
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 March 2023
Sec. Molecular and Cellular Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1002792
This article is part of the Research Topic Women in Molecular and Cellular Oncology Vol III: 2023 View all 8 articles
 Richa Malhotra1†
Richa Malhotra1† Vyomesh Javle1†
Vyomesh Javle1† Nishtha Tanwar1
Nishtha Tanwar1 Pooja Gowda1
Pooja Gowda1 Linu Varghese1
Linu Varghese1 Anju K1
Anju K1 Nandhitha Madhusudhan1
Nandhitha Madhusudhan1 Nupur Jaiswal1
Nupur Jaiswal1 Bhargavi K. S.1
Bhargavi K. S.1 Manjima Chatterjee1
Manjima Chatterjee1 Kumar Prabhash2
Kumar Prabhash2 Peddagangannagari Sreekanthreddy1
Peddagangannagari Sreekanthreddy1 Kshitij D. Rishi1
Kshitij D. Rishi1 Hitesh M. Goswami1
Hitesh M. Goswami1 Vidya H. Veldore1*‡
Vidya H. Veldore1*‡Introduction: The concept of personalized medicine in cancer has emerged rapidly with the advancement of genome sequencing and the identification of clinically relevant variants that contribute to disease prognosis and facilitates targeted therapy options. In this study, we propose to validate a whole exome-based tumor molecular profiling for DNA and RNA from formalin-fixed paraffin-embedded (FFPE) tumor tissue.
Methods: The study included 166 patients across 17 different cancer types. The scope of this study includes the identification of single-nucleotide variants (SNVs), insertions/deletions (INDELS), copy number alterations (CNAs), gene fusions, tumor mutational burden (TMB), and microsatellite instability (MSI). The assay yielded a mean read depth of 200×, with >80% of on-target reads and a mean uniformity of >90%. Clinical maturation of whole exome sequencing (WES) (DNA and RNA)- based assay was achieved by analytical and clinical validations for all the types of genomic alterations in multiple cancers. We here demonstrate a limit of detection (LOD) of 5% for SNVs and 10% for INDELS with 97.5% specificity, 100% sensitivity, and 100% reproducibility.
Results: The results were >98% concordant with other orthogonal techniques and appeared to be more robust and comprehensive in detecting all the clinically relevant alterations. Our study demonstrates the clinical utility of the exome-based approach of comprehensive genomic profiling (CGP) for cancer patients at diagnosis and disease progression.
Discussion: The assay provides a consolidated picture of tumor heterogeneity and prognostic and predictive biomarkers, thus helping in precision oncology practice. The primary intended use of WES (DNA+RNA) assay would be for patients with rare cancers as well as for patients with unknown primary tumors, and this category constitutes nearly 20–30% of all cancers. The WES approach may also help us understand the clonal evolution during disease progression to precisely plan the treatment in advanced stage disease.
Rapid advancements in sequencing technology and its easy access have brought down the cost of genome sequencing significantly in the last two decades, and hence, genomic or personalized medicine has become an integral part of cancer management.
A precise, comprehensive diagnostic workup in cancer requires advanced high-throughput technologies beyond microscopy, immunohistochemistry, and single gene tests to understand the disease complexity, particularly when the world is witnessing a rapid increase in the onset of cancers with increased mortality (GLOBOCAN 2020) (1). In recent clinical practice, next-generation sequencing (NGS) has emerged as a powerful approach that provides a holistic picture of the disease. Targeted NGS panels identify mutations in a limited number of genes and could miss a few rare yet important driver mutations, which are associated with the mechanism of resistance or