
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 September 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.993768
This article is part of the Research Topic Insights in Molecular and Cellular Oncology: 2022 View all 23 articles
Background: Nodular sclerosis classical Hodgkin lymphoma (NSCHL) is a rare disease in which Epstein–Barr virus (EBV) and CD20 can be detected. The clinical significance of EBV infection, CD20 expression and their relationship are still unclear in NSCHL presently. The aim of this research was to systematically explore the clinical significance of EBV infection, expression of CD20 and their relationship in NSCHL.
Methods: 109 NSCHL patients diagnosed in Qingdao University’s Affiliated Hospital were chosen from January 2010 to July 2019, and the clinical and survival data of all patients were collected retrospectively.
Results: Among 109 patients, 33 patients were assigned to the group of EBV-positives, following the results of the EBV-encoded RNA (EBER1). Compared with EBV-negative group patients, those in the group of EBV-positive were older (P=0.004) and their β2-microglobulin (β2-MG) levels were higher (P=0.006). The CD20 positivity rate in the group of EBV-positive was substantially higher than that in the EBV-negative group (54.5% vs 27.6%, P=0.007). Among 109 patients, EBV+ and CD20+ double positive patients acquired the least overall survival (OS), and patients with EBV- and CD20- double negative had the best OS (P < 0.001). Although old age, gender, EBV infection and CD20 positive were the risk factors for OS in NSCHL, multivariate analysis showed that CD20 positivity was the only characteristic that showed to be an independent risk factor for OS in NSCHL patients.
Conclusion: CD20 was found to be strongly expressed in NSCHL patients who had been infected with EBV, and it was found to be an independent risk factor for NSCHL patients’ survival.
Hodgkin lymphoma (HL) is a group of highly heterogeneous lymphoid neoplasia that originates from B cells, which generally has good clinical prognosis. The World Health Organization has a classification system for lymphoid neoplasms (2016 Version) (1); there are two main pathological subtypes of HL: classical HL (CHL) and HL dominated by nodular lymphocytes. CHL is further classified into the following four types: lymphocyte-rich, lymphocyte depletion, mixed cellularity, and nodular sclerosis. In China, nodular sclerosis CHL (NSCHL) is a common pathological subtype (2, 3).
EBV is a human herpesvirus with double-stranded DNA that belongs to the gamma herpesvirus subfamily. It has been reported that 90%-95% of people will be infected with the virus in their lifetime (4). At present, the development of several malignant tumors, including lymphomas, has been linked to EBV infection (5). However, the clinical significance of EBV infection and its exact pathogenesis in CHL patients remains unclear for now (6–10).
CD20 is a hallmark of B lymphocyte cells, but less than 40% of CHL patients are CD20 positive. At present, the clinical prognostic value of CD20 in patients with NSCHL remains controversial. In this study, to systematically investigate the clinical significance of EBV infection, CD20 expression and its relationship in patients with NSCHL, we retrospectively collected and analyzed the clinical data and survival data of 109 NSCHL patients admitted to Qingdao University’s affiliated hospital in the period of January 2010 to July 2019.
We reviewed the medical records of all NSCHL patients admitted to Qingdao University’s affiliated hospital from January 2010 to July 2019. All patients enrolled in this study satisfied the following criteria: 1) all patients were newly diagnosed and untreated adult NSCHL (age ≥ 18 years). Physicians from Qingdao University’s Affiliated Hospital’s Hematology Department diagnosed the pathologic diagnosis of all patients again; 2) Patients were given an ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) chemotherapy regimen as a first-line treatment and/or radiotherapy, and all patients received at least four cycles of this regimen according to risk stratification; 3) Clinical characteristics, laboratory data, and follow-up data of patients were available; 4) NSCHLs from central nervous system, gray zone lymphoma and immunodeficiency related lymphomas were excluded; 5) In view of the extensive differential diagnosis and high clinical relevance of EBV positive Hodgkin’s lymphoma, this study had taken some diseases as key differential diagnosis targets, such as Hodgkin-like angioimmunoblastic T-lymphomas and secondary EBV positive B cell proliferation. Patients with controversial pathological diagnosis were also excluded. In the end, 109 NSCHL patients were included in the study. The Ethical Committee of Qingdao University’s Affiliated Hospital approved this study, which was carried out in compliance with the Helsinki Declaration. Informed consent was obtained from patients.
