
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 21 September 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.995498
This article is part of the Research Topic GBM Stem Cells and the Brain Tumor Microenvironment View all 7 articles
Despite its growing use in cancer treatment, immunotherapy has been virtually ineffective in clinical trials for gliomas. The inherently cold tumor immune microenvironment (TIME) in gliomas, characterized by a high ratio of pro-tumor to anti-tumor immune cell infiltrates, acts as a seemingly insurmountable barrier to immunotherapy. Glioma stem cells (GSCs) within these tumors are key contributors to this cold TIME, often functioning indirectly through activation and recruitment of pro-tumor immune cell types. Furthermore, drivers of GSC plasticity and heterogeneity (e.g., reprogramming transcription factors, epigenetic modifications) are associated with induction of immunosuppressive cell states. Recent studies have identified GSC-intrinsic mechanisms, including functional mimicry of immune suppressive cell types, as key determinants of anti-tumor immune escape. In this review, we cover recent advancements in our understanding of GSC-intrinsic mechanisms that modulate GSC-TIME interactions and discuss cutting-edge techniques and bioinformatics platforms available to study immune modulation at high cellular resolution with exploration of both malignant (i.e., GSC) and non-malignant (i.e., immune) cell fractions. Finally, we provide insight into the therapeutic opportunities for targeting immunomodulatory GSC-intrinsic mechanisms to potentiate immunotherapy response in gliomas.
High-grade gliomas, including glioblastoma (GBM), are highly heterogeneous with a complex oncogenic microenvironment consisting of distinct tumor niches and remarkable cell heterogeneity (1, 2). A critical component of glioma malignancy derives from the distinct population of glioma stem cells (GSCs) that function to promote and maintain oncogenicity through their capacity for self-renewal, cellular adaptation, and multipotency (3–5). These stem-like cells engage in a synergistic relationship with the surrounding tumor microenvironment (TME) to promote tumor progression and contribute to the vast degree of intratumoral heterogeneity, immune-suppression, and therapy resistance encountered in gliomas (5). The plasticity of GSCs facilitates shifts between distinct tumorigenic stem-like states and allows for transitions along the spectrum of differentiation that characterizes glioma cells (6–8). These state transitions are brought on by various stemness-inducing stimuli (e.g., hypoxia, interaction with non-neoplastic cells, immune exposure, therapeutics, etc.) and carried out by reprogramming mechanisms that involve specific transcription factors and dynamic epigenetic modifications that alter transcriptional profiles and consequently, cellular and molecular phenotypes (9) (Figure 1).
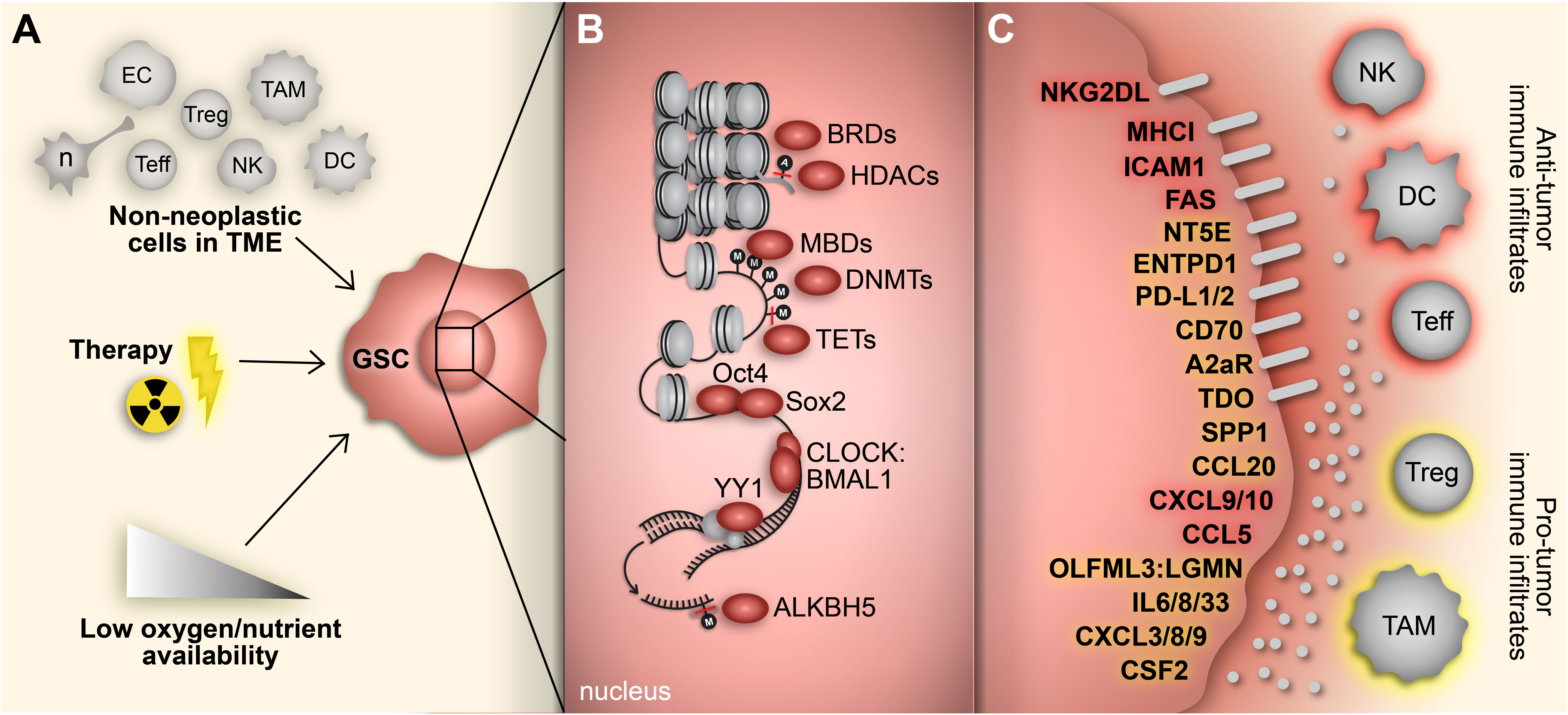
Figure 1 Adaptive responses drive immunosuppressive GSC mechanisms. (A) GBM cells face constant adaptive pressure due to stimuli in the surrounding TME including cell-cell interactions with non-neoplastic counterparts, standard-of-care therapeutics and availability of oxygen and nutrients. (B) These extrinsic stimuli induce reprogramming events, including acquisition of a stem-like state in GBM cells. Transition to a GSC state results from altered gene expression mediated by various transcriptional regulators (e.g., epigenetic modifiers, transcription factors) that regulate immunomodulatory mechanisms. (C) GSC-intrinsic immunomodulatory mechanisms occur through induction (yellow) or repression (red) of immune-related genes that play a key role in shaping the TIME in GBM. Cell types: EC, endothelial cell; n, neuron; Teff, effector T cell; Treg, regulatory T cell; NK; natural killer cell; TAM, tumor-associated macrophage/microglia; DC, dendritic cell.
A unique facet of cancer stem cell biology is the capacity of a tumor-propagating stem-like cancer cells to evolve from a non-stem-like state through a process known as dedifferentiation. Reprogramming transcription factors, collectively referred to as the Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc), play critical driving roles in this process (10, 11). In particular, Oct4 and Sox2 are sufficient to induce stem cell properties and in vivo tumor-propagating potential in differentiated and non-tumor-propagating GBM cells (12, 13). Additionally, our lab has identified multiple mechanisms downstream of Oct4 and Sox2 underlying GSC stemness and tumorigenicity (12–17). Furthermore, epigenetic modifications mediated by Sox2 and Oct4 have implications in GSC-immune interactions that contribute to an immunosuppressive TME (17).
In spite of the progress in our understanding of GSC biology, the inherent heterogeneity and plasticity of the GSC population and resulting phenotypic consequences have restricted the impact of ongoing therapeutic efforts. In this review, we cover the advancements in our understanding of GSC plasticity, GSC-intrinsic immunomodulatory mechanisms, and the capacity of GSCs to co-opt immunosuppressive cell phenotypes. Additionally, we discuss the application of evaluable and emerging cutting-edge techniques to study immunosuppressive GSC states and cell-cell interactions at high cellular resolution. Finally, we discuss potential therapeutic approaches aimed at exploiting epigenetic mechanisms and metabolic vulnerabilities associated with acquired immunosuppressive GSC states to enhance the efficacy of GBM immunotherapy.
A hallmark of GBM and significant barrier to immunotherapies is the immunosuppressive TME defined by relatively high numbers of suppressive, pro-tumor immune cell infiltrates (e.g., regulatory T cells (Tregs), tumor-associated microglia, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs)) and high prevalence of dysfunctional T cell states (e.g., exhaustion) (18–26). High infiltration of immunosuppressive myeloid and lymphoid cell populations negatively correlates with patient prognosis and therapy response in GBM (19, 25, 27–29). Notably, most infiltrating immune cells in GBM are of myeloid origin (e.g., monocyte-derived macrophages, brain-resident microglia, and MDSCs) and are defined by a variety of pro-tumor phenotypes that function to suppress the anti-tumor immune response (20, 24, 26, 30–32). Beyond their direct immune suppressive functions, pro-tumor myeloid populations (i.e., TAMs, microglia, and MDSCs) promote GBM growth, invasion, and angiogenesis (33–39). Moreover, TAMs have the capacity to induce a mesenchymal state in GBM, which is associated with therapy resistance and poor patient survival (40–42).
As mentioned above, GBM is highly heterogeneous and can be broadly classified by three molecularly defined subtypes – classical, proneural, or mesenchymal (43) – each with unique immunosuppressive characteristics. The diversity of myeloid cell states is demonstrated by the existence of myeloid phenotypes that preferentially associate with specific GBM subtypes and specific tumor niches. For example, mesenchymal tumors are enriched in blood-derived macrophages that possess a transcriptionally distinct immunosuppressive profile marked by upregulation of genes involved in chemokine signaling and lymphocyte chemotaxis and reside in microvascular and peri-necrotic regions (31).
GSCs also avoid recognition by the immune system through expression of checkpoint inhibitors, including PD-L1, CD70, A2aR and TDO, and downregulation of antigen-presentation molecules, specifically MHC class I (MHC-1) molecules (17, 44–46). Furthermore, GSCs can regulate the immune TME by recruiting and polarizing myeloid cells to an immunosuppressive state, producing T cell-suppressing cytokines, and monopolizing nutrients necessary for proper T cell function (47–51). For example, Wu et al. demonstrated GSC-mediated recruitment and polarization of macrophages and microglia to an M2-like phenotype through production of TGF-β1, MIC-1 and CSF-1 (47). From a metabolic standpoint, GSCs preferentially take up glucose from the microenvironment, leading to impaired T cell function due to glucose deprivation (49–51).
There are multiple contributors to the lack of immunotherapy efficacy in GBM. These include a relatively low gene mutation rate resulting in relatively few tumor-associated neoantigens (52), a unique TME comprised of an abundance of tumor-associated immunosuppressive M2-like macrophages (comprising up to 60% of immune cells) (20) and the immune-privileged brain environment (53). However, the fact that standard-of-care chemo/radiation for newly diagnosed GBM can lead to hypermutated recurrent tumors (54) unresponsive to immunotherapy and the efficacy of immunotherapy in cases of CNS metastases (e.g., melanoma) emphasize the potential dominant role for the GBM TME in driving immunotherapy resistance (55–58). For example, the TME of brain metastases and primary CNS tumors, including GBM, have distinct immune landscapes whereby brain metastases contain higher infiltration of leukocytes and fewer cells of monocytic-lineage (19).
Understanding the therapeutic consequences and vulnerabilities of GBM cell heterogeneity and plasticity is an ongoing effort in the scientific community. Despite the association between genetic aberrations and cell states in GBM (59), genomic alterations alone are insufficient to explain the highly adaptive and heterogeneous nature of GSCs (60). Epigenetic modifications mediate the acquisition of a stem-like state in cancer cells, including GBM, and recent studies have implicated epigenetic variability as a driving force in the adaptation of GBM to external stimuli throughout tumor evolution (4, 12, 14–16, 60–62).
Epigenetic mechanisms, including the addition and removal of activating or repressive marks to histone proteins, DNA, and RNA, serve to regulate gene expression in a reversible manner (62, 63). Notably, the balance between transcriptionally repressive methylation and activating demethylation of DNA, controlled by DNA methyltransferases (DNMTs) and ten-eleven translocation proteins (TETs), respectively, governs acquisition and maintenance of the GSC phenotype (12, 16). On the other hand, RNA methylation occurs post-transcriptionally to alter mRNA stability and impact stem cell differentiation (64–66). Histone proteins, which are essential for forming and stabilizing chromatin structure in either accessible (euchromatin) or inaccessible (heterochromatin) states, are also susceptible to modifications (e.g., methylation, acetylation, phosphorylation) which consequently alter chromatin architecture and lead to activation or repression of gene transcription (63). The progression from chromatin remodeling to transcription activation involves the activity of epigenetic readers, such as bromodomain and extraterminal domain (BET) proteins, that detect and bind histone marks. Readers recruit transcription regulatory factors and/or chromatin remodeling protein complexes to control gene expression (67). Collectively, dysregulation of epigenetic factors underlies the acquisition, maintenance, and plasticity of the GSC phenotype and represents a promising avenue for GSC-targeted therapeutics in GBM.
