
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 October 2022
Sec. Cancer Epidemiology and Prevention
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.998101
This article is part of the Research Topic Clinically Prediction Models for Gastrointestinal Cancer Diagnosis and Prognosis in the Era of Precision Oncology View all 8 articles
 Zhichao Zuo1†
Zhichao Zuo1† Yafeng Peng1†
Yafeng Peng1† Ying Zeng1†
Ying Zeng1† Shanyue Lin2
Shanyue Lin2 Weihua Zeng1
Weihua Zeng1 Xiao Zhou1
Xiao Zhou1 Yinjun Zhou1
Yinjun Zhou1 Bo Li1
Bo Li1 Jie Ma3
Jie Ma3 Mingju Long4
Mingju Long4 Shenghui Cao4*
Shenghui Cao4* Yang Liu5*
Yang Liu5*Objective: The standard treatment for stage II–III gastroesophageal junction adenocarcinoma (GEJA) remains controversial, and the role of radiotherapy (RT) in stage II–III GEJA is unclear. Herein, we aimed to evaluate the prognosis of different RT sequences and identify potential candidates to undergo neoadjuvant RT (NART) or adjuvant RT (ART).
Materials and methods: In total, we enrolled 3,492 patients with resectable stage II–III GEJA from the Surveillance, Epidemiology, and End Results (SEER) database, subsequently assigned to three categories: T1–2N+, T3–4N−, and T3–4N+. Survival curves were evaluated using the Kaplan–Meier method along with the log-rank test. We compared survival curves for NART, ART, and non-RT in the three categories. To further determine histological types impacting RT-associated survival, we proposed new categories by combining the tumor, node, and metastasis (TNM) stage with Lauren’s classification.
Results: ART afforded a significant survival benefit in patients with T1–2N+ and T3–4N+ tumors. In addition, NART conferred a survival advantage in patients with T3–4N+ and T3–4 exhibiting the intestinal type. Notably, ART and NART were both valuable in patients with T3–4N+, although no significant differences between treatment regimens were noted.
Conclusions: Both NART and ART can prolong the survival of patients with stage II–III GEJA. Nevertheless, the selection of NART or ART requires a concrete analysis based on the patient’s condition.
Previously, gastroesophageal junction adenocarcinoma (GEJA) and gastric cancer have been regarded as the same disease and treated using identical methods. Based on the current perspective, significant differences exist between GEJA and gastric cancer in terms of etiology and epidemiology. Therefore, it is necessary to examine GEJA as a distinct disease (1). Given the unique anatomical location of GEJA, the optimal treatment strategy remains controversial. Currently, surgery is considered the most effective treatment option for GEJA. However, patients with stage II and III GEJA reportedly exhibit poor survival rates with surgical intervention alone, and there is a high recurrence rate within 2 years, even after complete resection (2). Therefore, a perioperative treatment strategy is crucial for patients with GEJA, especially in advanced stages.
Based on the results of the Intergroup-0116 (INT-0116) trial conducted in the United States, adjuvant radiotherapy (ART) has become an important treatment strategy for resectable GEJA, and subgroup analysis revealed that individuals with Lauren’s classification-intestinal type were more likely to benefit from ART (3). However, the ARTIST and ARTIST-II trials performed in Korea have presented contrasting opinions, demonstrating that ART could not considerably reduce the incidence of recurrence post-D2 gastrectomy, which is also documented in the CRITICS trial (4–6). In addition, the subgroup analysis of ARTIST has afforded a similar result, indicating that ART failed to benefit patients with the intestinal type. Accordingly, the role of neoadjuvant radiotherapy (NART) in gastric cancer and GEJA has been explored. According to the findings of a meta-analysis, NART could improve the survival of patients with GEJA (7). This result has been supported by several randomized clinical trials (8–10). NART is a promising strategy to improve outcomes of advanced-stage GEJA. To provide additional data for clinical decision-making regarding the use of radiotherapy (RT) in advanced-stage GEJA, we compared the prognosis of different RT sequences and identified potential candidates for NART or ART.
