
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 September 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.997665
This article is part of the Research Topic Insights in Molecular and Cellular Oncology: 2022 View all 23 articles
We aimed to analyze the levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interleukin-12 (IL-12p70) in colorectal cancer and evaluate the predictive significance of clinical efficacy of patients with colorectal cancer treated with anti-vascular therapy combined with chemotherapy. A retrospective study of 162 patients with colorectal cancer in Fujian Medical University Hospital was conducted from January 2019 to December 2020. A comparative analysis of the levels of IL-6, TNF-α and IL-12p70 between the two groups were studied. The relationship between the levels and the clinical characteristics of patients was observed; the factors affecting the levels of IL-6, TNF-α, and IL-12p70 in colorectal cancer patients were analyzed, and the predictive validity of the efficacy of anti-vascular therapy was evaluated. We observed that the individual expression levels of IL-6, TNF-α and IL-12p70 in the patients with colorectal cancer are related to lymph node metastasis, TNM staging, and degree of differentiation (P<0.05); however, they are irrelevant to the age, sex, and tumor location of patients with colorectal cancer (P>0.05). The multiple stepwise regression analysis indicates that lymph node metastasis and TNM staging are independent risk factors that correlate with IL-6 and IL-12p70 levels in colorectal cancer patients (P<0.01). The degree of differentiation was found to be an independent risk factor connected to TNF- α levels of patients with colorectal cancer. The change of IL-12p70 level could predict the validity of anti-vascular treatment for advanced colorectal cancer. When evaluated for combined expression, IL-6 and IL-12p70 in patients with colorectal cancer closely related to lymph node metastasis and TNM staging. IL-12p70 can be used as a predictor of anti-vascular therapy with colorectal cancer.
The incidence of colorectal cancer is increasing in China (1). Early symptoms are not obvious and there is a lack of convenient and effective specific screening indicators (2). Most patients are already in the advanced stage when they undergo treatment (3). Cytokines such as tumor necrosis factor (TNF) and interleukins (ILs) are closely associated with the differentiation and proliferation of tumor cells and the formation of tumor neovascularization (4), which has an important impact on the progression of patients with tumor. They can be used as a reference index for the clinical outcome and follow-up of patients with colorectal cancer (5). The progression and metastasis of tumors depend on tumor blood vessels. VEGF, which is known to be the only growth factor acting specifically on vascular endothelial cells and directly involved in inducing tumor angiogenesis, strongly induces vascular growth. The over-expression of VEGF itself and its receptor is closely associated with tumor growth, invasion, and metastasis (6).
Tumor angiogenesis is controlled by pro-angiogenic and anti-angiogenic factors, which are secreted by tumor cells, stromal cells, inflammatory cells, or other circulating cells. The balance of cytokines and chemokines affects the efficacy of VEGF inhibitors (7, 8). In addition, VEGF inhibitors, which curb the secretion of vascular endothelial growth factor and complex interactions in the tumor micro-environment, affect the secretion levels of the above-mentioned various factors (9, 10). The baseline expression levels of cytokines and angiogenic factors affect drug activity that can be eventually utilized to predict the beneficiaries, determine the best drug dose, and clarify the mechanism of drug resistance. According to published studies, circulating endothelial cells, soluble VEGF receptor 2, and VEGF are all blood-based biomarkers and have been certificated to be utilized for evaluation of the efficacy of multiple VEGF receptor inhibitors (11, 12). It has been suggested that the baseline levels of circulating VEGF can be used to predict the clinical benefit or tumor response of these drugs, but whether cytokines can be used as a predictor of treatment efficacy needs further verification (13). Multiple technologies provide a non-invasive and convenient method to detect the expression of multiple cytokines based on a small amount of plasma, which offers the possibility of exploring the predictive potential of cytokines (14).
