- 1Department of General Surgery, Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Department of Thyroid and Breast Surgery, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Purpose: In our study, we aim to analyze the impact of clinicopathological factors on the recommendation of extended endocrine therapy (EET) in patients with ER+ breast cancer and to retrospectively validate the value of CTS5 in EET decision making.
Patients and methods: The retrospective analysis was performed in patients with ER+ breast cancer who have finished 4.5–5 years of adjuvant endocrine therapy and undergone MDT discussion from October 2017 to November 2019. Multivariate logistic regression was used to identify the independent factors for treatment recommendation. CTS5 was calculated for retrospective validation of the EET decision making.
Results: Two hundred thirty-five patients were received; 4.5–5 years of adjuvant endocrine therapy were included in the study. Multivariate analysis suggested that age (OR 0.460, 95% CI 0.219–0.965, p = 0.04), pN (OR 39.350, 95% CI 9.831–157.341, P < 0.001), and receipt of chemotherapy (OR 3.478, 95% CI 1.336–9.055, p = 0.011) were independent predictors for the recommendation of EET. In the previously selective estrogen receptor modulator (SERM)–treated subgroup, pN and receipt of chemotherapy were independent predictors for the recommendation of EET. In the previously AI-treated subgroup, age, pN, and receipt of chemotherapy were independent predictors. Adverse events did not affect the recommendation in patients previously treated with adjuvant endocrine treatment nor in the previously SERM or AI-treated subgroups. CTS5 (OR 21.887, 95% CI 2.846–168.309, p = 0.003) remained an independent predictor for the recommendation of EET.
Conclusions: Our study indicated that age, lymph nodal status, and receipt of chemotherapy were independent predictors for the recommendation of EET. The application of the CTS5 on EET decision making might be valuable among ER+ breast cancer patients.
Introduction
For decades, breast cancer has been the most frequently diagnosed malignant tumor in women globally. According to the latest global epidemiological cancer survey, 2.1 million new cases of breast cancer were diagnosed worldwide in 2018 (1). In estrogen receptor (ER)–positive early breast cancer, endocrine therapy plays an important role in its comprehensive treatment, and 5 years of treatment was considered the standard treatment duration traditionally (2–4).
However, recent studies have shown that among women with ER-positive breast cancer who were scheduled to receive 5 years of endocrine therapy, distant recurrences still have a steady rate for at least another 15 years after the end of the 5-year treatment (5–7). According to the results of several clinical trials regarding extended adjuvant endocrine therapy (ATLAS, aTTom, MA-17R, and NSABP B-42), the effect of extended endocrine therapy (EET) beyond 5 years to reduce the risk of late recurrence for ER+ breast cancer has been demonstrated (8–13). An EBCTCG meta-analysis also showed the efficacy of extending AI therapy compared with stopping AI after about 5 years of endocrine therapy in preventing disease recurrence and death from breast cancer (14). In the American Society of Clinical Oncology (ASCO) clinical practice guideline in 2018, EET was included among node-positive and some node-negative breast cancer patients with co-existing high-risk factors (15). However, controversies remain about the target population who may benefit from EET in clinical decision making.
In our study, we aim to analyze the impact of clinicopathological factors on the choice of follow-up treatment after 5 years of endocrine therapy in patients with ER-positive breast cancer and to retrospectively validate the value of CTS5 in EET decision making.
Patients and methods
Study population
The retrospective analysis was performed in patients who met the following eligibility criteria: (1) female gender; (2) post-surgery; (3) have received adjuvant endocrine therapy for 4.5–5 years; (4) have undergone Multiple Disciplinary Team (MDT) discussion regarding the use of EET in Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, between October 2017 and November 2019; (5) ER-positive. Patient information was extracted from Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB).
Histopathological evaluation
Tumor histopathologic result was independently performed by two experienced pathologists, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2) status, Ki-67 status, histological grade, and pathological type. ER-positivity (ER+) and PR-positivity (PR+) were defined as more than 1% positive invasive tumor cells with nuclear staining (16). HER-2 status was identified according to the 2013 ASCO/CAP guidelines (17) (the minority of patients’ HER-2 status diagnosed before 2013 was evaluated according to 2007 ASCO/CAP guidelines (18)). The median Ki-67 value for hormone receptor-positive disease in SJTU-BCDB was 15.0%, so we defined Ki-67 high as more than 15% positive invasive tumor cells with nuclear staining. TNM stage was based on the 7th edition of the American Joint Committee on Cancer (AJCC) (19). CTS5 was calculated for retrospective validation of the EET decision making, and patients were divided into two risk groups according to CTS5 score: low (< 3.13) and high (≥ 3.13) groups (20). (CTS5 = 0.438 × nodes + 0.988 × (0.093 × size(mm) - 0.001 × size (2) + 0.375 × grade + 0.017 × age).
Treatment decision
After the completion of 4.5–5 years of adjuvant endocrine therapy, the MDT meeting would be held to recommend extending the endocrine treatment regimen based on patients’ clinicopathological features and other related factors such as adverse events. Treatment choices on whether or not to extend endocrine therapy were decided through MDT meetings including surgical oncologists, medical oncologists, radiation oncologists, ultrasound physicians, pathologists, breast cancer specialized nurses, and other related specialists. The recommendation was first determined by each physician in the MDT team and then finally determined after MDT discussion and comprehensive opinions. The standard regimens for recommendation include stopping endocrine therapy, treating with aromatase inhibitors (AIs) for 3 or 5 years, and treating with selective estrogen receptor modulators (SERMs) for 5 years, with or without applying ovarian function suppression (OFS).
