- 1Ganzhou People’s Hospital, Ganzhou, China
- 2Liaocheng People’s Hospital, Liaocheng, China
- 3Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Library, Shandong First Medical University and Shandong Academy of Medical Sciences, Tai’an, China
Objective: To evaluate the safety and effectiveness of Iodine-125 (125I) brachytherapy combined with pre-operative transarterial chemoembolization in patients with locally advanced head and neck cancer.
Methods: In this study, a total of thirty-seven individuals suffering from locally advanced head and neck cancer were involved. The patients were subjected to transarterial chemoembolization as well as implantation of 125I seeds under the guidance of CT and ultrasonography. Follow-up was conducted for 36 months to study the following parameters: the local control rate, survival rate, and clinical complications.
Results: In total, thirty-six patients at the end of three months showed an objective response rate of 69.8% and disease control rate of 93.0%, respectively. The 1, 2, and 3-year cumulative overall survival rate was 89.2%, 73.0%, and 45.9%, respectively. The adverse events of the treatment included infection (n=1, Grade III), radiation brachial plexus injury (n=1, Grade III), leukopenia (n=1, Grade III), cerebrovascular embolism (n=1, Grade IV).
Conclusion: The combination of 125I brachytherapy and pre-operative transarterial chemoembolization was safe and effective in patients with locally advanced head and neck cancer.
Introduction
The current global health statistics list head and neck cancer (including lip, oral cavity, larynx, nasopharynx, oropharynx, hypopharynx, salivary glands, and thyroid) as the seventh highest prevailing cancer with an affected population of 1518,000 cases and a death number of 510,000 per year around the globe (1). The status of malignancies of the head and neck as a locally advanced disease makes them difficult to treat requiring a multidisciplinary approach that incorporates surgery, radiotherapy, and systematic therapy (2). Currently, local recurrence is still the main cause of failure after surgery and radiotherapy, and there is no ideal method for salvage treatment after recurrence (3). It is necessary to explore non-surgical treatment methods to preserve organ functions in order to live a full and healthy life because the head and neck are necessary for speech and other important functions.
The minimally invasive nature of brachytherapy offers superior dosimetric advantages over conventional external beam techniques as it focuses the irradiation on the target site leading to much more precise dosimetric calculations as well as protecting the nearby tissues from radiation due to a sharp dose fall-off when the distance from the radiation source increases (4). Brachytherapy is one of the treatments recommended by the American Brachytherapy Society and the Head and Neck Working Group of the European Brachytherapy Group for head and neck cancers (5, 6). Evidence from previous studies shows that locally advanced head and neck cancer can be treated safely and effectively by the implantation of Iodine-125 (125I) radioactive seeds, a key element of brachytherapy, as a form of palliative salvage therapy (7–9). The long-term function of organs and cosmetic outcomes of the treatment is usually excellent. A meta-analysis has reported brachytherapy as a boost provides good clinical efficacy in terms of progression-free survival as compared to external beam radiation therapy (EBRT) in prostate cancer (10). A comparative study of high-dose-rate brachytherapy with volumetric modulated arc therapy in head and neck cancer showed comparable target coverage with a lower dose to normal tissues with brachytherapy (11).
Tumor hemorrhage is an important complication of cancer treatment, particularly in advanced head and neck cancer owing to spontaneous bleeding, iatrogenic vessel injury, pseudoaneurysm rupture or treatment effects (12). As head and neck malignancies may be located in deep tissues, and identification of their accurate position is extremely necessary for treatment. Therefore, traditional hemostatic methods, such as local compression, nasal packing, bandaging and suture, and drug hemostasis, are often ineffective. The increase in blood loss may lead to asphyxia and shock, which is very difficult to deal with clinically (12, 13). An alternative is presented by endovascular approaches with selective arterial embolization, which has a minimal impact on surrounding tissues. Transarterial embolization is performed by catheter insertion and has been reported to improve clinical outcomes significantly in head and neck cancer (14). Interventional chemotherapy with embolization has been used as adjuvant or palliative treatment, and although it cannot significantly increase the local drug concentration in tumors or reduce adverse reactions, it can block the arterial blood supply, promote tumor necrosis and shrinkage, as well as improve the curative effect, thereby improving prognosis (14, 15). In addition, interventional embolization before particle implantation can prevent acute bleeding during puncture (16). This study used arterial chemoembolization combined with particle implantation for locally advanced head and neck tumors four years ago. The outcomes of this combination therapy were retrospectively reported in this paper, together with an assessment of its effectiveness and any associated side effects.