The first-line conventional therapy for metastatic colorectal cancer is bevacizumab (15). It is a re-combined humanized monoclonal antibody directed against VEGF-A. However, the effective rate is only about 60% (16). We selected 162 patients with colorectal cancer from Fujian Medical University Hospital to analyze the correlation between cytokine expression and clinical characteristics in patients at various stages, further analyzing the changes of cytokines before and after treatment of Bevacizumab. The study also analyzed the correlation of tumor regression of patients with late colorectal cancer and initially exploring the predictive significance of cytokines for the efficacy of treatment.
The study included 196 cases in Fujian Tumor Hospital between January 2019 and June 2020. The study was approved by the Ethics Committee of the Fujian Medical University (10-2018) and informed consent was obtained from all patients.
All patients were pathologically diagnosed as suffering from colorectal cancer. Stage I-III patients were treated with radical surgery and stage IV patients should meet the following conditions: (1) clinically, there are measurable lesions; (2) KPS equal to 80 or more; aged 18 years old or above; expected survival time above 3 months; (3) good bone marrow reservation function, normal heart, liver and kidney function and blood coagulation function; (4) no history of traumatic surgery within 4 weeks and no brain metastasis, with imaging showing no important vascular invasion, no uncontrollable hypertension (systolic blood pressure is 150 mmHg and/or diastolic blood pressure is below 100 mmHg), no active cardiovascular and cerebrovascular disease within 6 months; (5) at least after the treatment (3 -week plan for 2 cycles, 2 -week plan for 3 cycles).
162 cases met the selection criteria, including 77 males and 85 females aged 53-78, the medium age of which was 65.16 ± 4.25; 84 cases with left and right colon and sigmoid colon cancer, 78 cases with rectal cancer, 77 cases with tubular adenocarcinoma and 84 cases with mucinous adenocarcinoma; 52 cases with medium/high differentiation, 110 cases with poor differentiation; 113 cases under the treatment of radical surgery, 12 cases palliative surgery; 25 cases on the first-line chemotherapy on the basis of oxaliplatin; 24 cases on the foundation of irinotecan; 74 cases in stage I-II, 88 cases in stage III-IV, and 49 cases in stage IV.
The dose of Bevacizumab was 5mg/kg, intravenous drip for 30 to 90 minutes, once every 2 weeks. The dose of Bevacizumab was 7.5mg/kg, intravenous drip for 30 to 90 minutes, once every 3 weeks. FOLFOX6 regimen: Oxaliplatin (OXA) 100mg/m2, day 1: 2 hours of intravenous drip, Levoleucovorin (LV) 400 mg/m2, intravenous infusion for 2 hours; 5-fluorouracil (5 -FU) 400mg/m2, intravenous drip, the first day; 5-FU 2400~3000mg/m2, continuous pumping for 46 hours. Every 14 days constituted a cycle. FOLFIRI regiment: Irinotecan (CPT-11) 180 mg/m2, intravenous infusion for 90 minutes for the first day; LV 200mg/m2, intravenous infusion for 2 hours for the first day; LV 400 mg/m2, intravenous infusion, the first day; 5-FU 2400mg/m2, pumped continuously for 46 hours. 14 days constituted a period. XELOX regiment: Oxaliplatin (OXA) 100 mg/m2, intravenous drip for 2 hours on the first day; capecitabine (CAP) 1000 mg/m2 for the first fourteenth days (17). There were 21 days for a period. The chemotherapy regiment was administered simultaneously with bevacizumab.
Treatment of 3 weeks and 6 weeks, (1) taking heparin or EDTA anticoagulant whole blood (100 μl/part), set 12 × 75 mm in the special plastic test tube. (2) 20 μl of specific fluorescent monocompans was added per tube; the other with the transfection of a fluorescently labeled anti-parallel and staining at room temperature for 15 minutes. (3) Dissolve red blood cells with the help of a Q-Prep instrument, subsequently stabilizing and fixing white blood cells and locating for 5 minutes. (4) Detect with the upper flow cytometry.
