are its location and demographics. Specifically, we report herein the first such retrospective analysis consisting of North American patients, with a majority of patients whom identified ethnically as Hispanic/LatinX. Additionally, our cohort’s outcomes rank among the longest PFS and OS benefits recorded with post-IO ram+doc exposure to date. This result will require additional study with similar ethnic and geographic cohorts.
Our analysis has several limitations. First, this is a single-center, retrospective analysis, and as such these observations should be confirmed in a prospective fashion. The Phase II Lung-Map S1800A study evaluated ramucirumab with pembrolizumab combination therapy compared to standard of care chemotherapy, of which two-thirds of the control arm received ram+doc, and was found to demonstrate an OS benefit (17). Post-hoc analyses will be required to understand the true PFS and OS estimates seen in this sub-group, however. Additionally, the TREAT-LUNG observational study reported preliminary data for second- and third-line docetaxel vs. ram+doc in patients previously treated with both platinum-based chemotherapy and IO with a subset of patients demonstrating long-term responses (i.e., plateaus in Kaplan-Meier plots) (18). These findings merit closer attention once presented formally in the literature. The greatest limitation of our study is its small size. For example, a larger Japanese cohort of 1,439 patients utilized a propensity score analysis and did not find a PFS or OS advantage with this treatment strategy (19).
Overall, our institution’s experience with this combination chemo- and antiangiogenic-therapy strategy adds to the data related to ram+doc after IO exposure in mNSCLC. Interpretation should be limited given its retrospective timeframe and single-center patient population.
Ramucirumab RRID: AB_2911024.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Miami IRB Eprost 20170427. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
First authorship: SK. Equal contribution: KG, KK, DK, GL, ER. Senior/last authorship: RD. All authors contributed to the article and approved the submitted version.
We would like to acknowledge our patients; their families and caretakers; as well as our study collaborators for participating in the care necessary to perform this retrospective analysis. This study was presented in part at the virtual International Association for the Study of Lung Cancer 2020 World Conference on Lung Cancer.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. doi: 10.1016/S0025-6196(11)60735-0
2. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–Small-Cell lung cancer. New Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
3. Pérol M, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Quality of life results from the phase 3 REVEL randomized clinical trial of ramucirumab-plus-docetaxel versus placebo-plus-docetaxel in advanced/metastatic non-small cell lung cancer patients with progression after platinum-based chemotherapy. Lung Cancer (2016) 93:95–103. doi: 10.1016/j.lungcan.2016.01.007
4. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet (2014) 384(9944):665–73. doi: 10.1016/S0140-6736(14)60845-X
5. Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. the TAX 320 non-small cell lung cancer study group. J Clin Oncol (2000) 18(12):2354–62. doi: 10.1200/JCO.2000.18.12.2354
6. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Eng J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
7. Petrylak DP, de Wit R, Chi KN, Drakaki A, Sternberg CN, Nishiyama H, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol (2020) 21(1):105–20. doi: 10.1016/S1470-2045(19)30668-0
8. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol (2014) 15(11):1224–35. doi: 10.1016/S1470-2045(14)70420-6
9. Kawachi H, Tamiya M, Matsumoto K, Tamiya A, Yanase T, Tanizaki S, et al. Efficacy and safety of ramucirumab and docetaxel in previously treated patients with squamous cell lung cancer: a multicenter retrospective cohort study. Invest New Drugs (2022) 40(3):634–42. doi: 10.1007/s10637-022-01214-w
10. Kwon D, Reis IM. Simulation-based estimation of mean and standard deviation for meta-analysis via approximate Bayesian computation (ABC). BMC Med Res Methodol (2015) 12(15):61. doi: 10.1186/s12874-015-0055-5
11. Tozuka T, Kitazono S, Sakamoto H, Yoshida H, Amino Y, Uematsu S, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer (2020) 144:71–5. doi: 10.1016/j.lungcan.2020.04.021
12. Park K, Kim JH, Cho EK, Kang JH, Shih JY, Zimmermann AH, et al. East Asian Subgroup analysis of a randomized, double-blind, phase 3 study of docetaxel and placebo in the treatment of stage IV non-small cell lung cancer following disease progression after one prior platinum-based therapy (REVEL). Cancer Res Treat (2016) 48(4):1177–86. doi: 10.4143/crt.2015.401
13. Brueckl WM, Reck M, Rittmeyer A, Kollmeier J, Wesseler C, Wiest GH, et al. Efficacy of docetaxel plus ramucirumab as palliative second-line therapy following first-line chemotherapy plus immune-checkpoint-inhibitor combination treatment in patients with non-small cell lung cancer (NSCLC) UICC stage IV. Transl Lung Cancer Res (2021) 10(7):3093–105. doi: 10.21037/tlcr-21-197
14. Harada D, Takata K, Mori S, Kozuki T, Takechi Y, Moriki S, et al. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res (2019) 39(9):4987–93. doi: 10.21873/anticanres.13688
15. Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung1): a phase 3, double-blind, randomised control trial. Lancet Oncol (2014) 15(2):143–55. doi: 10.1016/S1470-2045(13)70586-2
16. Grohé C, Blau W, Gleiber W, Haas S, Hammerschmidt S, Krüger S, et al. Real-world efficacy of nintedanib plus docetaxel after progression on immune checkpoint inhibitors: results from the ongoing, non-interventional VARGADO study. Clin Oncol (R Coll Radiol) (2022) 34(7):459–68. doi: 10.1016/j.clon.2021.12.010
17. Metzenmacher M, Rizzo F, Kambartel K, Panse J, Schaufler D, Scheffler M, et al. Real-world efficacy of docetaxel plus nintedanib after chemo-immunotherapy failure in advanced pulmonary adenocarcinoma. Future Oncol (2021) 17(30):3965–76. doi: 10.2217/fon-2021-0424
18. Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-Lung-MAP S1800A. J Clin Oncol (2022) 40(21):2295–306. doi: 10.1200/JCO.22.00912
19. Pennell N, Clarke J, Liu SV, Gutierrez M, Batus M, Bauman JR, et al. CO145 ramucirumab+ docetaxel post immune checkpoint inhibitors (ICIS) and platinum-based chemotherapy (CHEMO) in advanced or metastatic non-small cell lung cancer (ANSCLC): Learning from the TREAT-LUNG observational study. Poster presented at International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 27th Annual Meeting; Washington, DC; May 15-18, 2022. (2022). Available at: https://statinmed.com/presentations/checkpoint-inhibitors-platinum-based-chemotherapy-nsclc-treat-lung-observational-study/.
Keywords: ramucirumab, docetaxel, platinum-based treatment resistance, metastatic NSCLC, REVEL
Citation: Kareff SA, Gawri K, Khan K, Kwon D, Rodriguez E, Lopes GdL and Dawar R (2023) Efficacy and outcomes of ramucirumab and docetaxel in patients with metastatic non-small cell lung cancer after disease progression on immune checkpoint inhibitor therapy: Results of a monocentric, retrospective analysis. Front. Oncol. 13:1012783. doi: 10.3389/fonc.2023.1012783
Received: 05 August 2022; Accepted: 08 February 2023;
Published: 21 March 2023.
Edited by:
Michele Simbolo, University of Verona, ItalyReviewed by:
Lorenzo Belluomini, University of Verona, ItalyCopyright © 2023 Kareff, Gawri, Khan, Kwon, Rodriguez, Lopes and Dawar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richa Dawar, cmljaGEuZGF3YXJAbWVkLm1pYW1pLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.