Data of patients diagnosed with GEJA were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database (diagnosed during 2000–2019) using the SEER*Stat software (version 8.3.6). Herein, enrollment criteria were as follows: a) patients with first primary malignancy, b) patients who underwent surgery and were histologically diagnosed with stage II–III GEJA, c) reassessed pathologic tumor stage [based on the collaborative stage (CS) site information provided by the SEER database and the number of positive lymph nodes cleared], corresponding to the American Joint Committee on Cancer (AJCC) 8th TNM staging system for gastric cancer (11). The exclusion criteria were as follows: a) patients with metastasis, unclear pathological tumor stage, and missing information regarding lymph node status; b) patients with survival durations of less than 30 days; and c) patients who received pre- and postoperative RT. Figure 1 presents a flowchart of the study.
We used the International Classification of Diseases for Oncology (ICD-O, third edition) to classify gastric adenocarcinoma according to Lauren’s classification, as follows: diffuse carcinoma (8,145), linitis plastica (8,142), and signet ring cell carcinoma (8,490); intestinal type includes adenocarcinoma, not otherwise specified (8,140), adenocarcinoma, intestinal type (8,144), adenocarcinoma, tubular (8,211), and carcinoma, not otherwise specified (8,010) (12).
We retrospectively reviewed the database to determine the demographic, clinical, and biological characteristics of enrolled patients. The main outcome variables were survival status and survival time. Survival time was measured as overall survival (OS), the period from the date of surgery to the date of death or the last follow-ups.
Survival curves were evaluated using the Kaplan–Meier method along with the log-rank test. We assigned patients to three categories (T1–2N+, T3–4N−, and T3–4N+) based on the initial T or N status following the 8th AJCC staging principle; subsequently, we compared survival curves of NART, ART, and non-RT in the three patient categories. To further determine histological types that impact RT-associated survival, we proposed new categories by combining the TNM stage with Lauren’s classification. A univariate Cox regression analysis was