Statistical analysis
All clinicopathological characteristics were analyzed as categorical variables by using logistic regression. Multivariate logistic regression was used to identify the independent factors for treatment recommendation. The chi-square test was used to evaluate the adverse events. Fisher’s exact tests were carried out if necessary. Data were analyzed using IBM SPSS statistics software version 23 (SPSS, Inc. Chicago, IL). Two-sided P < 0.05 were considered statistically significant.
Results
Clinical characteristics
A total of 252 patients participated in the multidisciplinary discussion, 235 patients who had received 4.5–5 years of adjuvant endocrine therapy were included in the study, and 17 patients were excluded because of information loss. One hundred forty-three patients (60.9%) were suggested to receive EET, and 92 patients (39.1%) were suggested to stop EET. The mean age of patients was 60 years old, and 136 (57.9%) patients were older than 50 years. There were 140 (59.6%) patients with T1 stage tumors and 105 (44.7%) patients with positive lymph nodes. The proportion of patients with PR-positive, Ki-67 ≥ 15%, or HER-2 positive was 81.3, 51.1, and 18.7%, respectively. The baseline characteristics of the participants are presented in Table 1.
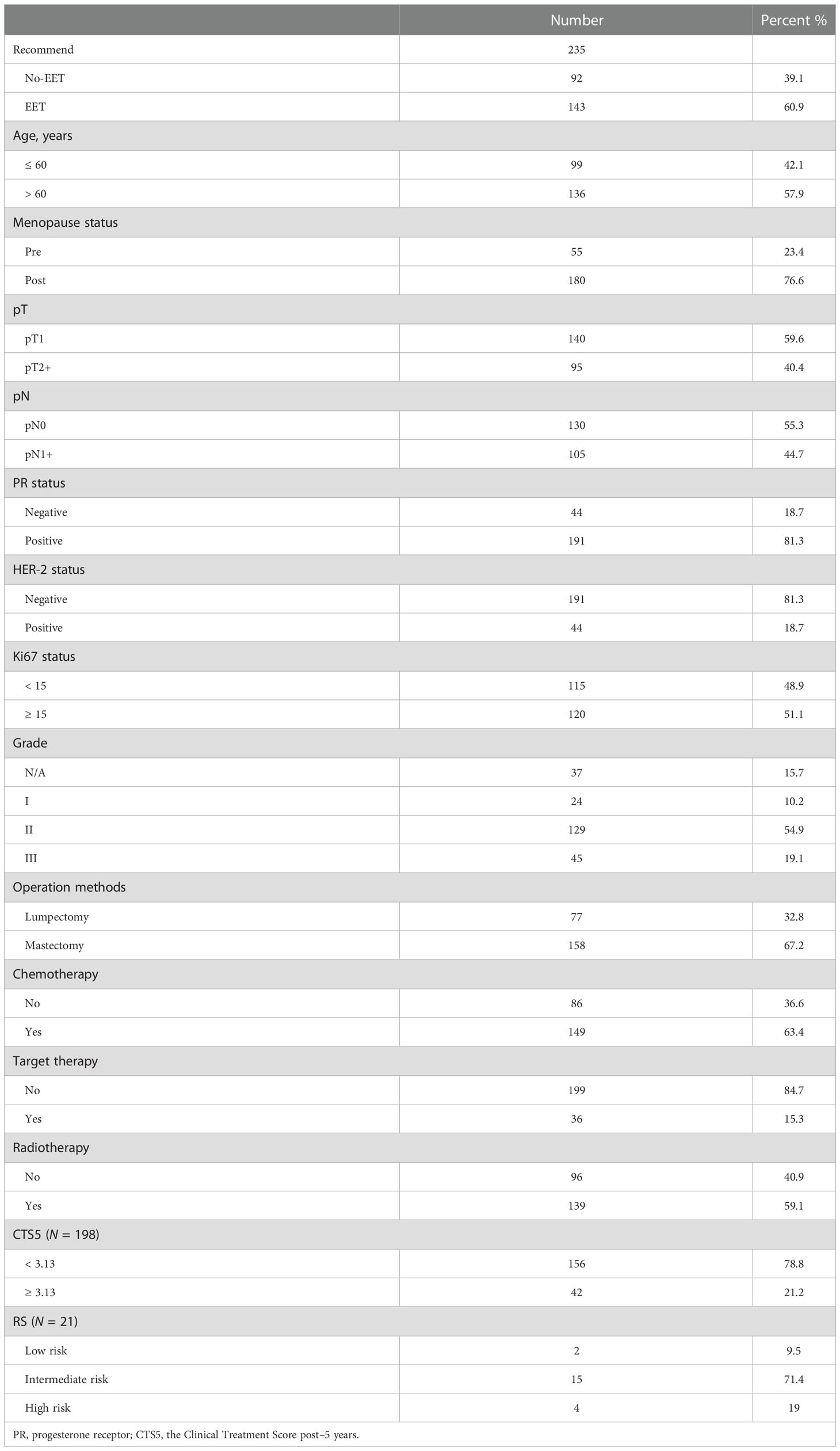
Table 1 Baseline characteristics of study participants and impact factors for extended endocrine therapy decision.
Impact factors on decision making in all patients
In univariate analysis, age (OR 5.19, 95% CI 0.301–0.895, p = 0.018), pT (OR 3.042, 95% CI 1.713–5.404, P < 0.001), pN (OR 26.444, 95% CI 1.713–5.404, p < 0.001), HER-2 (OR 2.989, 95% CI 1.361–6.562, p = 0.006), Ki-67 status (OR 2.574, 95% CI 1.500–4.415, p = 0.001), Grade (GII vs. GI: OR 1.994, 95% CI 0.828–4.802, p = 0.0124; and GIII vs. GI: OR 6.416, 95% CI 2.506–20.016, p = 0.001), receipt of chemotherapy (OR 9.288, 95% CI 5.042–17.108, p < 0.001), receipt of target therapy (OR 3.089, 95% CI 1.292–7.387, p = 0.011), and receipt of radiotherapy (OR 2.510, 95% CI 1.463–4.307, p = 0.001) were correlated with the recommendation of EET (Table 2).
