- 1Department of Hepatobiliary Surgery, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, China
- 2Department of Hepatobiliary Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Backgroud: At present, there is no definitive conclusion about the relative prognostic factors on intrahepatic cholangiocarcinoma perihilar large duct type (iCCAphl) and iCCA peripheral small duct type (iCCApps).
Aim of the study: To compare the prognoses of two different types of iCCA, and identify the independent risk factors affecting the long-term survival of patients undergoing radical resection for iCCA.
Methods: This study included 89 patients with iCCA who underwent radical resection at the Department of Hepatobiliary Surgery of the East Yard of the Shandong Provincial Hospital between January 2013 and March 2022. According to the tumor origin, these patients were divided into the iCCAphl group (n = 37) and iCCApps group (n = 52). The prognoses of the two groups were compared using Kaplan–Meier analysis, whereas the independent risk factors of their prognoses were identified using Cox univariate and multivariate regression analyses.
Results: In the iCCApps group, the independent risk factors for overall survival included diabetes history (p = 0.006), lymph node metastasis (p = 0.040), and preoperative carbohydrate antigen 19-9 (p = 0.035). In the iCCAphl group, the independent risk factors for overall survival included multiple tumors (p = 0.010), tumor differentiation grade (p = 0.008), and preoperative jaundice (p = 0.009).
Conclusions: Among the iCCA patients who underwent radical resection, the long-term prognosis of iCCApps maybe better than that of iCCAphl. The prognoses of these two types of iCCA were affected by different independent risk factors.
Introduction
Malignancies of the biliary tract can be categorized as intrahepatic cholangiocarcinoma (iCCA), hilar cholangiocarcinoma, carcinoma of common bile duct, or gallbladder carcinoma according to the location of the tumor. Hilar cholangiocarcinoma originates from where the bilateral hepatic ducts join the cystic duct and common hepatic duct, whereas iCCA originates from the second-order bile ducts and above (1). The iCCA is the second most common hepatic malignancy after hepatocellular carcinoma and has shown an escalating incidence in recent years (2, 3). Although the pathophysiology, genetic and epigenetic aberrations have not been fully discovered, it is currently believed that its pathogenesis may be associated with primary sclerosing cholangitis, parasitic infection, cholelithiasis, alcoholic liver disease, non-specific cirrhosis, diabetes, or asbestos exposure (4–6). Due to its delayed presentation of symptoms, difficulty in diagnosis, relatively delayed treatment initiation, easy recurrence, and lack of effective treatment methods other than surgery, the prognosis of iCCA is poorer than that of hepatocellular carcinoma (7–9). Some studies have suggested that factors associated with the long-term prognosis of patients with iCCA include preoperative lymph node metastasis, carbohydrate antigen 19-9 positivity, vascular invasion, and number of tumors (10–12).
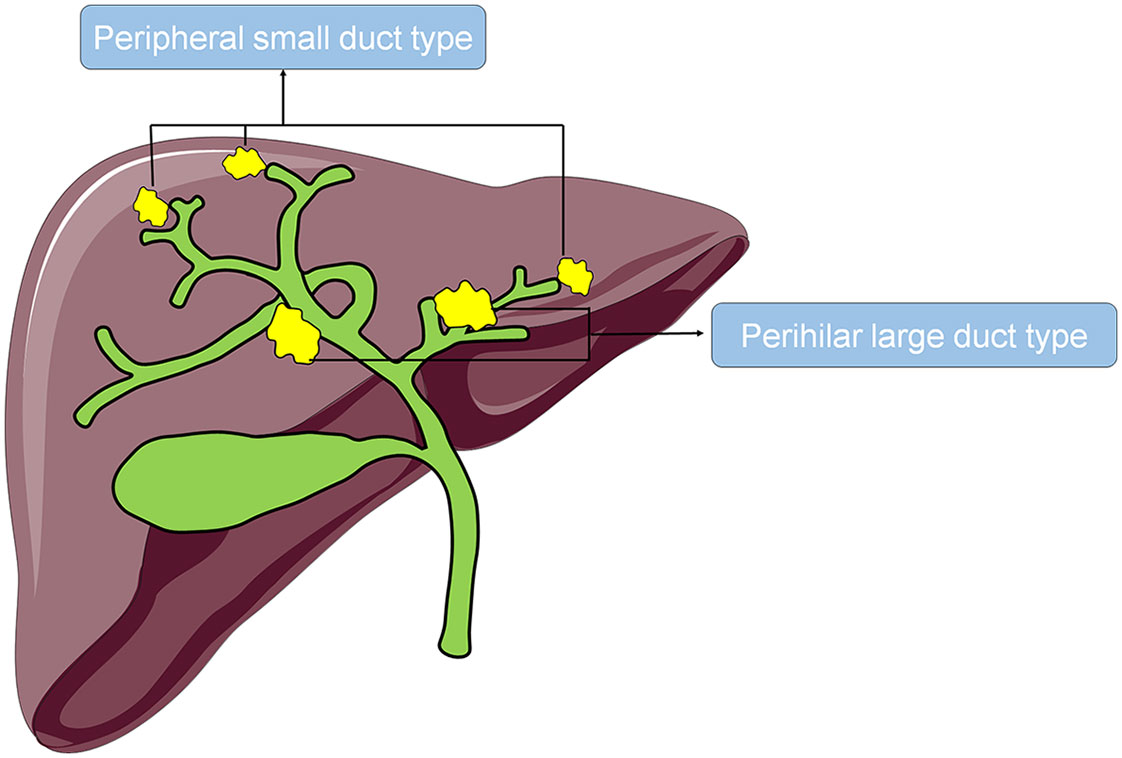
According to the origin of the tumor, iCCA can be categorized as the perihilar large duct type (iCCAphl) or the peripheral small duct type (iCCApps). ICCAphl refers to tumors originating from the large second-order bile ducts and above involving the peribiliary glands (Figure 1). They are composed of a large tubular or papillary proliferation of tall columnar epithelium with extracellular mucin production, often admixed with poorly differentiated carcinoma cells (13–16). On the other hand, iCCApps refers to tumors originating from the small bile ducts, such as Herling’s duct, which are usually smaller than the segmental branches (Figure 1). They are composed of a small tubular proliferation of small cuboidal epithelium with ductular pattern or closely packed cord-like structures, but lacking large glands with tall columnar cells (13–16). Previous studies have revealed several differences between these two subtypes in their histological characteristics, expression of interleukin 33, gene regulatory networks, and immune infiltration (17–20). However, the current research on the postoperative overall survival and disease-free survival or the associated risk factors of iCCApps and iCCAphl is limited. Therefore, this study aimed to compare the long-term prognosis of the two subtypes of iCCA and investigate their corresponding risk factors to provide references for the clinical diagnosis and treatment of iCCA.