(1) taking heparin or EDTA anticoagulated whole blood (100μl/part) and locating in a 12×75mm special plastic test tube. (2) Adding 50μl of specific monoclonal antibody (primary antibody) and incubating for 30minutes at room temperature. (3) Depositing on the Q-PREP instrument to dissolve red blood cells, stabilizing and fixing white blood cells. (4) Centrifuging (800-1000 rpm, 5 min) to discard the serum, washing the cells twice with PBS. (5) Adding 50μl of fluorescent (FITC or PE)-labeled secondary antibody, locating for 30minues in the darkness at room temperature. (6) Finally, detecting with the upper flow cytometry.
The short-term efficacy evaluation criteria of solid tumors refers to the RECIST 1.1 version (18), classified as complete remission (CR), partial remission (PR), disease progression (PD), stable disease (SD). Calculate the response rate (RR) with CR+PR and disease control rate (DCR) with CR+PR+SD. Adverse reactions were evaluated in accordance with the General Toxicity Criteria (NCI-CTC) version 3.0 set by the National Cancer Institute. (Because the median survival period has not reached as of the follow-up endpoint of this study, progression-free survival (PFS) is used as the main observational indicator).
In this study, telephone and imaging follow-up were used, with no loss of patients. Progression-free survival (PFS) is the time from the beginning of treatment to disease progression or death, while overall survival (OS) is that from the beginning of treatment to death or the last follow-up. The deadline for follow-up is November 31, 2020, with the follow-up time 23 months and the median follow-up time 11 ± 0.2 months.
We used SPSS17.0 statistical software to analyze data, Kaplan-Meier method to perform the survival analysis and the Pearson card testing to correlate analysis of data. The difference value (p <0.05) is statistically significant.
Our initial goal was to assess levels of cytokines individually in colorectal cancer patients. We started with the evaluation of levels of IL-6, TNF-α and IL-12P7, and observed that IL-6 levels are associated with lymph node metastasis and the TNM staging in patients with colorectal cancer (t = 6.705, -4.224, P <0.05). TNF-α levels, on the other hand, related to the degree of differentiation (t = -213, P <0.05) while IL-12P7 levels correlated with lymph node metastasis and TNM staging of patients (t = -2.713, 2.681, P <0.01). Also, IL-6, TNF-α and IL-12P7 levels did not correlate with age, gender, tumor site and histological classification (P> 0.05) (Table 1).

Table 1 The single factor analysis result of cytokine expression levels in patients with colorectal cancer.
After performing the evaluation of IL-6, TNF-α and IL-12P7 individually, we next checked for their combined expression. Multi-factor analysis of IL-6, TNF-α and IL-12P7 levels in patients refers to three factors (lymph node metastasis, historical score and TNM staging) as the argument, which uses the expression of IL-6, TNF-α and IL-12P7 as the dependent variable by multi-step regression analysis. The results indicated that lymph node metastasis and TNM staging are both independent risk factors connected to IL-6 and IL-12P7; the degree of differentiation being an independent risk factor (P <0.01) connected with TNF-α level (P <0.01) (Tables 2–4).
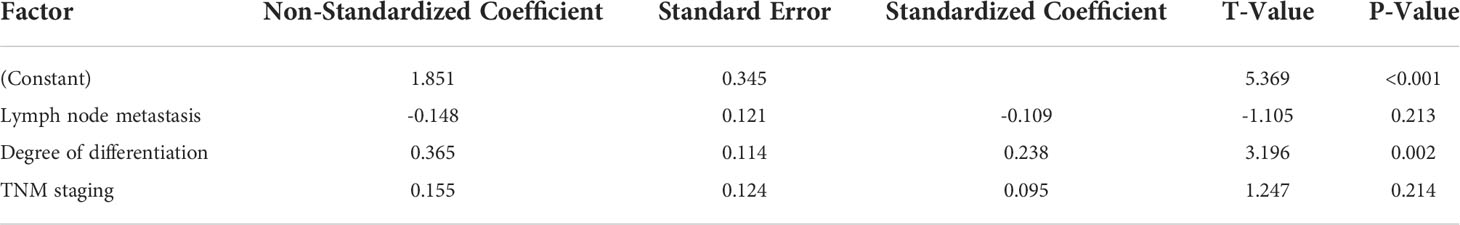
Table 3 Multi-factor analysis of the influencing factors of the TNF-α level in patients with colorectal cancer.