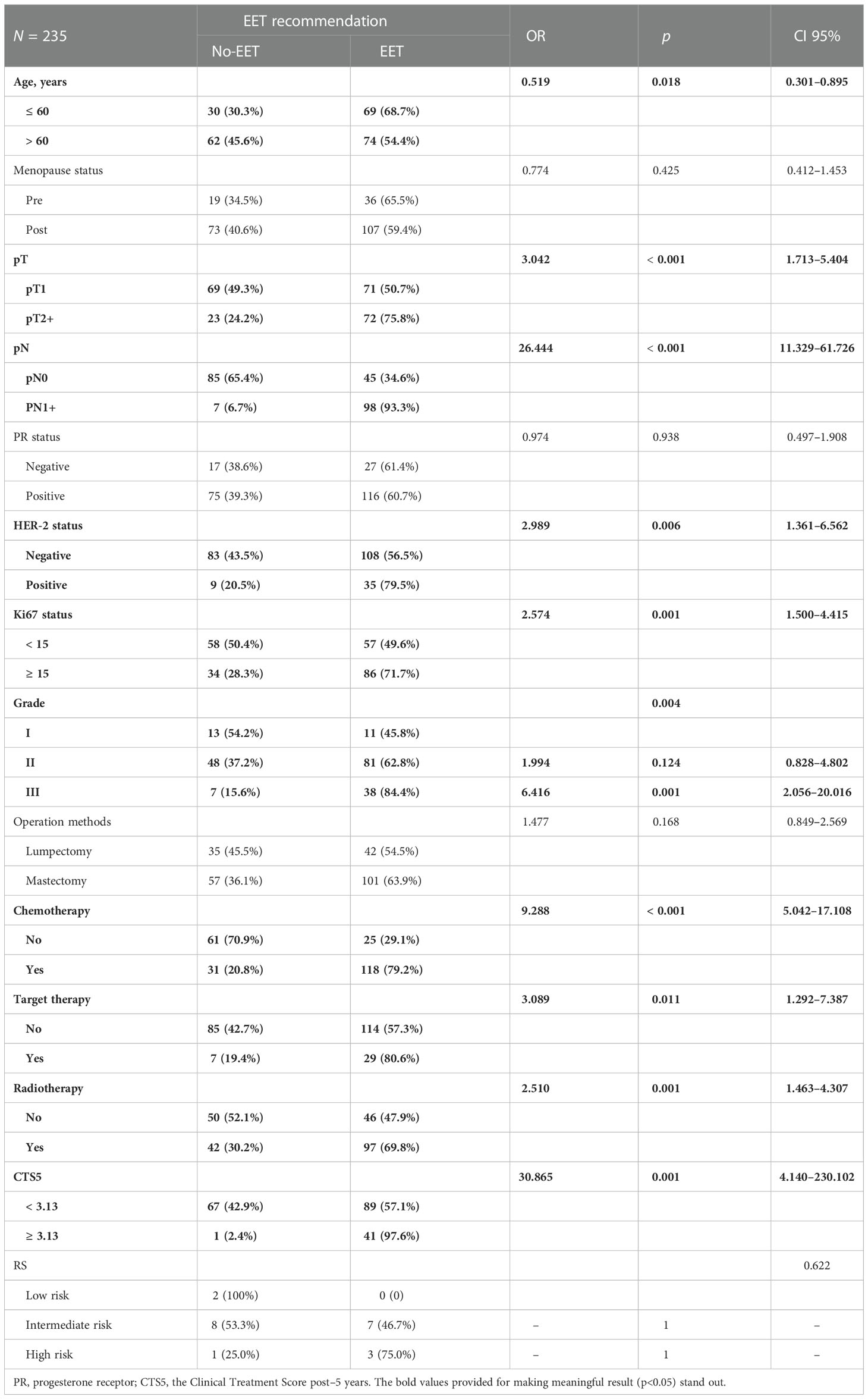
Table 2 Univariate analysis of impact factors for extended endocrine therapy recommendation in the whole-patients group.
Multivariate analysis suggested that age (OR 0.460, 95% CI 0.219–0.965, p = 0.04), pN (OR 39.350, 95% CI 9.831–157.341, p < 0.001), and receipt of chemotherapy (OR 3.478, 95% CI 1.336–9.055, p = 0.011) were independent predictors for the recommendation of EET (Table 3).
Impact factors of decision making in previously SERM/AI-treated subgroups
In univariate analysis of the previously SERM-treated group, pT (OR 4.000, 95% CI 1.136-14.085, p = 0.031), pN (OR 18.692, 95% CI 13.760–92.926, p < 0.001), Ki-67 index (OR 4.846, 95% CI 1.515–15.504, p = 0.008), receipt of chemotherapy (OR 16.333, 95% CI 4.281–62.310, p < 0.001) were correlated with EET (Table 4). Multivariate analysis suggested that pN (OR 10.811, 95% CI 1.937–60.346, p = 0.007) and receipt of chemotherapy (OR 9.396, 95% CI 2.155–40.980, p = 0.003) were independent predictors for the recommendation of EET (Table 3).