The time from the date of NSCHL diagnosis to the date of death from any cause or the last follow-up was defined as overall survival (OS).
In situ hybridization was used to detect EBV-encoded small nuclear RNA (EBER) oligonucleotide in paraffin-embedded specimens recovered from NSCHL patients, as previously described (11). The patient was considered as EBV-positive if the HRS cells expressed EBER1.
Immunohistochemistry staining workups included LCA, CD3, CD15, CD19, CD20, CD30, EMA, PAX-5, BOB.1 and OCT-2. According to a previously published article, in this study, if more than 10% of the lymphoma cells were stained, the specimen was considered positive (12).
The data was analyzed using IBM SPSS Statistics 23.0 software (IBM Corp., Armonk, NY, USA). If appropriate, chi-square test, Student’s t test, or Fisher’s exact test were used to compare clinical information and CD20 positivity rates between EBV-positive and EBV-negative patients. The Kaplan–Meier technique (Log-rank test) was used to assess the OS, and alive cases at the last follow-up were censored. The risk factors linked with OS in patients with NSCHL were investigated using Cox proportional hazards regression analysis (multivariable analysis). Differences were defined as significant when the P values (two sided) ≤ 0.01.
This study eventually enrolled a total of 109 NSCHL patients. The male to female ratio was 1.8:1.0, and the median age at diagnosis for all patients was 35 years (18-77 years). Based on the EBER1 analysis results, the EBV-positive group consisted of 33 patients, while the EBV-negative group consisted of 76 patients. We analyzed the clinical information of EBV-positive and EBV-negative patients, including gender, age, clinical stage, LDH levels, β2-MG levels, ESR levels, and B symptoms, and found that age (P=0.004) and β2-MG levels (P=0.006) were significantly different between the two groups (Table 1).
In the EBV-positive group, 54.5% (18/33) of patients had CD20 expression; however, only 27.6% (21/76) of patients were positive for CD20 in the EBV-negative group. Thus, the EBV-positive group had significantly more patients with CD20 expression, as compared to the patients in the EBV-negative group. Based on these observations, we can conclude that CD20 expression differed significantly between the two groups (P=0.007) with almost double the number of patients with CD20 expression in EBV-positive group, relative to EBV-negative group.
All patients were followed for a median of 3.8 years (range 0.9-10.7 years). Patients in the EBV-positive group had a significantly shorter OS (P=0.001) than those in the EBV-negative group (Figure 1), but neither group had a median OS. Similarly, whereas the median OS of CD20 positive patients was considerably lower than that of CD20 negative patients (P0.001), neither group’s median OS was attained (Figure 2). Then, we further explored the OS of patients with different EBV infection and CD20 expression status in our cohort. The results revealed that the patients with EBV positive and CD20 positive double positive had the worst OS, followed by patients with EBV negative combined with CD20 positive and patients with EBV positive combined with CD20 negative, and patients with double negative (EBV negative combined with CD20 negative) had the best OS (P<0.001) (Figure 3).