Patients and methods
Patients
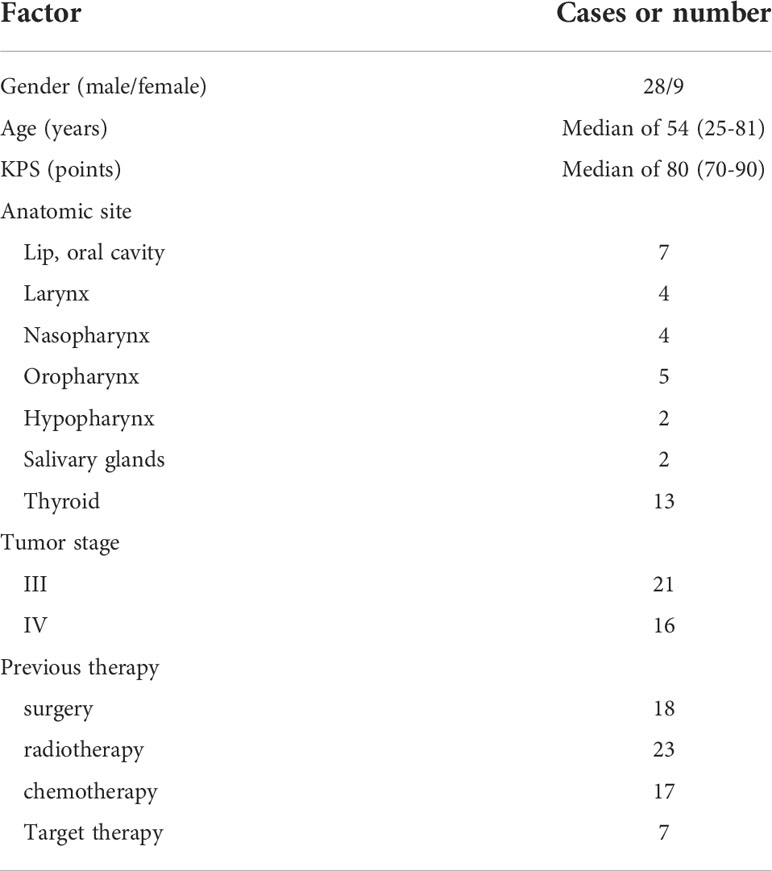
This retrospective investigation spanned from February 2018 to April 2022 and involved two hospitals covering thirty-seven individuals suffering from a malignant tumor of the head and neck. The main center provided most of the cases and only three cases were provided by the other hospital. The study protocol was approved by the Institutional ethical committee and all study procedures were consistent with the declaration of Helsinki. Table 1 shows all the general information about the participants. The following were the criteria for the selection of cases in this study (1): patients in the age range of eighteen to eighty-five years with Karnovsky Performance Status (KPS) > 70 (2), the presence of head and neck squamous carcinoma pathologically diagnosed as grade III or grade IV with a lesion diameter< 7 cm and no metastasis (3), a possible needles pathway detected through the CT scan capable of being used for 125I brachytherapy-based implantation (4), an anticipated survival rate of more than 3 months (5), the rejection or ineligibility for external beam radiotherapy or salvage surgery, which may occur due to disease extent or systemic status. The exclusion criteria were as follows (1): acute organ failure (2), coagulation dysfunction (3), active infection (4), mental disorder, and (5) extensive necrosis/ulcer formation/skin rupture. An informed consent form for brachytherapy and interventional therapy had been signed by each patient.
Transarterial chemoembolization
Seldinger technique was used to select an appropriate angiography catheter according to the vascular course, and a microcatheter was added for some patients. Selective arterial intubation was performed on different lesion sites, and drugs were injected after angiography to ensure no “dangerous anastomosis” and arteriovenous fistula. The chemotherapy regimen was docetaxel 60mg/m(2) with loplatin 30mg/m(2). The above drugs were diluted to 100 ml with normal saline and then slowly injected, respectively. After the infusion, embolization was performed with 300-500 um polyvinyl alcohol (PVA) particles.