Materials and methods
Data collection
This study included patients with iCCA who underwent surgical resection at the Department of Hepatobiliary Surgery of the East Yard of the Shandong Provincial Hospital between January 2013 and March 2022. The inclusion criteria were as follows: (1) history of radical resection for iCCA; (2) iCCA diagnosis on postoperative pathological analysis; and (3) availability of computed tomography (CT) or magnetic resonance imaging (MRI) data of the liver. The exclusion criteria were as follows: (1) only surgical or needle biopsy performed; (2) incomplete CT or MRI data; and (3) no follow-up information collected after discharge. In total, 89 patients were included, who were subsequently divided into the iCCAphl group (n = 37) and the iCCApps group (n = 52).
The demographic information of the patients was first collected during hospitalization. This included age, sex, length of hospitalization, drinking history, history of underlying diseases (hypertension, hyperglycemia, coronary heart disease, or history of abdominal surgery), preoperative jaundice, vascular invasion, number of tumors, surgical margin, maximum tumor diameter (>5 cm), tumor differentiation grade, lymph node metastasis, iCCA classification, preoperative carcinoembryonic antigen level, preoperative carbohydrate antigen 19-9 level, preoperative alpha-fetoprotein level, liver cirrhosis, intrahepatic bile duct stones, hepatitis B infection, use of blood transfusion products during surgery, surgical classification (whether >2 liver segments were removed), anesthesia classification, perioperative blood transfusion products, and postoperative chemotherapy. Furthermore, the overall survival and disease-free survival were recorded by following up on the telephone. The follow-up period ended either on May 1, 2022 or when the patient died or was lost to follow-up. This study was approved by the Ethics Committee of the Shandong Provincial Hospital, and informed consent was waived for all participants.
Outcome definition
Overall survival was defined as the time from surgery to death or when follow-up ended. Disease-free survival was defined as the time from surgery to the first discovery of recurrence or when follow-up ended. Patients were allocated to the iCCAphl group if their imaging findings revealed that the tumor was located in the large intrahepatic second-order bile ducts and above or was accompanied by the dilation of the distal bile ducts, and/or their histopathology revealed that the tumor was composed of a tubular or papillary component with tall columnar epithelium. Patients with tumors originating from branches other than the aforementioned branches were allocated to the iCCApps group, and/or their histopathology revealed that the tumor was composed of small tubules with cuboidal epithelium.
Statistical analysis
Statistical Product and Service Solutions, version 26 (IBM Corp, Armonk, New York), software was adopted for all statistical analyses. Pairwise comparison was performed between the two groups of iCCA patients. Variables conforming to normal distribution, variables not conforming to normal distribution, and categorical variables were analyzed using the student’s t-test, Mann–Whitney U test, and Fisher’s exact test, respectively; P-values <0.05 were considered statistically significant. The median overall survival, 1-year and 3-year survival rates, median disease-free survival, and 1-year and 3-year recurrence-free rates were calculated using the Kaplan–Meier analysis, followed by plotting a survival curve. Moreover, Cox univariate regression analysis was performed for the iCCAphl and iCCApps groups. Indicators with statistically significant differences identified in the univariate analysis (P < 0.05) were subsequently included in the Cox multivariate regression model to investigate the independent risk factors affecting the postoperative overall survival and disease-free survival of the patients of the two groups.