conducted to evaluate prognostic factors, and multivariate Cox models were constructed and assessed using the survival and “pec” packages. In addition, decision curve analysis (DCA) was performed using the “DCA” package. The calibration curves were plotted using the “rms” package to assess the calibration of the model. All analyses were performed using R version 4.0.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Herein, we enrolled 3,492 patients with resectable stage II–III GEJA who received NART or ART. In total, 2,825 (80.9%) patients were male, with a median age of 65 years (range, 20–90 years). Among these patients, 2,098 (60.3%) received RT (826 received ART, and 1,272 received NART). Factors associated with the RT sequence are presented in Table 1, including sex, age, marital status, race, grade, histological type, surgery, number of examined lymph nodes (ELNs), T stage, N stage, and chemotherapy.
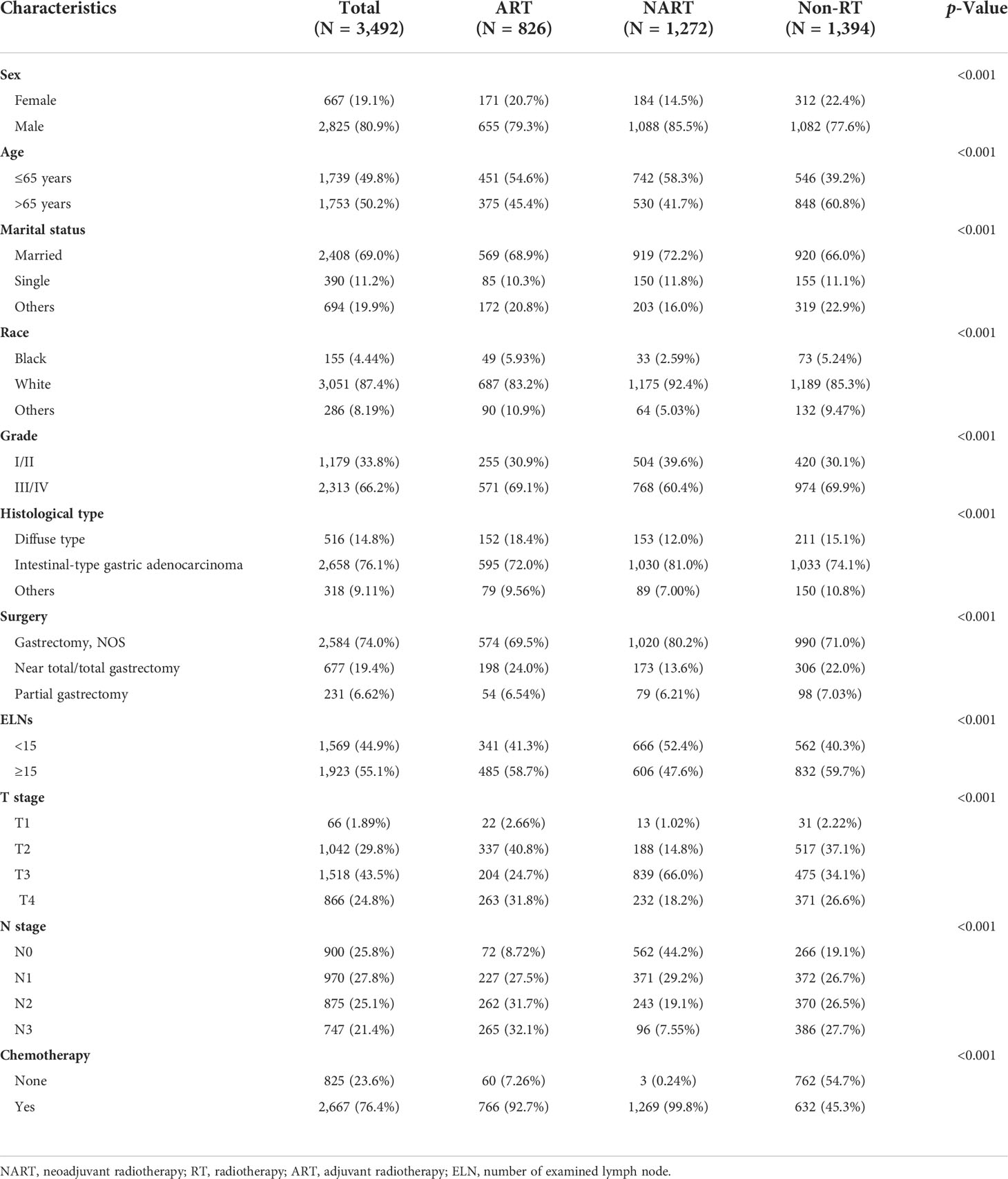
Table 1 Clinical and pathological features in patients with stage II–III gastroesophageal junction adenocarcinoma, stratified by RT sequence.
In the present study, the median and maximum follow-up periods were 104 and 191 months, respectively. The 5-year overall survival rates were 31.3%, 33.4%, and 23.1% for ART, NART, and non-RT, respectively. Considering that the T stage and nodal status can impact RT in gastric cancer (13), the patients were assigned to three categories: T1–2N+, T3–4N−, and T3–4N+. Based on the survival analysis, ART (p < 0.001) but not NART (p = 0.089) could benefit patients with the T1–2N+ stage, as presented in Figure 2. As shown in Figure 3, both ART and NART failed to afford survival benefits in patients with T3–4N− (both p = 0.200). As shown in Figure 4, both NART and ART conferred survival benefits in patients with T3–4N+ (both p < 0.001), although no significant differences were noted between the two RT sequences (p = 0.290).
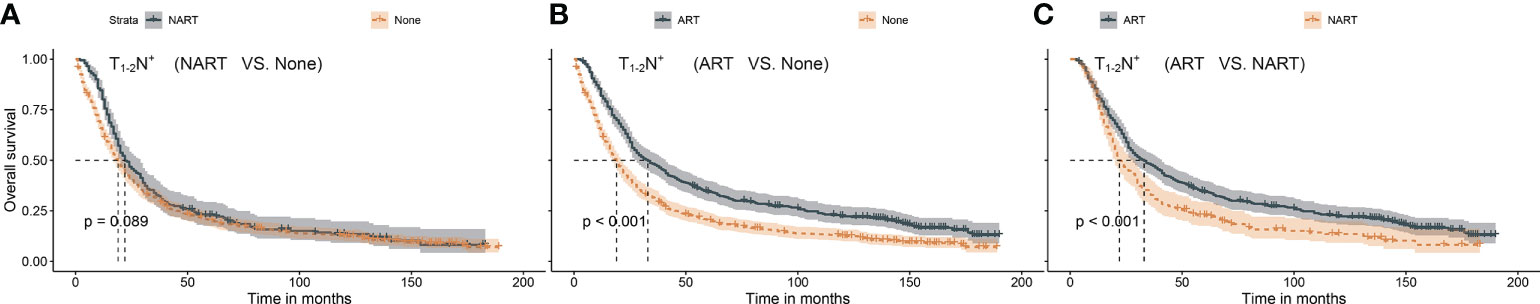
Figure 2 (A–C) Survival benefit after NART or ART in patients with T1–2N+. (A) NART failed to benefit patients with T1–2N+. (B) ART prolonged survival in patients with T1–2N+. (C) Overall survival after NART was worse than that after ART in patients with T1–2N+. NART, neoadjuvant radiotherapy; ART, adjuvant radiotherapy.
