
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 September 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.998346
This article is part of the Research TopicPolyphenols in Cancer Prevention and TherapyView all 4 articles
 Mohd Farhan1*
Mohd Farhan1* Asim Rizvi2
Asim Rizvi2 Ferasat Ali2
Ferasat Ali2 Aamir Ahmad3
Aamir Ahmad3 Mohammad Aatif4
Mohammad Aatif4 Arshi Malik5
Arshi Malik5 Mir Waqas Alam6
Mir Waqas Alam6 Ghazala Muteeb7
Ghazala Muteeb7 Saheem Ahmad8
Saheem Ahmad8 Awal Noor1
Awal Noor1 Farhan Asif Siddiqui9
Farhan Asif Siddiqui9Anthocyanidins are the most abundant polyphenols in pomegranate juice. This class of molecules includes Delphinidin (Del), Cyanidin (Cya), and Pelargonidin (Pel). Using prostate, breast and pancreatic cancer cell lines PC3, MDA-MB-231, BxPC-3 and MiaPaCa-2, we show that anthocyanidins inhibit cell proliferation (measured by MTT assay) and induce apoptosis like cell death (measured by DNA/Histone ELISA). Copper chelator neocuproine and reactive oxygen species scavengers (thiourea for hydroxyl radical and superoxide dismutase for superoxide anion) significantly inhibit this reaction thus demonstrating that intracellular copper reacts with anthocyanidins in cancer cells to cause DNA damage via ROS generation. We further show that copper-supplemented media sensitizes normal breast epithelial cells (MCF-10A) to Del-mediated growth inhibition as determined by decreased cell proliferation. Copper supplementation results in increased expression of copper transporters Ctr1 and ATP7A in MCF-10A cells, which is attenuated by the addition of Del in the medium. We propose that the copper mediated, ROS-induced mechanism of selective cell death of cancer cells may in part explain the anticancer effects of anthocyanidins.
Ancient systems of medicine, such as Greeko Arab Medicine (Unani Medicine) often advocate food products as medicine. Food, in these systems of medicine, exerts its therapeutic capability by its physiological and bio-molecular activity, amongst other factors. Thus the concept of dietary constituents as therapeutic agents is not new. According to epidemiological research, the consumption of fruits and vegetables is connected with a lower risk of cardiovascular illnesses and certain types of malignancies (1–4). Numerous fruits, vegetables, and nuts are abundant in polyphenolic substances such as flavonoids, tannins, curcuminoids, gallocatechins, stilbenes, and anthocyanidins, among others. The processes behind the wide variety of pharmacological characteristics possessed by plant polyphenols have generated considerable interest. They are identified as naturally occurring antioxidants and have been associated with anticancer properties (4). Recent studies have shown that plant polyphenolics such as curcumin (from turmeric), resveratrol (from red grapes and red wine), epigallocatechin-3-gallate (EGCG) (from green tea), and delphinidin (from pomegranate juice) induce apoptosis in numerous cancer cell lines (5–9). The finding that a number of these polyphenols, including EGCG, gallic acid, and resveratrol, elicit apoptotic cell death in diverse cell lines but not in normal cells is of particular relevance (6–8). Flavonoids (10), tannic acid and its structural constituent gallic acid (11), curcumin (12), gallocatechins (13), and resveratrol (14) have been shown to cause oxidative strand breakage in DNA either alone or in the presence of transition metal ions.
Copper is an essential metal ion present in chromatin and is intimately connected with DNA bases, particularly guanine (15). It is one of the most redox-active metal ions found in living cells. Copper elevation has been observed in a wide variety of malignancies (16), both in humans and in laboratory animals. Interestingly, for human malignancies the elevation of copper has been seen both in the serum and in the tissue (16). The elevation of copper in the malignancy does not depend on the tissue type, but is rather a metabolic characteristic of the malignancy itself (17). Whether this systemic elevation of copper is a cause or an effect of the malignant transformation remains open to debate and investigation.
Most of the pharmacological activities of plant polyphenols are believed to be derived from their capacity to scavenge endogenously generated oxygen radicals or those created by xenobiotics, radiation, etc. However, the antioxidant properties of polyphenolic compounds may not fully explain their chemopreventive benefits (18, 19). The majority of plant polyphenols exhibit both antioxidant and prooxidant characteristics (7, 10), and we have previously hypothesized that the prooxidant action of polyphenolics may be a significant mechanism behind their anticancer and apoptosis-inducing properties (19, 20). This mechanism for the lethal effect of these chemicals on cancer cells would include the mobilization of endogenous copper ions and the resulting prooxidant action.
In the present study, we demonstrate that anthocyanidins possess a significant oxidative damage-inducing capacity. We show that these characteristics of anthocyanidins in cancer cells are dependent upon the bioavailability of copper within the cell and its redox recycling. Figure 1 depicts the structures of the anthocyanidins used in these investigations.
The immortalized non-transformed breast cell line MCF-10A and cancer cell lines PC3, MDA-MB-231, BxPC-3, and MiaPaCa-2 were obtained from ATCC (Manassas, VA, USA). MDA-MB-231, BxPC-3, and MiaPaCa-2 cell lines were grown in DMEM (Invitrogen, Carlsbad, CA, USA), whilst PC3 cells were grown in RPMI (Invitrogen, Carlsbad, CA, USA). The medium was supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 g/mL streptomycin. All cells were cultivated, at 37°C and 5% CO2. Small aliquots of delphinidin, cyanidin, and pelargonidin stock solutions (50 mM) were kept at -80°C. Neocuproine (Neo), desferoxamine mesylate (DM), and histidine (His) stock solutions were prepared in PBS at a final concentration of 50 mM. MCF-10A, a normal breast epithelial cell line, was grown in DMEM/F12 (Invitrogen, Carlsbad, CA, USA) supplemented with 5% horse serum, 20 ng/mL EGF, 0.5 µg/mL hydrocortisone, 0.1 µg/mL cholera toxin, 10 µg/mL insulin, 100 units/mL penicillin, and 100 µg/mL streptomycin MCF-10A + Cu cells are MCF-10A cells that were grown for one month with 25 µM CuCl2 added to their standard culture medium (described above).
In 96-well microtiter culture plates, 2x103 cells per well were seeded. After an overnight incubation, the regular growth medium was replaced with a fresh medium containing various amounts of anthocyanidins diluted from a 50 mM stock. As specified in each study, various chelators were introduced to specific assays. After 3 days of incubation, 25 µl of 3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) solution (5 mg/mL in PBS) was added to each well, and the plates were incubated at 37°C and 5% CO2, for an additional 2 hours.
After 2 hours of incubation, the supernatant was discarded. MTT formazan from metabolically viable cells was dissolved in 100 µl of DMSO using a gyratory shaker for 30 minutes. The absorbance was calculated to be 595 nm using an Ultra Multifunctional Microplate Reader (TECAN, Durham, NC, USA). There were eight replicate wells for every treatment, and the concentration of DMSO in the response mixture never exceeded 0.1%. Each experiment was repeated three times.
The Cell Death Detection ELISA Kit (Roche, Palo Alto, CA, USA) was used to detect apoptosis in growth cells treated with different anthocyanidins. Cells were treated for 72 hours with polyphenols or DMSO as a control. After treatment, cytoplasmic histone and DNA from cells was extracted and incubated in microtiter plate modules coated with anti-histone antibody. Peroxidase-conjugated anti-DNA antibody was used to detect immobilized histone/DNA, followed by color development with peroxidase-specific ABTS substrate. Using Ultra Multifunctional Microplate Peruser (TECAN, Durham, NC, USA) at 405 nm, the spectrophotometric absorbance of the samples was measured.
In addition, specific metal ion chelators were employed during the processes. DM (50 µM) was employed for the chelation of Fe (II) particles, His (50 µM) was employed for Zn (II), and Neo (50 µM) was employed for the chelation of Cu (II) ions. Catalase 20 µg/mL, superoxide dismutase (SOD) 20 µg/mL, and thiourea (TU) 0.1 mM were used to study the involvement of reactive oxygen species in the reaction.
A cell migration assay was conducted using 24-well transwell permeable supports with 8 mm pores (Corning, NY, USA). The transwell embeds were planted with cells suspended in a solution devoid of serum. The lower wells were filled with media. After 24 hours, cells were stained with 4 mg/mL calcein AM (Invitrogen, Carlsbad, CA, USA) in PBS at 37 C for 1 hour and then detached from the inserts using trypsin. The fluorescence of the migrating cells was measured utilizing the ULTRA Multifunctional Microplate Peruser (TECAN, Durham, NC, USA). Cells were cultured in the presence and absence of delphinidin (50 µM) and neocuprione (50 µM) at various concentrations.
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was used to quantify mRNA expression. Sequences of primers for Ctr1 (forward: 5′-GCT GGA AGA AGG CAG TGG TA-3′; reverse: 5′-AAA GAG GAG CAA GAA GGG ATG-3′), ATP7A (forward: 5′-ACG AAT GAG CCG TTG GTA GTA-3′; reverse: 5′-CCT CCT TGT CTT GAA CTG GTG-3′) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward: 5′-TGG GTG TGA ACC ATG AGA AGT-3′; reverse: 5′-TGA GTC CTT CCA CGA TAC CAA-3′) were same as reported earlier (21, 22), and the amount of RNA was normalized to GAPDH expression.
siRNA transfections were carried out as previously described (22). Santa Cruz Biotechnology, Inc. was contacted for Ctr1-specific siRNA. A garbled siRNA was utilized as a control.