Next, we checked the patient prognosis. The average treatment time for patients was 52 days (14 to 84 days) in stage IV. Among the 49 patients, 32 had shrinkage of their tumors: 17 had tumor progression. The treatment efficiency was found to be 65.3%, the aggrandizement range being 6% -41% and the reduction range being 6% -53% (Figure 1).
The baseline level of IL-12p7 in patients was found to be associated with tumor reaction. We observed that, under IL-12P7 treatment, the variation is significantly associated with tumor reduction, and both are negatively correlated, which can be the marker for predicting treatment efficacy (Figure 2).
Inflammatory factors are mainly affected by changing cellular microenvironments to promote cell proliferation and induce cellular carcinogenic or tumor suppressor gene mutation (19). Many inflammatory factors exist in the tumor microenvironment, including IL-1, IL-6, IL-12, IL-17, TNF-α and TGF-β (20–22). They not only play the role of raising inflammatory cells to tumor parts; zooming in inflammation but also promoting tumor cell growth and metastasis; meantime accelerating tumor blood vessel and lymphatic production. Tumor angiogenesis and inflammatory factors promote each other, forming malicious tumor microenvironment (23). Therefore, by exploring the interrelation between anti-angiogenesis and cytokines, it is desirable to find possible anti-angiogenic effective biomarkers. This paper examines the interrelation among IL-6, TNF-α and IL-12P7 secreted by Th1 and Th2 with the attempt to indicate the relationship between tumor angiogenesis and tumor immunocellular environmental reprogramming.
IL-6 can induce the formation of signals, transduction protein and transcriptional activation factor-3 and makes joint efforts in TGF-β1 generation and epithelial conversion, inhibiting T-cell proliferation and killing effect and activating Treg, which plays the key role in the immunization of colorectal cancer and is related to the adverse prognosis (24). TNF-α has the function of modifying ribosome C (for biochemistry) which can improve the mismatched risk of chromosome DNA and inhibit the activity of mismatched repaired factor CYC. In addition, TNF-α can also increase the malignancy and adhesion of tumor cells to vascular endothelial or lymphatic endothelium, and tumor cells through lymphatic or blood-transfer risk (25, 26).
Our results show that the IL-6 levels of patients with neutral colorectal cancer in late clinical staging were higher (P <0.05) and the TNF-α levels of patients with low division were higher (P<0.05). In terms of installment, IL-12 levels of patients with no lymph node metastasis were higher (P<0.05). Studies have found that the level of expression of related cytokines of patients whose tumor diameters are longer has increased significantly, but the influence of cytokines on tumor diameters needs further examination (27). It is believed that the possible mechanism of cytokines affected the formation of new blood vessels, which therefore had an influence on the blood flow perfusion of local tumor lesions (28), however, it needs further study. According to the analysis of risk factor, we can also draw the conclusion that lymph node metastasis and TNM staging are statistically relevant to IL-6 and IL-12P7; in addition, the statistical correlation of degree of differentiation with TNF-α further suggests IL-6, TNF-α and IL-12P7 are closely related to the condition of patients. The above indicators may be involved in the progression of malignant tumors. By analyzing the factors for high expressions of IL-6 and TNF-α, it can promote transcriptional activity of tumor cell, promote the change of tumor cell biological characteristics, improve the infiltration of tumor cells to intestinal mucosal muscle layers or subcutaneous tissue, and further affect the occurrence and development of colorectal cancer.