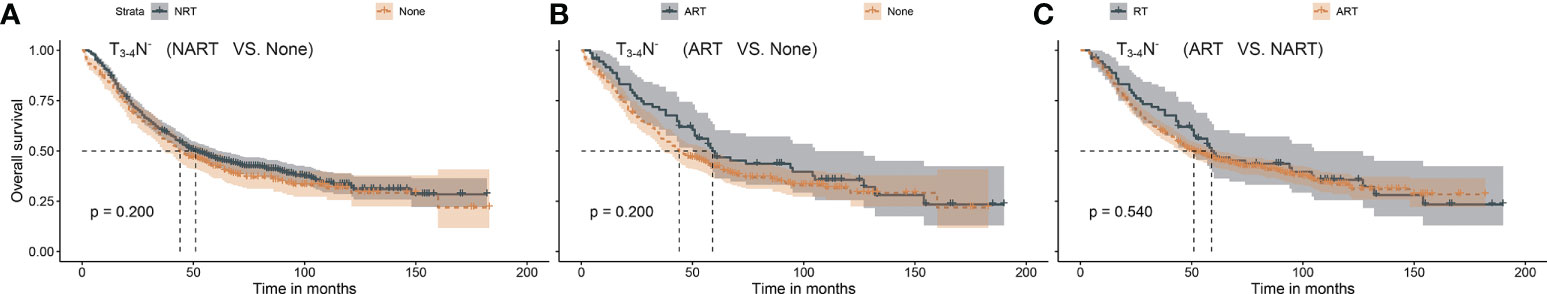
Figure 3 (A–C) Survival benefit after NART or ART in patients with T3–4N−. Patients with T3–4N− had no survival advantage after any ART or NART. NART, neoadjuvant radiotherapy; ART, adjuvant radiotherapy.
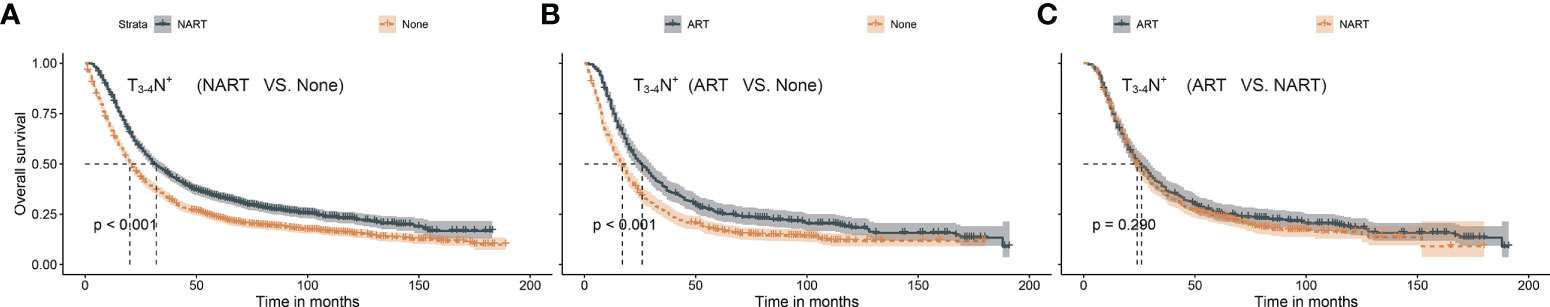
Figure 4 (A–C) Survival benefit after NART or ART in patients with T3–4N+. Survival benefit was obtained after both NART and ART but did not differ significantly between the two RT sequences. NART, neoadjuvant radiotherapy; ART, adjuvant radiotherapy.