Following the manufacturer’s instructions, transfections were carried out with Lipofectamine RNA iMAX Transfection Reagent (Invitrogen), 48 hours before to the experiment, Ctr1 was silenced by siRNA.
The results of all experiments are expressed as percentage of control ± Standard Error (S.E.) of triplicate determinations. p ≤ 0.01 is considered statistically significant.
Diverse cancer cell lines, PC3 (Prostate), MDA-MB-231 (Breast), and BxPC3 and MiaPaCa-2 (Pancreas), were treated with a range of concentrations of delphinidin, cyanidin, pelargonidin. MTT assay was used to assess the inhibition of cancer cell proliferation by anthocyanidins. All anthocyanidins inhibited cancer cell growth in a concentration-dependent manner (Figure 2). However, the inhibition was shown to be significantly stronger in delphinidin than in cyanidin and pelargonidin.
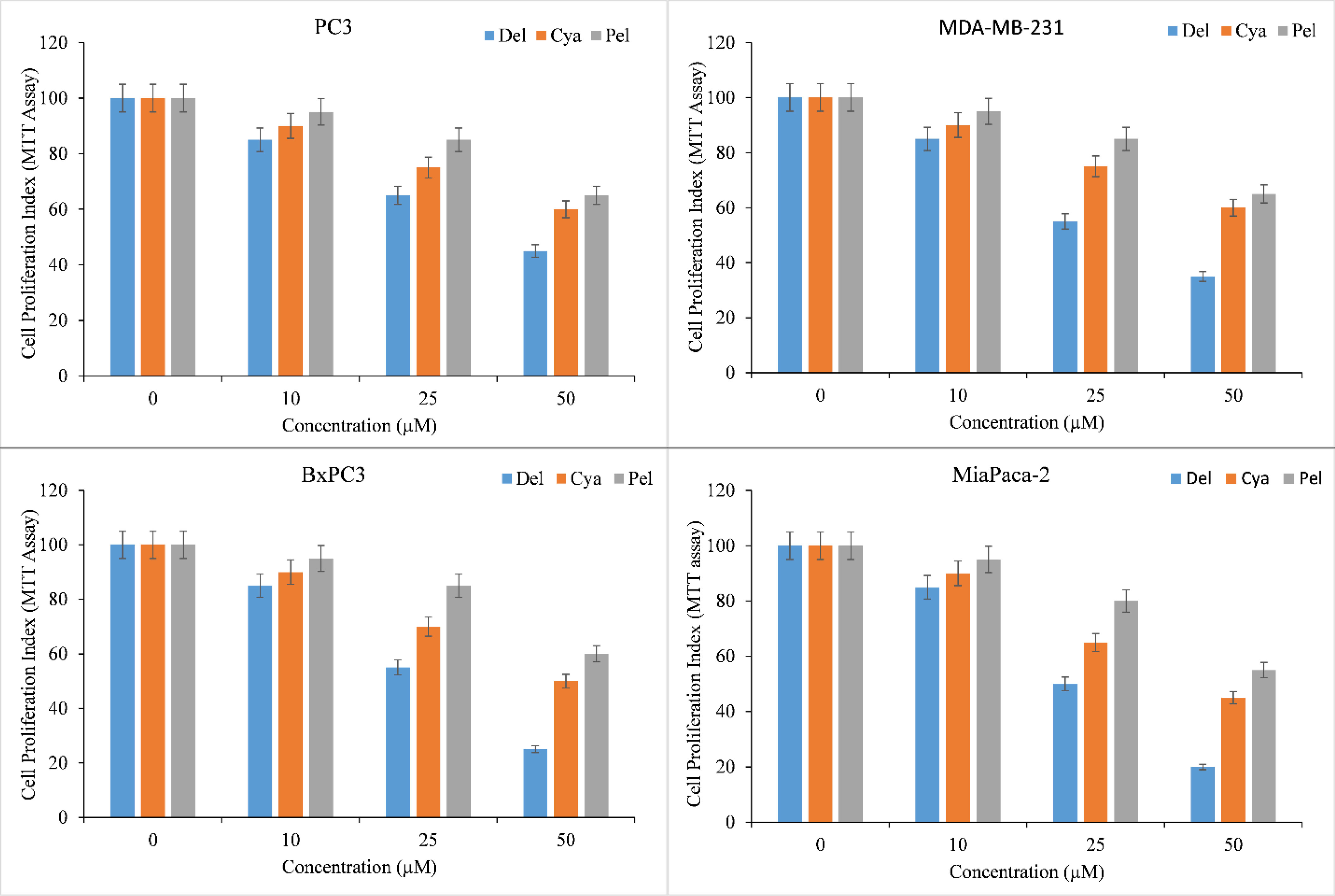
Figure 2 Effect of delphinidin, cyanidin and pelargonidin on cell growth in various cancer cell lines as revealed by MTT assay. Cells from PC3, MDA-MB-231, BxPC3, MiaPaCa-2 cancer cell lines were treated with stated concentrations of anthocyanidins (mentioned above) for 72 hours. The effect on cell proliferation was measured by performing MTT test as indicated in ‘Methods’. All data are expressed as percentage of control ± S.E of triplicate determinations. p ≤ 0.01 when compared to respective untreated control was treated as significant.
The induction of apoptosis by delphinidin, cyanidin, pelargonidin was evaluated by Histone/DNA ELISA to confirm these findings (Figure 3). Delphinidin was identified as the most potent molecule, confirming our previous findings. The most efficient inducer of apoptosis was also delphinidin followed by cyanidin and pelargonidin. We thus concluded that the cytotoxicity of anthocyanidins is dose-dependent. Delphinidin was the most effective molecule in this family. Thus for our mechanistic studies we only used Delphinidin.
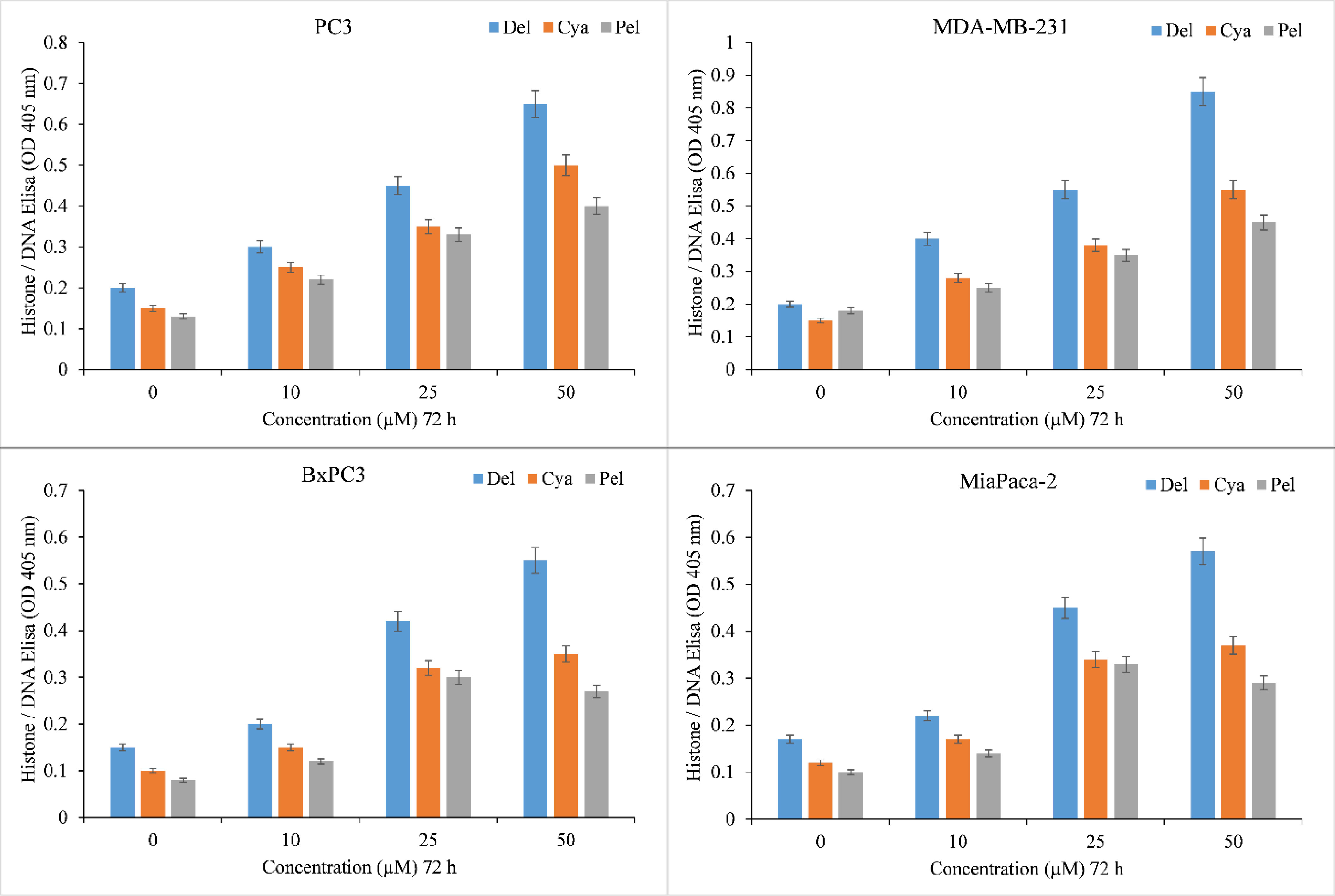
Figure 3 Induction of apoptosis by delphinidin, cyanidin and pelargonidin in several cancer cell lines. The Cell Death Detection ELISA Kit (Roche, Palo Alto, CA) was used to detect apoptosis in cancer cell lines after 72 hours of incubation with increasing concentrations of anthocyanidins (mentioned above), as depicted in the figure and detailed in the section titled “Methods.” The stated values are the S.E. of three separate tests. p ≤ 0.01 when compared to respective untreated control was treated as significant.