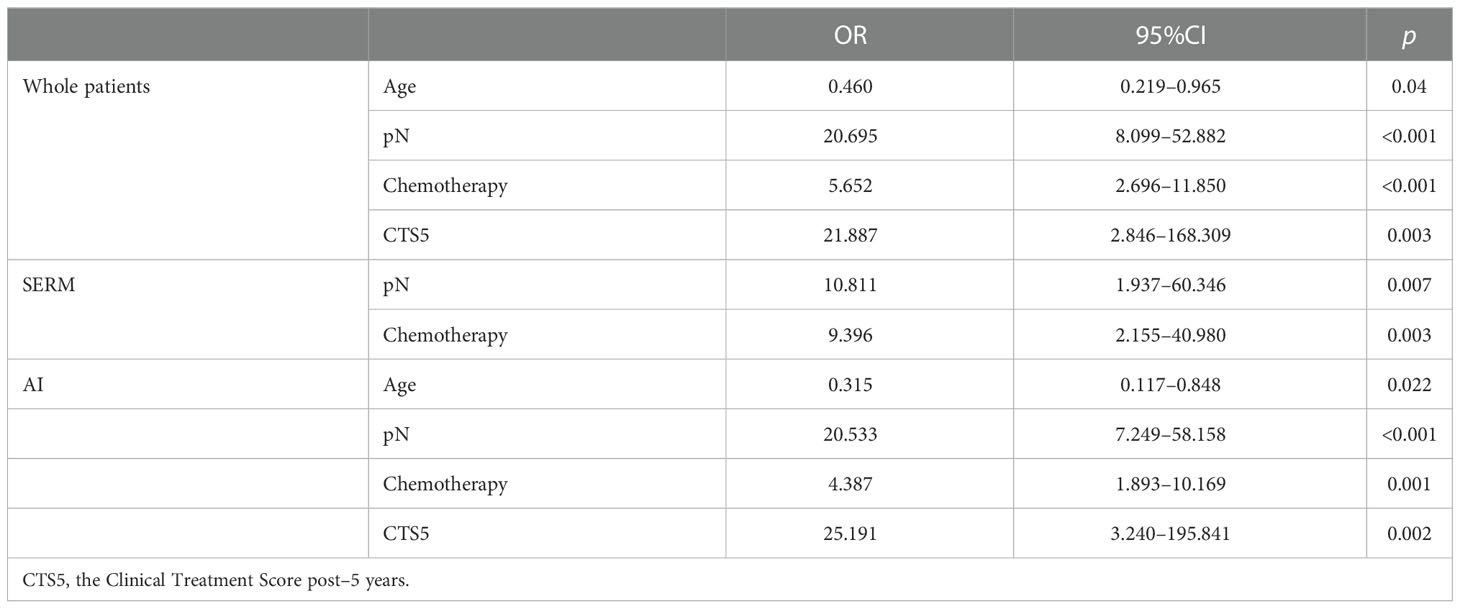
Table 3 Multivariate analysis of impact factors for extended endocrine therapy recommendation in whole-patients group and previously SERM or AI-treated subgroups.
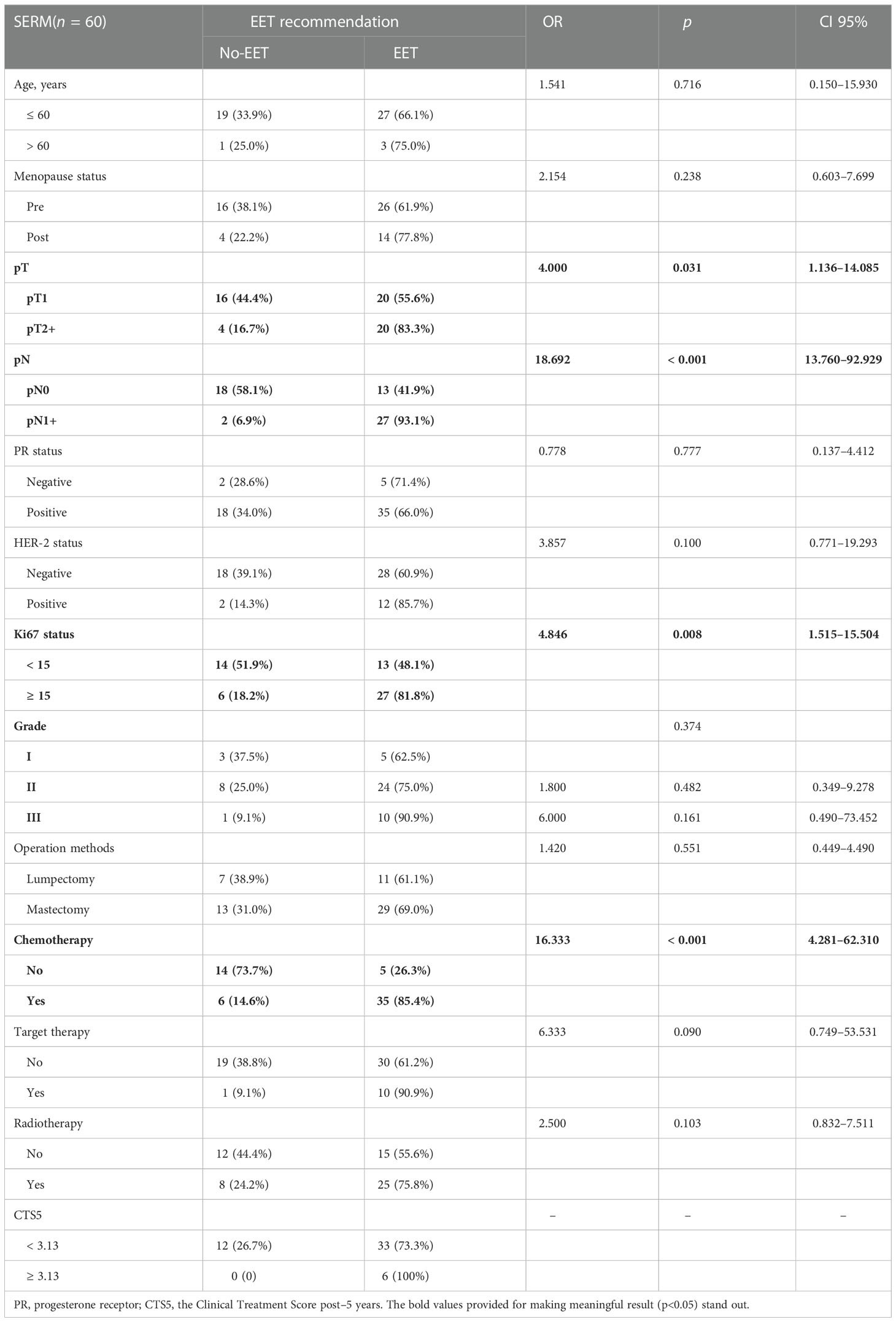
Table 4 Univariate analysis of impact factors for extended endocrine therapy recommendation in SERM group.