A relatively underappreciated aspect of dynamic GSC states is their contribution to the immunosuppressive tumor microenvironment. The role of the tumor microenvironment, specifically the immune compartment, in driving GBM and GSC states is being increasingly elucidated (7, 31, 41, 68, 69). However, improved understanding of how GSC adaptations influence the immune TME is crucial to overcome the immunosuppressive pressures that diminish the efficacy of immunotherapy in gliomas. As discussed above, the cell-intrinsic factors that modify and drive GSC states and transitions are abundant and include specialized reprogramming transcription factors, epigenetic modifiers, transcriptional regulators, cellular cycles, etc. In this section, we discuss findings that highlight the effects of GSC-intrinsic transcriptional variation and cellular transitions on their immunomodulatory role.
As mentioned previously, the most notable transcription factors associated with acquisition of a stem-like phenotype are the Yamanaka factors. Of particular interest in the context of glioma, especially related to the immune influence of GSCs, are Sox2 and Oct4. Sox2, which is a ubiquitously expressed transcription factor in cancer stem cells, has a direct effect on the immunosuppressive capacity of GSCs through induction of CD39 (ENTPD1), an ectonucleotidase responsible for hydrolyzing ATP towards adenosine and a critical mediator of immune response in cancer (70, 71). In GSCs, knockdown of Sox2 increased the extracellular ATP concentration and enhanced dendritic cell recruitment and phagocytic-ability as well as T-cell-mediated GSC lysis in co-cultures in direct association with a reduction in CD39 (70).
Another noteworthy stem cell-driving transcription factor, Oct4 (POU5F1), cooperates with Sox2 to induce an immunosuppressive transcriptome in GSCs, defined by induction of immune checkpoint inhibitory molecules (PD-L1, CD70, A2aR and TDO) alongside dysregulation of immune modulatory cytokines and chemokines including upregulation of SSP1, IL8, CXCL3, and CCL20 and downregulation of CCL5, CXCL9, and CXCL10. This GSC immune-suppressive phenotype was found to be mediated by and dependent on the BET protein BRD4 which is involved in directing chromatin remodeling in response to histone modifications (17).
Aside from the classical cancer stem cell reprogramming transcription factors, other transcription factors are involved in both the induction of a glioma stem-like state and acquisition of an immunosuppressive GSC phenotype. Yin Yang 1 (YY1), a zinc-finger transcription factor involved in polycomb protein recruitment and transcription regulation (72, 73), is necessary for maintenance of the stem cell phenotype in GBM, is associated with Sox2 and Oct4 expression across cancers (74–77), and mediates both chemotherapy and radiation resistance in GBM (74, 78). The YY1-CDK9 transcriptional complex in GBM cells promotes Treg infiltration, inhibits RNA methylation-dependent interferon responses, and reduces the efficacy of immune checkpoint inhibitor therapy in GBM (77), highlighting how transcription factors and epigenetic modifications cooperate to impart immunomodulatory function in GBM cells.
While we typically think of external pressures when discussing induction of stemness, pre-programmed endogenous processes such as the circadian rhythm may also drive and maintain the stem cell phenotype in cancer cells (79, 80). The circadian rhythm is a cell-autonomous, cyclical process composed of transcriptional-translational feedback loops that govern carefully timed adjustments in gene expression (81). The transcriptional variation attributed to circadian oscillations has a demonstrated capacity to alter cancer stem cell states, their interaction with the TME, and their susceptibility to therapeutic agents (79, 82–86). Disruption of the circadian rhythm leads to altered immune cell infiltration and T-cell exhaustion in a variety of cancers (85, 87, 88). In glioma, the CLOCK-BMAL1 complex, the main transcriptional component in the circadian rhythm, modulates the stem cell phenotype by enhancing self-renewal capacity and migration and regulating GSC metabolism (89–91). Furthermore, CLOCK, in partnership with BMAL1, upregulates LGMN via induction of OLFML3, a novel microglia-recruiting factor, in GSCs to enhance infiltration of microglia and polarize them to an immunosuppressive phenotype (89, 92). This finding, along with our understanding of circadian dysfunction in cancer, demonstrates the possibility of dynamic immunomodulatory GSC phenotypes mediated by cell-intrinsic processes.
As discussed previously, readers of histone modifications, e.g., BRDs, can direct acquisition of an immunosuppressive phenotype in GSCs (17). BRDs also have an immunomodulatory role in ATRX-deficient, IDH-mutant gliomas. Deficiency of ATRX, a histone protein involved in the chromatin remodeling SWI/SNF family, occurs in >80% of IDH-mutant grade II/III astrocytomas and >50% of secondary GBMs (93, 94). Hu et al. reports a BRD3/4-dependent induction of an immunosuppressive transcriptome, marked by upregulation of PD-L1/2, IL-33, CXCL8/9, CSF2, and IL-6, in ATRX-deficient glioma cells (95). Furthermore, this ATRX loss resulted in decreased T-cell-mediated glioma cell lysis, increased macrophage immune-suppressive M2-like polarization, and enhanced Treg tumor infiltration. Independently, Babikir et al. found increased infiltration of immunosuppressive monocyte-lineage cells in ATRX-mutant versus ATRX-wildtype IDH-mutant glioma (96). While the former findings were not in the context of cancer stem cells, ATRX-mutant IDH1-mutant GSCs have been shown to possess increased cell propagation capacity and upregulation of TGF-beta-associated pathways compared to ATRX-wildtype GSCs (97) suggesting that ATRX-deficiency enhances certain stemness characteristics in glioma in accordance with increased immunosuppressive effects.
Enzymes that remove histone acetylation marks, known collectively as histone deacetylases (HDACs), also regulate GSC-intrinsic immunomodulatory mechanisms (44, 98). A major factor in the poor immunogenicity of gliomas derives from the innate ability of GSCs to evade the immune response through dysregulation of major histocompatibility complex (MHC) molecule expression (44). Specifically, Yang et al. demonstrated HDAC-dependent MHC-1 downregulation in GSCs that consequently suppressed the function of tumor-infiltrating lymphocytes (TILs). Furthermore, pan-HDAC inhibition not only enhanced MHC-1 expression and the T-cell response, but it downregulated Sox2 and Oct4 protein expression and decreased the self-renewal capacity of GSCs. While the knowledge that HDAC inhibitors upregulate MHC-1 molecule expression in cancer is not new (99–103), this study extended the findings to gliomas and, more importantly, detailed the association between HDAC regulation of MHC1 expression and maintenance of the stem cell phenotype. Aside from MHC-1 expression, histone deacetylation via HDAC8 in glioma cells represses NKG2D ligand expression to evade natural killer (NK) cell-mediated tumor cell death (104). A study by Zhan et al. found that HDAC1 and HDAC2 promote GSC evasion of the suppressive interferon-mediated immune response through their involvement in the nucleosome remodeling and deacetylase (NuRD) complex (105). Zhan and colleagues show that methyl-CpG-binding domain 3 (MBD3) recruits and assembles the HDAC-containing NuRD complex at the promoter of STAT1 where deacetylation of H3K27 leads to repression of STAT1 transcription. From there, STAT1 repression desensitizes GSCs to interferon treatment. The driver of this mechanism, MBD3, also regulates proliferation, viability, and self-renewal capacity of GSCs and positively correlates with expression of Sox2, Olig2, and Nestin in GBM specimens (106). Together, these findings directly link glioma cell-intrinsic HDAC-mediated immunosuppression to the acquisition and maintenance of the stemness phenotype of GSCs.
The dynamics and consequences of DNA methylation in cancer biology have been vastly explored. Patterns of DNA methylation mediate repression of gene transcription thereby altering cell states and phenotypes and contributing to tumor progression (12, 16, 60). In the context of gliomas, levels of DNA methylation are associated with the IDH-mutation status of the tumor with IDH-mutant gliomas tending to acquire a hypermethylated phenotype, commonly referred to as the glioma CpG island methylator phenotype or G-CIMP (107). This CpG island hypermethylation has been implicated in controlling immunomodulatory effects of GSCs in both IDH-mutant and IDH-wildtype gliomas. Specifically, in IDH-mutant GSCs, increased methylation of the NKG2D ligand gene promoter suppresses its expression leading to the ability of GSCs to evade NK cell-mediated cytotoxicity (108). In line with evidence that decreasing DNA methylation in cancers enhances immunogenicity through upregulation of MHC-1 expression (109–112), GSCs are found to contain increased methylation along the regulatory regions of HLA genes (113). Similarly, a separate study discovered that methylation-dependent regulation of MHC-1 and ICAM1 expression regulates T cell-mediated GSC cytotoxicity in IDH-wildtype glioma (46). Gangoso et al. demonstrated emergence of a DNA methylation-dependent immune evasive phenotype highly associated with mesenchymal and mesenchymal-like GBM states (68). These GSCs were found to have extensive hypomethylation, especially along genes involved in immune-suppressive processes. Furthermore, immune evasive GSCs fostered an immunosuppressive TME in vivo characterized by enhanced infiltration of macrophages and myeloid-derived suppressor cells (MDSCs) and increased TIL dysfunction (68).
Unlike DNA methylation, RNA methylation is a relatively underappreciated regulator of the stem cell phenotype. In embryonic stem cells (ESCs), low levels of N (6)-methyladenosine (m6A methylation) promote pluripotency and protect against differentiation (64–66). Many transcripts of pluripotency genes, including Nanog, Sox2 and Klf2, are regulated by m6A methylation in both human and mouse ESCs (64, 65). In GSCs, Cui et al. found that METTL3, a catalytic subunit of the m6A methyltransferase complex, reduces cell proliferation and self-renewal capacity along with CD44 expression, through methylation of pluripotency-driving transcripts (114). However, conflicting roles of METTL3 in GSCs have been demonstrated by other studies (115–117). Visvanathan et al. showed that GSCs are enriched for METTL3 which functions to stabilized SOX2 expression and enhance self-renewal capacity (117). Moreover, METTL3 has been linked to TMZ resistance in GBM cells (115, 116). On the other hand, ALKBH5, an m6A RNA demethylase, is highly expressed in GSCs and associated with poor patient prognosis in GBM (118). Through demethylation, ALKBH5 enhances both self-renewal capacity and expression of stemness genes (i.e., Nestin, Sox2, Nanog, and Oct4) in GSCs (118). Furthermore, ALKBH5 can indirectly regulate the GBM cell-intrinsic immunosuppressive phenotype by demethylating the lncRNA NEAT1 which allows for NEAT1-mediated paraspeckle assembly (119). From there, relocation of transcriptional repressors to the newly assembled paraspeckles results in upregulation of CXCL8 and IL8. Consequently, ALKBH5-initiated CXCL8/IL8 induction improves tumor-associated macrophage infiltration and promotes tumor progression, demonstrating the impact of post-transcriptional epigenetic modifications in regulating the immunosuppressive GSC phenotype. These findings suggest the need for a more refined investigation of the role of m6A methylation in controlling the GSC phenotype, taking special care to account for and address the heterogeneous and dynamic states of GSCs.
A hallmark of cancer, metabolic remodeling permits cells to adapt and persist in conditions of depleted oxygen and/or nutrients by utilizing alternative metabolic pathways (120). Cancer stem cells in particular possess a great potential for metabolic reprogramming compared to more differentiated counterparts (121–123). In GBM, GSCs readily adjust their metabolic preferences to meet their energy demands (49, 123–125). Remodeling in response to hypoxic or low glucose environments occurs through various mechanisms including epigenetic modification (3, 126, 127), HIF-1α signaling (125, 128, 129), and altered expression of metabolic enzymes and transporters (49, 130). In general, metabolic profiles in GBM cells are spatially distinct and attributed to TME interactions and restricted nutrient availability (122, 125, 131, 132) with different GSC subsets having distinct metabolic preferences and dependencies (121, 133, 134).
Notably, metabolic reprogramming in GSCs modulates the anti-tumor immune response in favor of tumor progression. As mentioned previously, GSCs suppress T cell function and recruit and polarize microglia through glucose monopolization and circadian dysregulation, respectively (49, 89, 90). Additionally, GBM cells in spatial proximity to immune cells have distinct metabolic profiles associated with immune-suppressive transcriptional signatures. Similar to immune-responsive cells, hypoxia-responsive GBM cells, which are enriched for GSCs, reside in regions enriched in TAMs and T cells (including exhausted CD8+ T cells), linking hypoxia-induced metabolic remodeling to tumor-promoting immune interactions (135). Furthermore, Coy et al. identified increased levels of extracellular immune-suppressive adenosine in GBM attributed to high expression of CD73, which functions alongside CD39 to metabolize ATP into adenosine, in hypoxia-responsive GBM cells (131). Collectively, these findings demonstrate a connection between TME-driven metabolic reprogramming and GSC-mediated immune suppression, and prompt further investigation into therapeutically relevant metabolic vulnerabilities in these immune-suppressive cells.
Over the last decade or so, our understanding of the phenotypic plasticity of cancer cells has expanded to include the ability of cancer stem cells to behave like, or mimic, the function of other cells (Figure 2) (136). For example, GSCs can behave like vascular endothelial cells and pericytes to promote angiogenesis and increase oxygen and nutrient availability in a phenomenon referred to as vasculogenic mimicry (137–139). In many respects, this epitomizes the inherent plasticity of GSCs and is mediated by epigenetic mechanisms and influenced by conditions in the TME (136–141). Additionally, subsets of GSCs resemble neuronal and glial progenitor cells and engage in synaptic interactions to promote tumor invasion and progression (59, 142–146). Notably, a similar process affecting the GBM immune microenvironment has been identified whereby GSCs mimic immune cell function by co-opting transcriptional profiles typically associated with myeloid cells (68, 147). The aforementioned study by Gangoso et al. demonstrated acquisition of a myeloid-related immunosuppressive transcriptional profile in GSCs mediated by a DNA methylation-dependent immunoediting mechanism in response to repeated immune exposure (68). In particular, Irf8, Nt5e, and Cd274 were among the genes upregulated in immune evasive, mesenchymal-like GSCs due to DNA demethylation. Due to the often myeloid-specific expression of Irf8 and transcriptome-level induction of myeloid-related signatures, they concluded that altered DNA methylation governs acquisition of a myeloid-mimicking phenotype in GSCs (68). Furthermore, our lab has identified a Treg-like transcriptional profile in TGFBR2high GSCs driven by Oct4 and Sox2 that regulates the immunosuppressive TME by repressing T cell function through the action of effector genes canonically expressed by Tregs (e.g., CD274, ENTPD1, NT5E, LGALS1, TGFB1) (148). Collectively, these findings suggest that GSCs co-opt functions of non-neoplastic cells from the TME through dynamic state transitions to facilitate tumor progression and suppress the immune response.