aggressive disease biology (2). In contrast, whole exome sequencing (WES) has gained much more importance in large-scale population-based clinical research studies, thus remains an advanced solution to detect cancer predisposition, mutations associated with disease onset, and progression that facilitates access to the standard of care as well as novel targeted therapies (3, 4). Mutational signatures, tumor mutational burden (TMB), and microsatellite instability (MSI) are best uncovered using the whole exome-based NGS panels, as it provides the advantage of a complete footprint of the coding region (Figure 1) (5, 6). However, it is less clear that WES is advantageous over a targeted panel for MSI detection. MSI being a marker with different loci mutated in different cancers, broader gene panels are important. Based on the evidence from different clinical studies, it is very clear that NGS-based MSI detection requires cross-validation with standard clinical practices such as MMR-IHC and MSI-PCR demonstrating 100% accuracy (7–14). Taken together, the WES panel for tumor DNA and RNA helps to facilitate tailor-made treatment decisions in clinical practice in the most comprehensive manner.
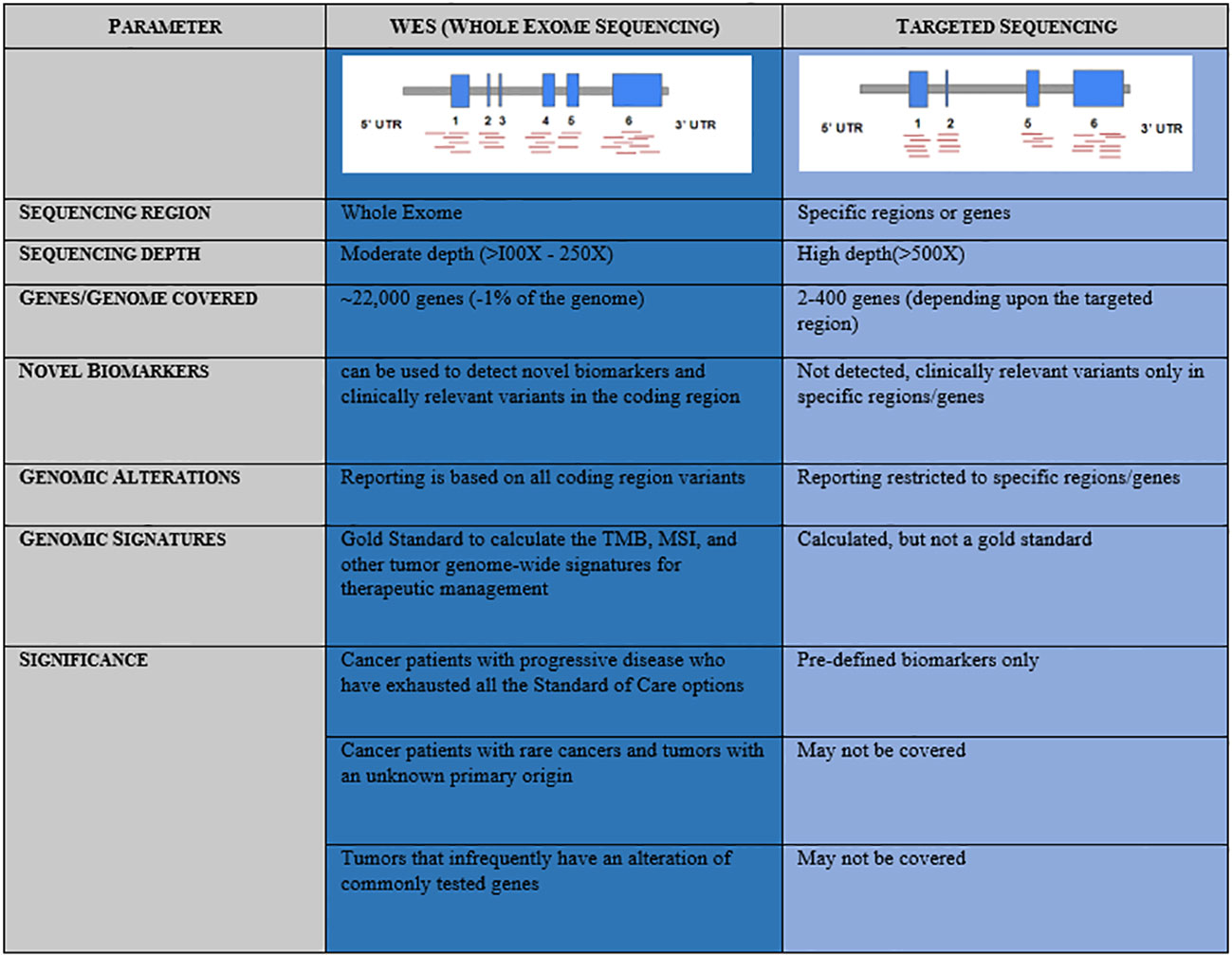
Figure 1 Comparative representation and advantage of Whole Exome Sequencing (WES) over Targeted Sequencing panels.
We present here a clinical validation of a whole exome (DNA and RNA) comprehensive NGS panel for tumor specimens [formalin-fixed paraffin-embedded (FFPE)] and its application in clinics with a reasonable turnaround time of 21 working days supporting physicians and cancer patients. The scope of the testing includes identifying single-nucleotide variants (SNVs), insertions/deletions (INDELS), copy number alterations (CNAs), gene fusions, TMB, and MSI. We believe that such a comprehensive approach could simplify the diagnostic algorithm, replacing the gene-specific or panel-based testing, and helps in better stratification of patients and their treatment management. The major application of this approach at the time of diagnosis may include tumors with rare histology as well as tumors where the primary origin could not be deciphered.
The present validation cohort constitutes a total of 166 samples across 17 cancer types, which includes: 1) a comprehensive set of representative clinical samples (N = 129) and 2) pre-characterized cancer cell lines, reference standards from Horizon Discovery (traceable from the National Institute of Standards and Technology), and proficiency testing material (N = 37) (Figure 2). The study was conducted according to the principles of the Declaration of Helsinki and as per the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice (GCP) guidelines (15, 16). Written informed consent was obtained from the enrolled patients for the use of de-identified data for research publications. The study was approved by an independent ethics committee and review board (Jehangir Clinical Development Center JCDC, India).
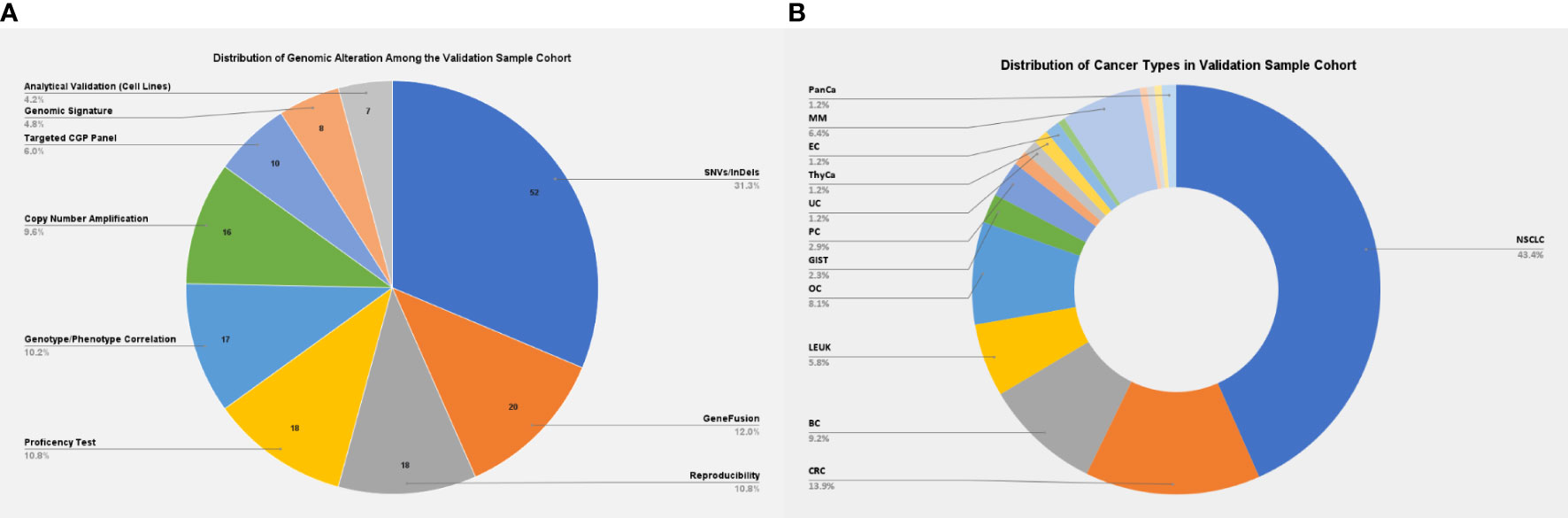
Figure 2 (A) Distribution of genomic alterations among the validation sample cohort. (B) Distribution of cancer types among the validation sample cohort. NSCLC, non-small cell lung carcinoma; CRC, colorectal cancer; BC, breast cancer; LEUK, leukemia; OC, ovarian cancer; GIST, gastrointestinal stromal tumors; PC, prostatic cancer; EC, endometrial cancer; MM, multiple myeloma; PanCa, pancreatic cancer.
Three different sample types were used, that includes FFPE DNA, Genomic DNA derived from blood and FFPE RNA. For the analytical validation, FFPE DNA were sourced from Horizon Discovery. The external quality assurance was performed using DNA obtained as part of proficiency testing.