We have previously established that the membrane permeable copper chelator neocuproine inhibits the delphinidin-induced oxidative breaking of cellular DNA in lymphocytes (23), indicating the involvement of endogenous copper in the process. Since lymphocytes differ from cancer cells, we wished to reproduce this effect in malignant cell lines.
We therefore, reproduced the investigation with malignant cells and discovered that only copper chelator Neo could significantly protect PC3, MDA-MB-231, and BxPC-3 cells from the growth-inhibiting effects of delphinidin (Figure 4). However, DM and His (iron and zinc chelators, respectively) failed to affect cell growth to any substantial degree, with the exception of PC3 and BxPC3 cells, in which DM and His show a protective effect against the growth suppression induced by delphinidin. However, this was significantly less than Neo’s inhibition (Figure 4).
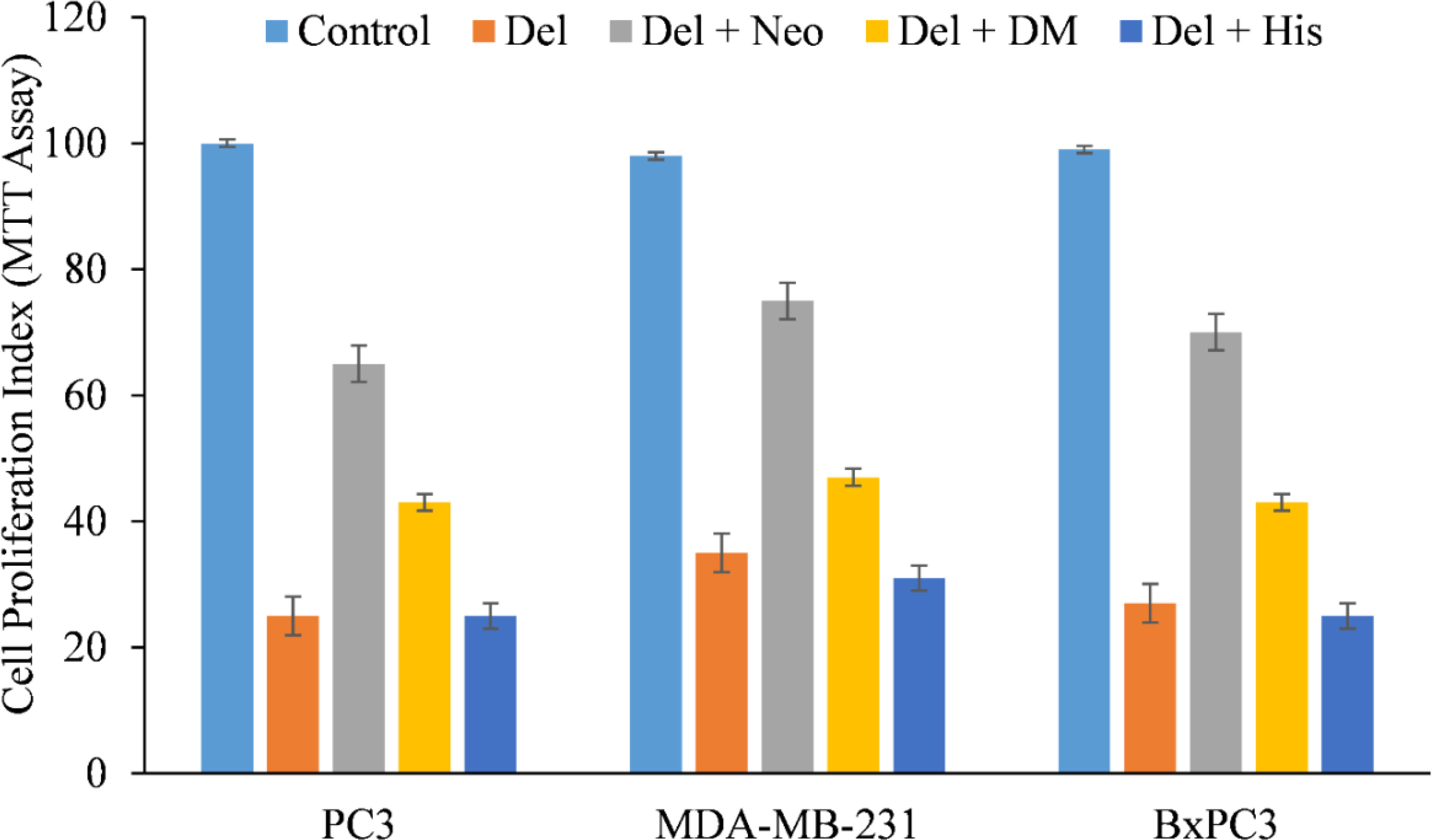
Figure 4 The effect of several metal-specific chelators on the antiproliferative activity of delphinidin in three cancer cell lines is depicted. As depicted in the picture, PC3, MDA-MB-231, and BxPC3 cancer cells were treated with 50 µM of delphinidin alone or in the addition of copper chelator neocuproine (Neo), iron chelator desferrioxamine mesylate (DM), or zinc chelator histidine (His). 50 µM was the concentration of metal chelators utilized. After 72 hours of treatment, the MTT assay was performed as indicated in “Methods.” The values reported are the S.E. of three separate trials. p ≤ 0.01 when compared to respective untreated control was treated as significant.
Additionally, the impact of several metal chelators on delphinidin-induced apoptosis was evaluated. This protection was not detected when iron or zinc chelators were used (Figure 5), corroborating the conclusion that the anticancer mechanism of the drug delphinidin includes mobilization of endogenous copper.
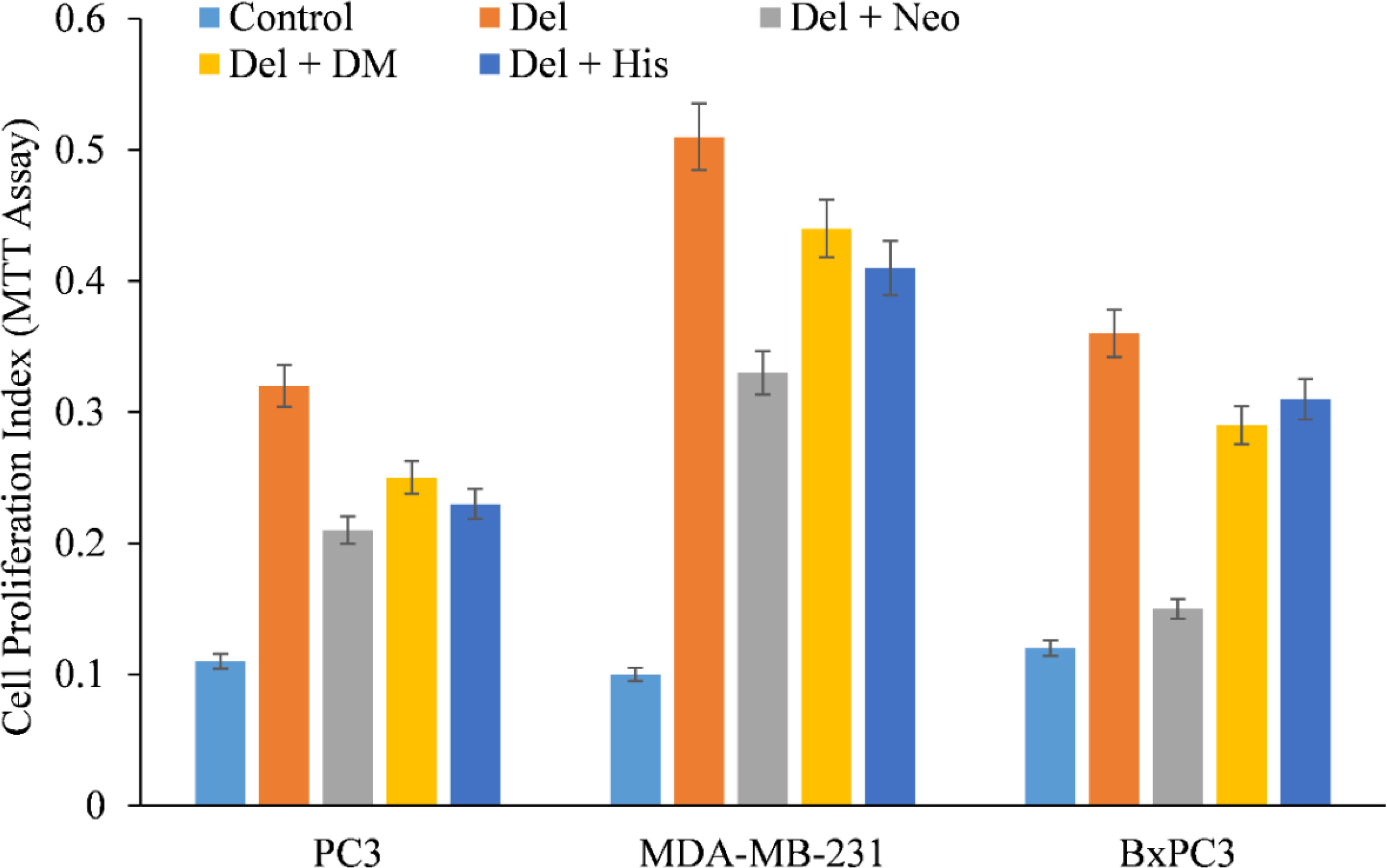
Figure 5 Effect of various metal chelators on delphinidin-induced apoptosis in three distinct cancer cell lines. As depicted in the figure, PC3, MDA-MB-231, and BxPC3 cancer cells were treated with 50 µM of delphinidin alone or in the presence of the copper chelator neocuproine (Neo), the iron chelator desferrioxamine mesylate (DM), or the zinc chelator histidine (His). The concentration of metal chelators utilized was 50 µM. After 72 hours of treatment, MTT assay was performed as indicated in “Methods.” p ≤ 0.01 when compared to respective untreated control was treated as significant.