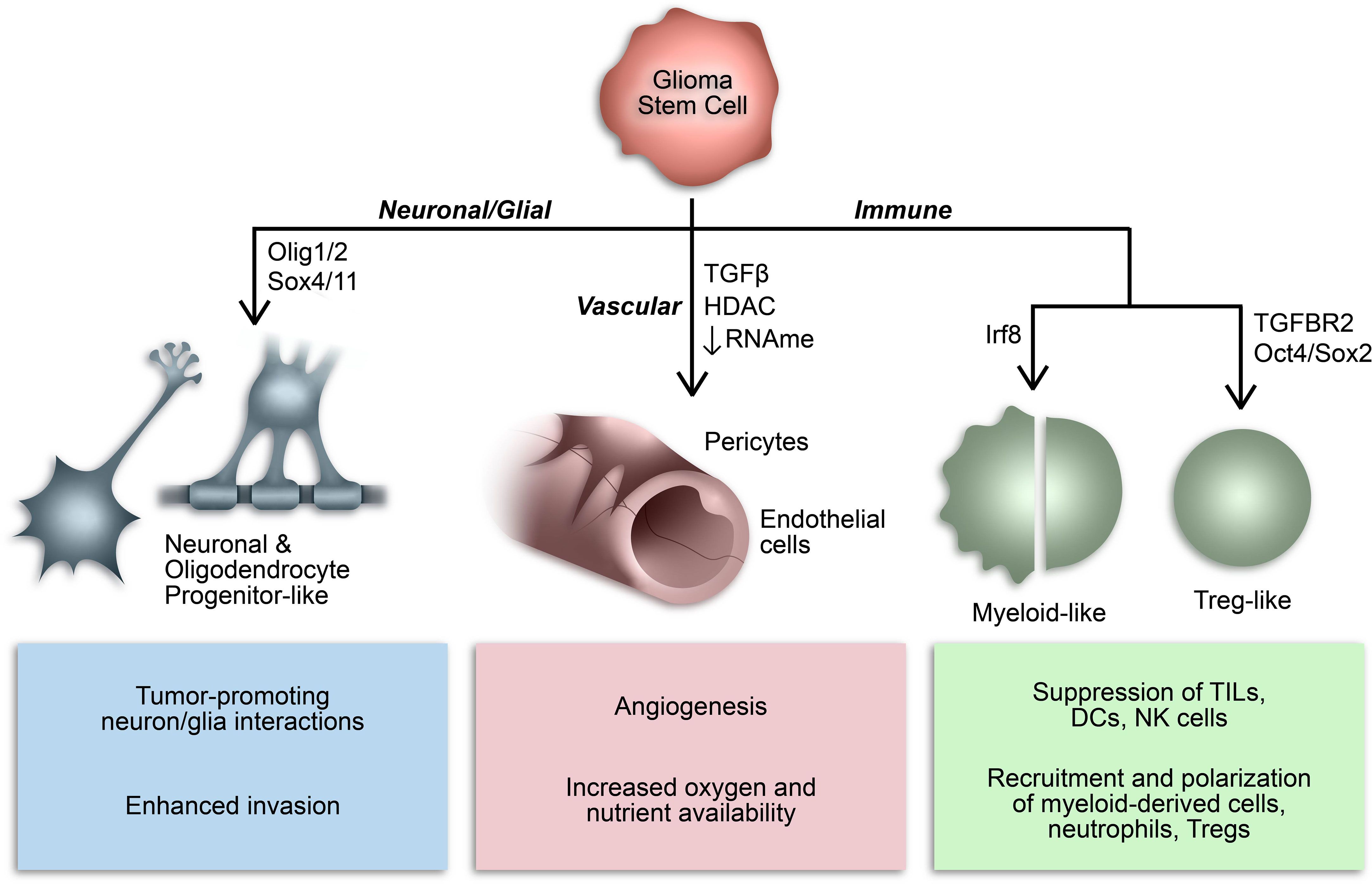
Figure 2 Consequences of GSC lineage plasticity. A defining characteristic of GSCs is their multipotency, or ability to differentiate into various cell types within a particular cell lineage. Cancer stem cells, including GSCs, can imitate cell types from multiple lineages by co-opting their transcriptional profiles and consequently mimicking their functions.
In summary, GSCs alter their immunomodulatory phenotype in response to stemness-regulating stimuli (Figure 1). A variety of gene expression regulators carry out these transformations, from pluripotency-driving transcription factors and circadian transcriptional complexes to epigenetic modifications (Table 1). While improving, our understanding of the immunosuppressive functions of GSCs as they relate to their heterogeneous and ever-changing states is limited. Unfortunately, many studies conducted in the context of GSC-immune interactions are restricted from the start to certain subpopulations of GSCs (e.g., gatekeeping GSCs by their CD133 expression). This not only sets the stage for potentially context-dependent findings, but inaccurately represents the diverse spectrum of GSC phenotypes found in patient specimens. Moving forward, it is essential to approach studies of GSC-intrinsic mechanisms from an unbiased starting point, especially in the context of TME interactions whereby both cell intrinsic and extrinsic influences constantly drive GSC adaptation and state plasticity. In the next section, we discuss cutting-edge techniques and bioinformatics tools available to study heterogeneous and dynamic GSC states at a high cellular resolution allowing for deep exploration of the bidirectional interaction between GSCs and cells of the TME that influence GSC-mediated immunomodulation (Table 2).
Whole tumor sequencing modalities, such as whole-genome and whole-exome sequencing, RNA-sequencing (RNA-seq), bisulfite sequencing, and assays for chromatin accessibility sequencing (ATAC-seq), have been widely used to classify tumors and explore context-specific cancer biology. However, these sequencing analyses conducted on dissociated bulk tumor specimens output an average value across all cells, placing limitations on the ability to distinguish which cell type or compartment is contributing to said output and, therefore, interfering with exploration of heterogeneous cell populations and phenotypes. Computational tools to deconvolute bulk-sequencing (bulk-seq) data have been developed, allowing one to take full advantage of large, pre-existing datasets of bulk-seq patient tumor specimens. These tools present an opportunity to infer cellular-level data from a vast number of patient tumors when the use of more advanced technologies is unfeasible or cost-prohibitive.
Computational deconvolution of bulk RNA-seq data has garnered interest in the realm of cancer immunology by granting insight into the composition of the immune compartment (e.g., cell type proportions and overall extent of immune infiltration) and transcriptional phenotypes within immune cell types based on associated expression of cell-specific markers (149–156). The degree of immune cell infiltration and tumor purity (i.e., proportion of tumor composed of neoplastic cells), calculated through ESTIMATE (149), have been associated with molecular phenotypes and trends in patient survival, with high immune cell infiltration and low tumor purity corresponding to mesenchymal tumors and shorter survival (193, 194). More in-depth analytic tools, such as CIBERSORTx (150), xCell (151), MCP-Counter (152), EPIC (153, 154), quanTIseq (155) and TIMER (156), go beyond the extent of immune infiltration and tumor purity to deduce absolute and relative cell proportions based on pre-set or user-defined cell signatures. The CIBERSORTx algorithm takes it a step further by allowing users to impute gene expression profiles (GEPs) for the estimated cell populations. However, this additional step is not recommended for low abundance cell types, such as tumor-infiltrating lymphocytes in GBM, due to insufficient statistical power. In populations with sufficient abundance, imputed GEPs can define cell-type specific transcriptional phenotypes in immune cells (e.g., M1/M2 macrophages, functional or exhausted T cells, activated natural killer or dendritic cells, etc.) and permit further exploration into cell-cell interactions based on ligand/receptor expression within respective cell types.
The value of bulk deconvolution algorithms is ultimately dependent upon two factors: (i) use of a validated, context-specific cell signature matrix and (ii) supportive findings through in vitro and/or in vivo experimentation. Varn et al. applied the CIBERSORTx platform to construct and validate a glioma cell signature matrix encompassing both neoplastic and non-neoplastic cell types derived from single-cell RNA-seq of IDH-wildtype and IDH-mutant patient gliomas (31). These efforts defined the temporal transitions in the composition of neoplastic cell populations and the associated changes in infiltrating immune cell types in newly diagnosed and recurrent tumors (31, 195). Moreover, a second CIBERSORTx cell signature matrix was constructed to infer proportions of GBM histological features within tumors by using gene expression profiles specific to Ivy GAP-defined histological features (e.g., leading-edge, infiltrating tumor, cellular tumor, microvascular proliferation, and pseudopalisading cells around necrosis) (196). After pathologist-based validation of the inferred proportions, Varn and colleagues were able to calculate correlations between cellular states and histological features. Together, utilization of CIBERSORTx software to estimate proportions of distinct cellular states and phenotypes and the extent of hallmark histological features (196, 197) allowed for comprehensive analysis of tumor cell interactions with and in relationship to the TME in a computational manner, setting the stage for downstream investigation of potentially therapeutically actionable cell states and mechanisms.
While tools for deconvolution of bulk sequencing still have their place in cancer research, especially due to their low cost and accessibility and the large volumes of publicly available data from patient tumors, the advent of cutting-edge single-cell sequencing technologies have turned what was once purely computational predictions into actual omics information at cellular-resolution.
The development of single-cell technologies to explore cellular states at the genomic, epigenomic, transcriptomic, proteomic, and metabolomic levels has led to unprecedented understanding of the inner workings of cancer (198–200). The resolution of single-cell sequencing allows for in-depth evaluation of rare cancer cell populations (e.g., GSC subsets and tumor-infiltrating immune cell subsets) within tumors that are otherwise lost in bulk analyses. The growing repertoire of single-cell technologies allows us to explore not only the constantly adapting cancer cell population but also the non-neoplastic cells in the surrounding TME, providing insight into the fluctuating & bidirectional communication between cell compartments.
In glioma, single-cell RNA-seq (scRNA-seq) analyses have broadened our knowledge of dynamic cellular and molecular phenotypes that represent the heterogeneous nature of gliomas, confer therapy resistance, and drive tumor recurrence in patients (1, 59, 134). Previously, GBMs were classified by their molecular subtype as determined by bulk sequencing of tumors (43). However, classification of tumor subtypes based on bulk transcriptome analyses masks the inherently high degree of intra-tumoral heterogeneity in GBM, demonstrated in early single-cell analyses by the representation of multiple molecular subtypes within individual tumors (1). Since then, scRNA-seq analyses conducted in patient tumors have defined distinct cellular states that vary in respect to their gene expression profiles (59, 60, 201), interaction with the TME (31, 41, 59, 68), and metabolic states (59, 135). Notably, Neftel et al. demonstrated that glioma cells can exist in and transition between four cellular states, oligodendrocyte-progenitor-like (OPC-like), neural progenitor-like (NPC-like), astrocyte-like (AC-like), and mesenchymal-like (MES-like), that differentially express genes involved in cell cycle, development, metabolism, and immune response, and in which all but one state (MES-like) resemble normal neurodevelopmental cell types (59). Cells in the MES-like state, which expresses higher levels of hypoxia-response and glycolytic genes relative to the other states, form synergistic relationships with TAMs to promote their respective mesenchymal-like, immunosuppressive phenotypes, demonstrated by Hara et al. (41).
Aside from scRNA-seq, one of the more commonly used single-cell techniques in the study of gliomas is cytometry by time-of-flight (CyTOF) (159, 160). CyTOF provides valuable information on cell proportions based on surface marker expression and has been used to characterize variation in immune cell landscapes between primary and metastatic CNS cancers (19), newly diagnosed and recurrent GBMs (20), and immune checkpoint inhibitor (ICI) refractory and responsive cancers and GBM mouse models (202). For example, Fu et al. identified a significant decrease in the proportion of tumor-promoting macrophages and microglia with recurrence whereas the frequencies of T cells, B cells, and NK cells were relatively unchanged (20). In another study, Simonds and colleagues compared immune cell proportions between ICI-refractory (GBM and sarcoma) and ICI-sensitive (renal cell carcinoma) cancers and found decreased T cells and DCs, but increased TAMs in ICI-refractory tumors (202). This aligns with the previously discussed finding that brain metastases, which are more sensitive to immunotherapy than GBM, have relatively more leukocytes and less myeloid cells in their TME, also assessed through CyTOF analysis (19).