The germline DNA was isolated from peripheral blood using QIAamp DNA Blood Kit (Cat. No. 51104). 200 ng of genomic DNA was used for Whole Exome library preparation using the standard procedures as per the manufacturer's instructions using the Agilent DNA Prep with Enrichment kit (Cat. No. 5191-6874).
The FFPE blocks with minimum tumor surface area ≥ 5 mm2 and tumor content ≥ 10% (i.e., roughly 150 viable tumor cells per high- power field (HPF) on microscopy as per histological examination) were processed for tumor genomic DNA and RNA extraction by using All Prep FFPE DNA/RNA kit Cat. No. 80234 (17) (Qiagen, Valencia, CA, USA). Quality control (QC)-qualified DNA/RNA samples were processed for library preparation, which includes fragmentation, adapter addition, amplification, and capturing of exonic regions of genomic DNA through overnight hybridization of exon-specific probes. Agilent DNA/RNA Prep with Enrichment kit (Cat. No. 5191-6874) (18) was used to prepare the DNA-exome and RNA-Seq (RNA-exome) libraries. The prepared libraries underwent QC analysis for the detection of library fragment size and concentration. A qualified NGS library had at least 10-nM concentration with a single distinct peak of approximately 300 bp (Figure S1). The qualified NGS libraries were subjected to paired-end (2 × 150 read length configuration) sequencing on the NextSeq™ Systems (Illumina Inc., San Diego, CA, USA) at a median coverage of 200X (Table S1).
The quality of the DNA and the NGS libraries are verified by Qubit and Bio-analyzer. Figure S1 provides the information that the QCs are measured using the Bio-analyzer rather than the standard spectrophotometric methods.
Raw sequencing reads from high-throughput sequencers were obtained in fastq formats. The reads were further analyzed using a customized bioinformatics pipeline (Figure 3) to detect genomic alterations (SNVs, INDELS, CNAs, and gene fusions). Illumina DRAGEN Somatic Pipeline (Illumina DRAGEN Bio-ITTM Platform v3.6) and Illumina DRAGEN RNA Pipeline (Illumina DRAGEN Bio-ITTM Platform v3.6) were used to analyze exome data for DNA and RNA, respectively. Figures S2A, B describe the workflow. High-quality short-read sequences were aligned to human reference sequence version hg19/GRCh37 using DRAGEN-aligner followed by flagging off the duplicate sequencing reads. The SNVs, INDELS, and CNAs (>6) were detected using DNA exome data, while gene fusions were detected using RNA exome data.
Variants were annotated using an in-house developed pipeline with modules of population databases (1000 Genomes and ExAC), in silico gene prioritization tools (CADD, SIFT, PolyPhen-2, and Mutation taster), and clinical databases (ClinVar, OMIM, and dbSNP) along with manually curated database from the National Comprehensive Cancer Network (NCCN), the US Food and Drug Administration (FDA), CIVIC, Precision Cancer Therapy-MD Anderson, OncoKB, Pharmacogenomics Knowledgebase (PharmGKB), and Clinical Trials and directed literature searches (19–36).
TMB is emerging as one of the potential biomarkers that help in predicting the response to Immune Checkpoint Inhibitor (ICI) therapy response. WES is considered to be the gold standard approach to arrive at the Tumor mutational burden burden in any cancer, and the same has been recommended by the Food and Drug Administration (FDA) in the approval of KEYTRUDA (pembrolizumab). A sequential three-level filtering approach was adapted from Parikh et al. (2020) for the removal of germline variants and retention of possible somatic mutation. In level 1 filtering (tolerant approach), global-population frequency and South-Asian frequency were used to remove the polymorphic variants (> 1% of the population) (5, 37) from the cohort. In level 2 filtering (stringent approach), the variant allele frequency was used to remove the germline heterozygous variants. In level 3 filtering (baseline approach), variants were filtered based on the in-house baseline (germline samples). The baseline (reference genome pattern) was created by pooling together the blood samples of healthy individuals. With the use of this algorithm, following a robust machine learning approach, the TMB pipeline was throughly verified with tumor-normal and tumor-only samples to derive the whole exome TMB pattern (38).
The microsatellite status was determined using a machine learning (ML)-based tool MSI-sensor2 (34). MSI occurs when mismatch repair (MMR) proteins are mutated, resulting in the accumulation of errors. The MSI status (based on the number of repeats) can be determined by polymerase chain reaction (PCR) on MMR immunohistochemistry (IHC) or by using the NGS technique. The presence of MSI-H in any tumor indicates a defect in the DNA repair mechanism. Based on our internal validation, the cutoff for high-TMB was considered 20 mutation/Mb, while patients with ≥15% MSI score were considered MSI-high (unpublished 4baseCare data).
Clinically relevant variants were prioritized and correlated to identify the markers associated with therapeutic response, prognosis, or supporting diagnostic evidence for any given patient’s tumor. These variants were interpreted and summarized in the report as per the American College of Medical Genetics and Genomics/American Society of Clinical Oncology/Association for Molecular Pathology/College of American Pathologists/European Society for Medical Oncology (ACMG/ASCO/AMP/CAP/ESMO) and NCCN guidelines (39, 40).
To estimate the analytical accuracy, the limit of detection, and sensitivity for small variant calling (SNVs/INDELS), we used the National Institute of Standards and Technology (NIST) traceable reference standard from Horizon Discovery HD832 and cancer cell lines (obtained from the National Centre for Cell Sciences (NCCS), Pune). The variant and the variant allele frequencies (VAFs) detected were compared to the known VAFs given by the manufacturer/reference standard. Table S2 summarizes results from Horizon reference standard HD832 demonstrating 100% concordance. Although genomic alterations below 5% were detected through the standard somatic pipeline from DRAGEN, at 1.9%, 2.8%, and 0.9%, the support of the mutant reads was less than 10. There was 98.7% concordance from the cell line data (Table S3). Similarly, gene fusion also showed 100% concordance using the Horizon reference standard (targeted FFPE RNA fusion reference standard; HD784-ST7) and 10 publicly available data from the Sequence Read Archive (SRA) database, which showed 100% concordance.