In univariate analysis of the previously-AI-treated group, age (OR 0.400, 95% CI 0.186-0.860, p = 0.019), pT (OR 2.844, 95% CI 1.483–5.454, p = 0.002), pN (OR 29.731, 95% CI 10.939–80.809, p < 0.001), HER-2 (OR 2.670, 95% CI 1.078–6.613, p = 0.034), Ki-67 status (OR 2.107, 95% CI 1.140–3.893, p = 0.017), Grade (GIII vs. GI: OR 7.778, 95% CI 2.032–29.773, p = 0.003), receipt of chemotherapy (OR 7.802, 95% CI 3.920–15.528, p < 0.001), and receipt of radiotherapy (OR 2.596, 95% CI 1.389–4.852, p = 0.003) were correlated with EET (Table 5). Multivariate analysis suggested that age (OR 0.315, 95% CI 0.117–0.848, p = 0.022), pN (OR 20.533, 95% CI 7.249–58.158, p < 0.001), and receipt of chemotherapy (OR = 4.387, 95% CI 1.893–10.169, p = 0.001) were independent predictors for the recommendation of EET (Table 3).
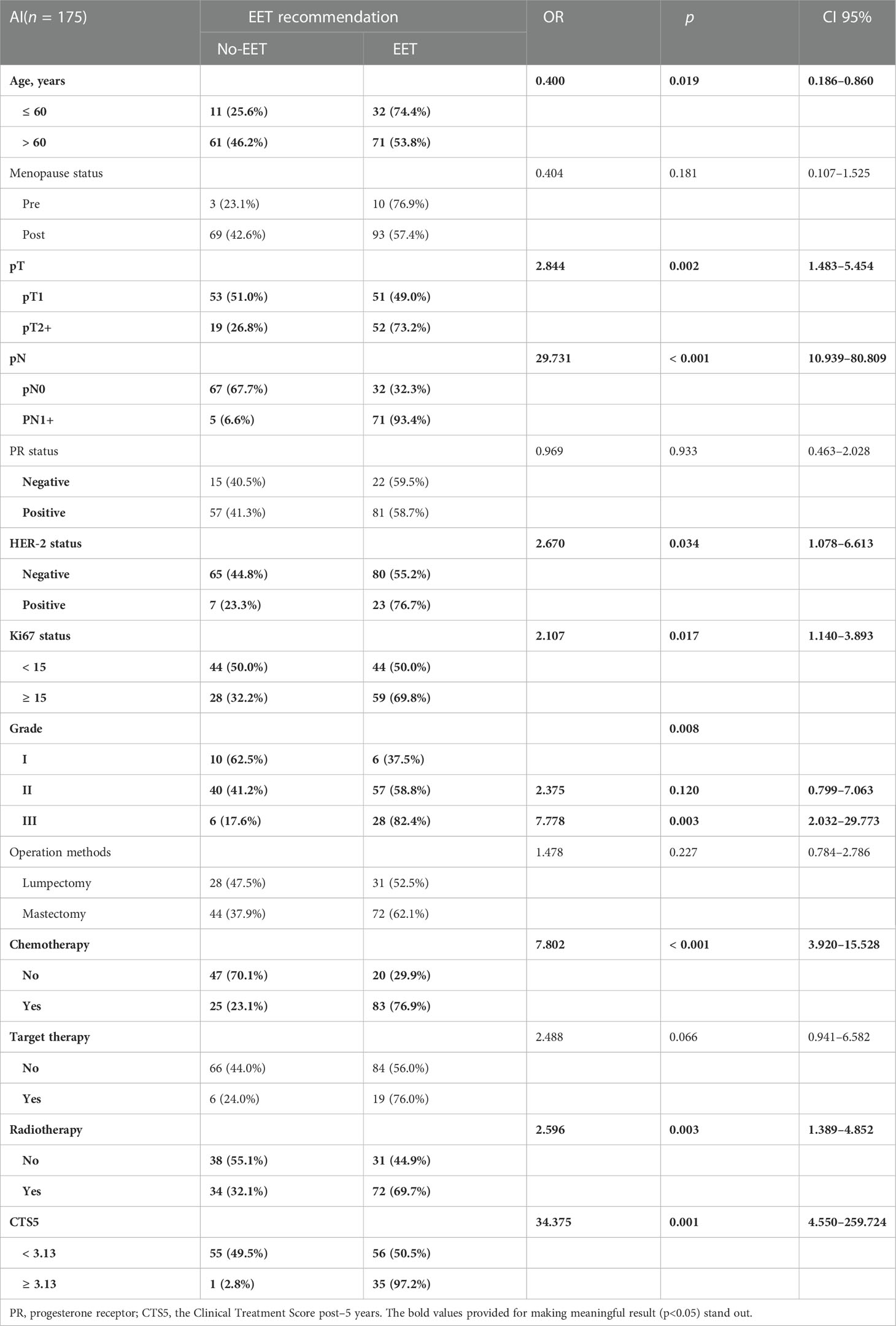
Table 5 Univariate analysis of impact factors for extended endocrine therapy recommendation in AI group.