Additional insight into cancer-immune interactions from the perspective of infiltrating immune cells can be obtained through techniques like CITE-seq (164, 165) and T cell receptor (TCR) sequencing (161) that provide information on surface marker-defined cell expression profiles and TCR variability and clonality, respectively. The use of CITE-seq, which simultaneously quantifies epitope-specific cell types and their associated transcriptomes via RNA sequencing, in glioma has identified novel markers for various transcriptionally defined subsets of TAMs with demonstrated susceptibility to macrophage-directed therapeutics (203). This exploration of the TAM population also unveiled a shift from microglia-derived to monocyte-derived TAMs in the TME with tumor recurrence (203). Notably, high infiltration of monocytic macrophages relative to resident microglia corresponds to increased malignancy in gliomas (19, 204). As for the tumor-infiltrating lymphoid population, TCR sequencing of newly diagnosed and recurrent GBMs revealed a diminished TCR repertoire with recurrence (22), which leads to suppressed T cell-mediated immune response via altered antigen-recognition machinery. Based on our understanding of how dynamic GSC states differentially express MHC molecules (44, 46, 113) and the fundamental knowledge that TCR repertoires are shaped in response to antigen exposure, one could infer that immunoediting mechanisms within GSCs directly alter T cell clonality during tumor progression, adding another facet to the immune evasive nature of glioma cells. However, this has yet to be conclusively demonstrated in the context of gliomas. Alternatively, a restricted TCR repertoire could signify T cell specification against the most actionable tumor antigens; however, those T cells are rendered ineffective for the reasons discussed in this review. Hypotheticals aside, these single-cell analyses expand on our understanding of immune TME changes with recurrence that suppress the immune response and facilitate glioma progression.
As discussed previously, epigenetic changes contribute significantly to the acquisition of an immune evasive phenotype in glioma cells in response to immune exposure, a mechanism widely referred to as immunoediting (68). These single-cell modalities to measure epigenetic changes can be combined with single-cell transcriptomics to provide unparalleled information on adaptive cell transitions within tumors. For example, a multimodal approach involving scRNA-seq and single-cell reduced representation bisulfite sequencing (scRRBS) in gliomas by Johnson et al. revealed DNA methylation-mediated induction of cell states associated with activation of stress response pathways and increased therapy resistance (60). Defined by distinct transcriptome and DNA methylome profiles, Johnson and colleagues identified three cell states referred to as stem-like, proliferating stem-like, and differentiated-like. Multi omic analysis revealed key transcriptional regulators in each cell state with differentiated-like cells having enhanced activity in transcription factors involved in hypoxia and stress response, demonstrating the value of epigenetic modifiers in stress-induced cell state plasticity (60).
Another technology amenable to single-cell implementation is the Assay for Transposase-Accessible Chromatin via Sequencing (ATAC-Seq (157)). Using this approach Guilhamon and colleagues found three GSC states, reactive, constructive, and invasive, distinguished by their chromatin accessibility profiles. Cells in the reactive state had increased accessibility to the promoters of immune-related genes, include genes associated with Tregs. Alternatively, the invasive GSC state had a demonstrated negative correlation with survival in orthotopic xenografts (201).
Currently, several technologies are being developed for multimodal single-cell application (Table 2) in addition to sophisticated computational tools for downstream analyses following single-cell sequencing. Often used in developmental biology but increasingly applied to cancer studies, these analyses can inform cellular hierarchies through pseudo-lineage tracing (e.g., pseudotime analysis (30, 205–207)), predicted cell state transitions (e.g., RNA Velocity (142)), and even metabolic phenotypes (e.g., Compass (208)). Since single-cell sequencing provides a point-in-time view of cell states, these algorithm-based insights into cellular transitions may reveal previously undetectable adaptive mechanisms in GSCs. Multi-plexing these state-of-the-art tools will permit interrogation of highly dimensional cell phenotypes at an unparalleled depth and can provide insight into clonal architecture and lineage tracing, regulators of GSC states, links between genetic and epigenetic profiles, drivers of adaptive responses to therapeutic or immune stimuli, and more (199). We envision these approaches being combined with scRNA-Seq to explore the role of the TME in promoting reversible, epigenetic-mediated GSC state transitions and, thus, build upon similar studies utilizing bulk multimodal analysis in patient-derived GSCs (61). Similar analyses at single-cell resolution would be invaluable in examining specific cell subsets and assessing their reciprocal impact on the TME.
Unfortunately, every rose has its thorn, and a major one for these cutting-edge single-cell sequencing technologies is the complete absence of spatial information. When it comes to understanding the influence of endogenous cell interactions, specifically GSC interactions with the surrounding TME, this information is critical, especially for pre-clinical studies relying on accurate representation of patient tumors. However, this information is lost with dissociative single-cell techniques, which is where newly generated spatially resolved omics technologies come into play.
While cell-cell interactions can be inferred to a degree through computational manipulation of scRNA-seq data (209–212), spatial omics provide next-level understanding of these interactions by accounting for endogenous tissue organization and cell-cell proximity (213). Interrogation of RNA localization in tissue specimens using spatial transcriptomics techniques, broadly classified as either high-plex RNA imaging or spatial barcoding (199, 213), is arguably the most used spatial analysis as of now. High-plex imaging techniques include in situ sequencing (e.g., STARmap (170), FISSEQ (171)) and fluorescent in situ hybridization (e.g. RNAScope (172), MERFISH (173, 174), and seqFISH (175–177)) that provide single-cell resolution spatial gene expression with the limitation of relying on predetermined target transcripts and quality imaging instrumentation. On the other hand, spatial barcoding or in situ capture techniques, including Slide-seq (178, 179), 10X Visium, and high-definition spatial transcriptomics (180), provide unbiased, transcriptome-wide expression output. However, data is collected at multi-cellular spot resolution which requires additional deconvolution analysis to appropriately distinguish individual cell expression.
Aside from gene expression data, spatially resolved proteomic, metabolomic, and lipidomic information can be gathered from tissue specimens using imaging mass spectrometry or cytometry techniques. Unbiased coverage of biochemical features can be achieved through semi-quantitative mass spectrometry imaging (MSI) methods including time-of-flight secondary ion mass spectrometry (ToF-SIMS) (181, 182) and matrix-assisted laser desorption/ionization (MALDI) time-of-flight (MALDI-TOF) or fourier transform ion cyclotron resonance (MALDI-FTICR) mass spectrometry (183). Alternatively, imaging mass cytometry (IMC) can detect and quantify surface protein profiles using isotope-coupled antibody panels (184) in a method analogous to a spatially resolved CyTOF analysis. While many of the above spatial technologies have yet to be applied in the context of GBM, RNAScope and MSI methods have been utilized with the former identifying expression-based prognostic markers from GBM tissues (214, 215). On the other hand, MSI methods have investigated GBM heterogeneity based on metabolic and proteomic profiles and drug distribution in tumors (131, 216–220).
Integration of spatial and single-cell omics data grants unprecedented understanding of cellular and molecular phenotypes of cancer cells within their native environment. Ravi et. Al. define spatially distinct cellular phenotypes distinguished by transcriptional and metabolic programs through integration of spatial transcriptomics, MALDI-FTICR mass spectrometry, and mass cytometry proteomics (132). These five phenotypes were classified as radial glia, spatial OPC, neural development, reactive hypoxia, and reactive immune. Furthermore, cell phenotypes overlapped with previously established GSC states and were further distinguished by their spatial localization and proximity to immune cell infiltrates. Specifically, the reactive immune cell state, which has similarities to both the MES1 and AC-like states defined by Neftel et al. (59), had significant interaction with both myeloid and lymphoid infiltrating immune cells. Moreover, T cells near reactive-immune cells expressed higher levels of PD-1, representative of T cell exhaustion (132). Overall, multi-modal in situ sequencing of sequential tumor tissue slices paints a spatially resolved, complete picture of native cellular states, paving the way for mechanistic validation and identification of potentially translatable therapeutic vulnerabilities within specified cell types.
While single-cell and spatial analyses cannot classify GSCs by their defining functional traits (i.e., self-renewal capacity, multipotency, and tumor propagation capacity), these technologies are advantageous in their own rights for characterization of GSCs. Notably, chromatin accessibility profiles and validated GSC-specific transcriptomes can be used to delineate between GSCs and differentiated GBM cells in silico (134, 157, 201, 221–223). Additionally, high resolution omics data provides information at a remarkable depth necessary for proper investigation of dynamic and heterogeneous GSC states. Overall, the degree of insight into dynamic GSC biology afforded by multi-dimensional single-cell and spatial technologies has the potential to elucidate not only the factors that coordinate adaptive and immunosuppressive GSC mechanisms but their associated therapeutic vulnerabilities. Meticulous experimental design and utilization of spatially resolved single-cell technologies could conceivably inform novel treatment approaches capable of eliciting substantial anti-tumor response, a feat yet to be achieved in recurrent GBM.
The existence of dynamic GSC states shaped by cell-intrinsic factors, the tumor microenvironment, and therapeutic pressures demand a revised approach to GBM therapy development that’s directed at disrupting the bidirectional interactions between GSCs and the TME that promote GBM malignancy, immune evasion, and tumor recurrence. Given the pervasive involvement of epigenetic modifications in GSC plasticity and acquisition of immunosuppressive phenotypes, using epigenetic inhibitors to augment the response of GBM to available immunotherapies warrants investigation (Figure 3). Notably, clinical attempts to treat newly diagnosed and recurrent GBM with epigenetic inhibitors (plus chemoradiation and surgery) have had negligible anti-tumor effects compared to standard-of-care treatment alone (186–191). However, preclinical studies investigating the immune effects of epigenetic inhibitors demonstrate increased immunogenicity, indicating that combining epigenetic and immunostimulatory treatment modalities may have potent anti-tumor effects (Table 3) (17, 44, 46, 104, 185). Two early phase clinical trials combining anti-PD1 checkpoint inhibitors with azacytidine (DNA methyltransferase inhibitor) or vorinostat (HDAC inhibitor) plus standard chemoradiation are currently underway in IDH-mutant gliomas (NCT03684811) and newly diagnosed GBM (NCT03426891), respectively. Additionally, several clinical trials testing combination epigenetic and immune therapy have been conducted or are currently underway in a variety of cancers, however, durable clinical effects are rare and often restricted to small subsets of patients (224–236). However, ongoing clinical trials may provide insight into mechanisms of resistance and opportunities for therapeutic improvement. Given the knowledge that immune cell states are also intimately controlled by epigenetic modifications (237), the lack of response from combination epigenetic-immune therapies may be due in part to unwanted effects on systemic immune cell pools that alter their phenotypic profiles and potentially induce dysfunctional states. Thus, strategies for targeting immune suppressive mechanism specifically within the TME while avoiding counterproductive systemic effects are needed. Despite these early phase results, the immunogenic effects of epigenetic inhibitors and combo epigenetic-immune therapy in preclinical GBM studies are promising (17, 44, 46, 104, 185, 192) and deserve to be explored to their full extent in clinical trials. Notably, increased immune recognition and resultant cytolysis of tumor cells by T cells and NK cells has been achieved through HDAC inhibitors and DNA hypomethylating agents (e.g., decitabine) (44, 46, 104, 185). Additionally, BRD4 inhibition attenuated an immunosuppressive GSC phenotype to enhance the anti-tumor immune response (17). Furthermore, BRD4 was implicated in an acquired immunosuppressive gene expression profile in GSCs refractory to CAR-T cell treatment. Targeting BRD4 in a mouse model of GBM effectively potentiated CAR-T cell therapy, highlighting the potential for clinical success with combination epigenetic and immunotherapy in GBM (192).
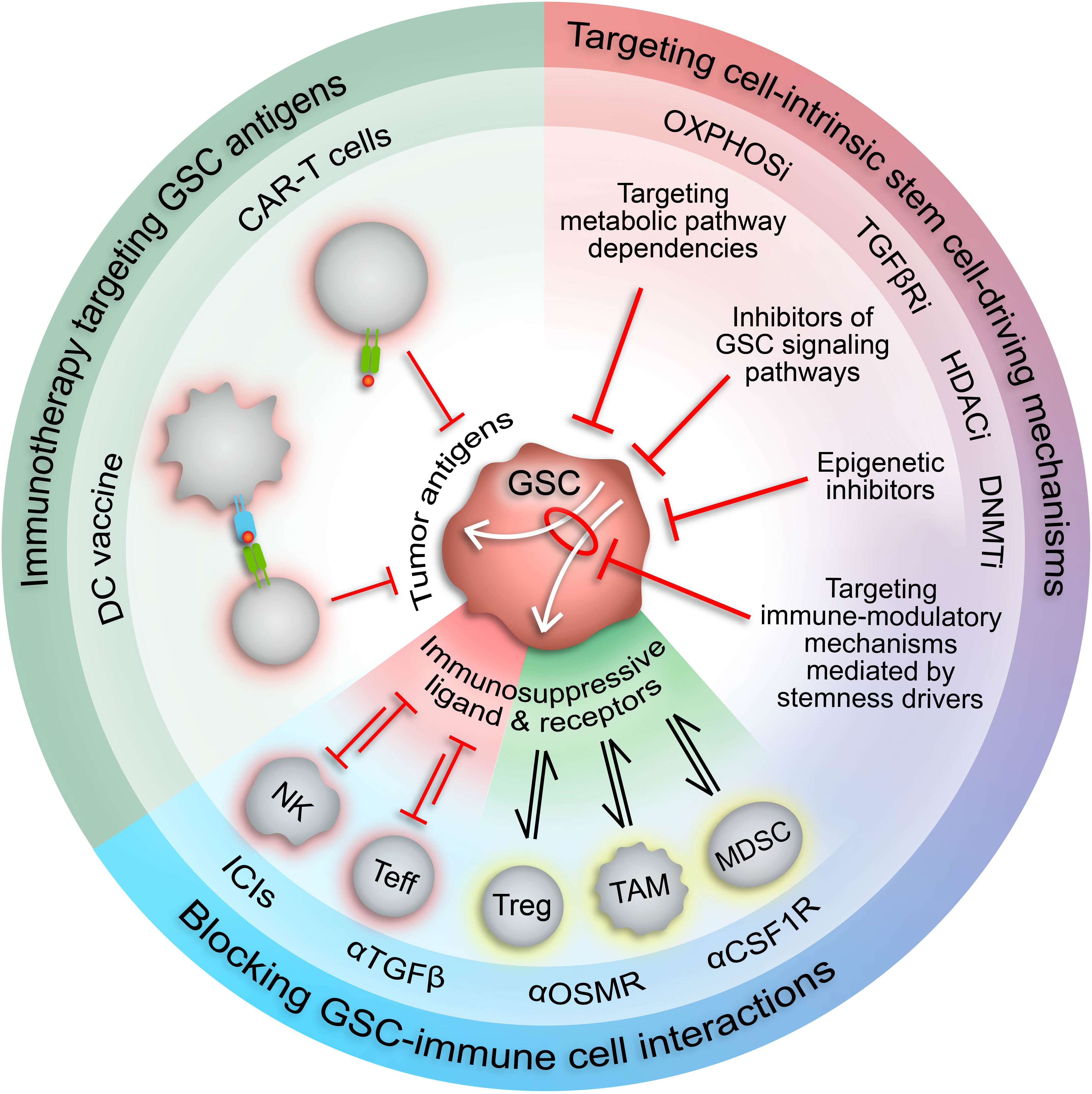
Figure 3 Therapeutic approaches to target immunosuppressive GSCs. Increased understanding of the malignant properties of GSCs and their inherent plasticity and heterogeneity has designated GSCs as desirable therapeutic targets. The role of GSCs in driving and maintaining an immunosuppressive TME suggests that GSC-targeted therapies could potentiate current immunotherapies. Targeting stemness mechanisms that also mediate immunosuppressive mechanisms in GSCs (white arrows) has the potential to augment immunotherapy response in GBM by increasing expression of tumor-specific antigens and repressing immunosuppressive cell interactions. CAR-T cell, chimeric antigen receptor T cell; DC, dendritic cell; NK, natural killer cell; Teff, effector T cell; Treg, regulatory T cell; TAM, tumor-associated macrophage/microglia; MDSC, myeloid-derived suppressor cell; ICIs, immune checkpoint inhibitors; HDACi, HDAC inhibitors; DNMTi, DNMT inhibitors; TGFβRi, TGFβ receptor inhibitors; OXPHOSi, oxidative phosphorylation inhibitors.