Iodine-125 brachytherapy
Before treatment, a spiral CT scan was performed, and tumor level data were input into a computer 3-D treatment planning system (TPS) to outline the tumor and the tumor target volume was simulated. The prescribed dose was set at 150Gy. The general principle of dose optimization was to achieve a clinical target volume (CTV) reaching prescription dose (PD) as far as possible while ensuring as low as possible exposure dose to normal tissues, and as few needles as possible. The specific limitations included (17) (1): determining the puncture trajectory to avoid vascularity, ventricles, and important structures (2). CTV100 (the CTV covered by more than 100% of PD) should be greater than 90% of the CTV (3). Organs at risk (OARs) were more than 1 cm away from the 100% PD isodose line. In this process, the radio-oncologist needs to adjust the needles and seeds repeatedly to meet those requirements. Dose-volume histogram (DVH) and real-time isodose contours were utilized for optimization. Patients were instructed to fast two hours before the operation and were given sedatives and lidocaine for local anesthesia before the operation, and their vital signs were monitored during the operation. Under the guidance of CT and B-ultrasound, the seed source was implanted into the tumor according to TPS. After the operation, the implant needle was pulled out and bandaged. Antibiotics and hemostatic drugs were routinely used three days after the operation to prevent postoperative infection and bleeding.
Follow-up
The participants underwent a routine follow-up after a span of three months in the first year following 125I brachytherapy treatment, and 6 months in the later years. The patients underwent head and neck CT and enhanced MR, chest and abdomen multiphase CT, and laboratory examinations during each follow-up visit. The images obtained in the first and third months after 125I brachytherapy were utilized to analyze the response of the tumor to the treatment. The CT scan at the first follow-up was used for postoperative dose verification on the TPS and could be supplemented with seed implantation therapy when the dose was unevenly distributed and insufficient.
Evaluations
The revised RECIST guideline (version: 1.1) was used as a reference to analyze the response of the tumor to the treatment in different aspects such as partial response (PR), complete response (CR), disease progression (PD), and stable disease (SD) at the first and second follow-up (18). The arterial phase enhancement lesion was detected using MRI and the selected lesion was identified as the target. This information was used to compute the objective response rate (ORR) and disease control rate (DCR) as follows:
The parameters, such as progression-free survival (PFS), the time between the initiation of treatment to the progress of the disease or death or even the time that the follow-up ends, and overall survival (OS), the duration of time survived from the initiation of treatment to either death or even the end of follow-up, were measured. The Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) were used to perform toxicity scoring of the radiation with reference to their toxicity criteria (19). The Common Terminology Criteria for Adverse Events v5.0 was utilized for analyzing the procedure-related side effects and complications associated with chemoembolization (20).
Statistical analysis
SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analysis of the data in the Windows operating system. The tumor response was calculated in percentage, whereas the Kaplan-Meier model was utilized to measure the survival rate. The number of different adverse events associated with the procedure was analyzed.
Results
Short-term efficacy
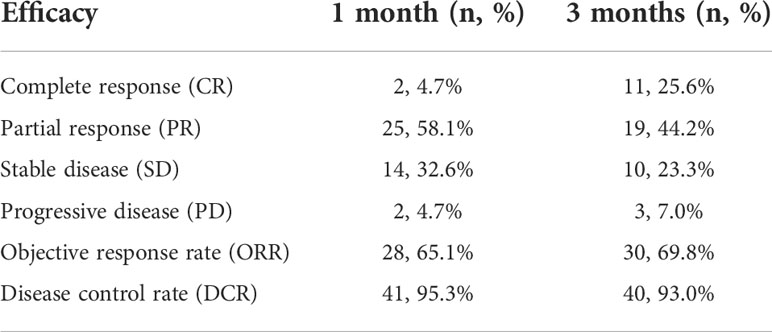
The locally assessable lesions in forty-three cases were treated with the help of a multidisciplinary approach combining arterial chemoembolization with the implantation of 125I seeds. Thirty-five lesions were successfully implanted at one time in line with TPS requirements, whereas eight lesions failed to meet the TPS requirements, so seeds were re-implanted one month after the first operation. The range of 125I seeds implanted in a single lesion was 9 ~ 45, with an average of 26 seeds. Local control rates were 95.3% and 93.0% at one and three months after treatment, respectively (Table 2).