Polyphenol induced apoptosis of cancer cells is caused by ROS mediated DNA breakage (24, 25). To determine whether anthocyanidin-induced DNA damage in cancer cell lines also involved ROS, the effect of several ROS scavengers (such as catalase, thiourea, and superoxide dismutase) on delphinidin-induced death of cancer cells was also investigated. All three ROS scavengers inhibited delphinidin-induced apoptosis in diverse cancer cell lines (Table 1), with TU exhibiting the greatest degree of inhibition. These results validated the significance of reactive oxygen species (ROS) as effectors of anthocyanidin-induced apoptosis, perhaps by a Fenton-type physiologically active reaction, as described previously (26, 27).
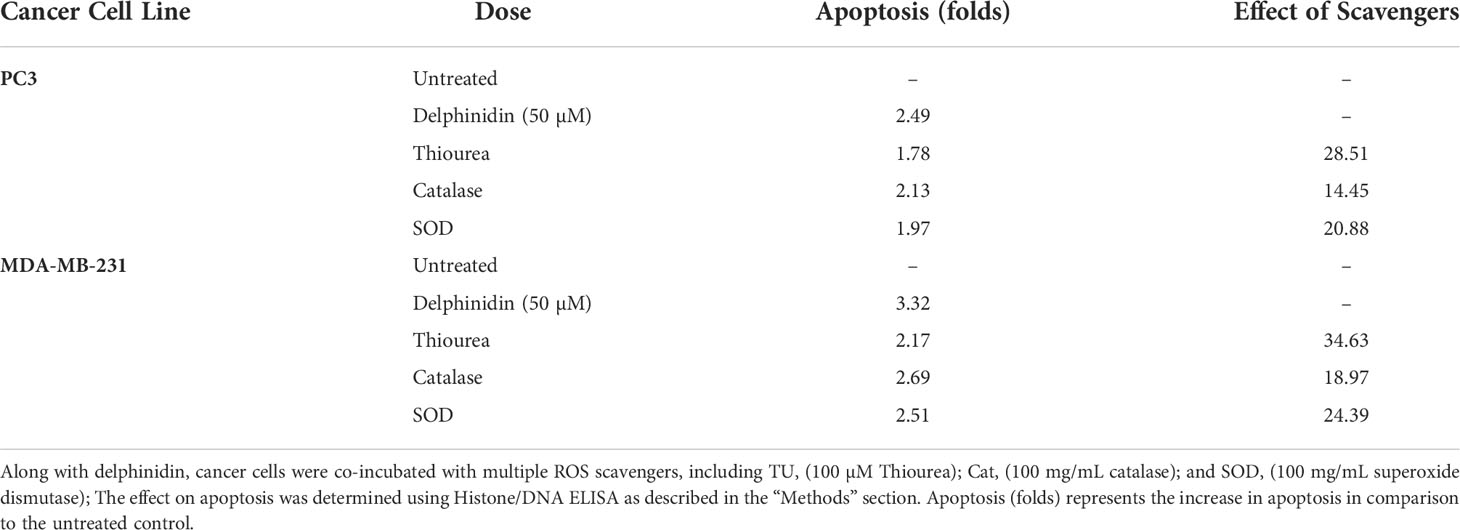
Table 1 The effect of ROS scavengers on the activation of apoptosis in two distinct cancer cell lines by delphinidin.
Malignant cells are characterized by their migration and invasion of secondary locations via metastasis. We found that delphinidin decreased the migratory capacity of PC3 and MDA-MB-231, cells (Figure 6), making them less susceptible to metastasis. Interestingly, when copper was chelated from the cells by the membrane permeable copper chelator neocuprione in the presence of delphinidin, the cells regained their metastatic potential (Figure 6).
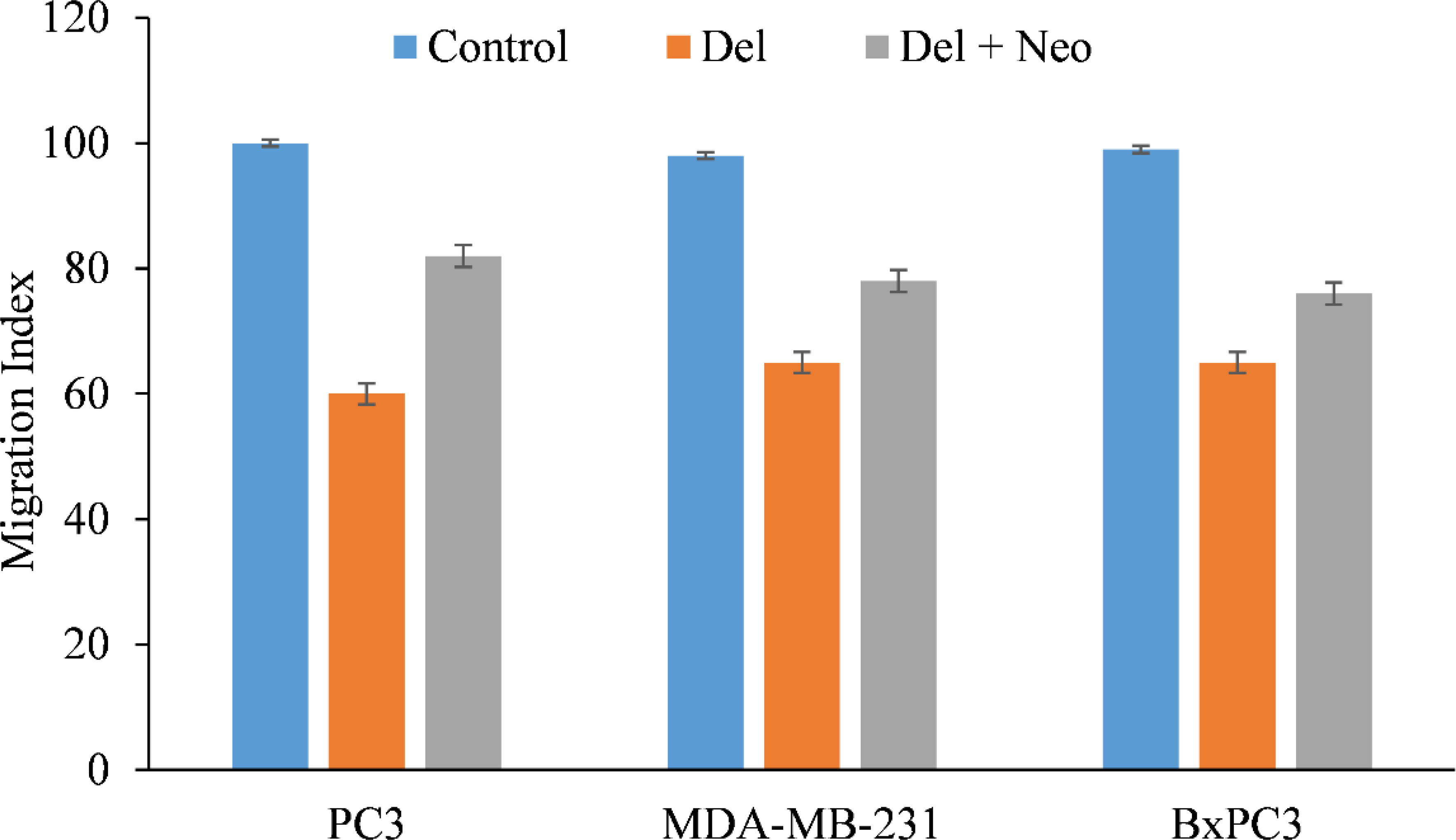
Figure 6 In the presence of the copper chelating agent neocuproine, the effect of delphinidin on the migration of PC3 (prostate), MDA-MB-231 (breast), and BxPC3 (pancreatic) cancer cells was examined. As detailed in “Methods,” a cell migration test was conducted utilizing 24-well transwell permeable supports with 8-mm pores (Corning). In the presence and absence of delphinidin (50 µM) and neocuprione (50 µM), the cells were developed. Ultra Multifunctional Microplate Reader was used to analyze the fluorescence of the migrating cells (TECAN). The values reported are the S.E. of three separate trials. p ≤ 0.01 when compared to respective untreated control was treated as significant.