Results
Pairwise comparison of the two groups
The iCCAphl group included 37 patients (average age, 60.35 ± 9.85 years), comprising 18 (48.6%) male patients. The iCCApps group included 52 patients (average age, 59.33 ± 9.8 years), comprising 27 (51.9%) male patients. The analysis of the demographic data revealed no significant differences in the age (60.35 ± 9.85 vs. 59.33 ± 9.80), man (18 [48.6%] vs. 27 [51.9%]), drinking history (10 [27.0%] vs. 18 [34.6%]), and history of diabetes (4 [10.8%] vs. 9 [17.3%]), hypertension (4 [10.8%] vs. 9 [17.3%]), coronary heart disease (4 [10.8%] vs. 3 [5.8%]), or abdominal surgery (8 [21.6%] vs. 6 [11.5%]) between the two groups.
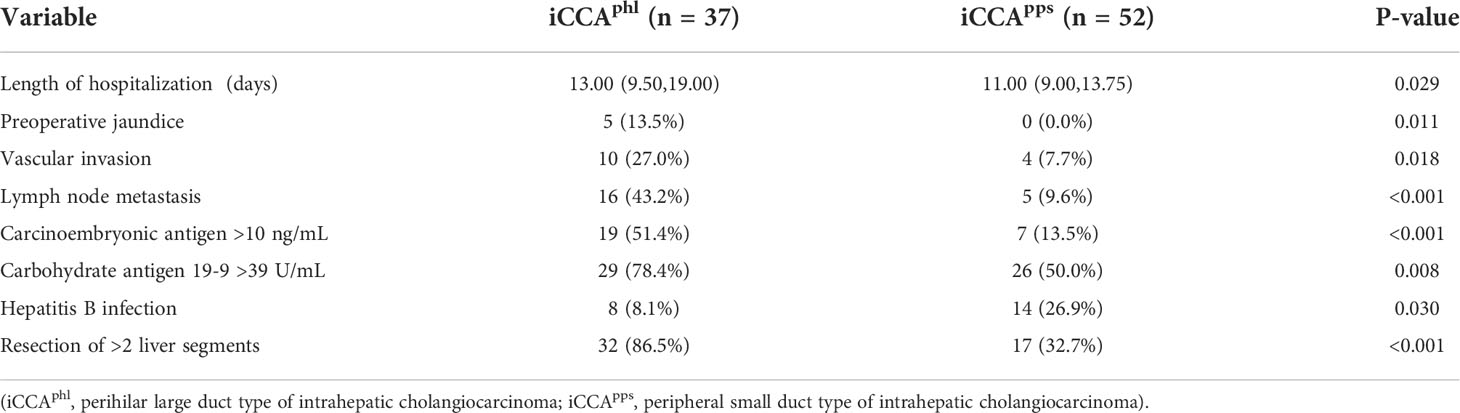
The average length of hospitalization differed significantly between the iCCAphl and iCCApps groups (13.00 [9.50–19.00] vs. 11.00 [9.00–13.75]). Moreover, the iCCAphl and iCCApps groups showed significant differences in the proportion of cases with preoperative jaundice (5 [13.5%] vs. 0 [0.0%]), hepatic vascular invasion (10 [27.0%] vs. 4 [7.7%]), lymph node metastasis (16 [43.2%] vs. 5 [9.6%]), preoperative carcinoembryonic antigen >10 ng/mL (19 [51.4%] vs. 7 [13.5%]), preoperative carbohydrate antigen 19-9 >39 U/mL (29 [78.4%] vs. 26 [50.0%]), hepatitis B infection (8 [8.1%] vs. 14 [26.9%]), and resection of >2 liver segments (32 [86.5%] vs. 17 [32.7%]). However, the remaining indicators did not show significant pairwise differences (Table 1; Figure 2).
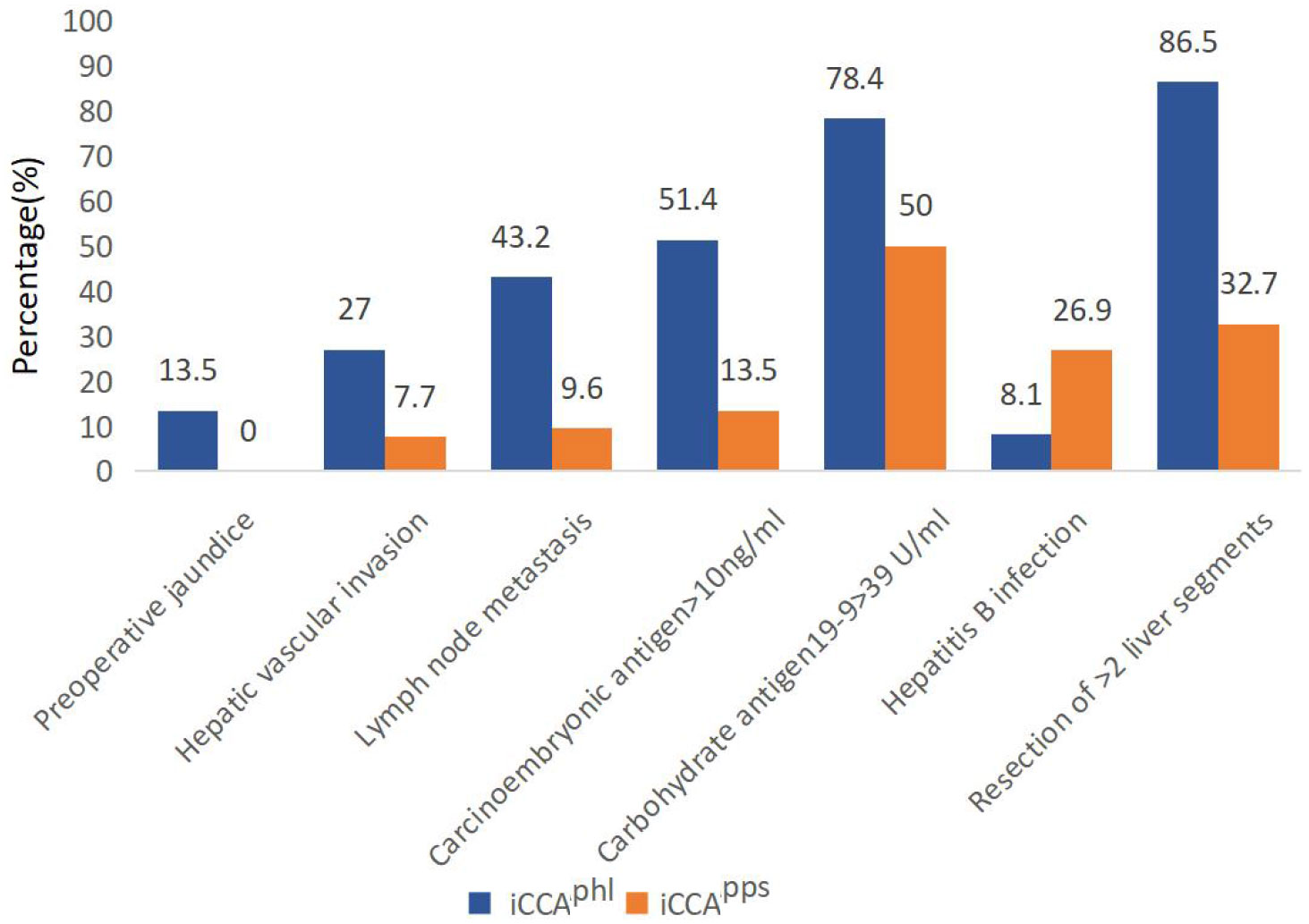
Figure 2 Pairwise comparison of the two types of intrahepatic cholangiocarcinoma (P < 0.05). The bars in the graph indicate the number of cases in each group for each risk factor. iCCAphl, perihilar large duct type of intrahepatic cholangiocarcinoma; iCCApps, peripheral small duct type of intrahepatic cholangiocarcinoma.