The retrospective validation of CTS5 for EET decision making
In this study, 198 patients had data on CTS5. The distribution of CTS5 was 78.8% and 21.2% for the low (< 3.13) and high-risk (≥ 3.13) groups, respectively. Overall, CTS5 (OR 36.865, 95% CI 2.846–168.309, p = 0.001) was correlated with EET in univariate analysis (Table 2). After excluding the factors involved in the CTS5 formula, CTS5 (OR 21.887, 95% CI 2.846–168.309, p = 0.003) remained an independent predictor for the recommendation of EET in multivariate analysis (Table 3).
For patients previously SERM-treated, all patients were suggested to extend the endocrine therapy when their CTS5 status was indicated as high risk (Table 4).
For patients previously AI-treated, 35 (97.2%) patients were recommended EET in the previously AI-treated group when their CTS5 status was indicated as high risk. In the univariate analysis of the previously AI-treated group, CTS5 (OR 34.375, 95% CI 4.550–259.724, p = 0.001) was correlated with EET (Table 5). In multivariate analysis, CTS5 (OR 25.191, 95% CI 3.240–195.841, p = 0.002) remained an independent predictor for the recommendation of EET (Table 3).
Impact of adverse events on decision making
In our study, the following common adverse events after endocrine therapy were recorded and analyzed: endometrial thickening, endometrial cancer, musculoskeletal symptoms, T-score < -2, fracture, hot flash (≥ G3), libido decreased (≥ G2), depression or anxiety, and dyslipidemia.
Of all patients who participated in the multidisciplinary discussion (n = 235), 14 patients had endometrial thickening (6%), one patient had endometrial cancer (0.4%), 19 patients had musculoskeletal symptoms (8.1%), 46 patients had osteoporosis (19.6%), 14 patients had fracture(6%), nine patients had hot flash (3.8%), six patients had libido decreased (≥ G2) (2.6%), 43 patients had depression or anxiety(18.3%), and 58 patients had dyslipidemia (24.7%) (Table 6). None of these AEs were significantly correlated with the recommendation of EET in univariate analysis. In patients previously treated with SERM or AI, similar results were found that none of these adverse events were correlated with treatment decisions (Table 6).
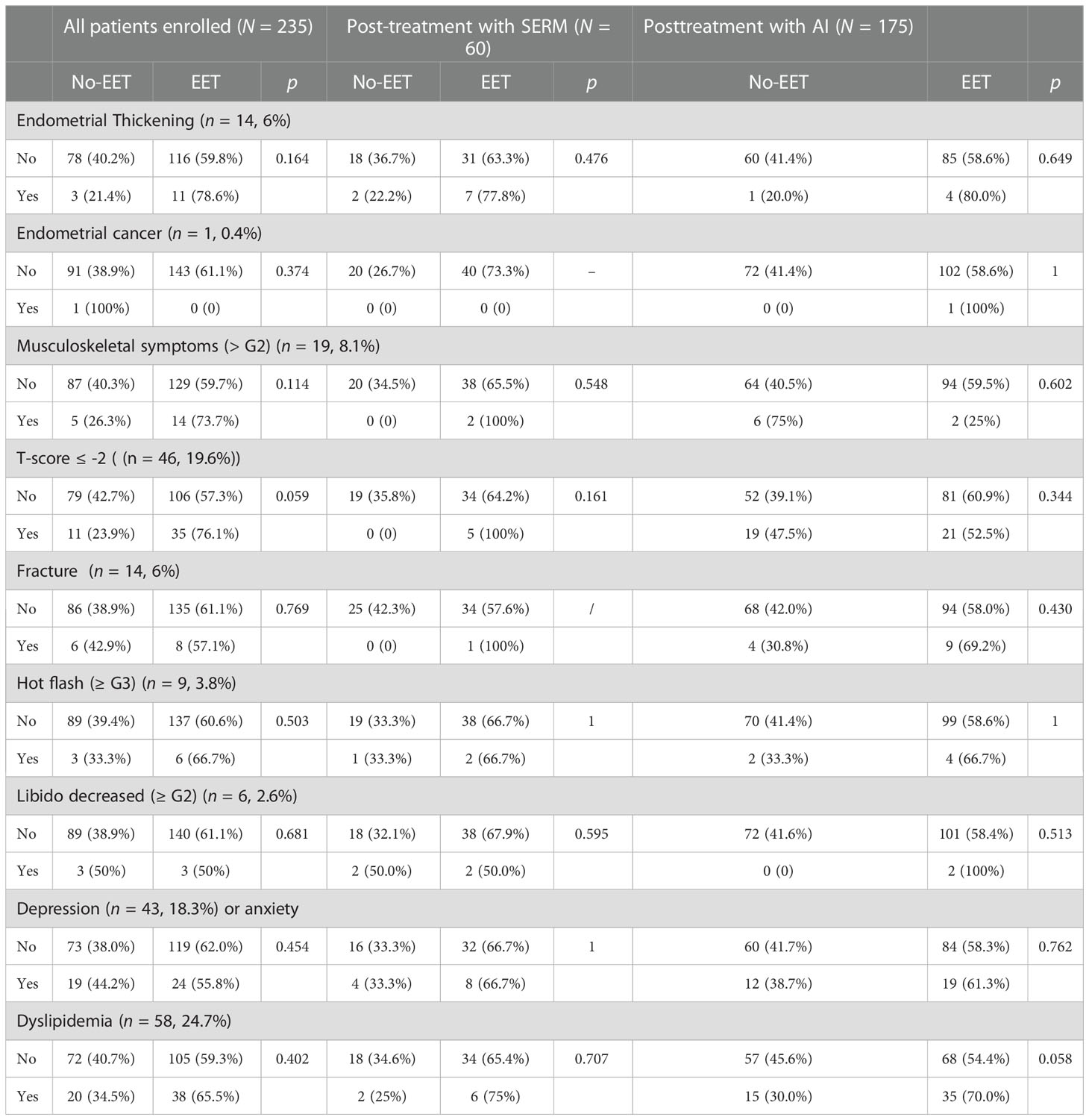
Table 6 The distribution of side effects in enrolled breast cancer patients and the correlation between AEs and extended endocrine therapy in whole patients enrolled and patients previously treated with SERM or AI.