In our validation, when we generated libraries for HD832 using 200 ng as input DNA, we noticed that at 100X, we were unable to pick up any mutant rates that could confidently call for an EGFR exon 19 deletion. However, as we increased the depth of sequencing to 200X, we were able to pick up mutant reads correlating to exon 19 deletion as listed by Horizon Discovery. This enabled us to decide the minimum depth of coverage required to detect the INDELS in any given sample. We noticed that as we go lower in terms of the percentage of INDELS below 10%, allele frequency correlation or the mutant allelic burden correlation with high concordance was not achievable with adequate mutant read support, and hence, we had to limit our sensitivity/limit of detection (LOD) to 10% for INDELS. This could be due to the intrinsic limitation of the technology or the intrinsic nature of the reference/clinical sample or any other factor that could not be measurable or quantifiable. Hence, we have created a threshold for calling a variant a true variant provided that the variant has at least 10 good-quality mutant reads supporting the alternate read depth. The same threshold has been used throughout the analysis for all the patient data, reference material, and proficiency testing samples for any exonic deletions.
In our validation, the scope for detection of deletions is observed to be <20 bps. The sensitivity of detection of deletions from FFPE DNA depends to a large extent on the quality of the input DNA provided; if the input DNA is heavily fragmented, then the PCR amplifiability is affected and hence the library preparation.
The gene fusions are prioritized based on the confidence score provided by the DRAGEN workflow. Those gene fusions, which are true, have their scores listed with high confidence and minimum read support as five. Subsequently, as part of our in-house pipeline, the fusion reads are clinically annotated using public domain fusion databases such as FusionGDB and ChimeraDB. The well-studied functional fusion with diagnostic/prognostic/predictive biomarkers is further considered for clinical reporting. Those gene fusions, where the open reading frame (ORF) is lost, are not considered for further analysis.
Table S3 demonstrates analytical accuracy for MSI and TMB calculation using an NGS- based approach on whole exome data. Six cancer cell lines (C33A, DU145, HCT-15, HCT-116, Jurkat6, and MOLT-4) with MSI-high status were found to have >22% MSI score (≥15% MSI score is considered as MSI-High), which corroborates with literature evidence (41–46). In addition to this, cell lines with high-MSI scores were also found to have TMB scores of >18 mutations/Mb. The T-47D breast cancer cell line was found to have a 7.58% MSI score (MSI-low or microsatellite stable (MSS)) and a TMB score of 3 mutation/Mb (TMB-low), which aligns with the literature evidence (46). Although the number of cancer cell lines used in this validation, with known MMR/MSI status, was less/limited, we have attempted to cover a wide range of primary tumor sources.
The clinical validation of the WES (DNA and RNA)- based assay was established by comparing the result of the 106 assays with the corresponding conventional orthogonal methods [IHC, real-time PCR, Sanger sequencing, and fluorescence in situ hybridization (FISH)] and targeted NGS panels. This study of 62 SNVs/INDELS (biomarkers: EGFR, KRAS, BRAF, KIT, PDGFRA, MLH1, MSH2, MSH6, PMS2, ATM, BRCA1, BRCA2, FANCA, CHEK1, ATR, PALB2, PBRM1, POLE, POLD1, PTEN, B2M, JAK1, JAK2, MDM2, MDM4, and DNMT3A); 16 CNAs (biomarkers: ERBB2, MET, CDK4, MDM2, BCL2L1, and MCL1 amplification) and 10 gene fusions (ALK, ROS, RET, and NTRK fusions) depicted 100% concordance when compared to conventional testing and targeted NGS panels (Table 1 and Tables S4-S6).
We compared MSI calculation from eight clinical samples (using an in-house approach on exome data) with results from conventional IHC tests on four genes, namely, MLH1, MSH2, MSH6, and PMS2, which are used to detect the status of MMR in these patients. Our results showed 100% concordance with the results of IHC (Table S7). Moreover, our NGS assay focuses on loci of multiple microsatellite regions throughout the genome of the tumor cells, rather than selected short tandem repeats. The landscape of the mutational signatures depicted that TMB and MSI showed similar trends as detected by NGS in cancer patients’ cohort [unpublished 4baseCare data from 200 patients; the percentage of patients with high TMB was approximately 14% and that of high MSI was approximately 3% (Figure 4D)]. Identification of mutational signatures in sporadic cancer patients was useful in establishing prognosis and was predictive of response to certain chemotherapeutic regimens as well as immunotherapy.
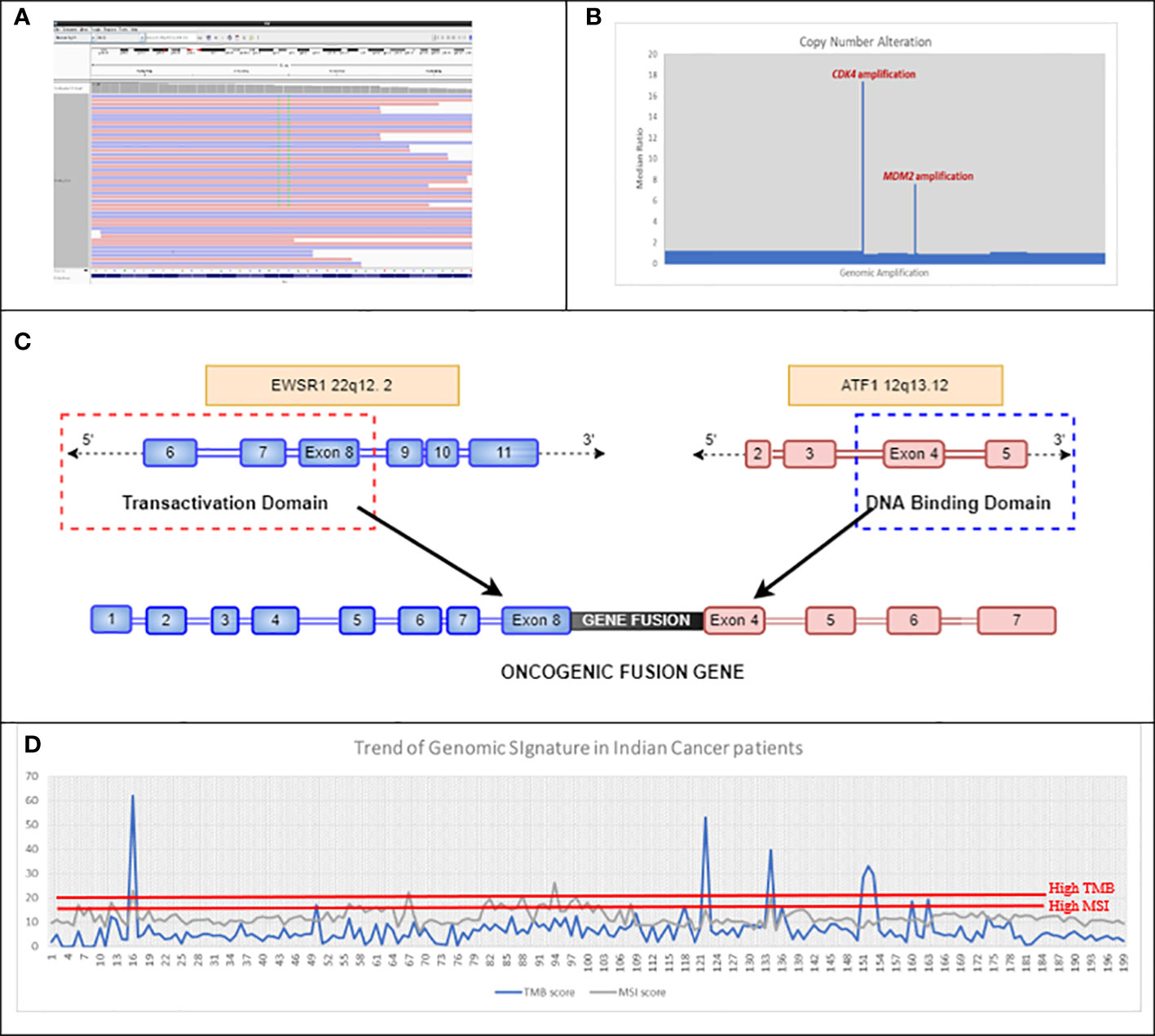