Kaplan–Meier analysis revealed that the median overall survival in the iCCAphl group was 12.0 (95% confidence interval [CI]: 3.09–20.91) months, and the 1-year and 3-year overall survival rates were 49.3% and 33.2%, respectively. In contrast, the median overall survival in the iCCApps group was 25.0 (95% CI: 11.45–38.55) months, with 1-year and 3-year overall survival rates of 73.5%, and 41.2%, respectively. Additionally, the pairwise differences between the two groups were statistically significant (P = 0.019) (Figure 3). It showed that the hazard ratio (HR) of the two tumor subtypes was 1.780, 95% CI: 0.770–4.113 and P=0.177 after adjustment with multivariate cox regression analysis of the variables with statistical differences in univariate analysis and the two tumor subtypes. The median disease-free survival in the iCCAphl group was 6.0 (95% CI: 0.00–14.12) months, and the 1-year and 3-year recurrence-free rates were 44.1% and 27.6%, respectively. The median disease-free survival in the iCCApps group was 17.0 (95% CI: 7.65–26.35) months, with 1-year and 3-year recurrence-free rates of 54.5% and 27.2%, respectively. However, pairwise differences between the two groups were not significant (P = 0.191) (Figure 4).
Figure 3 Pairwise comparison of the overall survival between the two types of intrahepatic cholangiocarcinoma. iCCAphl, perihilar large duct type of intrahepatic cholangiocarcinoma; iCCApps, peripheral small duct type of intrahepatic cholangiocarcinoma; M [95% CI], median [95% confidence interval].