The involvement of GSCs in modulation of the anti-tumor immune response has prompted investigation into GSC-targeted immune-related therapies (Figure 3). Proposed methods include chimeric antigen recognition T cells (CAR-T cells) or dendritic cell vaccines directed at GSC-specific surface markers and antigens (53). However, the immunosuppressive effects of GSCs may attenuate the functionality of these engineered immune cells, thereby impeding therapeutic efficacy. Alternatively, forced differentiation of GSCs into a cellular state responsive to current therapeutic regimens holds promise (238), especially in combination with immunotherapy, but fails to consider how controlled differentiation would oppose plasticity mechanisms induced by standard-of-care treatments and/or potentially select for a more immunosuppressive GSC state. Therefore, we propose combining immunotherapy with approaches that disrupt mechanisms governing the transition of GSCs to immunosuppressive states and/or exploit cellular and molecular vulnerabilities inherent in these cell states (Figure 3). Identifying actionable therapeutic targets by incorporating high-dimensional, spatially aware single-cell analyses of patient tumor specimens and patient-derived GSC models into preclinical studies allows for development of therapeutics with high potential for clinical success.
Conceptualization: ALJ, JL, and HL-B.; Writing – Original Draft: ALJ; Writing – Review & Editing: ALJ, JL, and HL-B; Funding Acquisition: JL, and HL-B; Supervision: JL, and HL-B. All authors contributed to the article and approved the submitted version.
This study is funded by the National Institute of Neurological Disorders and Stroke (NINDS) grants 1R01NS110087, 1R01NS096754 (JL), 1R01NS120949 (HLB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell rna-seq highlights intratumoral heterogeneity in primary glioblastoma. Science (2014) 344(6190):1396–401. doi: 10.1126/science.1254257
2. Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A (2013) 110(10):4009–14. doi: 10.1073/pnas.1219747110
3. Gimple RC, Bhargava S, Dixit D, Rich JN. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev (2019) 33(11-12):591–609. doi: 10.1101/gad.324301.119
4. Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, et al. Adaptive chromatin remodeling drives glioblastoma stem cell plasticity and drug tolerance. Cell Stem Cell (2017) 20(2):233–46.e7. doi: 10.1016/j.stem.2016.11.003
5. Schonberg DL, Lubelski D, Miller TE, Rich JN. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol Aspects Med (2014) 39:82–101. doi: 10.1016/j.mam.2013.06.004
6. Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell (2010) 17(4):362–75. doi: 10.1016/j.ccr.2009.12.049
7. Dirkse A, Golebiewska A, Buder T, Nazarov PV, Muller A, Poovathingal S, et al. Stem cell-associated heterogeneity in glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat Commun (2019) 10(1):1787. doi: 10.1038/s41467-019-09853-z
8. Lan X, Jorg DJ, Cavalli FMG, Richards LM, Nguyen LV, Vanner RJ, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature (2017) 549(7671):227–32. doi: 10.1038/nature23666
9. Li Y, Laterra J. Cancer stem cells: Distinct entities or dynamically regulated phenotypes? Cancer Res (2012) 72(3):576–80. doi: 10.1158/0008-5472.CAN-11-3070
10. Liu X, Huang J, Chen T, Wang Y, Xin S, Li J, et al. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res (2008) 18(12):1177–89. doi: 10.1038/cr.2008.309
11. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell (2006) 126(4):663–76. doi: 10.1016/j.cell.2006.07.024
12. Lopez-Bertoni H, Lal B, Li A, Caplan M, Guerrero-Cazares H, Eberhart CG, et al. Dnmt-dependent suppression of microrna regulates the induction of gbm tumor-propagating phenotype by Oct4 and Sox2. Oncogene (2015) 34(30):3994–4004. doi: 10.1038/onc.2014.334
13. Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, et al. C-met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A (2011) 108(24):9951–6. doi: 10.1073/pnas.1016912108
14. Lopez-Bertoni H, Kotchetkov IS, Mihelson N, Lal B, Rui Y, Ames H, et al. A Sox2:Mir-486-5p axis regulates survival of gbm cells by inhibiting tumor suppressor networks. Cancer Res (2020) 80(8):1644–55. doi: 10.1158/0008-5472.CAN-19-1624
15. Lopez-Bertoni H, Lal B, Michelson N, Guerrero-Cazares H, Quinones-Hinojosa A, Li Y, et al. Epigenetic modulation of a mir-296-5p:Hmga1 axis regulates Sox2 expression and glioblastoma stem cells. Oncogene (2016) 35(37):4903–13. doi: 10.1038/onc.2016.22
16. Lopez-Bertoni H, Johnson A, Rui Y, Lal B, Sall S, Malloy M, et al. Sox2 induces glioblastoma cell stemness and tumor propagation by repressing Tet2 and deregulating 5hmc and 5mc DNA modifications. Signal Transduct Target Ther (2022) 7(1):37. doi: 10.1038/s41392-021-00857-0
17. Ma T, Hu C, Lal B, Zhou W, Ma Y, Ying M, et al. Reprogramming transcription factors Oct4 and Sox2 induce a brd-dependent immunosuppressive transcriptome in gbm-propagating cells. Cancer Res (2021) 81(9):2457–69. doi: 10.1158/0008-5472.CAN-20-2489
18. Ohue Y, Nishikawa H, Regulatory T (Treg). Cells in cancer: Can treg cells be a new therapeutic target? Cancer Sci (2019) 110(7):2080–9. doi: 10.1111/cas.14069
19. Friebel E, Kapolou K, Unger S, Nunez NG, Utz S, Rushing EJ, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell (2020) 181(7):1626–42.e20. doi: 10.1016/j.cell.2020.04.055
20. Fu W, Wang W, Li H, Jiao Y, Huo R, Yan Z, et al. Single-cell atlas reveals complexity of the immunosuppressive microenvironment of initial and recurrent glioblastoma. Front Immunol (2020) 11:835. doi: 10.3389/fimmu.2020.00835
21. Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell (2017) 32(1):42–56.e6. doi: 10.1016/j.ccell.2017.06.003
22. Mohme M, Schliffke S, Maire CL, Runger A, Glau L, Mende KC, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res (2018) 24(17):4187–200. doi: 10.1158/1078-0432.CCR-17-2617
23. Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res (2018) 24(17):4175–86. doi: 10.1158/1078-0432.CCR-17-1846
24. Dubinski D, Wolfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, et al. Cd4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol (2016) 18(6):807–18. doi: 10.1093/neuonc/nov280
25. Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished Cd4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res (2006) 66(6):3294–302. doi: 10.1158/0008-5472.CAN-05-3773
26. Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol (2011) 13(6):591–9. doi: 10.1093/neuonc/nor042
27. Elliott LH, Brooks WH, Roszman TL. Activation of immunoregulatory lymphocytes obtained from patients with malignant gliomas. J Neurosurg (1987) 67(2):231–6. doi: 10.3171/jns.1987.67.2.0231
28. Haddad AF, Chen JS, Oh T, Pereira MP, Joshi RS, Aghi MK. Higher cytolytic score correlates with an immunosuppressive tumor microenvironment and reduced survival in glioblastoma. Sci Rep (2020) 10(1):17580. doi: 10.1038/s41598-020-73793-8
29. Marinari E, Allard M, Gustave R, Widmer V, Philippin G, Merkler D, et al. Inflammation and lymphocyte infiltration are associated with shorter survival in patients with high-grade glioma. Oncoimmunology (2020) 9(1):1779990. doi: 10.1080/2162402X.2020.1779990
30. Landry AP, Balas M, Alli S, Spears J, Zador Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci Rep (2020) 10(1):19542. doi: 10.1038/s41598-020-76657-3
31. Varn FS, Johnson KC, Martinek J, Huse JT, Nasrallah MP, Wesseling P, et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell (2022) 185(12):2184–99.e16. doi: 10.1016/j.cell.2022.04.038
32. Grabowski MM, Sankey EW, Ryan KJ, Chongsathidkiet P, Lorrey SJ, Wilkinson DS, et al. Immune suppression in gliomas. J Neurooncol (2021) 151(1):3–12. doi: 10.1007/s11060-020-03483-y
33. Bayik D, Zhou Y, Park C, Hong C, Vail D, Silver DJ, et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discovery (2020) 10(8):1210–25. doi: 10.1158/2159-8290.CD-19-1355
34. Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol (2002) 103(4):351–5. doi: 10.1007/s00401-001-0472-x
35. Carvalho da Fonseca AC, Wang H, Fan H, Chen X, Zhang I, Zhang L, et al. Increased expression of stress inducible protein 1 in glioma-associated Microglia/Macrophages. J Neuroimmunol (2014) 274(1-2):71–7. doi: 10.1016/j.jneuroim.2014.06.021
36. Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, et al. Rage expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res (2014) 74(24):7285–97. doi: 10.1158/0008-5472.CAN-14-1240
37. Otvos B, Silver DJ, Mulkearns-Hubert EE, Alvarado AG, Turaga SM, Sorensen MD, et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells (2016) 34(8):2026–39. doi: 10.1002/stem.2393
38. Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, et al. Microglia-derived tgf-beta as an important regulator of glioblastoma invasion–an inhibition of tgf-Beta-Dependent effects by shrna against human tgf-beta type ii receptor. Oncogene (2008) 27(7):918–30. doi: 10.1038/sj.onc.1210683
39. Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-associated Microglia/Macrophages enhance the invasion of glioma stem-like cells Via tgf-Beta1 signaling pathway. J Immunol (2012) 189(1):444–53. doi: 10.4049/jimmunol.1103248
40. Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, et al. Mesenchymal differentiation mediated by nf-kappab promotes radiation resistance in glioblastoma. Cancer Cell (2013) 24(3):331–46. doi: 10.1016/j.ccr.2013.08.001
41. Hara T, Chanoch-Myers R, Mathewson ND, Myskiw C, Atta L, Bussema L, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell (2021) 39(6):779–92.e11. doi: 10.1016/j.ccell.2021.05.002
42. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell (2006) 9(3):157–73. doi: 10.1016/j.ccr.2006.02.019
43. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in pdgfra, Idh1, egfr, and Nf1. Cancer Cell (2010) 17(1):98–110. doi: 10.1016/j.ccr.2009.12.020
44. Yang W, Li Y, Gao R, Xiu Z, Sun T. Mhc class I dysfunction of glioma stem cells escapes from ctl-mediated immune response Via activation of Wnt/Beta-catenin signaling pathway. Oncogene (2020) 39(5):1098–111. doi: 10.1038/s41388-019-1045-6
45. Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by pd-L1 on glioblastoma-derived extracellular vesicles. Sci Adv (2018) 4(3):eaar2766. doi: 10.1126/sciadv.aar2766
46. Riccadonna C, Yacoub Maroun C, Vuillefroy de Silly R, Boehler M, Calvo Tardon M, Jueliger S, et al. Decitabine treatment of glioma-initiating cells enhances immune recognition and killing. PLoS One (2016) 11(8):e0162105. doi: 10.1371/journal.pone.0162105
47. Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive Macrophages/Microglia. Neuro Oncol (2010) 12(11):1113–25. doi: 10.1093/neuonc/noq082
48. Cosset E, Ilmjarv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, et al. Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell (2017) 32(6):856–68.e5. doi: 10.1016/j.ccell.2017.10.016
49. Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci (2013) 16(10):1373–82. doi: 10.1038/nn.3510
50. Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell (2015) 162(6):1217–28. doi: 10.1016/j.cell.2015.08.012
51. Siska PJ, van der Windt GJ, Kishton RJ, Cohen S, Eisner W, MacIver NJ, et al. Suppression of Glut1 and glucose metabolism by decreased Akt/Mtorc1 signaling drives T cell impairment in b cell leukemia. J Immunol (2016) 197(6):2532–40. doi: 10.4049/jimmunol.1502464
52. Hodges TR, Ott M, Xiu J, Gatalica Z, Swensen J, Zhou S, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: Implications for immune checkpoint immunotherapy. Neuro Oncol (2017) 19(8):1047–57. doi: 10.1093/neuonc/nox026
53. Esparza R, Azad TD, Feroze AH, Mitra SS, Cheshier SH. Glioblastoma stem cells and stem cell-targeting immunotherapies. J Neurooncol (2015) 123(3):449–57. doi: 10.1007/s11060-015-1729-x
54. Kim H, Zheng S, Amini SS, Virk SM, Mikkelsen T, Brat DJ, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res (2015) 25(3):316–27. doi: 10.1101/gr.180612.114
55. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-Small-Cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol (2016) 17(7):976–83. doi: 10.1016/S1470-2045(16)30053-5
56. Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase ii trial. J Clin Oncol (2019) 37(1):52–60. doi: 10.1200/JCO.18.00204
57. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol (2018) 19(5):672–81. doi: 10.1016/S1470-2045(18)30139-6