MCF-10A normal (non-cancerous) breast epithelial cells were grown in medium containing 25 µM copper. When copper-supplemented MCF-10A cells (MCF-10A+Cu) were treated with delphinidin, a substantial decrease in cell proliferation was found as compared to non-copper-supplemented MCF-10A cells (Figure 7).
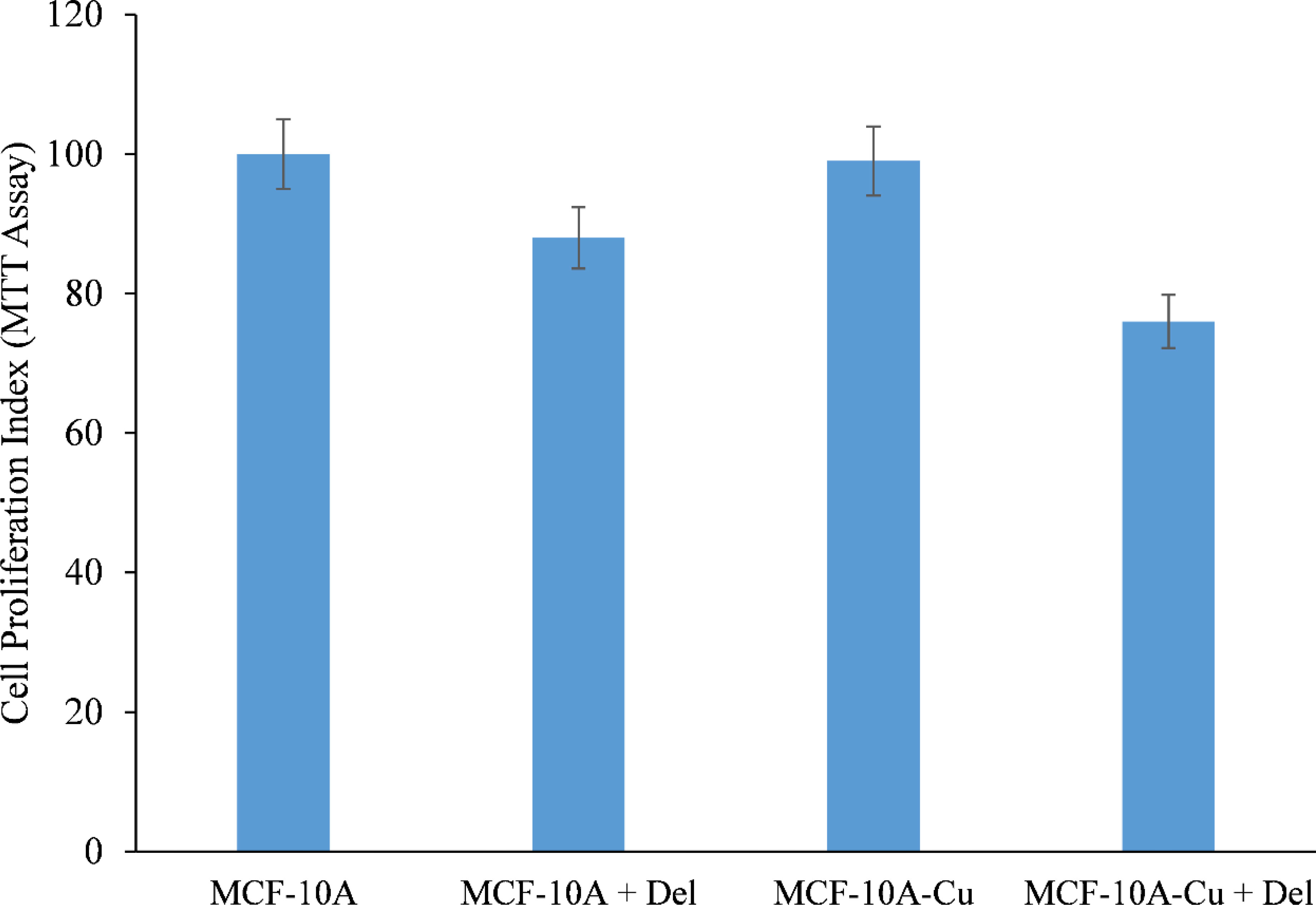
Figure 7 Effect of delphinidin on cell growth suppression in MCF-10A (normal breast epithelial cells) and MCF-10A cells cultivated in Cu (II)-supplemented medium (MCF-10A-Cu). MCF-10A and MCF-10A-Cu (normal cells cultivated in a medium containing 25 µM CuCl2) were treated with delphinidin 50 µM for 72 hours. The proliferation of cells was then measured using the MTT test, as stated in “Methods.” The stated values are the S.E. of three separate experiments. p ≤ 0.01 when compared to respective untreated control was treated as significant.
Since malignant transformation is followed by a dramatic increase in intracellular copper levels (24, 26, 27), it is plausible to conclude that the delphinidin-induced inhibition of malignant cell proliferation is a result of its interaction with cellular copper. The addition of exogenous copper to non-malignant epithelial cells sensitizes these non-malignant cells to delphinidin-induced cell growth suppression (Figure 7).
We showed that the growth inhibition elicited by delphinidin is a result of its interaction with intracellular copper in both malignant cells (Figures 4, 5) and non-malignant epithelial cells cultured in media enriched with copper (Figure 7). Since malignant cells express copper transporter Ctr1 at a higher level (28), we next examined whether copper supplementation led to an increase in copper transporter expression in non-malignant epithelial cells. Copper supplementation in the growth media of MFC-10A cells led to a significant increase in the expression of copper transporters Ctr1 and ATP7A, (Figure 8) (28, 29). Adding more delphinidin to the medium decreased the expression of both copper transporters (Figure 8), indicating that delphinidin had an influence on the copper metabolism of cancer cells.
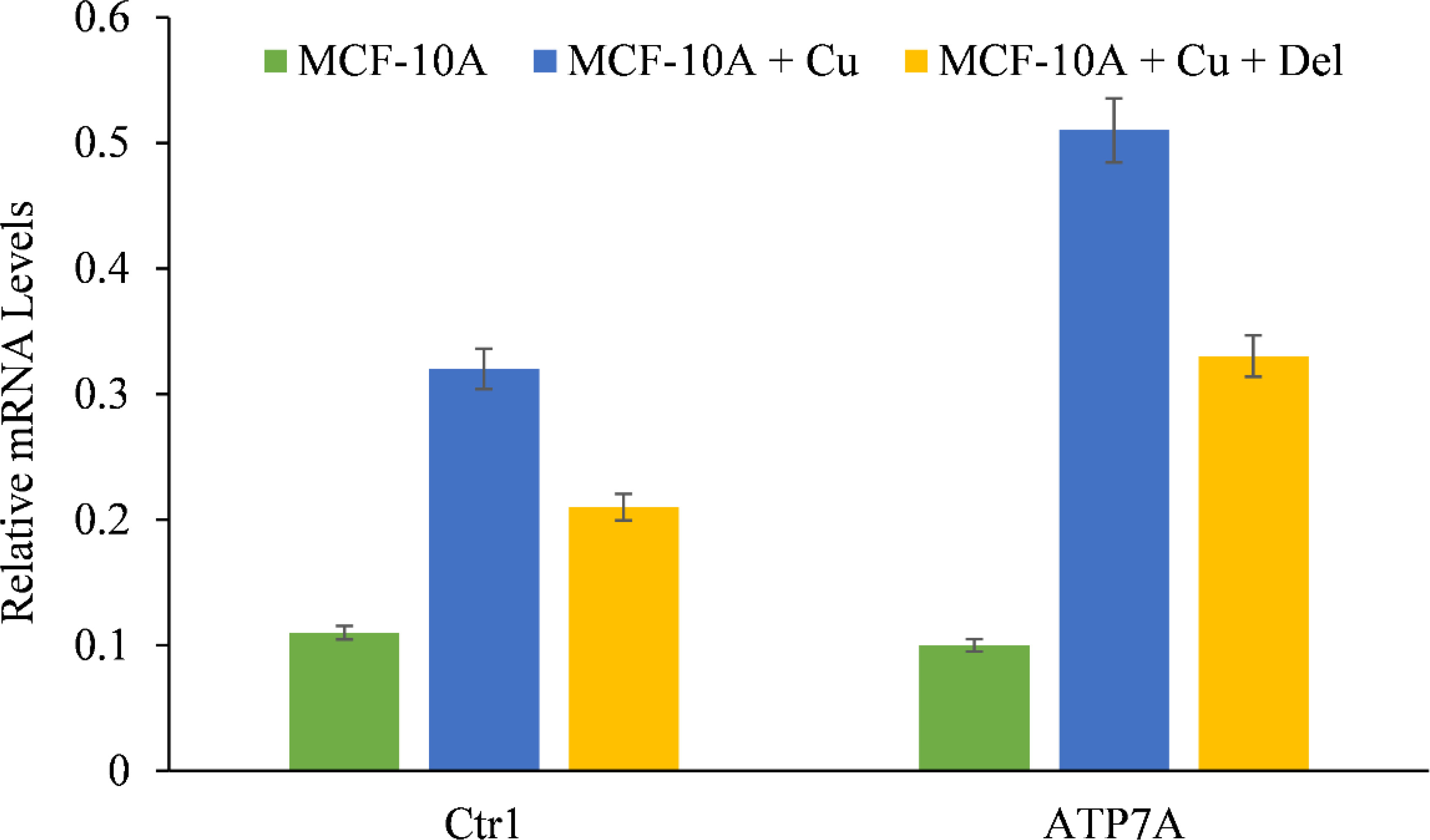
Figure 8 The effect of delphinidin on the elevated mRNA transcript levels of copper transporters Ctr1 and ATP7A in MCF-10A-Cu cells compared to their parental MCF-10A cells. TRIzol (Invitrogen) was used to isolate total RNA in accordance with the manufacturer’s instructions. As described in “Methods,” Ctr1 and ATP7A mRNA expression was quantified using real-time PCR. The values reported are the S.E. of three separate trials. p ≤ 0.01 when compared to respective untreated control was treated as significant.