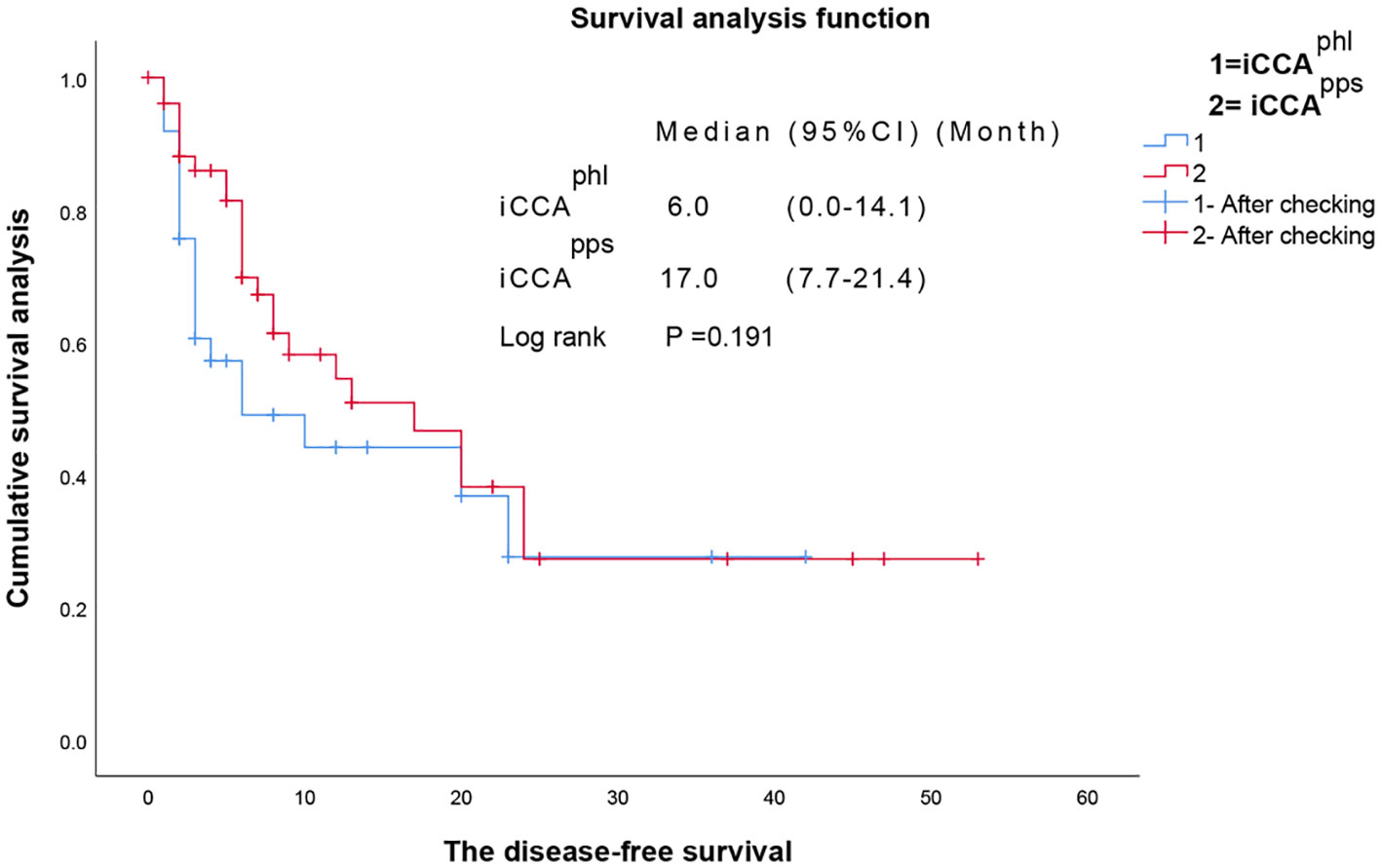
Figure 4 Pairwise comparison of the disease-free survival between the two types of intrahepatic cholangiocarcinoma. iCCAphl, perihilar large duct type of intrahepatic cholangiocarcinoma; iCCApps, peripheral small duct type of intrahepatic cholangiocarcinoma; CI, confidence interval.
Risk factor analysis of the prognosis of radical resection for iCCApps
Cox univariate regression analysis of the overall survival in the iCCApps group revealed that length of hospitalization, age, diabetes history, vascular invasion, multiple tumors, lymph node metastasis, preoperative carbohydrate antigen 19-9 >39 U/mL, intrahepatic bile duct stones, surgical resection of >2 liver segments, postoperative complication grade, and hepatitis B infection were the risk factors. Subsequent multivariate regression analysis showed that diabetes history (HR: 11.768, 95% CI: 2.003–69.136), lymph node metastasis (HR: 6.180, 95% CI: 1.090–35.038), and preoperative carbohydrate antigen 19-9 >39 U/mL (HR: 3.140, 95% CI: 1.081–9.121) were independent risk factors. Additionally, length of hospitalization (HR: 1.287, 95% CI: 1.060–1.562) exhibited a statistically significant effect (Table 2).