Figure 4 (A) Double mutation detected in exon 12 of KRAS, which led to an MNV change of KRAS G12F in Case 4BC-19. (B) Representative image of CNA detected using in-house bioinformatics pipeline, which showed amplification of CDK4 and MDM2 genes in Case number 4BC-63. (C) Schematic of the EWSR1::ATF1 fusion gene detected in case number 4BC-146. The EWSR1::ATF1 rearrangement is caused by the fusion of the N-terminal transactivation domain of EWSR1 gene with the C-terminal DNA binding domain of ATF1 gene. (D) Trend of mutational signatures (TMB and MSI) in 200 Indian cancer patients (unpublished 4baseCare data). The cutoff for MSI-H is >15 and that for TMB-H is >20. MNV, Muti-Nucleotide Variant; CNA, Copy Number Alteration; TMB, Tumor Mutational Burden; MSI, Microsatellite Instability.
Reproducibility was measured by sequencing Horizon libraries along with four independent clinical libraries. In line with requirements for clinical validation, we here demonstrated the reproducibility using horizon reference standard and along with four independent clinical samples to detect the SNVs (5% LOD), INDELS (10% LOD), and gene fusions. The experiments included 20 SNVs/INDELS and five gene fusions, which were reproduced in all the libraries that depict 100% reproducibility of the assay (Table S8).
As part of external quality assurance, 18 samples (Table S11) from proficiency testing programs for different cancer types (breast, lung, pancreatic, prostate, and ovarian) demonstrated 100% concordance using an in-house pipeline (unpublished 4baseCare data). In addition, inter-laboratory comparison with other NGS panels for 10 clinical samples showed 100% concordance in results (Tables S4, S7).
Four families (10 individuals) with a family history of hereditary cancer syndromes had undergone WES DNA blood based assay along with Sanger sequencing (4BC-113 to 4BC-122). Both the test results revealed the presence of pathogenic BRCA1 frameshift mutation in two families and a pathogenic missense variant in RAD50 gene in the third family, which is associated with hereditary breast and ovarian cancer (HBOC) syndrome. The fourth family had STK11 pathogenic mutation, which is associated with the Peutz–Jeghers syndrome (Table S10 and Figure S3). In addition to this genotype–phenotype correlation in hereditary cancer syndrome, we also identified pathogenic mutations (SNVs/INDELS and gene fusions) in seven patients with sporadic cancers that correlated with the clinical presentation (Table S9). In sporadic cancers, this extensive approach of CGP facilitates the discovery of rare mechanisms of resistance in different cancers such as hormone-positive breast cancer, non-small cell lung cancer, and castration-resistant prostate cancer in disease progression, that could help in prognostication as well as changing the treatment modality. We also identified some of the rare driver mutations in non-small cell lung cancer that could explain how genomics could help in predicting the treatment efficacy/inefficacy.
The advantages of the WES approach for tumor DNA and RNA, over targeted NGS panel or RT-PCR, includes the identification of the broad spectrum of mutations (both driver and passenger) that helps in understanding tumor complexity and hence provides a comprehensive picture of the disease biology. As an outcome of this validation, we could detect the classical actionable driver mutations [EGFR exon 19 deletions in non-small cell lung cancer (NSCLC) patients (Figure S4)] and complex mutations and gene fusions. A few interesting case examples are described below from the validation cohort.
NGS as a technology stands out as more accurate for clinical management, as it can detect rare/complex muti-nucleotide variants (MNVs) that could be missed by conventional RT-PCR/genotyping techniques (predetermined assays). KRAS codon 12 (G12C) is one of the recently approved biomarkers for targeted therapy in lung cancer. The approval for sotorasib was based on CodeBreaK 100, a multicenter, single-arm, open-label clinical trial (NCT03600883) that included patients with locally advanced or metastatic NSCLC with KRAS G12C mutations. The overall response rate (ORR) observed in patients with KRAS G12C-mutated NSCLC treated with sotorasib (n = 124) was 36% [95% confidence interval (CI), 28–45]. In our study (case 4BC-19), real-time PCR of a rectal adenocarcinoma patient showed the expression of two different clonal mutations (G12V and G12C) at the same codon position (codon 12) of KRAS gene (Figure 4A). However, NGS revealed the presence of two neighboring nucleotides from the same codon 12 being mutated, thus depicting a complex event, resulting in an amino acid change from glycine to phenylalanine (G12F). This identification of complex nucleotide substitution and subsequent change in an amino acid is crucial in order to make treatment-related decisions for targeted therapy options.
Gene fusions in FGFR2, FGFR3, NTRK1, NTRK2, NTRK3, ROS1, ALK, and RET are frequently observed in NSCLC. However, case 4BC-146 revealed the presence of rare fusions of EWSR1::ATF1 in NSCLC patient (Figure 4C). In this case, the outcome from the NGS is more toward understanding the molecular drivers associated with disease onset and its phenotype, since EWSR1 fusions do not have targeted therapy drugs approved (47). These data elucidate the importance of RNA exome sequencing to identify rare/novel driver mutations associated with the disease (59). Such an exercise could also provide leads for novel targeted therapy as well as chemotherapy options.