Discussion
Extending the duration of the endocrine therapy to 10 years has now proved to reduce the risk of late recurrence in selected ER+ breast cancer patients (4, 5, 10, 21). However, controversies remain about the target population who may benefit from the EET in clinical decision making. In our study, there were 235 ER-positive patients participated in the multidisciplinary discussion, and we found that age, lymph node status, and receipt of chemotherapy were independently associated with the recommendation of EET.
Among classic clinicopathological factors, nodal status is the strongest predictor of early recurrence (22). The study by HongChao and colleagues included 62,923 ER+ breast cancer patients and reported that the annual risk of distant recurrence was strongly related to nodal status (P < 0.001), and recurrence increased with the number of metastatic lymph nodes (20-year risk with N0, N1, and N2: 22%, 31%, and 52%) (5). 2018 ASCO guideline also recommended that women with node-positive breast cancer receive extended therapy, including AI, for up to a total of 10 years of adjuvant endocrine treatment (11). In our study, lymph node status turned out to be the strongest factor associated with therapy recommendation in all clinicopathological indicators. This indicated that clinicians would pay more attention to lymph node status when they make a recommendation on whether to use EET or not.
In our study, we found that age did not affect the recommendation for extended SERMs in the previously SERM-treated group, but it was the independent predictor for recommendation in the previously AI-treated group and older patients are less likely to be recommended for extended AIs.
At present, there is no evidence that age is related to the risk of recurrence (2). A meta-analysis in 2017 shows that there was no statistically significant benefit from extended therapy in the age subgroup (23). Therefore, for those who were previously treated with SERMs, age will not affect the choice of doctors. However, in patients previously treated with AI, the proportion of elderly patients (age > 60) is relatively large (75%) in our data. In the face of those patients, considering the physical condition, tolerance, and the lack of evidence to prove the validity of EET, clinicians tend to make the relatively conservative decision.
CTS5 (ATAC), including tumor size, number of positive nodes, histologic grade, and age, is a simple tool that was validated as highly prognostic for late recurrence (7, 24). In Dowsett’s research, the prognostic value of CTS5 was tested using data from the ATAC trial and validated with data from the BIG 1-98 trial (20). Furthermore, populations of those clinical trials are all postmenopausal patients and may behave differently to real-life patients. In their follow-up study, CTS5 demonstrated clinical validity for predicting late recurrence in unselected postmenopausal patients but less so in premenopausal patients (25). In our study, CTS5 was used for retrospective validation of the physician’s clinical recommendations about EET. Consistent with the experimental conclusion mentioned previously, we found that CTS5 was strongly associated with clinician recommendations, especially in the previously AI-treated group, and the higher the value, the more likely EET would be recommended. In addition, from the perspective of the OR values in the multivariate analysis, the CTS5 score was a more valuable guiding factor for the EET recommendation than lymph nodes and other independent clinicopathological factors.
Endocrine therapy causes some side effects, most of which were non-life threatening. In the IBIS-II trial, John et al. reported the side-effect profiles in breast cancer patients who had completed 5 years of endocrine therapy, including fractures (9%), arthralgia (57%), and osteoporosis (7%) with anastrozole and gynecological cancers (1.9%), vaginal symptoms (28%), and deep vein thromboses (1%) with tamoxifen (26). There are also some studies showing a possible side effect of tamoxifen with raise in the triglycerides level (27, 28). In our study, the side effects we counted were mainly T-score< -2 (26.7%), musculoskeletal symptoms (5.3%), and fracture (8.7%) with AI and endometrial thickening (15.5%) and dyslipidemia (13.3%) with tamoxifen.
EET would also increase the incidence of some side effects. In the NSABP B-14 trial, the risk of endometrial cancer was raised in the extended tamoxifen group [RR:2.0 (0.7–6.6)]. As reported by MA-17R, extended letrozole significantly increased the risk of osteoporosis (12%:9%, p = 0.01) and fracture (14%:9%, p = 0.001) (10, 29, 30). In our study, there was no influence of adverse events on the treatment decision. First, this might be related to the fact that the population enrolled in this study was able to tolerate 5 years of basic adjuvant endocrine therapy and was likely to endure further EET. Secondly, we consider that if complications would occur, priority would be given to the change of treatment or to treat complications aggressively rather than stopping EET.
There are some limitations to our study: one is that it was a retrospective analysis, which needs further validation. Next in importance, our data included cases from October 2017 to December 2019. Since then, the publication of clinical trial results and the update of clinical guidelines would lead to a change in decision making.