We wished to validate the crucial involvement of copper in the growth inhibition mediated by delphinidin. To this end we silenced the copper transporter Ctr1 using targeted siRNA (Figure 9). Ctr1 mediates copper uptake in cells, and as established before (Figure 8), its expression makes MCF-10A cells more susceptible to delphinidin-induced growth suppression. We discovered that silencing copper transporter Ctr1 decreased MCF-10A cells’ susceptibility to delphinidin when cultured in copper-enriched media.
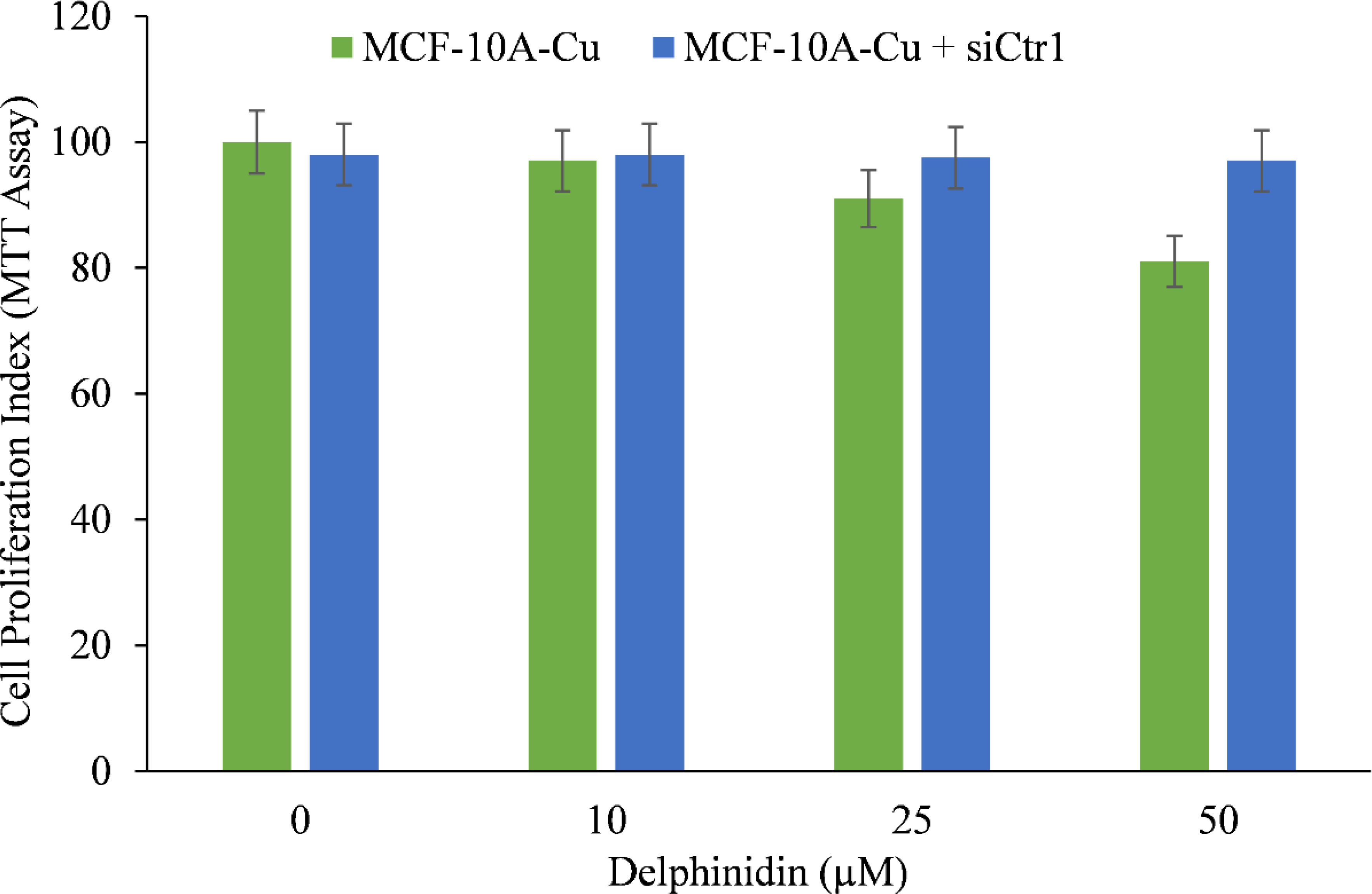
Figure 9 The effect of delphinidin on the elevated mRNA transcript levels of copper transporters Ctr1 and ATP7A in MCF-10A-Cu cells compared to their parental MCF-10A cells. TRIzol (Invitrogen) was used to isolate total RNA in accordance with the manufacturer’s instructions. As described in “Methods,” Ctr1 and ATP7A mRNA expression was quantified using real-time PCR. To determine the effect of delphinidin on mRNA expression, only MCF-10A-Cu cells (normal MCF-10A cells cultured in a medium containing 25 µM CuCl2) with enhanced mRNA expression of copper transporters were treated with 50 µM delphinidin. The values reported are the S.E. of three separate trials. p ≤ 0.01 when compared to respective untreated control was treated as significant.
This demonstrates conclusively that delphinidin interacts with cellular copper and that cellular copper is essential for the growth-inhibiting effect of delphinidin on cancer cells.
Similar to other classes of plant polyphenols, the anthocyanidin (delphinidin, cyanidin and pelargonidin) are capable of causing (i) cellular DNA degradation in human cancer cell lines either alone or in the presence of copper ions (ii) such DNA breakage is caused by the production of reactive oxygen species (ROS) and (iii) delphinidin is most capable of causing DNA breakage (among the anthocyanidins tested) in the cancer cell lines tested.
Pomegranate has been utilized in traditional medicine for millennia, and is now recognized as a possible anticancer agent. In animal models, pomegranate juice has been demonstrated to be cytotoxic against skin, prostate, lung, breast, colon, and blood malignancies. On consumption of pomegranate juice, a variety of proteins and transcription factors implicated in apoptosis induction pathways are up- or down-regulated (30). The principal anthocynanidin in pomegranate juice, delphinidin, has been demonstrated to cause apoptosis in human pro-myelocytic leukemia (HL-60) cells (9). It was demonstrated that a JNK activation pathway driven by oxidation may be implicated in delphinidin-induced apoptosis. In addition, delphinidin caused the cells to create hydrogen peroxide, and antioxidants such as N-acetylcysteine and catalase reduced JNK phosphorylation, caspase-3 activation, and DNA fragmentation induced by delphinidin. According to structure–activity studies, the presence of orthodihydroxy phenolic groups on the B-ring of anthocyanidins is important for inducing apoptosis. It is noteworthy that we have previously demonstrated that such a structural configuration of hydroxyl groups in flavonoids and tannins is crucial for the oxidative destruction of DNA in the presence of copper ions (11, 31–33). Gallic acid (which contains ortho dihydroxy phenolic groups) is also active in lymphocyte DNA destruction, although its modified derivative syringic acid is not. Antioxidants, such as catalase, superoxide dismutase, and hydroxyl radical scavengers, also suppress this type of cellular DNA breaking (34, 35).