A comprehensive NGS approach can also help in the identification of biomarkers associated with disease progression as depicted in case 4BC-63 with liposarcoma that revealed CDK4 and MDM2 amplification (Figure 4B). This corroborates the study by Ricciotti et al., where the authors showed that high levels of MDM2 and CDK4 amplification were associated with decreased disease-free survival (DFS) (p = 0.0168 and p = 0.0169, respectively) and disease-specific survival (DSS) (p = 0.0082 and p = 0.0140, respectively) (60).
In some patients whose tumor is defined by complex histologies, and or with rare tumor types, where there are no standard of care guidelines for clinical management, tailor-made targeted therapy based on the findings from comprehensive genomic profiling of the patient’s tumor is being explored as options in a single subject clinical trial setting (48). In addition to genomic alteration status, WES remains a gold standard for the derivation of mutational signatures, particularly, TMB and MSI, which are known immunotherapy biomarkers. The correlation between TMB and MSI is highly debatable. The majority of MSI-high (MSI-H) tumors happened to be TMB-high (TMB-H); however, not all TMB-H tumors are essentially MSI-H (52). In this study, we observed that MSI-H cell lines were found to have TMB scores on the higher side.
We have used DRAGEN Bio-IT™ platform, which is faster than the GATK with the BWA-MEM2 pipeline earlier established by Broad Institute. DRAGEN uses a type of hardware called field-programmable gate arrays (FPGAs) to deliver phenomenal speed-ups to their GATK-based short (SNV/INDELS) variant discovery pipeline (53). The time taken for exome analysis is less than 30 min on DRAGEN as compared to a few hours on the GATK (BWA-MEM2) workflow (61); also, DRAGEN has an advantage of improved sensitivity and specificity over the latter.
As part of the validation beyond the conversion of FASTQ to VCF using DRAGEN, we developed a pipeline for variant annotation, MSI, and TMB calculation along with curation for gene fusion. Most of the algorithms and database source here were publicly available. However, as per the CAP-AMP recommendation, any laboratory-developed test, or RUO NGS panel require analytical and clinical validation before it gets implemented for clinical use. Our validation study demonstrated here is one such effort.
In brief, WES (DNA and RNA)- based assay is a comprehensive NGS panel that targets approximately 22,000 unique genes to detect tumor genomic alterations. The assay is well validated with 97.5% specificity, 100% sensitivity, 98.2% accuracy, and 100% reproducibility to detect low-frequency somatic variants (Table S12). The customized in-house bioinformatics pipeline and data curation along with Illumina DRAGEN workflow for detection, annotation, and classification of genomic alterations provide a complete end-to-end workflow. The clinical report summarizes the genomic alterations as per the standard recommendations.
Together, this study summarizes a robust NGS testing that could provide the most comprehensive genomic information from the tumor DNA and RNA, describing the potential application of CGP in the clinic and correlating the clinical phenotype with the molecular findings for better disease stratification and treatment decisions for individual cancer patients.
A major limitation of our validation process is that there is no globally available guideline for WGS/WES in the clinic. The infrastructure access, together with trained resources (with technical and scientific capabilities) for performing such a complex genome sequencing assay, is a limitation in many community hospitals or tertiary care settings. To bridge the gap and to bring this service available to each cancer patient, we would require a strong collaboration between the industry, academia, and the government to support such an effort. Also, the major interfering substances that would impact the assay performance includes: a) cold ischemic time b) tissue fixation time, c) buffer used for tissue fixation neutral buffered formalin (NBF) and its pH, d) quality of wax used, e) presence of necrosis, and f) decalcification due to the presence of bony component.
Although the cost of NGS for whole exome was a bottleneck in extending the application of this tool in the clinical setting for patients of all economic strata, major technology leaders such as Illumina have constantly been striving to bring down the cost of genome sequencing to support cancer care and management. Owing to their developments, WES has become an economically viable option in the last two years with the cost being less than $500/exome. Beyond the ability to decipher the complex tumor heterogeneity that is inherent in many cancers, the adaptation of exome sequencing in the routine clinics could help the clinical community in better management of rare cancers at presentation as well as when the patient reaches a roadblock after multiple lines of therapy as per the standard of care.
The datasets presented in this study can be found in online repositories. The name of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Informed consent was obtained from the enrolled patients for the use of de-identified data for research publications. The study was conducted according to the principles of the Declaration of Helsinki and as per the ICH and GCP guidelines. The study was approved by an independent ethics committee and review board (JCDC, India). The patients/participants provided their written informed consent to participate in this study.
Conception and design: VV. Provision of study material or patients: in-house walk-in patients at 4baseCare, HG and KR. Collection and assembly of data: VJ, NT, LV, AK, PS, NM, and NJ. Data analysis and interpretation: RM, VJ, MC, BS, NT, and VV. Manuscript writing: RM, VJ, MC, NT, KP, and VV. Final approval of manuscript: all authors. Accountable for all aspects of the work: VV and HG.
The authors declare that this study received funding from 4baseCare Onco Solutions Pvt. Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of the article, or the decision to submit it for publication.
The authors acknowledge all the patients for giving their consent to use the de-identified data for scientific publications. The authors acknowledge Archana N for providing the pedigree chart used in the Supplementary Information. The authors thank Dr. Giridharan P for his critical review and valuable comments.
Authors RM, VJ, MC, SP,NT, LV, AK, PS, NM, NJ, KR, HG, VV and BS are the employees of 4baseCare Onco Solutions Pvt. Ltd.