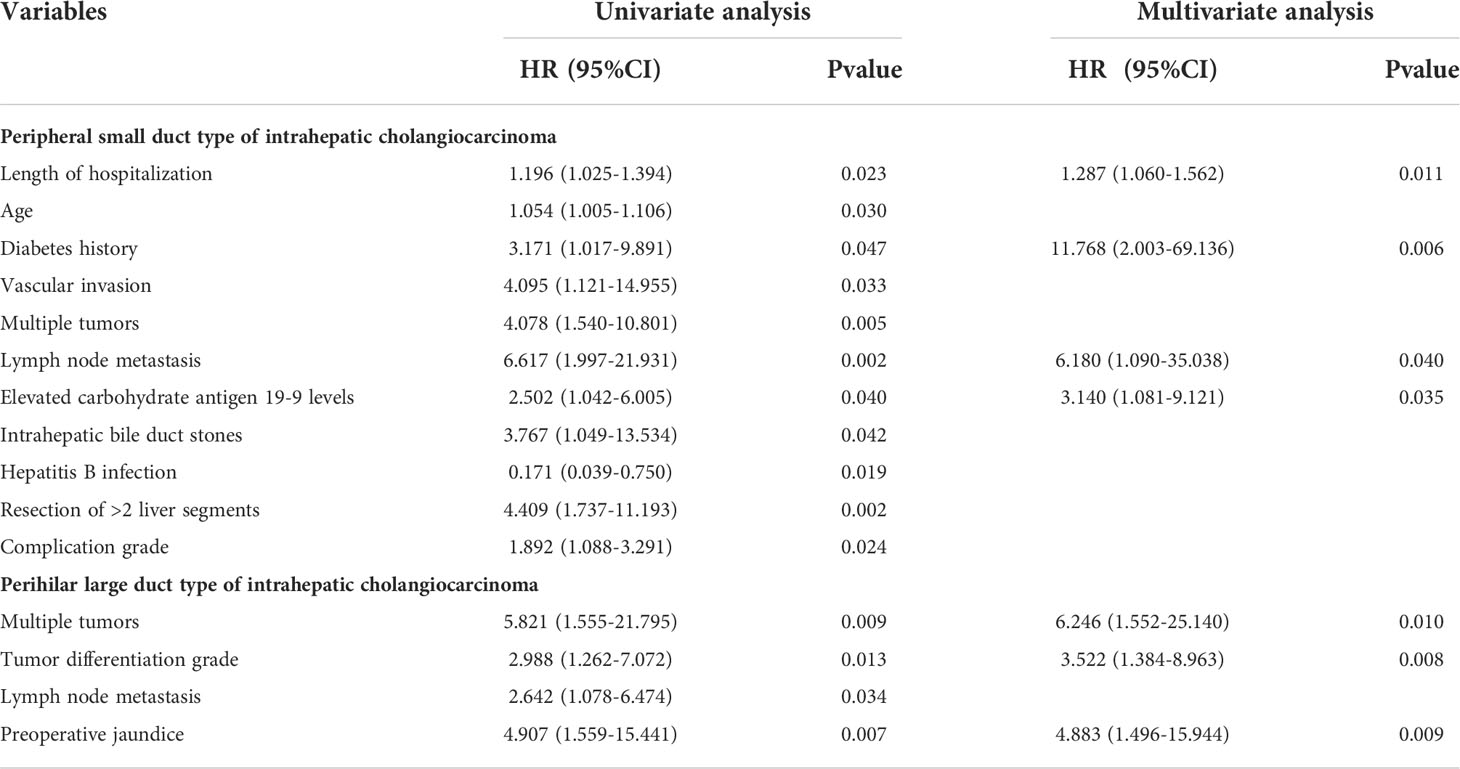
Table 2 Risk factor analysis of the prognosis of radical resection for the two types of intrahepatic cholangiocarcinoma.
Cox univariate regression analysis of the disease-free survival in the iCCApps group showed that lymph node metastasis (HR: 11.496, 95% CI: 2.962–44.623) and preoperative carbohydrate antigen 19-9 >39 U/mL (HR: 3.031, 95% CI: 1.322–6.946) were risk factors. Subsequent Cox multivariate analysis suggested that both these were independent risk factors for the disease-free survival in the iCCApps group (lymph node metastasis, HR: 7.765, 95% CI: 1.961–30.750; preoperative carbohydrate antigen 19-9 >39 U/mL, HR: 2.555, 95% CI: 1.074–6.082).
Risk factor analysis of the prognosis of radical resection for iCCAphl
Cox univariate regression analysis showed that the risk factors affecting the overall survival in the iCCAphl group were multiple tumors, tumor differentiation grade, lymph node metastasis, and preoperative jaundice. Subsequent multivariate analysis showed that multiple tumors (HR: 6.246, 95% CI: 1.552–25.140), tumor differentiation grade (HR: 3.522, 95% CI: 1.384–8.963), and preoperative jaundice (HR: 4.883, 95% CI: 1.496–15.944) were independent risk factors for the overall survival of patients with iCCAphl (Table 2).
Furthermore, Cox univariate regression analysis showed that preoperative jaundice (HR: 4.498, 95% CI: 1.540–13.134) was the only risk factor of disease-free survival in the iCCAphl group.
Discussion
Recently, the incidence of iCCA has been on the rise. Moreover, the diagnosis may be delayed due to its hidden onset in most cases, and the window for surgery may have already been missed due to the severe disease progression, thereby leading to poor prognosis. Multiple studies have reported that elevated preoperative carbohydrate antigen 19-9 and carcinoembryonic antigen levels and lymph node metastasis are important prognostic indicators in iCCA patients (21–25). Recent studies have shown that iCCA can be categorized as iCCAphl and iCCApps, and these two subtypes differ significantly in their pathological morphology and survival prognosis (14, 15, 26). Our study showed that the length of hospitalization was longer and the incidence of preoperative jaundice, vascular invasion, lymph node metastasis, and elevated preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels were more common in the iCCAphl patients than in the iCCApps patients. In univariable analysis, the median postoperative overall survival was shorter in the iCCAphl group than in the iCCApps group. However, after adjustment with multivariate cox regression analysis, it revealed no significant differences in postoperative survival time between the two tumor subtypes. This may be related to the low incidence of the disease and the low number of patients enrolled. Therefore, further large-sample prospective studies are needed to identify the differences in prognosis between iCCAphl and iCCApps. Therefore, it was speculated that iCCAphl maybe more aggressive than iCCApps and results in a poor overall survival, which corroborated the findings of Shinichi et al. (26). Thus, in clinical practice, it would be possible to predict the patient’s prognosis early based on the preoperative imaging findings. Subsequently, early interventions can be introduced for the treatment of iCCAphl, thereby reducing the risk of postoperative recurrence and prolonging the overall survival of the patient.