The bioavailability of delphinidin in mammalian systems warrants discussion. It has been observed that delphinidin and cyanidin decrease vascular endothelial growth factor (VEGF) expression in vascular smooth muscle cells at doses as low as 10 µM (36). It has not escaped our notice that 10 µM delphinidin (without additional copper) is capable of causing considerable DNA damage in MCF-10A cancer cell line (Figure 7). The concentration of cyanidin and delphinidin in red wine is approximately 150 µM (37) and the bioavailability of cyanidin and delphinidin has been estimated to be approximately 60% in healthy volunteers after red wine consumption, and therefore such concentrations are likely to be achieved in the blood after red wine consumption (38). Another future area of research can be to determine if anthocyanidins, used in this study can produce a synergistic chemotherapeutic effect with other naturally derived molecules or chemotherapeutic drugs. Other areas where research on dietary polyphenols and their anticancer effects can be extrapolated has been discussed by us elsewhere in great details (25).
The elevation of copper in malignancies is a well-established fact, which is supported by adequate experimental and clinical evidence. This idea is also validated by complementary and alternative systems of medicine, such as Greeko Arab Medicine (Unani Medicine), where it is postulated that elevation in physiologically available metals can increase the propensity of a tumor mass to be cancerous.
We have previously shown (19, 20) that the prooxidant activity of plant polyphenols involving the mobilization of endogenous copper ions may be a key mechanism underlying their anticancer activities. According to our hypothesis, the preferential cytotoxicity of plant polyphenols towards cancer cells is explained by the observation made decades earlier that serum (39, 40), tissue (41), and intracellular copper levels in cancer cells (42) are significantly elevated in a variety of cancers. Since cancer cells possess elevated levels of copper, they may be more susceptible to electron transfer with polyphenols to produce reactive oxygen species (43). As a result of higher intracellular copper levels in cancer cells, it is possible to expect that the lethal concentrations of polyphenols necessary for these cells would be lower than for normal cells (44, 45).
In animal studies, plant polyphenols have been demonstrated to trigger tumor regression (46–48). Our own in vivo studies on hepatocellular carcinoma show that EGCG is capable of inducing cell death of malignant cells (49), a similar mechanism has also been demonstrated by Rizvi et al. using Vitamin D (27).
Thus, plant polyphenols with anticancer and apoptosis-inducing qualities might mobilize endogenous copper ions. Our experiments here demonstrate that thiourea, a chelator of hydroxyl radical, superoxide dismutase, a scavenger of superoxide free radical and catalase, a scavenger of hydrogen peroxide, reduces the ability of anthocyanidins to cause copper mediated cell death, possibly, by reducing the generation of ROS near DNA (50, 51).
This mechanism would thus essentially be an alternate, non-enzymatic copper-dependent mechanism for the cytotoxic activity of some anticancer molecules capable of mobilizing endogenous copper. Indeed, a shared mechanism, common to all polyphenols, such as the mechanism elucidated here, better explains the anticancer effects of polyphenols with varied chemical structures.
In live cells, the most redox-active metal ions are Fe3+ and Cu2+. Although iron is substantially more prevalent in biological systems, copper and zinc are the predominant ions in the nucleus (52). Burkitt et al. (53) listed various reasons why Cu2+, and not Fe3+, may be responsible for OP-stimulated DNA fragmentation in isolated nuclei.
ROS production in normal cells is tightly regulated by homeostasis (54). Numerous variables, including metals, medicines, and prooxidants such as H2O2, can increase ROS production, leading in oxidative stress and the activation of apoptosis. Several investigations have shown that the activation of apoptosis by polyphenols and other anticancer drugs is independent of caspases and mitochondria (55, 56) and is associated by an increase in the intracellular levels of reactive oxygen species (ROS) (57–59).
Copper transporters enable intracellular copper levels to increase. This plays a critical role in the anticancer activity of anthocyanidins and plant-derived polyphenols in general. This study adds a new dimension to the designing of future mechanism-based anticancer lead molecules, which target the tumor microenvironment, specifically increased cellular copper, in order to achieve the desired efficacy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project Number GRANT 1370).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer (2006) 54:111–42. doi: 10.1207/s15327914nc5401_13
3. Temple NJ, Gladwin KK. Fruit, vegetable and the prevention of cancer: research challenges. Nutrition (2003) 19:467–70. doi: 10.1016/S0899-9007(02)01037-7
4. Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals–promising cancer chemopreventive agents in humans? a review of their clinical properties. Int J Cancer (2007) 120:451–8. doi: 10.1002/ijc.22419
5. Jaruga E, Salvioli S, Dobrucki J. Apoptosis like reversible changes in plasma membrane asymmetry and permeability and transient modifications in mitochondrial membrane potential induced by curcumin in rat hepatocytes. FEBS Lett (1998) 433:287–93. doi: 10.1016/S0014-5793(98)00919-3
6. Clement M, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signalling dependant apoptosis in human tumor cells. Blood (1998) 92:996–1002. doi: 10.1182/blood.V92.3.996
7. Inoue M, Suzuki R, Koide T, Sakaguchi N, Ogihara Y, Yabu Y. Antioxidant, gallic acid induces apoptosis in HL 60 r cells. Biochem Biophys Res Commun (1994) 204:898–904. doi: 10.1006/bbrc.1994.2544
8. Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst (1997) 89:1881–6. doi: 10.1093/jnci/89.24.1881
9. Hou DX, Ose T, Lin S, Harazoro K, Imamura I, Kubo M, et al. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: structure–activity relationship and mechanisms involved. Int J Onocol. (2003) 23:705–12. doi: 10.3892/ijo.23.3.705
10. Ahmad MS, Fazal F, Rahman A, Hadi SM, Parish JH. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): correlation with the generation of active oxygen species. Carcinogenesis (1992) 13:605–8. doi: 10.1093/carcin/13.4.605
11. Khan NS, Hadi SM. Structural features of tannic acid important for DNA degradation in the presence of Cu(II). Mutagenesis (1998) 13:271–4. doi: 10.1093/mutage/13.3.271
12. Ahsan H, Hadi SM. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett (1998) 124:23–30. doi: 10.1016/S0304-3835(97)00442-4
13. Malik A, Azam S, Hadi N, Hadi SM. DNA Degradation by water extract of green tea in the presence of copper ions: implications for anticancer properties. Phytother. Res (2003) 17:358–63. doi: 10.1002/ptr.1149
14. Ahmad A, Asad SF, Singh S, Hadi SM. DNA Breakage by reservatrol and Cu(II): reaction mechanism and bacteriophage inactivation. Cancer Lett (2000) 154:29–37. doi: 10.1016/S0304-3835(00)00351-7
15. Kagawa TF, Geierstanger BH, Wang AHJ, Ho PS. Covalent modification of guanine bases in double stranded DNA: the 1:2-AZ-DNA structure of dc(CACACG) in the presence of CuCl2. J Biol Chem (1991) 226:20175–84. doi: 10.1016/S0021-9258(18)54906-1
16. Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev (2009) 35(1):32–46. doi: 10.1016/j.ctrv.2008.07.004
17. Rizvi A, Furkan M, Naseem I. Physiological serum copper concentrations found in malignancies cause unfolding induced aggregation of human serum albumin in vitro. Arch Biochem Biophysics (2017) 636:71–8. doi: 10.1016/j.abb.2017.11.001
18. Gali HU, Perchellet EM, Klish DS, Hohnson JM, Perchellet JP. Hydrolysable tannins: potent inhibitors of hydroperoxide production and tumor promotion in mouse skin treated with 12-o-tetradecanoyl phorbol-13-acetate. Int J Cancer (1992) 51:425–32. doi: 10.1002/ijc.2910510315
19. Hadi SM, Asad SF, Singh S, Ahmad AA. Putative mechanism for anticancer and apoptosis inducing properties of plant-derived polyphenolic compounds. IUBMB Life (2000) 50:1–5. doi: 10.1080/152165400300001471
20. Hadi SM, Bhat SH, Azmi AS, Hanif S, Shamim U, Ullah MF. Oxidative breakage of cellular DNA by plant polyphenols: a putative mechanism for anticancer properties. Semin Cancer Biol (2007) 17:370–6. doi: 10.1016/j.semcancer.2007.04.002