The remining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1002792/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, et al. Guidelines for validation of next-generation sequencing–based oncology panels: A joint consensus recommendation of the association for molecular pathology and college of American pathologists. J Mol Diagn (2017) 19(3):341–65. doi: 10.1016/j.jmoldx.2017.01.011
3. Rusch M, Nakitandwe J, Shurtleff S, Newman S, Zhang Z, Edmonson MN, et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat Commun (2018) 9(1):1–3. doi: 10.1038/s41467-018-06485-7
4. Malone ER, Oliva M, Sabatini PJ, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med (2020) 12(1):1–9. doi: 10.1186/s13073-019-0703-1
5. Cha, lmers ZR, CF C, Fabrizio D, Gay L, SM A, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med (2017) 9(1):1–4. doi: 10.1186/s13073-017-0424-2
6. Wu HX, Wang ZX, Zhao Q, Wang F, Xu RH. Designing gene panels for tumor mutational burden estimation: The need to shift from 'correlation' to 'accuracy'. J Immunother Canc. (2019) 7(1):206. doi: 10.1186/s40425-019-0681-2
7. Johansen AFB, Kassentoft CG, Knudsen M, Laursen MB, Madsen AH, Iversen LH, et al. Validation of computational determination of microsatellite status using whole exome sequencing data from colorectal cancer patients. BMC Cancer (2019) 19:971. doi: 10.1186/s12885-019-6227-7
8. Kang SY, Kim DG, Ahn S, Ha SY, Jang KT, Kim KM. Comparative analysis of microsatellite instability by next-generation sequencing, MSI PCR and MMR immunohistochemistry in 1942 solid cancers. Pathol Res Pract (2022) 233:153874. doi: 10.1016/j.prp.2022.153874
9. Renault V, Tubacher E, How-Kit A. Assessment of microsatellite instability from next-generation sequencing data. Adv Exp Med Biol (2022) 1361:75–100. doi: 10.1007/978-3-030-91836-1_5
10. Ebili HO, Agboola AO, Rakha E. MSI-WES: A simple approach for microsatellite instability testing using whole exome sequencing. Future Oncol (2021) 17(27):3595–606. doi: 10.2217/fon-2021-0132
11. Cortes-Ciriano I, Lee S, Park WY, Kim T-M, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun (2017) 8:15180. doi: 10.1038/ncomms15180
12. Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol (2017) 2017:PO.17.00073. doi: 10.1200/PO.17.00073
13. Dedeurwaerdere F, Claes KB, Van Dorpe J, Rottiers I, Van der Meulen J, Breyne J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep (2021) 11:12880. doi: 10.1038/s41598-021-91974-x
14. Yu F, Makrigiorgos A, Leong KW, Makrigiorgos GM. Sensitive detection of microsatellite instability in tissues and liquid biopsies: Recent developments and updates. Comput Struct Biotechnol J (2021) 19:4931–40. doi: 10.1016/j.csbj.2021.08.037
15. World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053
16. Jokstad A. The approval of clinical research by an independent ethics committee–a compulsory requirement and not a matter of the investigator's choosing. Clin Exp Dent Res (2017) 3(5):163. doi: 10.1002/cre2.92
17. Dedeurwaerdere F, Claes K, Van Dorpe J, Rottiers I, van der Meulen J, Breyne J, et al. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci Rep (2021) 11(1):1–5. doi: 10.1038/s41598-021-91974-x
18. Siggillino A, Ulivi P, Pasini L, Reda MS, Chiadini E, et al. Detection of EGFR mutations in plasma cell-free tumor DNA of TKI-treated advanced-NSCLC patients by three methodologies: Scorpion-ARMS, PNAClamp, and digital PCR. Diagnostics (2020) 10(12):1062. doi: 10.3390/diagnostics10121062
19. Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet (2013) 76(1):7–20. doi: 10.1002/0471142905.hg0720s76
20. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. The 1000 genomes project consortium. A Global Ref. Hum Genet Variat. Nat (2015) 526(7571):68–74.
21. Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, et al. The ExAC browser: Displaying reference data information from over 60 000 exomes. Nucleic Acids Res (2017) 45(D1):D840–5. doi: 10.1093/nar/gkw971
22. González-Pérez A, López-Bigas N. Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, condel. Am J Hum Genet (2011) 88(4):440–9. doi: 10.1016/j.ajhg.2011.03.004
23. Ng PC, Henikoff S. SIFT. predicting amino acid changes that affect protein function. Nucleic Acids Res (2003) 31(13):3812–4. doi: 10.1093/nar/gkg509
24. Islam SA, Huether R, Kudalkar E. (2020). Identification of novel druggable fusions enabled through the use of an automated RNA fusion prioritization pipeline. Cancer Research 80(16_Supplement), 5474–5474.
25. Thorn CF, Klein TE, Altman RB. PharmGKB: The pharmacogenomics knowledge base, in pharmacogenomics. Totowa, NJ: Humana Press (2013) p. 311–20.
26. Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci association with indices of cardivascular disease. J Acta Nat Genet (2009) 41(6):712.
27. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res (2001) 29(1):308–11. doi: 10.1093/nar/29.1.308
28. Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharm Therap (2012) 92(4):414–7. doi: 10.1038/clpt.2012.96
29. Kurnit KC, Bailey AM, Zeng J, Johnson AM, Shufean MA, Brusco L, et al. “Personalized cancer therapy”: A publicly available precision oncology resource. Cancer Res (2017) 77(21):e123–6. doi: 10.1158/0008-5472.CAN-17-0341
30. Sherry ST, Ward M, Sirotkin K. dbSNP–database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res (1999) 9(8):677–9. doi: 10.1101/gr.9.8.677
31. Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res (2018) 46(D1):D1062–7. doi: 10.1093/nar/gkx1153
32. Chakravarty D, Gao J, Phillips S, Kundra R, Zhang H, Wang J, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol (2017) 1:1–6. doi: 10.1200/PO.17.00011
33. Hamosh A, Scott AF, Amberger J, Bocchini C, Valle D, McKusick VA. Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res (2002) 30(1):52–5. doi: 10.1093/nar/30.1.52
34. Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: Microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics (2014) 30(7):1015–6. doi: 10.1093/bioinformatics/btt755
35. Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res (2019) 47(D1):D886–94. doi: 10.1093/nar/gky1016
36. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat Methods (2014) 11(4):361–2. doi: 10.1038/nmeth.2890
37. The 1000 genomes project consortium. an integrated map of genetic variation from 1,092 human genomes. Nature (2012) 491:56–65.