58. Tawbi HA, Forsyth PA, Hodi FS, Lao CD, Moschos SJ, Hamid O, et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (Checkmate 204). Neuro Oncol (2021) 23(11):1961–73. doi: 10.1093/neuonc/noab094
59. Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell (2019) 178(4):835–49.e21. doi: 10.1016/j.cell.2019.06.024
60. Johnson KC, Anderson KJ, Courtois ET, Gujar AD, Barthel FP, Varn FS, et al. Single-cell multimodal glioma analyses identify epigenetic regulators of cellular plasticity and environmental stress response. Nat Genet (2021) 53(10):1456–68. doi: 10.1038/s41588-021-00926-8
61. Baysan M, Woolard K, Bozdag S, Riddick G, Kotliarova S, Cam MC, et al. Micro-environment causes reversible changes in DNA methylation and mrna expression profiles in patient-derived glioma stem cells. PloS One (2014) 9(4):e94045. doi: 10.1371/journal.pone.0094045
62. Chaligne R, Gaiti F, Silverbush D, Schiffman JS, Weisman HR, Kluegel L, et al. Epigenetic encoding, heritability and plasticity of glioma transcriptional cell states. Nat Genet (2021) 53(10):1469–79. doi: 10.1038/s41588-021-00927-7
63. Kouzarides T. Chromatin modifications and their function. Cell (2007) 128(4):693–705. doi: 10.1016/j.cell.2007.02.005
64. Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, et al. M(6)a rna modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell (2014) 15(6):707–19. doi: 10.1016/j.stem.2014.09.019
65. Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, et al. Stem cells. M6a mrna methylation facilitates resolution of naive pluripotency toward differentiation. Science (2015) 347(6225):1002–6. doi: 10.1126/science.1261417
66. Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol (2014) 16(2):191–8. doi: 10.1038/ncb2902
67. Marmorstein R, Zhou MM. Writers and readers of histone acetylation: Structure, mechanism, and inhibition. Cold Spring Harb Perspect Biol (2014) 6(7):a018762. doi: 10.1101/cshperspect.a018762
68. Gangoso E, Southgate B, Bradley L, Rus S, Galvez-Cancino F, McGivern N, et al. Glioblastomas acquire myeloid-affiliated transcriptional programs Via epigenetic immunoediting to elicit immune evasion. Cell (2021) 184(9):2454–70.e26. doi: 10.1016/j.cell.2021.03.023
69. Pine AR, Cirigliano SM, Nicholson JG, Hu Y, Linkous A, Miyaguchi K, et al. Tumor microenvironment is critical for the maintenance of cellular states found in primary glioblastomas. Cancer Discovery (2020) 10(7):964–79. doi: 10.1158/2159-8290.CD-20-0057
70. Liu B, Cao Y, Li Y, Ma H, Yang M, Zhang Q, et al. Glioma stem cells upregulate Cd39 expression to escape immune response through Sox2 modulation. Cancers (Basel) (2022) 14(3). doi: 10.3390/cancers14030783
71. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by Cd39 and Cd73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204(6):1257–65. doi: 10.1084/jem.20062512
72. Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor Yy1: Structure, function, and therapeutic implications in cancer biology. Oncogene (2006) 25(8):1125–42. doi: 10.1038/sj.onc.1209080
73. Wilkinson FH, Park K, Atchison ML. Polycomb recruitment to DNA in vivo by the Yy1 repo domain. Proc Natl Acad Sci U.S.A. (2006) 103(51):19296–301. doi: 10.1073/pnas.0603564103
74. Jia B, Liu W, Gu J, Wang J, Lv W, Zhang W, et al. Mir-7-5p suppresses stemness and enhances temozolomide sensitivity of drug-resistant glioblastoma cells by targeting yin yang 1. Exp Cell Res (2019) 375(1):73–81. doi: 10.1016/j.yexcr.2018.12.016
75. Kaufhold S, Garban H, Bonavida B. Yin yang 1 is associated with cancer stem cell transcription factors (Sox2, Oct4, Bmi1) and clinical implication. J Exp Clin Cancer Res (2016) 35:84. doi: 10.1186/s13046-016-0359-2
76. Li J, Song J, Guo F. Mir-186 reverses cisplatin resistance and inhibits the formation of the glioblastoma-initiating cell phenotype by degrading yin yang 1 in glioblastoma. Int J Mol Med (2019) 43(1):517–24. doi: 10.3892/ijmm.2018.3940
77. Qiu Z, Zhao L, Shen JZ, Liang Z, Wu Q, Yang K, et al. Transcription elongation machinery is a druggable dependency and potentiates immunotherapy in glioblastoma stem cells. Cancer Discovery (2022) 12(2):502–21. doi: 10.1158/2159-8290.CD-20-1848
78. Gu J, Mu N, Jia B, Guo Q, Pan L, Zhu M, et al. Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma. Neuro Oncol (2021) 24(7):1056–70. doi: 10.1093/neuonc/noab288
79. De A, Beligala DH, Sharma VP, Burgos CA, Lee AM, Geusz ME. Cancer stem cell generation during epithelial-mesenchymal transition is temporally gated by intrinsic circadian clocks. Clin Exp Metastasis (2020) 37(5):617–35. doi: 10.1007/s10585-020-10051-1
80. Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, et al. Core circadian clock genes regulate leukemia stem cells in aml. Cell (2016) 165(2):303–16. doi: 10.1016/j.cell.2016.03.015
81. Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol (2014) 24(2):90–9. doi: 10.1016/j.tcb.2013.07.002
82. Geusz ME, Malik A, De A. Insights into oncogenesis from circadian timing in cancer stem cells. Crit Rev Oncog (2021) 26(4):1–17. doi: 10.1615/CritRevOncog.2021041960
83. Hadadi E, Acloque H. Role of circadian rhythm disorders on emt and tumour-immune interactions in endocrine-related cancers. Endocr Relat Cancer (2021) 28(2):R67–80. doi: 10.1530/ERC-20-0390
84. Hassan SA, Ali AAH, Sohn D, Flogel U, Janicke RU, Korf HW, et al. Does timing matter in radiotherapy of hepatocellular carcinoma? an experimental study in mice. Cancer Med (2021) 10(21):7712–25. doi: 10.1002/cam4.4277
85. Huang Z, He A, Wang J, Lu H, Zhang R, Wu L, et al. The circadian clock is associated with prognosis and immune infiltration in stomach adenocarcinoma. Aging (Albany NY) (2021) 13(12):16637–55. doi: 10.18632/aging.203184
86. Lee Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp Mol Med (2021) 53(10):1529–38. doi: 10.1038/s12276-021-00681-0
87. de Assis LVM, Kinker GS, Moraes MN, Markus RP, Fernandes PA, Castrucci AML. Expression of the circadian clock gene Bmal1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Front Oncol (2018) 8:185. doi: 10.3389/fonc.2018.00185
88. He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity (2018) 49(6):1175–90.e7. doi: 10.1016/j.immuni.2018.10.007
89. Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, et al. Circadian regulator clock recruits immune-suppressive microglia into the gbm tumor microenvironment. Cancer Discovery (2020) 10(3):371–81. doi: 10.1158/2159-8290.CD-19-0400
90. Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discovery (2019) 9(11):1556–73. doi: 10.1158/2159-8290.CD-19-0215
91. Li A, Lin X, Tan X, Yin B, Han W, Zhao J, et al. Circadian gene clock contributes to cell proliferation and migration of glioma and is directly regulated by tumor-suppressive mir-124. FEBS Lett (2013) 587(15):2455–60. doi: 10.1016/j.febslet.2013.06.018
92. Xuan W, Hsu WH, Khan F, Dunterman M, Pang L, Wainwright DA, et al. Circadian regulator clock drives immunosuppression in glioblastoma. Cancer Immunol Res (2022) 10(6):770–84. doi: 10.1158/2326-6066.CIR-21-0559
93. Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, idh, and tert promoter mutations in tumors. N Engl J Med (2015) 372(26):2499–508. doi: 10.1056/NEJMoa1407279
94. Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, et al. Mutational landscape and clonal architecture in grade ii and iii gliomas. Nat Genet (2015) 47(5):458–68. doi: 10.1038/ng.3273
95. Hu C, Wang K, Damon C, Fu Y, Ma T, Kratz L, et al. Atrx loss promotes immunosuppressive mechanisms in Idh1 mutant glioma. Neuro Oncol (2022) 24(6):888–900. doi: 10.1093/neuonc/noab292
96. Babikir H, Wang L, Shamardani K, Catalan F, Sudhir S, Aghi MK, et al. Atrx regulates glial identity and the tumor microenvironment in idh-mutant glioma. Genome Biol (2021) 22(1):311. doi: 10.1186/s13059-021-02535-4
97. Shin K, Shin H, Cho HJ, Kang H, Lee JK, Seo YJ, et al. Sphere-forming culture for expanding genetically distinct patient-derived glioma stem cells by cellular growth rate screening. Cancers (Basel) (2020) 12(3). doi: 10.3390/cancers12030549
98. Yelton CJ, Ray SK. Histone deacetylase enzymes and selective histone deacetylase inhibitors for antitumor effects and enhancement of antitumor immunity in glioblastoma. Neuroimmunol Neuroinflamm (2018) 5. doi: 10.20517/2347-8659.2018.58
99. Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discovery (2014) 13(9):673–91. doi: 10.1038/nrd4360
100. Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol (2007) 27(22):7886–94. doi: 10.1128/MCB.01547-07
101. Setiadi AF, Omilusik K, David MD, Seipp RP, Hartikainen J, Gopaul R, et al. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res (2008) 68(23):9601–7. doi: 10.1158/0008-5472.CAN-07-5270
102. Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RC, et al. Epigenetic therapy ties myc depletion to reversing immune evasion and treating lung cancer. Cell (2017) 171(6):1284–300.e21. doi: 10.1016/j.cell.2017.10.022
103. Woan KV, Sahakian E, Sotomayor EM, Seto E, Villagra A. Modulation of antigen-presenting cells by hdac inhibitors: Implications in autoimmunity and cancer. Immunol Cell Biol (2012) 90(1):55–65. doi: 10.1038/icb.2011.96
104. Mormino A, Cocozza G, Fontemaggi G, Valente S, Esposito V, Santoro A, et al. Histone-deacetylase 8 drives the immune response and the growth of glioma. Glia (2021) 69(11):2682–98. doi: 10.1002/glia.24065
105. Hu G, Wade PA. Nurd and pluripotency: A complex balancing act. Cell Stem Cell (2012) 10(5):497–503. doi: 10.1016/j.stem.2012.04.011
106. Zhan X, Guo S, Li Y, Ran H, Huang H, Mi L, et al. Glioma stem-like cells evade interferon suppression through Mbd3/Nurd complex-mediated Stat1 downregulation. J Exp Med (2020) 217(5). doi: 10.1084/jem.20191340
107. Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, et al. Idh1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature (2012) 483(7390):479–83. doi: 10.1038/nature10866
108. Zhang X, Rao A, Sette P, Deibert C, Pomerantz A, Kim WJ, et al. Idh mutant gliomas escape natural killer cell immune surveillance by downregulation of Nkg2d ligand expression. Neuro Oncol (2016) 18(10):1402–12. doi: 10.1093/neuonc/now061
109. Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-Aza-2'-Deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother (2009) 58(4):589–601. doi: 10.1007/s00262-008-0582-6
110. Falahat R, Berglund A, Putney RM, Perez-Villarroel P, Aoyama S, Pilon-Thomas S, et al. Epigenetic reprogramming of tumor cell-intrinsic sting function sculpts antigenicity and T cell recognition of melanoma. Proc Natl Acad Sci U S A (2021) 118(15). doi: 10.1073/pnas.2013598118
111. Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi F, et al. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-Aza-2'-Deoxycytidine in cutaneous melanoma: Immunotherapeutic implications. Clin Cancer Res (2007) 13(11):3333–8. doi: 10.1158/1078-0432.CCR-06-3091
112. Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, et al. Rexpression of hla class I antigens and restoration of antigen-specific ctl response in melanoma cells following 5-Aza-2'-Deoxycytidine treatment. Int J Cancer (2001) 94(2):243–51. doi: 10.1002/ijc.1452
113. Sese B, Iniguez-Munoz S, Ensenyat-Mendez M, Llinas-Arias P, Ramis G, Orozco JIJ, et al. Glioblastoma embryonic-like stem cells exhibit immune-evasive phenotype. Cancers (Basel) (2022) 14(9). doi: 10.3390/cancers14092070
114. Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. M(6)a rna methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep (2017) 18(11):2622–34. doi: 10.1016/j.celrep.2017.02.059
115. Li F, Chen S, Yu J, Gao Z, Sun Z, Yi Y, et al. Interplay of M(6) a and histone modifications contributes to temozolomide resistance in glioblastoma. Clin Transl Med (2021) 11(9):e553. doi: 10.1002/ctm2.553
116. Shi J, Chen G, Dong X, Li H, Li S, Cheng S, et al. Mettl3 promotes the resistance of glioma to temozolomide Via increasing mgmt and anpg in a M(6)a dependent manner. Front Oncol (2021) 11:702983. doi: 10.3389/fonc.2021.702983
117. Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, et al. Essential role of Mettl3-mediated M(6)a modification in glioma stem-like cells maintenance and radioresistance. Oncogene (2018) 37(4):522–33. doi: 10.1038/onc.2017.351
118. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. M(6)a demethylase Alkbh5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining Foxm1 expression and cell proliferation program. Cancer Cell (2017) 31(4):591–606.e6. doi: 10.1016/j.ccell.2017.02.013
119. Dong F, Qin X, Wang B, Li Q, Hu J, Cheng X, et al. Alkbh5 facilitates hypoxia-induced paraspeckle assembly and Il8 secretion to generate an immunosuppressive tumor microenvironment. Cancer Res (2021) 81(23):5876–88. doi: 10.1158/0008-5472.CAN-21-1456
120. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
121. Hoang-Minh LB, Siebzehnrubl FA, Yang C, Suzuki-Hatano S, Dajac K, Loche T, et al. Infiltrative and drug-resistant slow-cycling cells support metabolic heterogeneity in glioblastoma. EMBO J (2018) 37(23). doi: 10.15252/embj.201798772
122. Shakya S, Gromovsky AD, Hale JS, Knudsen AM, Prager B, Wallace LC, et al. Altered lipid metabolism marks glioblastoma stem and non-stem cells in separate tumor niches. Acta Neuropathol Commun (2021) 9(1):101. doi: 10.1186/s40478-021-01205-7
123. Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A (2011) 108(38):16062–7. doi: 10.1073/pnas.1106704108
124. Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J, Clement-Schatlo V, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev (2012) 26(17):1926–44. doi: 10.1101/gad.188292.112
125. Kathagen A, Schulte A, Balcke G, Phillips HS, Martens T, Matschke J, et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol (2013) 126(5):763–80. doi: 10.1007/s00401-013-1173-y
126. Dong Z, Cui H. Epigenetic modulation of metabolism in glioblastoma. Semin Cancer Biol (2019) 57:45–51. doi: 10.1016/j.semcancer.2018.09.002
127. Heddleston JM, Wu Q, Rivera M, Minhas S, Lathia JD, Sloan AE, et al. Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ (2012) 19(3):428–39. doi: 10.1038/cdd.2011.109
128. Heiland DH, Gaebelein A, Borries M, Worner J, Pompe N, Franco P, et al. Microenvironment-derived regulation of hif signaling drives transcriptional heterogeneity in glioblastoma multiforme. Mol Cancer Res (2018) 16(4):655–68. doi: 10.1158/1541-7786.MCR-17-0680
129. Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the Hif1alpha-Zeb1 axis. Cancer Lett (2015) 359(1):107–16. doi: 10.1016/j.canlet.2015.01.010
130. Xu X, Wang L, Zang Q, Li S, Li L, Wang Z, et al. Rewiring of purine metabolism in response to acidosis stress in glioma stem cells. Cell Death Dis (2021) 12(3):277. doi: 10.1038/s41419-021-03543-9
131. Coy S, Wang S, Stopka SA, Lin JR, Yapp C, Ritch CC, et al. Single cell spatial analysis reveals the topology of immunomodulatory purinergic signaling in glioblastoma. Nat Commun (2022) 13(1):4814. doi: 10.1038/s41467-022-32430-w
132. Ravi VM, Will P, Kueckelhaus J, Sun N, Joseph K, Salie H, et al. Spatially resolved multi-omics deciphers bidirectional tumor-host interdependence in glioblastoma. Cancer Cell (2022) 40(6):639–55.e13. doi: 10.1016/j.ccell.2022.05.009
133. Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1a3. Proc Natl Acad Sci U S A (2013) 110(21):8644–9. doi: 10.1073/pnas.1221478110
134. Richards LM, Whitley OKN, MacLeod G, Cavalli FMG, Coutinho FJ, Jaramillo JE, et al. Gradient of developmental and injury response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity. Nat Cancer (2021) 2(2):157–73. doi: 10.1038/s43018-020-00154-9
135. Garofano L, Migliozzi S, Oh YT, D'Angelo F, Najac RD, Ko A, et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat Cancer (2021) 2(2):141–56. doi: 10.1038/s43018-020-00159-4
136. Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer (2021) 20(1):7. doi: 10.1186/s12943-020-01288-1
137. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell (2013) 153(1):139–52. doi: 10.1016/j.cell.2013.02.021
138. Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization Via endothelial differentiation of glioblastoma stem-like cells. Nature (2010) 468(7325):824–8. doi: 10.1038/nature09557
139. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature (2010) 468(7325):829–33. doi: 10.1038/nature09624
140. Pastorino O, Gentile MT, Mancini A, Del Gaudio N, Di Costanzo A, Bajetto A, et al. Histone deacetylase inhibitors impair vasculogenic mimicry from glioblastoma cells. Cancers (Basel) (2019) 11(6). doi: 10.3390/cancers11060747
141. Tao M, Li X, He L, Rong X, Wang H, Pan J, et al. Decreased rna M(6)a methylation enhances the process of the epithelial mesenchymal transition and vasculogenic mimicry in glioblastoma. Am J Cancer Res (2022) 12(2):893–906.
142. Couturier CP, Ayyadhury S, Le PU, Nadaf J, Monlong J, Riva G, et al. Single-cell rna-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat Commun (2020) 11(1):3406. doi: 10.1038/s41467-020-17186-5
143. Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature (2019) 573(7775):532–8. doi: 10.1038/s41586-019-1564-x
144. Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature (2019) 573(7775):539–45. doi: 10.1038/s41586-019-1563-y
145. Yuan J, Levitin HM, Frattini V, Bush EC, Boyett DM, Samanamud J, et al. Single-cell transcriptome analysis of lineage diversity in high-grade glioma. Genome Med (2018) 10(1):57. doi: 10.1186/s13073-018-0567-9
146. Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wissmann N, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell (2022) 185(16):2899–917.e31. doi: 10.1016/j.cell.2022.06.054
147. Gao R, He B, Huang Q, Wang Z, Yan M, Lam EW, et al. Cancer cell immune mimicry delineates onco-immunologic modulation. iScience (2021) 24(10):103133. doi: 10.1016/j.isci.2021.103133
148. Amanda Johnson JK, Laterra J, Lopez-Bertoni H. Stem-03. Oct4/Sox2 drive an immunosuppressive gsc phenotype by inducing T-reg effector genes Via Tgfbr2 signaling. Neuro-Oncology (2021) 23:vi21–vi2. doi: 10.1093/neuonc/noab196.079
149. Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun (2013) 4:2612. doi: 10.1038/ncomms3612
150. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol (2019) 37(7):773–82. doi: 10.1038/s41587-019-0114-2
151. Aran D, Hu Z, Butte AJ. Xcell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol (2017) 18(1):220. doi: 10.1186/s13059-017-1349-1
152. Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol (2016) 17(1):218. doi: 10.1186/s13059-016-1070-5
153. Racle J, de Jonge K, Baumgaertner P, Speiser DE, Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife (2017) 6. doi: 10.7554/eLife.26476
154. Racle J, Gfeller D. Epic: A tool to estimate the proportions of different cell types from bulk gene expression data. Methods Mol Biol (2020) 2120:233–48. doi: 10.1007/978-1-0716-0327-7_17
155. Finotello F, Mayer C, Plattner C, Laschober G, Rieder D, Hackl H, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of rna-seq data. Genome Med (2019) 11(1):34. doi: 10.1186/s13073-019-0638-6
156. Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. Timer2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res (2020) 48(W1):W509–W14. doi: 10.1093/nar/gkaa407
157. Buenrostro JD, Corces MR, Lareau CA, Wu B, Schep AN, Aryee MJ, et al. Integrated single-cell analysis maps the continuous regulatory landscape of human hematopoietic differentiation. Cell (2018) 173(6):1535–48.e16. doi: 10.1016/j.cell.2018.03.074
158. Guo H, Zhu P, Wu X, Li X, Wen L, Tang F. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res (2013) 23(12):2126–35. doi: 10.1101/gr.161679.113
159. Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, et al. Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-Flight mass spectrometry. Anal Chem (2009) 81(16):6813–22. doi: 10.1021/ac901049w
160. Gadalla R, Noamani B, MacLeod BL, Dickson RJ, Guo M, Xu W, et al. Validation of cytof against flow cytometry for immunological studies and monitoring of human cancer clinical trials. Front Oncol (2019) 9:415. doi: 10.3389/fonc.2019.00415
161. Pai JA, Satpathy AT. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods (2021) 18(8):881–92. doi: 10.1038/s41592-021-01201-8
162. Bartosovic M, Kabbe M, Castelo-Branco G. Single-cell Cut&Tag profiles histone modifications and transcription factors in complex tissues. Nat Biotechnol (2021) 39(7):825–35. doi: 10.1038/s41587-021-00869-9
163. Zhang B, Srivastava A, Mimitou E, Stuart T, Raimondi I, Hao Y, et al. Characterizing cellular heterogeneity in chromatin state with Sccut&Tag-pro. Nat Biotechnol (2022) 40(8):1220–30. doi: 10.1038/s41587-022-01250-0
164. Peterson VM, Zhang KX, Kumar N, Wong J, Li L, Wilson DC, et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol (2017) 35(10):936–9. doi: 10.1038/nbt.3973
165. Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods (2017) 14(9):865–8. doi: 10.1038/nmeth.4380
166. Cao J, Cusanovich DA, Ramani V, Aghamirzaie D, Pliner HA, Hill AJ, et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science (2018) 361(6409):1380–5. doi: 10.1126/science.aau0730
167. Bian S, Hou Y, Zhou X, Li X, Yong J, Wang Y, et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science (2018) 362(6418):1060–3. doi: 10.1126/science.aao3791
168. Hou Y, Guo H, Cao C, Li X, Hu B, Zhu P, et al. Single-cell triple omics sequencing reveals genetic, epigenetic, and transcriptomic heterogeneity in hepatocellular carcinomas. Cell Res (2016) 26(3):304–19. doi: 10.1038/cr.2016.23
169. Clark SJ, Argelaguet R, Kapourani CA, Stubbs TM, Lee HJ, Alda-Catalinas C, et al. Scnmt-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat Commun (2018) 9(1):781. doi: 10.1038/s41467-018-03149-4
170. Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science (2018) 361(6400). doi: 10.1126/science.aat5691
171. Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, et al. Highly multiplexed subcellular rna sequencing in situ. Science (2014) 343(6177):1360–3. doi: 10.1126/science.1250212
172. Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. Rnascope: A novel in situ rna analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn (2012) 14(1):22–9. doi: 10.1016/j.jmoldx.2011.08.002
173. Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. Rna imaging. spatially resolved, highly multiplexed rna profiling in single cells. Science (2015) 348(6233):aaa6090. doi: 10.1126/science.aaa6090
174. Xia C, Fan J, Emanuel G, Hao J, Zhuang X. Spatial transcriptome profiling by merfish reveals subcellular rna compartmentalization and cell cycle-dependent gene expression. Proc Natl Acad Sci U S A (2019) 116(39):19490–9. doi: 10.1073/pnas.1912459116
175. Eng CL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, et al. Transcriptome-scale super-resolved imaging in tissues by rna seqfish. Nature (2019) 568(7751):235–9. doi: 10.1038/s41586-019-1049-y
176. Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L. Single-cell in situ rna profiling by sequential hybridization. Nat Methods (2014) 11(4):360–1. doi: 10.1038/nmeth.2892
177. Shah S, Lubeck E, Zhou W, Cai L. In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron (2016) 92(2):342–57. doi: 10.1016/j.neuron.2016.10.001
178. Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science (2019) 363(6434):1463–7. doi: 10.1126/science.aaw1219
179. Stickels RR, Murray E, Kumar P, Li J, Marshall JL, Di Bella DJ, et al. Highly sensitive spatial transcriptomics at near-cellular resolution with slide-Seqv2. Nat Biotechnol (2021) 39(3):313–9. doi: 10.1038/s41587-020-0739-1
180. Vickovic S, Eraslan G, Salmen F, Klughammer J, Stenbeck L, Schapiro D, et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods (2019) 16(10):987–90. doi: 10.1038/s41592-019-0548-y
181. Gularyan SK, Gulin AA, Anufrieva KS, Shender VO, Shakhparonov MI, Bastola S, et al. Investigation of inter- and intratumoral heterogeneity of glioblastoma using tof-sims. Mol Cell Proteomics (2020) 19(6):960–70. doi: 10.1074/mcp.RA120.001986
182. Jungnickel H, Laux P, Luch A. Time-of-Flight secondary ion mass spectrometry (Tof-sims): A new tool for the analysis of toxicological effects on single cell level. Toxics (2016) 4(1). doi: 10.3390/toxics4010005
183. Spraggins JM, Rizzo DG, Moore JL, Noto MJ, Skaar EP, Caprioli RM. Next-generation technologies for spatial proteomics: Integrating ultra-high speed maldi-tof and high mass resolution maldi fticr imaging mass spectrometry for protein analysis. Proteomics (2016) 16(11-12):1678–89. doi: 10.1002/pmic.201600003
184. Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods (2014) 11(4):417–22. doi: 10.1038/nmeth.2869