In summary, ART conferred significant survival benefits in patients with T1–2N+ and T3–4N+, whereas survival advantages after NART were only noted in patients with T3–4N+. In a previous report (14), patients with Lauren’s classification-intestinal type were found to exhibit a response to NART. Therefore, we proposed a new category based on the intestinal type of Lauren’s classification in patients with T3–4. As shown in Figure 5, patients with T3–4 presenting intestinal type benefited from NART (p < 0.001) rather than ART (p = 0.160).
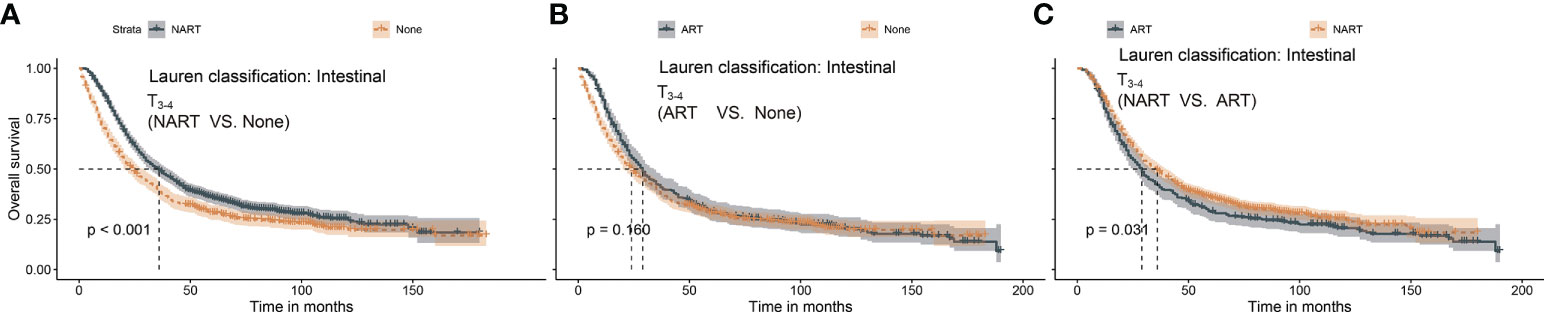
Figure 5 (A–C) Survival benefit after NART or ART in T3–4 patients with intestinal type. (A) NART prolonged survival in T3–4 patients with intestinal type. (B) ART failed to benefit survivors in T3–4 patients with intestinal type. (C) Overall survival after ART was worse than that after NART in T3–4 patients with intestinal type. NART, neoadjuvant radiotherapy; ART, adjuvant radiotherapy.
The results of univariate Cox regression analysis revealed that several clinical and biological characteristics, such as RT sequence, age, tumor grade, histological type, surgery, ELNs, TNM stage, and chemotherapy, were associated with prognosis. Based on the subsequent multivariate Cox regression analysis, patients who received NART or ART, ≤65 years of age, marital status (married), lower tumor grade, partial gastrectomy, ELNs ≥ 15, intestinal type, and chemotherapy were protective factors, whereas advanced TNM stage was a significant risk factor (Figure 6; Table 2). A superior risk threshold probability of 5% to 22% was observed in the DCA of the net benefit when compared with the baseline (Figure 7).
The Cox model well-fitted the observed data, as shown by the calibration plot, in which the calibration curve overlapped with the diagonal of the reference line of perfect calibration (Figure 8).
Previously, patients with gastric cancer patients and those with GEJA have been considered the same population, given that both patient groups received identical treatments. Subsequently, growing data have suggested that GEJA should be considered an independent disease (15, 16). Accordingly, in the present study, we aimed to identify a precise treatment strategy for patients with stage II–III GEJA. Herein, we employed a large population-based cohort that included representative demographic, clinical, and biological characteristics to examine the prognostic significance of NART and ART in patients with stage II–III GEJA. Our study evaluated the differences in survival in patients who underwent NART or ART, grouped by TNM stage and histological type. These results may be valuable for clinicians in screening potential candidates who would benefit from NART or ART.