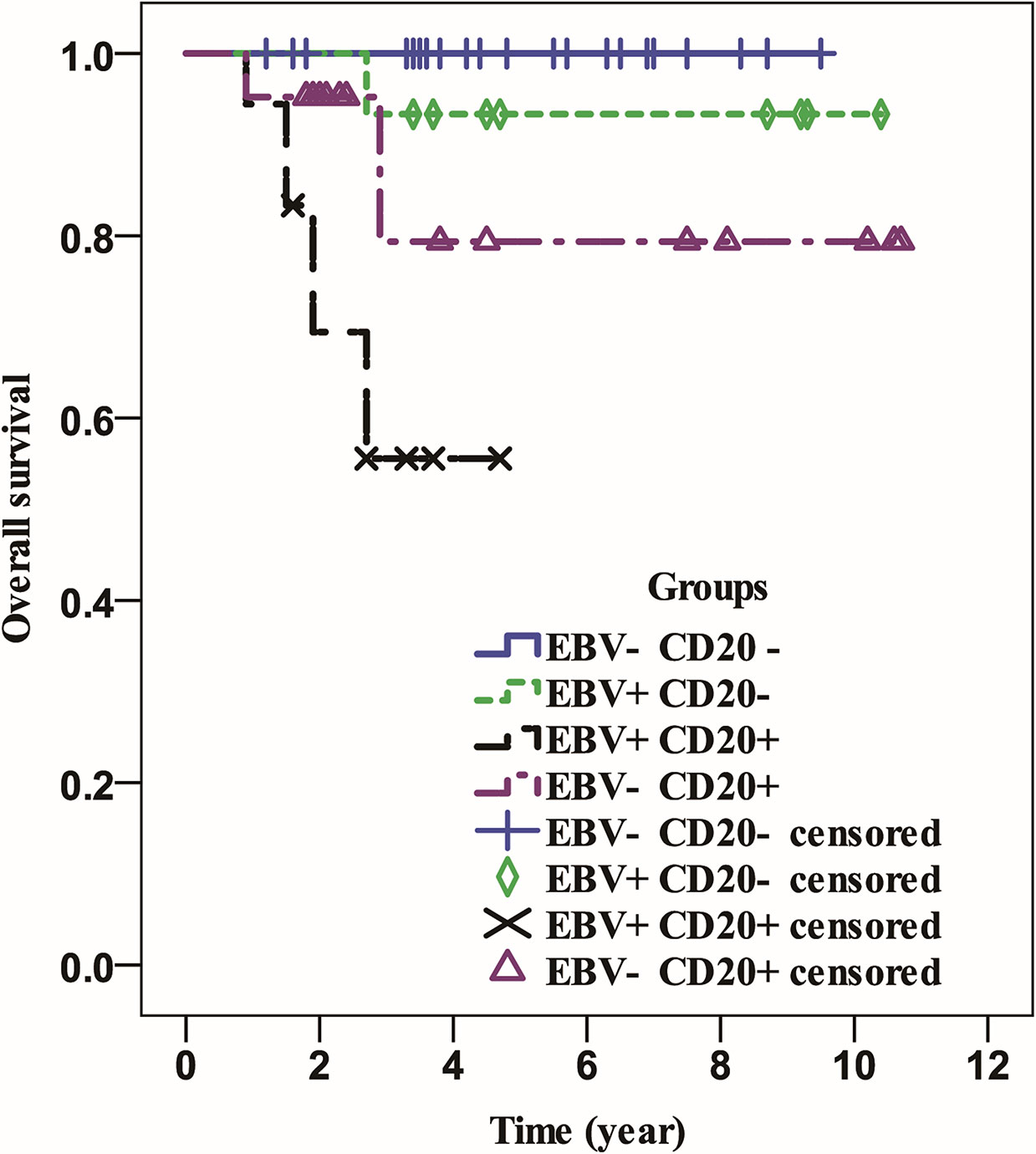
Figure 3 The overall survival of patients with different status of EBV infection and CD20 expression.
In this section, we firstly investigated the risk factors, including gender, age, clinical stage, LDH levels, β2-MG levels, ESR levels, B symptom, EBV status and CD20 expression. The Kaplan–Meier technique (Log-rank test) was used to calculate the odds of survival in 109 NSCHL patients, and the results revealed that age (P=0.001), EBV status (P=0.001), and CD20 expression (P0.001) were all risk factors. Then, we conducted multivariate regression analysis on the above three risk factors (age, EBV status and CD20 expression). CD20 positivity was found to be an independent risk factor for OS in NSCHL patients (Table 2).
CHL is a hematological malignant cancer that accounts for less than 10% of lymphomas in Asia, including China (13, 14). Hodgkin and Reed-Sternberg cells, a hallmark of CHL, disperse in the reactive inflammatory microenvironment of lymphoid tissues. Although it has been reported that up to 40% of HL patients had EBV infection, the function of EBV infection in the clinical outcome of HL patients is still debated (15). Some studies revealed that EBV infection could be a good prognostic factor for HL patients (16, 17). However, it was also reported that EBV infection could be an adverse risk factor for worse survival times in HL patients in some articles (18). In addition, other studies have displayed that there were no significant effects on the clinical prognosis of HL patients infected with EBV (9, 19). In this study, in NSCHL patients, EBV infection was a poor prognostic factor for survival. We considered that this conclusion may be associated with following two factors. First, some studies have confirmed that CHL patients with age more than 50 years had a poorer clinical prognosis, and the median age of patients in EBV-positive group was 13 years older than that in EBV-negative group (47 years versus 34 years) (20, 21). Secondly, CD20 positivity was significantly greater in the EBV-positive group than in the EBV-negative group, and CD20 positivity was confirmed as an independent risk factor for OS in NSCHL patients in this study.