21. Gao C, Zhu L, Zhu F, Sun J, Zhu Z. Effects of different sources of copper on Ctr1, ATP7A, ATP7B, MT and DMT1 protein and gene expression in caco-2 cells. J Trace Elem Med Biol (2014) 28:344–50. doi: 10.1016/j.jtemb.2014.04.004
22. Ahmad A, Maitah MY, Ginnebaugh KR, Li Y, Bao B, Gadgeel SM, et al. Inhibition of hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J Hematol Oncol (2013) 6:77. doi: 10.1186/1756-8722-6-77
23. Hanif S, Shamim U, Ullah MF, Azmi AS, Bhat SH, Hadi SM. The anthocyanidin delphinidin mobilizes endogenous copper ions from human lymphocytes leading to oxidative degradation of cellular DNA. Toxicology (2008) 249:19–25. doi: 10.1016/j.tox.2008.03.024
24. Farhan M, Rizvi A, Ahmad A, Aatif M, Alam MW, Hadi SM. Structure of some green tea catechins and the availability of intracellular copper influence their ability to cause selective oxidative DNA damage in malignant cells. Biomedicines (2022) 10(3):664. doi: 10.3390/biomedicines10030664
25. Farhan M, Rizvi A. Understanding the prooxidant action of plant polyphenols in the cellular microenvironment of malignant cells: Role of copper and therapeutic implications. Front Pharmacol (2022) 13. doi: 10.3389/fphar.2022.929853
26. Farhan M, Khan HY, Oves M, Al-Harrasi A, Rehmani N, Arif H, et al. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins (Basel) (2016) 8:37. doi: 10.3390/toxins8020037
27. Rizvi A, Farhan M, Naseem I, Hadi SM. Calcitriol–copper interaction leads to non enzymatic, reactive oxygen species mediated DNA breakage and modulation of cellular redox scavengers in hepatocellular carcinoma. Apoptosis (2016) 21:997–1007. doi: 10.1007/s10495-016-1261-2
28. Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer (2021) 30:25. doi: 10.1038/s41568-021-00417-2
29. Shanbhag V, Jasmer-McDonald K, Zhu S, Martin AL, Gudekar N, Khan A, et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc Natl Acad Sci USA (2019) 116(14):6836–41. doi: 10.1073/pnas.1817473116
30. Syed DN, Afaq F, Mukhtar H. Pomegranate derived products for cancer prevention. Semin Cancer Biol (2007) 17:377–85. doi: 10.1016/j.semcancer.2007.05.004
31. Jain A, Martin MC, Parveen N, Khan NU, Parish JH, Hadi SM. Reactivities of flavonoids with different hydroxyl substituents for the cleavage of DNA in the presence of Cu(II). Phytother Res (1999) 13:609–12. doi: 10.1002/(SICI)1099-1573(199911)13:7<609::AID-PTR566>3.0.CO;2-I
32. Arif H, Rehmani N, Farhan M, Ahmad A, Hadi SM. Mobilization of copper ions by flavonoids in human peripheral lymphocytes leads to oxidative DNA breakage: A structure activity study. Int J Mol Sci (2015) 16:26754–69. doi: 10.3390/ijms161125992
33. Arif H, Sohail A, Farhan M, Rehman AA, Ahmad A, Hadi SM. Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int J Biol Macromol (2018) 106:569–78. doi: 10.1016/j.ijbiomac.2017.08.049
34. Azmi AS, Bhat SH, Hadi SM. Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: implications for anticancer properties. FEBS Lett (2005) 579:3131–5. doi: 10.1016/j.febslet.2005.04.077
35. Azmi AS, Bhat SH, Hanif S, Hadi SM. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS Lett (2006) 580:533–8. doi: 10.1016/j.febslet.2005.12.059
36. Oak MH, Bedoui JE, Madeira SVF, Chalupsky K, Schini-Kerth VB. Delphinidin and cyanidin inhibit PDGFAB-induced VEGF release in vascular smooth muscle cells by preventing activation of p38 MAPK and JNK. Br J Pharmacol (2006) 149:283–90. doi: 10.1038/sj.bjp.0706843
37. Nyman NA, Kumpulainen JT. Determination of anthocyanidins in berries and red wine by high-performance liquid chromatography. J Agric Food Chem (2001) 49:4183–7. doi: 10.1021/jf010572i
38. Frank T, Netzel M, Strass G, Bitsch R, Bitsch I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can J Physiol Pharmacol (2003) 81:423–35. doi: 10.1139/y03-038
39. Ebadi M, Swanson S. The status of zinc, copper and metallothionien in cancer patients. Prog Clin Biol Res (1988) 259:161–75.
40. Margalioth EJ, Udassin R, Cohen C, Maor J, Anteby SO, Schenker JG. Serum copper level in gynaecologic malignancies. Am J Obstet Gynaecol (1987) 157:93–6. doi: 10.1016/S0002-9378(87)80353-8
41. Yoshida D, Ikeda Y, Nakazawa S. Quantitative analysis of copper, zinc and copper/zinc ratio in selective human brain tumors. J Neurooncol (1993) 16:109–15. doi: 10.1007/BF01324697
42. Ebara M, Fukuda H, Hanato R, Saisho H, Nagato Y, Suzuki J, et al. Relationship between zinc, copper and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol (2000) 33:415–22. doi: 10.1016/S0168-8278(00)80277-9
43. Zheng LF, Wei QY, Cai YJ, Fang JG, Zhou B, Yang L, et al. DNA Damage induced by resveratrol and its synthetic analogs in the presence of Cu(II) ions: mechanism and structure–activity relationship. Free Radic Biol Med (2006) 41:1807–16. doi: 10.1016/j.freeradbiomed.2006.09.007
44. Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin-3-gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett (1998) 129:173–9. doi: 10.1016/S0304-3835(98)00108-6
45. Lu J, Ho CT, Ghai G, Chen KY. Differential effects of theaflavinmonogallates on cell growth, apoptosis, and cox-2 gene expression in cancerous versus normal cells. Cancer Res (2000) 60:6465–71.
46. Orsolic N, Terzic S, Sver L, Basic I. Honey-bee products in the prevention and therapy of murine transplantable tumors. J Sci Food Agric (2005) 85:363–370. doi: 10.1002/jsfa.2041
47. Mukhtar H, Katiyar SK, Agarwal R. Green tea and skin-anticarcinogenic effects. J Invest Dermatol (1994) 102:3–7. doi: 10.1111/1523-1747.ep12371720
48. Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci (2001) 98:10350–5. doi: 10.1073/pnas.171326098
49. Farhan M, Rizvi A, Naseem I, Hadi SM, Ahmad A. Targeting increased copper levels in diethylnitrosamine induced hepatocellular carcinoma cells in rats by epigallocatechin-3-gallate. Tumor Biol (2015) 36:8861–7. doi: 10.1007/s13277-015-3649-y
50. Pryor WA. Why is hydroxyl radical the only radical that commonly adds to DNA? hypothesis: it has rare combination of high electrophilicity, thermochemical reactivity and a mode of production near DNA. Free Radic Biol Med (1988) 4:219–33. doi: 10.1016/0891-5849(88)90043-3
51. Chevion M. Site-specific mechanism for free radical induced biological damage. the essential role of redox-active transition metals. Free Radic Biol Med (1988) 5:27–37. doi: 10.1016/0891-5849(88)90059-7
52. Zubair H, Azim S, Khan HY, Ullah MF, Wu D, Singh AP, et al. Mobilization of Intracellular Copper by Gossypol and Apogossypolone Leads to Reactive Oxygen Species-Mediated Cell Death: Putative Anticancer Mechanism. International J Molecular Sciences (2016) 17:973. doi: 10.3390/ijms17060973
53. Burkitt MJ, Milne L, Nicotera P, Orrenius S. 1,10-phenanthroline stimulates internucleosomal DNA fragmentation in isolated rat liver nuclei by promoting redox activity of endogenous copper ions. Biochem J (1996) 313:163–9. doi: 10.1042/bj3130163
54. Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest (2003) 111:785–93. doi: 10.1172/JCI200318182
55. Piwocka K, Zablocki K, Weickowski MR, Skierski J, Feiga I, Szopa J, et al. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp Cell Res (1999) 249:299–307. doi: 10.1006/excr.1999.4480
56. Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol (2001) 2:589–98. doi: 10.1038/35085008
57. Yoshino M, Haneda M, Naruse M, Htay HH, Tsuboushi R, Qiao SL, et al. Prooxidant activity of curcumin: copperdependent formation of 8-hydroxy-2-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol In Vitro (2004) 18:783–8. doi: 10.1016/j.tiv.2004.03.009
58. Noda C, He J, Takano T, Tanaka C, Kondo T, Tohyama K, et al. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid b cells. Biochem Biophys Res Commun (2007) 362:951–7. doi: 10.1016/j.bbrc.2007.08.079
Keywords: anthocyanidin, copper, reactive oxygen species, prooxidant, DNA damage, cell death
Citation: Farhan M, Rizvi A, Ali F, Ahmad A, Aatif M, Malik A, Alam MW, Muteeb G, Ahmad S, Noor A and Siddiqui FA (2022) Pomegranate juice anthocyanidins induce cell death in human cancer cells by mobilizing intracellular copper ions and producing reactive oxygen species. Front. Oncol. 12:998346. doi: 10.3389/fonc.2022.998346
Received: 19 July 2022; Accepted: 17 August 2022;
Published: 06 September 2022.
Edited by:
Mohd Wasim Nasser, University of Nebraska Medical Center, United StatesReviewed by:
Nagaraj Nagathihalli, University of Miami, United StatesCopyright © 2022 Farhan, Rizvi, Ali, Ahmad, Aatif, Malik, Alam, Muteeb, Ahmad, Noor and Siddiqui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd Farhan, bWZhcmhhbkBrZnUuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.