Complete resection of the primary tumor regional lymph nodes is pivotal for improving the prognosis of patients with resectable GEJA. GEJA has a distinct lymph node drainage pathway in esophageal and gastric cancers (15). The extent of lymph node resection remains debatable, posing a challenge for the surgeon to ensure R0 resection in patients with stage II–III GEJA. Historically, RT has been the standard adjuvant treatment for gastric cancer, based on reported randomized controlled trials (3). The Korean ARTIST-1 study has demonstrated that adjuvant chemoradiotherapy can improve disease-free survival when compared with adjuvant chemotherapy alone in gastric/gastroesophageal junction adenocarcinomas (16). Kim et al. (17) have shown that chemoradiation could improve locoregional recurrence-free survival in stage III gastric cancer treated with R0 gastrectomy and D2 lymph node dissection when compared with chemotherapy. Few studies have investigated the effect of ART in patients with stage II–III GEJA. Herein, we found that ART afforded significant survival benefits in patients with T1–2N+ and T3–4N+ tumors. Thus, we speculated that lymph node status might play a predominant role in mediating the positive effects of ART, and the results of the present study corroborate those of previous studies (18). However, whether a patient undergoes complete postoperative RT should be determined based on the patient’s physical condition, such as chronic underlying diseases, limited functional capacity, and poor clinical condition.
Considering the low completion rate of ART, it is of considerable importance to explore the impact of NART in stages II–III GEJA. NART can help reduce the preoperative stage and improve the likelihood of R0 resection. Moreover, patients exhibit better tolerance to the full course of NART than to ART. Based on the findings of our study, survival advantages afforded by NART could be noted in patients with T3–4N+ and T3–4 with intestinal type, which was similar to the findings of a previous study (14), which failed to consider the implications of ART in GEJA. According to Shridhar et al., NART could improve survival rates in patients with lymph node involvement; however, the authors failed to perform subgroup analysis by T stage stratification (19). In the present study, NART did not afford survival benefits in patients with T3–4N− and T1–2N+ stages. This finding might indicate that benefits conferred by NART may fail to significantly surpass NART-induced side effects in patients with a low primary tumor burden or those without regional lymph node metastasis (7). In brief, both NART and ART could provide additional survival benefits in the T3–4N+ GEJA subgroup. For T3–4N− and T1–2N+ stages, the application of NART needs to be further explored, considering new RT techniques, different dosages, and fractionation schemes.
In the prognosis analysis, multivariate Cox regression identified that receiving NART or ART, ≤65 years of age, married, lower tumor grade, partial gastrectomy, ELNs ≥ 15, intestinal type, and chemotherapy were protective factors, whereas an advanced TNM stage was a significant risk factor. A low number of ELNs was associated with decreased overall survival, consistent with previous studies (18, 19). In addition, our study revealed that being married could be a protective factor for better outcomes. Wang et al. (20) suggested that married patients potentially experienced less distress and depression after a cancer diagnosis and received spousal encouragement, enabling them to accept treatment better. Moreover, diffuse-type gastric adenocarcinoma exhibited an overall poorer prognosis than the intestinal type, consistent with a study by Tang et al. (21).
However, the limitations of this study need to be addressed. First, this study lacked accurate information regarding RT dosage, irradiation site, and RT techniques. Second, surgical margins are a critical factor affecting patient outcomes; we could not obtain this information from the SEER database. Finally, we failed to verify the results externally by assessing non-database cases. Therefore, the conclusions of this study require further validation.