Currently, differentiation of the iCCA types has not been widely implemented clinically. The Cox multivariate regression analysis in this study suggested that the length of hospitalization, diabetes history, lymph node metastasis, and elevated carbohydrate antigen 19-9 levels were independent risk factors for the prognosis of iCCApps, whereas multiple tumors, tumor differentiation, and preoperative jaundice were independent risk factors for the prognosis of iCCAphl. Since these two subtypes have completely different risk factors, prognosis prediction based solely on the risk factors for iCCA may not match the actual situation and could be inaccurate. Therefore, it is necessary to clinically differentiate between iCCApps and iCCAphl, evaluate the corresponding risk factors, predict the prognosis early, and introduce appropriate and timely treatment.
This study found that the effect of hepatitis B infection on the patients’ overall survival was statistically significant only in the univariate analysis, but not in the multivariate analysis. Currently, the effect of hepatitis B infection on the prognosis of iCCA remains controversial. A study by Jeong et al. indicated that hepatitis B infection was a strong predictor of the good prognosis of iCCA (27, 28), whereas Ahn et al. found that it was not an independent prognostic factor of iCCA (29, 30). Therefore, the influence of hepatitis B infection on the prognosis of iCCA requires further verification via prospective studies.
The strength of this study is that both univariate and multivariate cox regression analyses were performed for the two types of iCCA to identify the differences in their prognostic factors. Moreover, unlike the study by Shinichi et al., tumors >5 cm in size were included in this study, thereby increasing the differentiation by tumor size and consequently reducing the selection bias caused by deliberately excluding large-sized tumors. The limitation of this study is that this was a single-center study, and only patients admitted to the Department of Hepatobiliary Surgery of the East Yard of the Shandong Provincial Hospital were included. Furthermore, only those patients who underwent radical resection were evaluated; therefore, further investigations are warranted to conclude whether the findings of this study are applicable to iCCA patients who do not undergo surgery.
In conclusion, this study showed that iCCAphl maybe more aggressive than iCCApps, and the overall survival maybe differ between them. In clinical practice, individualized early interventions should be introduced in patients with iCCA according to the disease subtype to improve their prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong Provincial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZY: Writing-original draf, data curation. QN: Investigation, methodology, writing review and editing, conceptualization. HJ: Writing review and editing. HG: Investigation, methodology, writing review and editing. FY: Methodology, writing review and editing. HZ: Investigation, methodology, writing review and editing. FL: Investigation, methodology, conceptualization. JW: Investigation, supervision. XZ: Investigation, supervision, methodology. JL: Investigation, conceptualization, methodology, supervision. HC: Investigation, conceptualization, methodology, supervision. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Shandong Provincial Natural Science Foundation, Grant/Award Number: ZR2020MH054.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am (2019) 28:587–99. doi: 10.1016/j.soc.2019.06.002
2. Entezari P, Riaz A. Intrahepatic cholangiocarcinoma. Semin Intervent Radiol (2020) 37:475–83. doi: 10.1055/s-0040-1719188
3. Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg (2008) 248:84–96. doi: 10.1097/SLA.0b013e318176c4d3
4. Brandi G, Tavolari S. Asbestos and intrahepatic cholangiocarcinoma. Cells (2020) 9(2):421. doi: 10.3390/cells9020421
5. Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: A 25-year single-centre experience. Eur J Gastroenterol Hepatol (2012) 24:1051–8. doi: 10.1097/MEG.0b013e3283554bbf
6. Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology (2005) 128:620–6. doi: 10.1053/j.gastro.2004.12.048
7. Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol (2009) 16:14–22. doi: 10.1245/s10434-008-0180-z
8. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72:353–63. doi: 10.1016/j.jhep.2019.10.009
9. Bekki Y, Von Ahrens D, Takahashi H, Schwartz M, Gunasekaran G. Recurrent intrahepatic cholangiocarcinoma - review. Front Oncol (2021) 11:776863. doi: 10.3389/fonc.2021.776863
10. Lurje G, Bednarsch J, Czigany Z, Lurje I, Schlebusch IK, Boecker J, et al. The prognostic role of lymphovascular invasion and lymph node metastasis in perihilar and intrahepatic cholangiocarcinoma. Eur J Surg Oncol (2019) 45:1468–78. doi: 10.1016/j.ejso.2019.04.019