CHL is a malignant tumor derived from B lymphocytes. To the best of our knowledge, the vast majority of CHL cells express CD15 and CD30 antigens. However, only 20% ~ 40% of CHL patients have CD20 positive cells (22). To date, the role of CD20 positive in the clinical prognosis of CHL is still unclear. Greaves et al. discovered that there was no statistical significance between CD20 positive and the prognosis of patients with HL (23). However, some studies demonstrated that CD20 positive was closely associated with favorable OS and PFS in CHL patients (24, 25). Other studies showed that the clinical prognosis of CHL patients with CD20 positive was poorer (26, 27). In our study, CD20 positive was a poor prognostic factor for the OS NSCHL patients. We speculate that age may be an important factor associated with the poor prognosis of NSCHL CD20-positive patients, as the median age was 12 years older for NSCHL CD20-compared to CD20-negative NSCHL patients.
At present, chemotherapy and radiotherapy are still the first-line therapy strategies for CHL patients. The vast majority of CHL patients achieve complete remission after the initial treatment. However, 34% of CHL patients with stage III/IV disease and 15% of CHL patients with stage I/II disease eventually experience relapse after the first-line treatment regimens (28). For those patients, with the development of medical technology, novel treatment strategies can be chosen at the time of definite diagnosis, such as checkpoint blocking therapy, chemoimmunotherapy and anti-EBV proteome antibody therapy (29–32). CD20 positivity was shown to be significantly greater in EBV-positive patients with NSCHL in this study. Therefore, we propose that clinical efficacy to therapy in EBV-positive patients with NSCHL may be further improved by incorporating an anti-CD20 monoclonal antibody (rituximab) into the first-line chemotherapy regimen.
The study has some limitations that need to be addressed. To begin with, this is a retrospective, single-centered study. Secondly, the sample size of this study is small, particularly for EBV-positive patients with NSCHL. Therefore, in order to verify the reliability of the conclusions of this study, we will further increase the sample size of EBV-positive patients with NSCHL by conducting a multi-center study in China in the future.
In conclusion, EBV-positive NSCHL may be a distinct disease entity characterized by advanced age, higher levels of β2-MG, and dismal prognosis. CD20 is highly expressed in EBV-positive patients with NSCHL, In NSCHL patients, this was an independent risk factor for OS.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The Ethical Committee of Qingdao University’s Affiliated Hospital. The patients/participants provided their written informed consent to participate in this study.
XZ and YM collected data and performed analysis. HB analyzed data. ZL supervised study and wrote first draft. XZ, YM, HB and ZL edited manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the China Postdoctoral Science Foundation (Grant No.2020M682128 to XZ) and the project of the Affiliated Hospital of Qingdao University (Grant No. QDFY201929 to ZL).
We would like to show our deepest gratitude to the professors from division of Hematopathology in the Affiliated Hospital of Qingdao University, who had provided us with valuable guidance in pathologic diagnosis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
b2–MG, b2–microglobulin; CHL, classical HL; CI, confidence interval; EBV, Epstein–Barr virus; ESR, erythrocyte sedimentation rate; HL, Hodgkin lymphomas; HR, hazard ratio; LDH, lactate dehydrogenase; NSCHL, Nodular sclerosis classical Hodgkin lymphoma; OS, Overall survival.
1. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
2. Li XQ, Li GD, Gao ZF, Zhou XG, Zhu XZ. Distribution pattern of lymphoma subtypes in China: A nationwide multicenter study of 10002 cases. J Diagn Concepts Pract (2012) 11:111–5. doi: 10.3969/j.issn.1671-2870.2012.02.006
3. Yang M, Ping L, Liu W, Xie Y, Aliya, Liu Y, et al. Clinical characteristics and prognostic factors of primary extranodal classical Hodgkin lymphoma: a retrospective study. Hematology (2019) 24:413–9. doi: 10.1080/16078454.2019.1598678
4. Chang CM, Yu KJ, Mbulaiteye SM, Hildesheim A, Bhatia K. The extent of genetic diversity of Epstein-Barr virus and its geographic and disease patterns: a need for reappraisal. Virus Res (2009) 143:209–21. doi: 10.1016/j.virusres.2009.07.005
5. Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infect Agent Cancer (2014) 9:38. doi: 10.1186/1750-9378-9-38
6. Myriam BD, Sonia Z, Hanene S, Teheni L, Mounir T. Prognostic significance of Epstein-Barr virus (EBV) infection in Hodgkin lymphoma patients. J Infect Chemother (2017) 23:121–30. doi: 10.1016/j.jiac.2016.09.004
7. Krugmann J, Tzankov A, Gschwendtner A, Fischhofer M, Greil R, Fend F, et al. Longer failure-free survival interval of Epstein-Barr virus-associated classical hodgkin's lymphoma: a single-institution study. Mod Pathol (2003) 16:566–73. doi: 10.1097/01.MP.0000071843.09960.BF
8. Keegan TH, Glaser SL, Clarke CA, Gulley ML, Craig FE, Digiuseppe JA, et al. Epstein-Barr Virus as a marker of survival after hodgkin's lymphoma: a population-based study. J Clin Oncol (2005) 23:7604–13. doi: 10.1200/JCO.2005.02.6310
9. Stark GL, Wood KM, Jack F, Angus B, Proctor SJ, Taylor PR, et al. Hodgkin's disease in the elderly: a population-based study. Br J Haematol (2002) 119:432–40. doi: 10.1046/j.1365-2141.2002.03815.x
10. Herling M, Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP, Kliche KO, Nadali G, et al. Expression of Epstein-Barr virus latent membrane protein-1 in Hodgkin and reed-sternberg cells of classical hodgkin's lymphoma: associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res (2003) 9:2114–20.
11. Gulley ML, Glaser SL, Craig FE, Borowitz M, Mann RB, Shema SJ, et al. Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting Epstein-Barr virus in Hodgkin lymphoma. Am J Clin Pathol (2002) 117:259–67. doi: 10.1309/MMAU-0QYH-7BHA-W8C2
12. Sakatani A, Igawa T, Okatani T, Fujihara M, Asaoku H, Sato Y, et al. Clinicopathological significance of CD79a expression in classic Hodgkin lymphoma. J Clin Exp Hematop (2020) 60:78–86. doi: 10.3960/jslrt.20010
13. Makita S, Maruyama D, Maeshima AM, Taniguchi H, Miyamoto K, Kitahara H, et al. Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma. Int J Hematol (2016) 104:236–44. doi: 10.1007/s12185-016-2007-1
14. Meng J, Chang C, Pan H, Zhu F, Xiao Y, Liu T, et al. Epidemiologic characteristics of malignant lymphoma in hubei, China: A single-center 5-year retrospective study. Medicine (2018) 97:e12120. doi: 10.1097/MD.0000000000012120
15. Bakkalci D, Jia Y, Winter JR, Lewis JE, Taylor GS, Stagg HR. Risk factors for Epstein Barr virus-associated cancers: a systematic review, critical appraisal, and mapping of the epidemiological evidence. J Glob Health (2020) 10:10405. doi: 10.7189/jogh.10.010405
16. Flavell KJ, Billingham LJ, Biddulph JP, Gray L, Flavell JR, Constandinou CM, et al. The effect of Epstein-Barr virus status on outcome in age- and sex-defined subgroups of patients with advanced hodgkin's disease. Ann Oncol (2003) 14:282–90. doi: 10.1093/annonc/mdg065
17. Vassallo J, Metze K, Traina F, de Souza CA, Lorand-Metze I. Expression of Epstein-Barr virus in classical hodgkin's lymphomas in Brazilian adult patients. Haematologica (2001) 86:1227–8.