Both NART and ART can prolong the survival rate of patients with stage II–III GEJA. ART may afford survival benefits in patients presenting the T1–2N+ stage. Patients in stage T3–4 with intestinal type could benefit from NART. Moreover, NART and ART could positively impact the prognosis of patients with T3–4N+ GEJA.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
YL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ZZ, YP and YZ. Acquisition, analysis, or interpretation of data: ZZ, SL, WZ and XZ. Drafting of the manuscript: ZZ, YJZ, BL, JM and ML. Statistical analysis: ZZ, YP, YZ, SL, WZ and XZ. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol (2013) 23:3–9. doi: 10.1016/j.semradonc.2012.09.008
2. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol (2014) 32:385–91. doi: 10.1200/JCO.2013.51.2186
3. Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol (2012) 30:2327–33. doi: 10.1200/JCO.2011.36.7136
4. Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol (2012) 30:268–73. doi: 10.1200/JCO.2011.39.1953
5. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A randomized phase III trial comparing adjuvant single-agent S1, s-1 with oxaliplatin, and postoperative chemoradiation with s-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial (☆). Ann Oncol (2021) 32:368–74. doi: 10.1016/j.annonc.2020.11.017
6. Dikken JL, van Sandick JW, Maurits Swellengrebel H, Lind PA, Putter H, Jansen EP, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer (2011) 11:329. doi: 10.1186/1471-2407-11-329
7. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol (2011) 12:681–92. doi: 10.1016/S1470-2045(11)70142-5
8. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
9. Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer (2015) 15:532. doi: 10.1186/s12885-015-1529-x
10. Slagter AE, Jansen EPM, van Laarhoven HWM, van Sandick JW, van Grieken NCT, Sikorska K, et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer (2018) 18:877. doi: 10.1186/s12885-018-4770-2
11. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg (2017) 6:119e30. doi: 10.21037/acs.2017.03.14
12. Tang CT, Zeng L, Yang J, Zeng C, Chen Y. Analysis of the incidence and survival of gastric cancer based on the Lauren classification: A Large population-based study using SEER. Front Oncol (2020) 10:1212. doi: 10.3389/fonc.2020.01212
13. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med (2001) 345:725–30. doi: 10.1056/NEJMoa010187
14. Zhou YJ, Lu XF, Meng JL, Wang XY, Zhang QW, Chen JN, et al. Neo-adjuvant radiation therapy provides a survival advantage in T3-T4 nodal positive gastric and gastroesophageal junction adenocarcinoma: a SEER database analysis. BMC Cancer (2021) 21:771. doi: 10.1186/s12885-021-08534-9
15. Pedrazzani C, de Manzoni G, Marrelli D, Giacopuzzi S, Corso G, Minicozzi AM, et al. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J Thorac Cardiovasc Surg (2007) 134:378–85. doi: 10.1016/j.jtcvs.2007.03.034
16. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol (2015) 33:3130–6. doi: 10.1200/JCO.2014.58.3930
17. Kim TH, Park SR, Ryu KW, Kim YW, Bae JM, Lee JH, et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys (2012) 84:e585-92. doi: 10.1016/j.ijrobp.2012.07.2378
18. Wang P, Zhou H, Han G, Ni Q, Dai S, Huang J, et al. Assessment of the value of adjuvant radiotherapy for treatment of gastric adenocarcinoma based on pattern of post-surgical progression. World J Surg Oncol (2021) 19:205. doi: 10.1186/s12957-021-02304-4
19. Shridhar R, Dombi GW, Finkelstein SE, Meredith KL, Hoffe SE. Improved survival in patients with lymph node-positive gastric cancer who received preoperative radiation: an analysis of the surveillance, epidemiology, and end results database. Cancer (2011) 117:3908–16. doi: 10.1002/cncr.25995
20. Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: An analysis of the surveillance, epidemiology and end results (SEER) database. Cancer Epidemiol (2018) 54:119–24. doi: 10.1016/j.canep.2018.04.007
Keywords: neoadjuvant radiotherapy, adjuvant radiotherapy, survival, gastric cancer, gastroesophageal junction adenocarcinoma (GEJA)
Citation: Zuo Z, Peng Y, Zeng Y, Lin S, Zeng W, Zhou X, Zhou Y, Li B, Ma J, Long M, Cao S and Liu Y (2022) Survival benefit after neoadjuvant or adjuvant radiotherapy for stage II–III gastroesophageal junction adenocarcinoma: A large population-based cohort study. Front. Oncol. 12:998101. doi: 10.3389/fonc.2022.998101
Received: 19 July 2022; Accepted: 30 September 2022;
Published: 20 October 2022.
Edited by:
Irene X. Y. Wu, Central South University, ChinaReviewed by:
Weishi Liang, Beijing Chaoyang Hospital, ChinaCopyright © 2022 Zuo, Peng, Zeng, Lin, Zeng, Zhou, Zhou, Li, Ma, Long, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenghui Cao, ODM2NDIxNTQ4QHFxLmNvbQ==; Yang Liu, bGl1eWFuZ2d4bXVAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.