38. Meléndez B, Van Campenhout C, Rorive S, Remmelink M, Salmon I, D’Haene N. Methods of measurement for tumor mutational burden in tumor tissue. Transl Lung Cancer Res (2018) 7(6):661. doi: 10.21037/tlcr.2018.08.02
39. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med (2015) 17(5):405–23. doi: 10.1038/gim.2015.30
40. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the association for molecular pathology, American society of clinical oncology, and college of American pathologists. J Mol Diagn (2017) 19(1):4–23. doi: 10.1016/j.jmoldx.2016.10.002
41. Seneviratne D, Ma J, Tan X, Kwon YK, Muhammad E, Melhem M, et al. Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis. Gastroenterology (2015) 148(1):181–91. doi: 10.1053/j.gastro.2014.09.019
42. Panyutin IG, Panyutin IV, Powell-Castilla I, Felix L, Neumann RD. Single nucleotide variations in cultured cancer cells: Effect of mismatch repair. Mutat Res - Fundam Mol Mech Mutagen (2017) 803:22–5. doi: 10.1016/j.mrfmmm.2017.07.003
43. Gayet J, Zhou XP, Duval A, Rolland S, Hoang JM, Cottu P, et al. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene (2001) 20(36):5025–32. doi: 10.1038/sj.onc.1204611
44. Brimmell M, Mendiola R, Mangion J, Packham G. BAX frameshift mutations in cell lines derived from human haemopoietic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene (1998) 16(14):1803–12. doi: 10.1038/sj.onc.1201704
45. Inoue K, Kohno T, Takakura S, Hayashi Y, Mizoguchi H, Yokota J. Frequent microsatellite instability and BAX mutations in T cell acute lymphoblastic leukemia cell lines. Leuk Res (2000) 24(3):255–62. doi: 10.1016/S0145-2126(99)00182-4
46. Lu Y, Soong TD, Elemento O. A novel approach for characterizing microsatellite instability in cancer cells. PloS One (2013) 8(5):e63056. doi: 10.1371/journal.pone.0063056
47. Möller E, Praz V, Rajendran S, Dong R, Cauderay A, Xing Y-H, et al. EWSR1-ATF1 dependent 3D connectivity regulates oncogenic and differentiation programs in clear cell sarcoma. Nat Commun (2022) 13:2267. doi: 10.1038/s41467-022-29910-4
48. Suda K, Mitsudomi T. Emerging oncogenic fusions other than ALK, ROS1, RET, and NTRK in NSCLC and the role of fusions as resistance mechanisms to targeted therapy. Transl Lung Cancer Res (2020) 9(6):2618. doi: 10.21037/tlcr-20-186
49. Gokden M, Ariza A, Arnaoutakis K. Molecular markers in the diagnosis and treatment of cancer. BioMed Res Int (2015), 105217. doi: 10.1155/2015/105217
50. Zhang GC, Liao N, Chen B, Lin J, Lai J, Xiao W, et al. Next-generation sequencing (NGS) identifies a new breast cancer subtype with HER2 low-amplification status as a candidate for targeted therapy. Journal of Clinical Oncology 38 (15_suppl), 553–553. doi: 10.1200/JCO.2020.38.15_suppl.553
51. Dumbrava EE, Balaji K, Raghav K, Hess K, Javle M, Blum-Murphy M, et al. Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol (2019) 3:1–2. doi: 10.1200/PO.18.00345
52. Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol Res (2019) 7(10):1570–3. doi: 10.1158/2326-6066.CIR-19-0149
53. Bonneville R, Krook MA, Chen HZ, Smith A, Samorodnitsky E, Wing MR, et al. Detection of microsatellite instability biomarkers via next-generation sequencing. In: Biomarkers for immunotherapy of cancer. New York, NY: Humana (2020). p. 119–32.
54. Nakamura K, Aimono E, Oba J, Hayashi H, Tanishima S, Hayashida T, et al. Estimating copy number using next-generation sequencing to determine ERBB2 amplification status. Med Oncol (2021) 38(4):1–8. doi: 10.1007/s12032-021-01482-1
55. Heydt C, Wölwer CB, Velazquez Camacho O, Wagener-Ryczek S, Pappesch R, Siemanowski J, et al. Detection of gene fusions using targeted next-generation sequencing: A comparative evaluation. BMC Med Genomics (2021) 14(1):1–4. doi: 10.1186/s12920-021-00909-y
56. Hehir-Kwa JY, Koudijs MJ, Verwiel ET, Kester LA, van Tuil M, Strengman E, et al. Improved gene fusion detection in childhood cancer diagnostics using RNA sequencing. JCO Precis Oncol (2022) 6:e2000504. doi: 10.1200/PO.20.00504
57. Boyle TA, Mondal AK, Saeed-Vafa D, Ananth S, Ahluwalia P, Kothapalli R, et al. Guideline-adherent clinical validation of a comprehensive 170-gene DNA/RNA panel for determination of small variants, copy number variations, splice variants, and fusions on a next-generation sequencing platform in the CLIA setting. Front Genet (2021) 12:503830. doi: 10.3389/fgene.2021.503830
58. Shrestha E, Coulter JB, Guzman W. Oncogenic gene fusions in nonneoplastic precursors as evidence that bacterial infection can initiate prostate cancer. PNAS (2021) 118(32):e2018976118. doi: 10.1073/pnas.2018976118
59. Wang S, Chen R, Tang Y, Yu Y, Fang Y, Huang H, et al. Comprehensive genomic profiling of rare tumors: Routes to targeted therapies. Front Oncol (2020) 10:536. doi: 10.3389/fonc.2020.00536
60. Ricciotti RW, Baraff AJ, Jour G, Kyriss M, Wu Y, Liu Y, et al. High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet (2017) 218:69–80. doi: 10.1016/j.cancergen.2017.09.005
61. Cheng YW, Stefaniuk C, Jakubowski MA. Real-time PCR and targeted next-generation sequencing in the detection of low level EGFR mutations: Instructive case analyses. Respir Med Case Rep (2019) 28:100901. doi: 10.1016/j.rmcr.2019.100901
Keywords: comprehensive genomic profiling (CGP), microsatellite instability (MSI), RNA exome, tumor mutational burden (TMB), whole exome sequencing (WES)
Citation: Malhotra R, Javle V, Tanwar N, Gowda P, Varghese L, K A, Madhusudhan N, Jaiswal N, K S B, Chatterjee M, Prabhash K, Sreekanthreddy P, D Rishi K, M Goswami H and H Veldore V (2023) An absolute approach to using whole exome DNA and RNA workflow for cancer biomarker testing. Front. Oncol. 13:1002792. doi: 10.3389/fonc.2023.1002792
Received: 25 July 2022; Accepted: 24 January 2023;
Published: 13 March 2023.
Edited by:
Avaniyapuram Kannan Murugan, King Faisal Specialist Hospital & Research Centre, Saudi ArabiaReviewed by:
Sophia Yohe, University of Minnesota Twin Cities, United StatesCopyright © 2023 Malhotra, Javle, Tanwar, Gowda, Varghese, K, Madhusudhan, Jaiswal, K S, Chatterjee, Prabhash, Sreekanthreddy, D Rishi, M Goswami and H Veldore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vidya H. Veldore, dmlkeWFANGJhc2VjYXJlLmNvbQ==
†These authors share first authorship
‡ORCID: Vidya H. Veldore, orcid.org/0000-0001-5989-4836
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.