Conclusions
Our study indicated that age, lymph nodal status, and receipt of chemotherapy were independent predictors for the recommendation of EET. The application of the CTS5 on EET decision making might be valuable among ER+ breast cancer patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WLC: Conceptualization, Writing - Original Draft, Formal analysis, Statistical Analysis. JW: Methodology, Visualization. YZ, JHH: Investigation. XC: Data Curation. OH, JRH: Validation. YL, WGC, KS: Project administration, Supervision. LZ: Writing- Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the assistant from Yidong Du for her significant contribution during the follow-up. Special thanks should be given to the Shanghai Jiaotong University Breast Cancer Database (SJTU-BCDB).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EET, extended endocrine therapy; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; SERM, selective estrogen receptor modulators; AI, aromatase inhibitors; OFS, ovarian function suppression; MDT, Multiple Disciplinary Team.References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol (2016) 34(14):1689–701. doi: 10.1200/JCO.2015.65.9573
3. NCCN Breast Cancer Clinical Practice Guidelines in Oncology. (2006). Available at: http://www.nccn.org/profess.
4. Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol (2003) 21:3357–65. doi: 10.1200/JCO.2003.04.576
5. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. Engl J Med (2017) 377:1836–46. doi: 10.1056/NEJMoa1701830
6. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet (2015) 386(10001):1341–52. doi: 10.1016/S0140-6736(15)61074-1
7. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol (2010) 11(12):1135e41. doi: 10.1016/S1470-2045(10)70257-6
8. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (2013) 381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1
9. Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol (2013) 31(suppl; abstr5). doi: 10.1200/jco.2013.31.15_suppl.5
10. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med (2016) 375(3):209–19. doi: 10.1056/NEJMoa1604700
11. Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer CE Jr, Rastogi P, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP b-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2019) 20(1):88–99. doi: 10.1016/S1470-2045(18)30621-1
12. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2009) 30(10):1674. doi: 10.1093/annonc/mdz189
13. NCCN. NCCN clinical practice guidelines in oncology: Breast cancer . Available at: http://www.nccn.org.
14. Gray R. Early Breast Cancer Trialists' Collaborative Group. Effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: An EBCTCG meta-analysis of individual patient data from 12 randomized trials including 24,912 women. J. Cancer research (2019) 79(4_Supplement):GS3-03-GS3-03.
15. Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol (2019) 37(5):423–38. doi: 10.1200/JCO.18.01160
16. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S. Et al: American society of clinical oncology/college of American pathologist’s guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol (2010) 28(16):2784–95. doi: 10.1200/JCO.2009.25.6529
17. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical Oncology/College of America pathologists clinical practice guideline update. J Clin Oncol (2013) 31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984
18. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol (2007) 25(33):5287–312. doi: 10.1200/JCO.2007.14.2364
19. Edges B, Compton CC. The American joint committee on cancer: The 7th edition of the ajcc cancer staging manual and the future of TNM. Ann Surg Oncol (2010) 17(6):1471–14 174. doi: 10.1245/s10434-010-0985-4
20. Dowsett M, Sestak I, Regan MM, Dodson A, Viale G, Thürlimann B, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor–positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol (2018) 36:1941–8. doi: 10.1200/JCO.2017.76.4258
21. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst (2005) 97(17):1262–71. doi: 10.1093/jnci/dji250
22. Khoshnoud MR, Fornander T, Johansson H, Rutqvist LE. Long-term pattern of disease recurrence among patients with early-stage breast cancer according to estrogen receptor status and use of adjuvant tamoxifen. Breast Cancer Res Treat (2008) 107(1):71–8. doi: 10.1007/s10549-007-9520-0
23. Goldvaser H. Efficacy of extended adjuvant therapy with aromatase inhibitors in early breast cancer among common clinicopathologically-defined subgroups: A systematic review and meta-analysis. Cancer Treat Rev (2017) 60:53–7. doi: 10.1016/j.ctrv.2017.08.008
24. Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet (2002) 359(9324):2131–9. doi: 10.1016/s0140-6736(02)09088-8
25. Richman J, Ring A, Dowsett M, Sestak I. Clinical validity of clinical treatment score 5 (CTS5) for estimating risk of late recurrence in unselected, non-trial patients with early oestrogen receptor-positive breast cancer. Breast Cancer Res Treat (2021) 186(1):115–23. doi: 10.1007/s10549-020-06013-6
26. Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomized controlled trial. Lancet (2016) 387(10021):866–73. doi: 10.1016/S0140-6736(15)01129-0
27. Hozumi Y, Kawano M, Saito T, Miyata M. Effect of tamoxifen on serum lipid metabolism. J Clin Endocrinol Metab (1998) 83(5):1633–5. doi: 10.1210/jcem.83.5.4753
28. Liu CL, Yang TL. Sequential changes in serum triglyceride levels during adjuvant tamoxifen therapy in breast cancer patients and the effect of dose reduction. Breast Cancer Res Treat (2003) 79(1):11–6. doi: 10.1023/A:1023348021773
29. Goldvaser H, Barnes TA, Seruga B, Cescon DW, Ocaña A, Ribnikar D, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst (2018) 110(1):31–9. doi: 10.1093/jnci/djx141
Keywords: breast malignancy, extended endocrine therapy, multidisciplinary team, estrogen receptor positive (ER+), CTS5
Citation: Chen W, Wu J, Zhu Y, Huang J, Chen X, Huang O, He J, Li Y, Chen W, Shen K and Zhu L (2023) Impact of clinicopathological factors on extended endocrine therapy decision making in estrogen receptor–positive breast cancer. Front. Oncol. 12:996522. doi: 10.3389/fonc.2022.996522
Received: 17 July 2022; Accepted: 14 December 2022;
Published: 12 January 2023.
Edited by:
Dayanidhi Raman, University of Toledo, United StatesReviewed by:
Liu Yiqiang, Beijing Cancer Hospital, ChinaEkta Khattar, SVKM’s Narsee Monjee Institute of Management Studies, India
Copyright © 2023 Chen, Wu, Zhu, Huang, Chen, Huang, He, Li, Chen, Shen and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhu, emh1bGk4QHllYWgubmV0
†These authors have contributed equally to this work
 Weilin Chen1†
Weilin Chen1† Jiayi Wu
Jiayi Wu Jiahui Huang
Jiahui Huang Xiaosong Chen
Xiaosong Chen Kunwei Shen
Kunwei Shen Li Zhu
Li Zhu