185. Ma R, Rei M, Woodhouse I, Ferris K, Kirschner S, Chandran A, et al. Decitabine increases neoantigen and cancer testis antigen expression to enhance T cell-mediated toxicity against glioblastoma. Neuro Oncol (2022). doi: 10.1093/neuonc/noac107
186. Galanis E, Anderson SK, Miller CR, Sarkaria JN, Jaeckle K, Buckner JC, et al. Phase I/Ii trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of alliance N0874/Abtc 02. Neuro Oncol (2018) 20(4):546–56. doi: 10.1093/neuonc/nox161
187. Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase ii trial of vorinostat in recurrent glioblastoma multiforme: A north central cancer treatment group study. J Clin Oncol (2009) 27(12):2052–8. doi: 10.1200/JCO.2008.19.0694
188. Peters KB, Lipp ES, Miller E, Herndon JE 2nd, McSherry F, Desjardins A, et al. Phase I/Ii trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol (2018) 137(2):349–56. doi: 10.1007/s11060-017-2724-1
189. Puduvalli VK, Wu J, Yuan Y, Armstrong TS, Vera E, Wu J, et al. A Bayesian adaptive randomized phase ii multicenter trial of bevacizumab with or without vorinostat in adults with recurrent glioblastoma. Neuro Oncol (2020) 22(10):1505–15. doi: 10.1093/neuonc/noaa062
190. Iwamoto FM, Lamborn KR, Kuhn JG, Wen PY, Yung WK, Gilbert MR, et al. A phase I/Ii trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American brain tumor consortium study 03-03. Neuro Oncol (2011) 13(5):509–16. doi: 10.1093/neuonc/nor017
191. Lee EQ, Reardon DA, Schiff D, Drappatz J, Muzikansky A, Grimm SA, et al. Phase ii study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol (2015) 17(6):862–7. doi: 10.1093/neuonc/nou350
192. Xia L, Liu JY, Zheng ZZ, Chen YJ, Ding JC, Hu YH, et al. Brd4 inhibition boosts the therapeutic effects of epidermal growth factor receptor-targeted chimeric antigen receptor T cells in glioblastoma. Mol Ther (2021) 29(10):3011–26. doi: 10.1016/j.ymthe.2021.05.019
193. Jiang Z, Shi Y, Zhao W, Zhang Y, Xie Y, Zhang B, et al. Development of an immune-related prognostic index associated with glioblastoma. Front Neurol (2021) 12:610797. doi: 10.3389/fneur.2021.610797
194. Qiu H, Li Y, Cheng S, Li J, He C, Li J. A prognostic microenvironment-related immune signature Via estimate (Promise model) predicts overall survival of patients with glioma. Front Oncol (2020) 10:580263. doi: 10.3389/fonc.2020.580263
195. Consortium G. Glioma through the looking glass: Molecular evolution of diffuse gliomas and the glioma longitudinal analysis consortium. Neuro Oncol (2018) 20(7):873–84. doi: 10.1093/neuonc/noy020
196. Puchalski RB, Shah N, Miller J, Dalley R, Nomura SR, Yoon JG, et al. An anatomic transcriptional atlas of human glioblastoma. Science (2018) 360(6389):660–3. doi: 10.1126/science.aaf2666
197. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 who classification of tumors of the central nervous system: A summary. Neuro Oncol (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
198. Stuart T, Satija R. Integrative single-cell analysis. Nat Rev Genet (2019) 20(5):257–72. doi: 10.1038/s41576-019-0093-7
199. Nam AS, Chaligne R, Landau DA. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat Rev Genet (2021) 22(1):3–18. doi: 10.1038/s41576-020-0265-5
200. Suva ML, Tirosh I. Single-cell rna sequencing in cancer: Lessons learned and emerging challenges. Mol Cell (2019) 75(1):7–12. doi: 10.1016/j.molcel.2019.05.003
201. Guilhamon P, Chesnelong C, Kushida MM, Nikolic A, Singhal D, MacLeod G, et al. Single-cell chromatin accessibility profiling of glioblastoma identifies an invasive cancer stem cell population associated with lower survival. Elife (2021) 10. doi: 10.7554/eLife.64090
202. Simonds EF, Lu ED, Badillo O, Karimi S, Liu EV, Tamaki W, et al. Deep immune profiling reveals targetable mechanisms of immune evasion in immune checkpoint inhibitor-refractory glioblastoma. J Immunother Cancer (2021) 9(6). doi: 10.1136/jitc-2020-002181
203. Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci (2021) 24(4):595–610. doi: 10.1038/s41593-020-00789-y
204. Yeo AT, Rawal S, Delcuze B, Christofides A, Atayde A, Strauss L, et al. Single-cell rna sequencing reveals evolution of immune landscape during glioblastoma progression. Nat Immunol (2022) 23(6):971–84. doi: 10.1038/s41590-022-01215-0
205. Setty M, Kiseliovas V, Levine J, Gayoso A, Mazutis L, Pe'er D. Characterization of cell fate probabilities in single-cell data with palantir. Nat Biotechnol (2019) 37(4):451–60. doi: 10.1038/s41587-019-0068-4
206. Street K, Risso D, Fletcher RB, Das D, Ngai J, Yosef N, et al. Slingshot: Cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics (2018) 19(1):477. doi: 10.1186/s12864-018-4772-0
207. Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol (2014) 32(4):381–6. doi: 10.1038/nbt.2859
208. Wagner A, Wang C, Fessler J, DeTomaso D, Avila-Pacheco J, Kaminski J, et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell (2021) 184(16):4168–85.e21. doi: 10.1016/j.cell.2021.05.045
209. Browaeys R, Saelens W, Saeys Y. Nichenet: Modeling intercellular communication by linking ligands to target genes. Nat Methods (2020) 17(2):159–62. doi: 10.1038/s41592-019-0667-5
210. Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, et al. Analysis of single-cell rna-seq identifies cell-cell communication associated with tumor characteristics. Cell Rep (2018) 25(6):1458–68.e4. doi: 10.1016/j.celrep.2018.10.047
211. Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DB, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell (2019) 178(3):714–30.e22. doi: 10.1016/j.cell.2019.06.029
212. Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature (2018) 563(7731):347–53. doi: 10.1038/s41586-018-0698-6
213. Longo SK, Guo MG, Ji AL, Khavari PA. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat Rev Genet (2021) 22(10):627–44. doi: 10.1038/s41576-021-00370-8
214. Yuan Q, Cai HQ, Zhong Y, Zhang MJ, Cheng ZJ, Hao JJ, et al. Overexpression of Igfbp2 mrna predicts poor survival in patients with glioblastoma. Biosci Rep (2019) 39(6). doi: 10.1042/BSR20190045
215. Yuan Q, Wang SQ, Zhang GT, He J, Liu ZD, Wang MR, et al. Highly expressed of Serpina3 indicated poor prognosis and involved in immune suppression in glioma. Immun Inflammation Dis (2021) 9(4):1618–30. doi: 10.1002/iid3.515
216. Cornett DS, Frappier SL, Caprioli RM. Maldi-fticr imaging mass spectrometry of drugs and metabolites in tissue. Anal Chem (2008) 80(14):5648–53. doi: 10.1021/ac800617s
217. Dilillo M, Ait-Belkacem R, Esteve C, Pellegrini D, Nicolardi S, Costa M, et al. Ultra-high mass resolution maldi imaging mass spectrometry of proteins and metabolites in a mouse model of glioblastoma. Sci Rep (2017) 7(1):603. doi: 10.1038/s41598-017-00703-w
218. Gomez-Caudillo L, Ortega-Lozano AJ, Martinez-Batallar AG, Rosas-Vargas H, Minauro-Sanmiguel F, Encarnacion-Guevara S. Principal component analysis on Lcms/Ms and 2demalditof in glioblastoma cell lines reveals that mitochondria act as organelle sensors of the metabolic state in glioblastoma. Oncol Rep (2020) 44(2):661–73. doi: 10.3892/or.2020.7625
219. Park CK, Jung JH, Park SH, Jung HW, Cho BK. Multifarious proteomic signatures and regional heterogeneity in glioblastomas. J Neurooncol (2009) 94(1):31–9. doi: 10.1007/s11060-009-9805-8
220. Petre G, Durand H, Pelletier L, Poulenard M, Nugue G, Ray PF, et al. Rapid proteomic profiling by maldi-tof mass spectrometry for better brain tumor classification. Proteomics Clin Appl (2020) 14(5):e1900116. doi: 10.1002/prca.201900116
221. Gomez NC, Hepperla AJ, Dumitru R, Simon JM, Fang F, Davis IJ. Widespread chromatin accessibility at repetitive elements links stem cells with human cancer. Cell Rep (2016) 17(6):1607–20. doi: 10.1016/j.celrep.2016.10.011
222. Stergachis AB, Neph S, Reynolds A, Humbert R, Miller B, Paige SL, et al. Developmental fate and cellular maturity encoded in human regulatory DNA landscapes. Cell (2013) 154(4):888–903. doi: 10.1016/j.cell.2013.07.020
223. Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell (2014) 157(3):580–94. doi: 10.1016/j.cell.2014.02.030
224. Bever KM, Thomas DL 2nd, Zhang J, Diaz Rivera EA, Rosner GL, Zhu Q, et al. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin Epigenet (2021) 13(1):25. doi: 10.1186/s13148-021-01014-8
225. Chien KS, Kim K, Nogueras-Gonzalez GM, Borthakur G, Naqvi K, Daver NG, et al. Phase ii study of azacitidine with pembrolizumab in patients with intermediate-1 or higher-risk myelodysplastic syndrome. Br J Haematol (2021) 195(3):378–87. doi: 10.1111/bjh.17689
226. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in Relapsed/Refractory acute myeloid leukemia: A nonrandomized, open-label, phase ii study. Cancer Discovery (2019) 9(3):370–83. doi: 10.1158/2159-8290.CD-18-0774
227. Di Giacomo AM, Covre A, Finotello F, Rieder D, Danielli R, Sigalotti L, et al. Guadecitabine plus ipilimumab in unresectable melanoma: The nibit-M4 clinical trial. Clin Cancer Res (2019) 25(24):7351–62. doi: 10.1158/1078-0432.CCR-19-1335
228. Holmberg-Thyden S, Dufva IH, Gang AO, Breinholt MF, Schejbel L, Andersen MK, et al. Epigenetic therapy in combination with a multi-epitope cancer vaccine targeting shared tumor antigens for high-risk myelodysplastic syndrome - a phase I clinical trial. Cancer Immunol Immunother (2022) 71(2):433–44. doi: 10.1007/s00262-021-02993-6
229. Kuang C, Park Y, Augustin RC, Lin Y, Hartman DJ, Seigh L, et al. Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: A single-arm phase 2 trial and correlative biomarker analysis. Clin Epigenet (2022) 14(1):3. doi: 10.1186/s13148-021-01226-y
230. Levy BP, Giaccone G, Besse B, Felip E, Garassino MC, Domine Gomez M, et al. Randomised phase 2 study of pembrolizumab plus cc-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur J Cancer (2019) 108:120–8. doi: 10.1016/j.ejca.2018.11.028
231. Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, et al. Improved clinical outcome in a randomized phase ii study of anti-Pd-1 camrelizumab plus decitabine in Relapsed/Refractory Hodgkin lymphoma. J Immunother Cancer (2021) 9(4). doi: 10.1136/jitc-2021-002347
232. Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, et al. Addition of low-dose decitabine to anti-Pd-1 antibody camrelizumab in Relapsed/Refractory classical Hodgkin lymphoma. J Clin Oncol (2019) 37(17):1479–89. doi: 10.1200/JCO.18.02151
233. Ny L, Jespersen H, Karlsson J, Alsen S, Filges S, All-Eriksson C, et al. The pemdac phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat Commun (2021) 12(1):5155. doi: 10.1038/s41467-021-25332-w
234. Palomba ML, Cartron G, Popplewell L, Ribrag V, Westin J, Huw LY, et al. Combination of atezolizumab and tazemetostat in patients with Relapsed/Refractory diffuse Large b-cell lymphoma: Results from a phase ib study. Clin Lymphoma Myeloma Leuk (2022) 22(7):504–12. doi: 10.1016/j.clml.2021.12.014
235. Saxena K, Herbrich SM, Pemmaraju N, Kadia TM, DiNardo CD, Borthakur G, et al. A phase 1b/2 study of azacitidine with pd-L1 antibody avelumab in Relapsed/Refractory acute myeloid leukemia. Cancer (2021) 127(20):3761–71. doi: 10.1002/cncr.33690
236. Taylor K, Loo Yau H, Chakravarthy A, Wang B, Shen SY, Ettayebi I, et al. An open-label, phase ii multicohort study of an oral hypomethylating agent cc-486 and durvalumab in advanced solid tumors. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-000883
237. Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol (2020) 17(2):75–90. doi: 10.1038/s41571-019-0266-5
Keywords: cancer stem cell, immunomodulation, multi omics, cancer therapy, cellular mimicry, spatial analysis, single-cell sequencing
Citation: Johnson AL, Laterra J and Lopez-Bertoni H (2022) Exploring glioblastoma stem cell heterogeneity: Immune microenvironment modulation and therapeutic opportunities. Front. Oncol. 12:995498. doi: 10.3389/fonc.2022.995498
Received: 15 July 2022; Accepted: 02 September 2022;
Published: 21 September 2022.
Edited by:
Roger Abounader, University of Virginia, United StatesReviewed by:
Tao Jiang, Zhejiang Chinese Medical University, ChinaCopyright © 2022 Johnson, Laterra and Lopez-Bertoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Laterra, Laterra@kennedykrieger.org; Hernando Lopez-Bertoni, Lopezbertoni@kennedykrieger.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.