The baseline level of IL-12 has the best correlation with tumor reaction, which is likely to be labeled as an important predictive indicator and is the main regulator of the Th1 immune responses and the known vascular production inhibitor. As far as we know, this is the reaction predictor of the cyclic IL-12 which accepts an anti-angiogenic TKI of patients. In summary, levels of IL-6, TNF-α and IL-12P7 in plasma of patients with advanced colorectal cancer increased. In addition, IL-6 and IL-12P7 are associated with TNM staging and lymph node metastasis, while TNF-α is related to differentiation. The elevation of the expressional level can predict effective outcome of antivascular therapy of colorectal cancer. Preliminary findings of this study are that there is correlation between plasma cytokines and advanced colorectal cancer, which may be a predictive indicator of anti-visional therapeutic efficacy. However, this study is to take a further clinical trial in the future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of the Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
JZ, JY, and ZZ collected patient data, JZ, XW, JY, and ZZ analyzed data and prepared manuscript, ZG supervised study and arranged experiments. All authors contributed to the article and approved the submitted version.
Startup Fund for scientific research, Fujian Medical University (No. 2018QH1224); Fujian Provincial Health Technology Project (No. 2019-2-6); Fujian Provincial Health Technology project (No. 2020J011111); Fujian Innovation project (No. 2019-CX-4).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang Y, Han Z, Li X, Huang A, Shi J, Gu J. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res (2020) 32:729–41. doi: 10.21147/j.issn.1000-9604.2020.06.06
2. Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, et al. Treatment of patients with late-stage colorectal cancer: ASCO resource-stratified guideline. JCO Glob Oncol (2020) 6:414–38. doi: 10.1200/JGO.19.00367
3. Wierdak M, Pisarska M, Kusnierz-Cabala B, Kisielewski M, Major P, Witowski JS, et al. Use of inflammatory markers in the early detection of infectious complications after laparoscopic colorectal cancer surgery with the ERAS protocol. Wideochir Inne Tech Maloinwazyjne (2018) 13:315–25. doi: 10.5114/wiitm.2018.75846
4. Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther (2020) 11:232. doi: 10.1186/s13287-020-01755-y
5. Schietroma M, Pessia B, Colozzi S, Carlei F, Clementi M, Amicucci G, et al. Septic complications after resection for middle or low rectal cancer: Role of gut barrier function and inflammatory serum markers. Dig Surg (2017) 34:507–17. doi: 10.1159/000475847
6. Nikolinakos PG, Altorki N, Yankelevitz D, Tran HT, Yan S, Rajagopalan D, et al. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res (2010) 70:2171–9. doi: 10.1158/0008-5472.CAN-09-2533
7. Maloney JP, Gao L. Proinflammatory cytokines increase vascular endothelial growth factor expression in alveolar epithelial cells. Mediators Inflammation (2015) 2015:387842. doi: 10.1155/2015/387842
8. Geindreau M, Bruchard M, Vegran F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancers (Basel) (2022) 14(10):2446. doi: 10.3390/cancers14102446
9. Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res (2008) 68:521–9. doi: 10.1158/0008-5472.CAN-07-3217
10. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell (2009) 15:232–9. doi: 10.1016/j.ccr.2009.01.021
11. Hanrahan EO, Ryan AJ, Mann H, Kennedy SJ, Langmuir P, Natale RB, et al. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res (2009) 15:3600–9. doi: 10.1158/1078-0432.CCR-08-2568
12. Watanabe T, Kobunai T, Yamamoto Y, Matsuda K, Ishihara S, Nozawa K, et al. Gene expression of vascular endothelial growth factor a, thymidylate synthase, and tissue inhibitor of metalloproteinase 3 in prediction of response to bevacizumab treatment in colorectal cancer patients. Dis Colon Rectum (2011) 54:1026–35. doi: 10.1097/DCR.0b013e31821c44af
13. Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia (2000) 2:306–14. doi: 10.1038/sj.neo.7900102
14. Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol (2010) 11:1172–83. doi: 10.1016/S1470-2045(10)70232-1
15. Burge M, Price T, Karapetis CS. First-line therapy for metastatic colorectal cancer: Current perspectives and future directions. Asia Pac J Clin Oncol (2019) 15(Suppl 1):3–14. doi: 10.1111/ajco.13119
16. Stein A, Schwenke C, Folprecht G, Arnold D. Effect of application and intensity of bevacizumab-based maintenance after induction chemotherapy with bevacizumab for metastatic colorectal cancer: A meta-analysis. Clin Colorectal Cancer (2016) 15:e29–39. doi: 10.1016/j.clcc.2015.12.005
17. Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother (2004) 38:1258–64. doi: 10.1345/aph.1D470
18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
19. Sharma T, Gupta A, Chauhan R, Bhat AA, Nisar S, Hashem S, et al. Cross-talk between the microbiome and chronic inflammation in esophageal cancer: potential driver of oncogenesis. Cancer Metastasis Rev (2022) 41:281–99. doi: 10.1007/s10555-022-10026-6
20. Xu J, Ye Y, Zhang H, Szmitkowski M, Makinen MJ, Li P, et al. Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Med (Baltimore) (2016) 95:e2502. doi: 10.1097/MD.0000000000002502
21. Huang Q, Duan L, Qian X, Fan J, Lv Z, Zhang X, et al. IL-17 promotes angiogenic factors IL-6, IL-8, and vegf production via Stat1 in lung adenocarcinoma. Sci Rep (2016) 6:36551. doi: 10.1038/srep36551
22. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev (2002) 13:143–54. doi: 10.1016/S1359-6101(01)00033-8
23. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939
24. Gunawardene A, Dennett E, Larsen P. Prognostic value of multiple cytokine analysis in colorectal cancer: a systematic review. J Gastrointest Oncol (2019) 10:134–43. doi: 10.21037/jgo.2018.07.11
25. Waljee AK, Higgins PDR, Jensen CB, Villumsen M, Cohen-Mekelburg SA, Wallace BI, et al. Anti-tumour necrosis factor-alpha therapy and recurrent or new primary cancers in patients with inflammatory bowel disease, rheumatoid arthritis, or psoriasis and previous cancer in Denmark: a nationwide, population-based cohort study. Lancet Gastroenterol Hepatol (2020) 5:276–84. doi: 10.1016/S2468-1253(19)30362-0
26. Yoshimatsu Y, Wakabayashi I, Kimuro S, Takahashi N, Takahashi K, Kobayashi M, et al. TNF-alpha enhances TGF-beta-induced endothelial-to-mesenchymal transition via TGF-beta signal augmentation. Cancer Sci (2020) 111:2385–99. doi: 10.1111/cas.14455
27. Lasek W, Zagozdzon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother (2014) 63:419–35. doi: 10.1007/s00262-014-1523-1
28. Hoving S, Seynhaeve AL, van Tiel ST, aan de Wiel-Ambagtsheer G, de Bruijn EA, Eggermont AM, et al. Early destruction of tumor vasculature in tumor necrosis factor-alpha-based isolated limb perfusion is responsible for tumor response. Anticancer Drugs (2006) 17:949–59. doi: 10.1097/01.cad.0000224450.54447.b3
Keywords: IL-6, colorectal cancer, tumor necrosis factor-alpha, il-12, anti-vascular therapy
Citation: Zheng J, Wang X, Yu J, Zhan Z and Guo Z (2022) IL-6, TNF-α and IL-12p70 levels in patients with colorectal cancer and their predictive value in anti-vascular therapy. Front. Oncol. 12:997665. doi: 10.3389/fonc.2022.997665
Received: 19 July 2022; Accepted: 30 August 2022;
Published: 26 September 2022.
Edited by:
Aamir Ahmad, University of Alabama at Birmingham, United StatesReviewed by:
C. Sykora, Australian National University, AustraliaCopyright © 2022 Zheng, Wang, Yu, Zhan and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengqing Guo, Z3pxXzAwNUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.