18. Clarke CA, Glaser SL, Dorfman RF, Mann R, DiGiuseppe JA, Prehn AW, et al. Epstein-Barr Virus and survival after Hodgkin disease in a population-based series of women. Cancer (2001) 91:1579–87. doi: 10.1002/1097-0142(20010415)91:8<1579::aid-cncr1169>3.0.co;2-l
19. Enblad G, Sandvej K, Sundström C, Pallesen G, Glimelius B. Epstein-Barr Virus distribution in hodgkin's disease in an unselected Swedish population. Acta Oncol (1999) 38:425–9. doi: 10.1080/028418699431942
20. Jarrett RF, Stark GL, White J, Angus B, Alexander FE, Krajewski AS, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood (2005) 106:2444–51. doi: 10.1182/blood-2004-09-3759
21. Diepstra A, van Imhoff GW, Schaapveld M, Karim-Kos H, van den Berg A, Vellenga E, et al. Latent Epstein-Barr virus infection of tumor cells in classical hodgkin's lymphoma predicts adverse outcome in older adult patients. J Clin Oncol (2009) 27:3815–21. doi: 10.1200/JCO.2008.20.5138
22. Santos M, Lima MM. CD20 role in pathophysiology of hodgkin's disease. Rev Assoc Med Bras (2017) 63:810–3. doi: 10.1590/1806-9282.63.09.810
23. Greaves P, Clear A, Coutinho R, Wilson A, Matthews J, Owen A, et al. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J Clin Oncol (2013) 31:256–62. doi: 10.1200/JCO.2011.39.9881
24. Horvat M, Kloboves Prevodnik V, Lavrencak J, Jezersek Novakovic B. Predictive significance of the cut-off value of CD20 expression in patients with b-cell lymphoma. Oncol Rep (2010) 24:1101–7.
25. Tzankov A, Krugmann J, Fend F, Fischhofer M, Greil R, Dirnhofer S. Prognostic significance of CD20 expression in classical Hodgkin lymphoma: a clinicopathological study of 119 cases. Clin Cancer Res (2003) 9:1381–6.
26. Aldred V, Vassallo J, Froes M Campos AH, Augusto Soares F. CD20 expression by Hodgkin-Reed-Sternberg cells in classical Hodgkin lymphoma is related to reduced overall survival in young adult patients. Leuk Lymphoma (2008) 49:2198–202. doi: 10.1080/10428190802239170
27. Portlock CS, Donnelly GB, Qin J, Straus D, Yahalom J, Zelenetz A, et al. Adverse prognostic significance of CD20 positive reed-sternberg cells in classical hodgkin's disease. Br J Haematol (2004) 125:701–8. doi: 10.1111/j.1365-2141.2004.04964.x
28. DeVita VT Jr, Costa J. Toward a personalized treatment of hodgkin's disease. N Engl J Med (2010) 362:942–3. doi: 10.1056/NEJMe0912481
29. Liu Z, Jarrett RF, Hjalgrim H, Proietti C, Chang ET, Smedby KE, et al. Evaluation of the antibody response to the EBV proteome in EBV-associated classical Hodgkin lymphoma. Int J Cancer (2020) 147:608–18. doi: 10.1002/ijc.32741
30. Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol (2016) 17:1283–94. doi: 10.1016/S1470-2045(16)30167-X
31. Strati P, Fanale MA, Oki Y, Turturro F, Fayad LE, Bartlett NL, et al. ABVD plus rituximab versus ABVD alone for advanced stage, high-risk classical Hodgkin lymphoma: a randomized phase 2 study. Haematologica (2019) 104:e65–7. doi: 10.3324/haematol.2018.199844
Keywords: CD20, Epstein–Barr virus, nodular sclerosis, Hodgkin lymphoma, overall survival
Citation: Zhao X, Ma Y, Bian H and Liu Z (2022) CD20 expression is closely associated with Epstein–Barr virus infection and an inferior survival in nodular sclerosis classical Hodgkin lymphoma. Front. Oncol. 12:993768. doi: 10.3389/fonc.2022.993768
Received: 14 July 2022; Accepted: 17 August 2022;
Published: 06 September 2022.
Edited by:
Aamir Ahmad, University of Alabama at Birmingham, United StatesReviewed by:
Gerald Schwan, University of Texas Southwestern Medical Center, United StatesCopyright © 2022 Zhao, Ma, Bian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihe Liu, emhpaGVsaXVAcWR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.