11. Asaoka T, Kobayashi S, Hanaki T, Iwagami Y, Tomimaru Y, Akita H, et al. Clinical significance of preoperative CA19-9 and lymph node metastasis in intrahepatic cholangiocarcinoma. Surg Today (2020) 50:1176–86. doi: 10.1007/s00595-020-01992-x
12. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
13. Yamada M, Yamamoto Y, Sugiura T, Kakuda Y, Ashida R, Tamura S, et al. Comparison of the clinicopathological features in small bile duct and bile ductular type intrahepatic cholangiocarcinoma. Anticancer Res (2019) 39:2121–7. doi: 10.21873/anticanres.13325
14. Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J Hepatobil Pancreat Sci (2015) 22:94–100. doi: 10.1002/jhbp.154
15. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol (2010) 2:419–27. doi: 10.4254/wjh.v2.i12.419
16. Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int (2019) 39 Suppl 1:7–18. doi: 10.1111/liv.14093
17. Song G, Shi Y, Meng L, Ma J, Huang S, Zhang J, et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat Commun (2022) 13:1642. doi: 10.1038/s41467-022-29164-0
18. Sawada R, Ku Y, Akita M, Otani K, Fujikura K, Itoh T, et al. Interleukin-33 overexpression reflects less aggressive tumour features in large-duct type cholangiocarcinomas. Histopathology (2018) 73:259–72. doi: 10.1111/his.13633
19. Akita M, Sofue K, Fujikura K, Otani K, Itoh T, Ajiki T, et al. Histological and molecular characterization of intrahepatic bile duct cancers suggests an expanded definition of perihilar cholangiocarcinoma. HPB (Oxford) (2019) 21:226–34. doi: 10.1016/j.hpb.2018.07.021
20. Yamashita YI, Wang H, Kurihara T, Tsujita E, Nishie A, Imai K, et al. Clinical significances of preoperative classification of intrahepatic cholangiocarcinoma: Different characteristics of perihilar vs. peripheral ICC. Anticancer Res (2016) 36:6563–9. doi: 10.21873/anticanres.11260
21. Juntermanns B, Kaiser GM, Itani Gutierrez S, Heuer M, Buechter M, Kahraman A, et al. CA19-9 in intrahepatic cholangiocarcinoma: A diagnostic and prognostic armamentarium? Chirurg (2018) 89:466–71. doi: 10.1007/s00104-018-0636-z
22. Moro A, Mehta R, Sahara K, Tsilimigras DI, Paredes AZ, Farooq A, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol (2020) 27:2888–901. doi: 10.1245/s10434-020-08350-8
23. Jolissaint JS, Soares KC, Seier KP, Kundra R, Gönen M, Shin PJ, et al. Intrahepatic cholangiocarcinoma with lymph node metastasis: Treatment-related outcomes and the role of tumor genomics in patient selection. Clin Cancer Res (2021) 27:4101–8. doi: 10.1158/1078-0432.CCR-21-0412
24. Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol (2019) 29:3725–35. doi: 10.1007/s00330-019-06142-7
25. Li H, Feng Y, Liu C, Li J, Li J, Wu H, et al. Importance of normalization of carbohydrate antigen 19-9 in patients with intrahepatic cholangiocarcinoma. Front Oncol (2021) 11:780455. doi: 10.3389/fonc.2021.780455
26. Shinichi A, Yousuke K, Yunosuke N, Tomohiro I, Kenichi T, Akinobu T, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: Clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol (2007) 31(7):1059–67. doi: 10.1097/PAS.0b013e31802b34b6
27. Zhou HB, Hu JY, Hu HP. Hepatitis b virus infection and intrahepatic cholangiocarcinoma. World J Gastroenterol (2014) 20:5721–9. doi: 10.3748/wjg.v20.i19.5721
28. Jeong S, Luo G, Wang ZH, Sha M, Chen L, Xia Q. Impact of viral hepatitis b status on outcomes of intrahepatic cholangiocarcinoma: A meta-analysis. Hepatol Int (2018) 12:330–8. doi: 10.1007/s12072-018-9881-y
29. Seo JW, Kwan BS, Cheon YK, Lee TY, Shim CS, Kwon SY, et al. Prognostic impact of hepatitis b or c on intrahepatic cholangiocarcinoma. Korean J Intern Med (2020) 35:566–73. doi: 10.3904/kjim.2018.062
Keywords: iCCA, iCCApps, iCCAphl, prognostic analysis, radical resection
Citation: Yu Z, Ni Q, Jia H, Gao H, Yang F, Zhu H, Liu F, Wang J, Zhou X, Chang H and Lu J (2022) Prognostic analysis of radical resection for iCCAphl and iCCApps: A retrospective cohort study. Front. Oncol. 12:992606. doi: 10.3389/fonc.2022.992606
Received: 12 August 2022; Accepted: 24 October 2022;
Published: 21 November 2022.
Edited by:
Marco Rengo, Sapienza University of Rome, ItalyReviewed by:
Christian Cotsoglou, Ospedale di Vimercate - ASST Brianza, ItalyTommaso Stecca, ULSS2 Marca Trevigiana, Italy
Copyright © 2022 Yu, Ni, Jia, Gao, Yang, Zhu, Liu, Wang, Zhou, Chang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Lu, bHVqdW5Ac2RmbXUuZWR1LmNu; Hong Chang, Y2hhbmdob25nQHNkZm11LmVkdS5jbg==
†These authors share first authorship
 Zetao Yu1†
Zetao Yu1† Qingqiang Ni
Qingqiang Ni Hengjun Gao
Hengjun Gao Huaqiang Zhu
Huaqiang Zhu Jun Lu
Jun Lu