Long-term efficacy
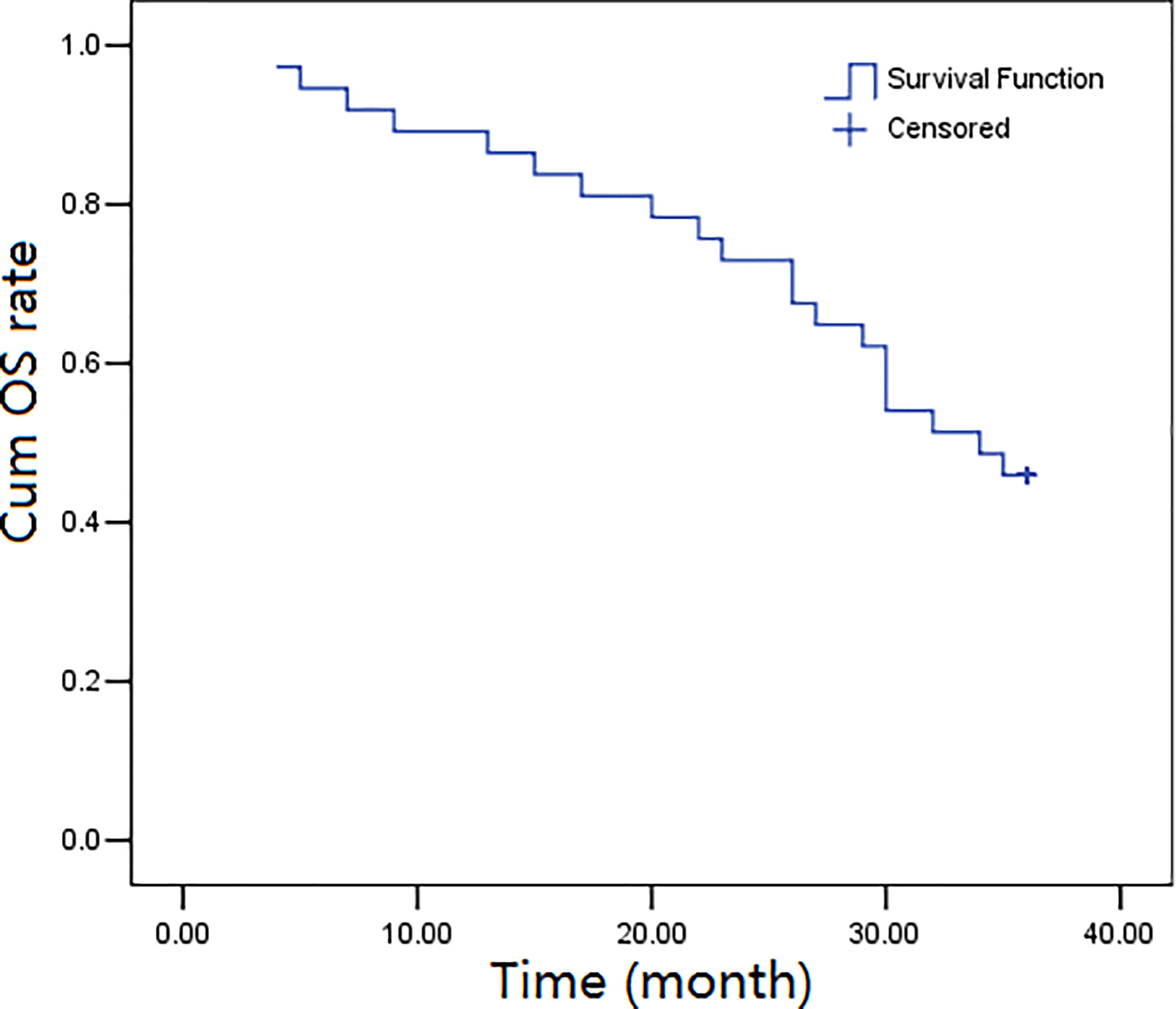
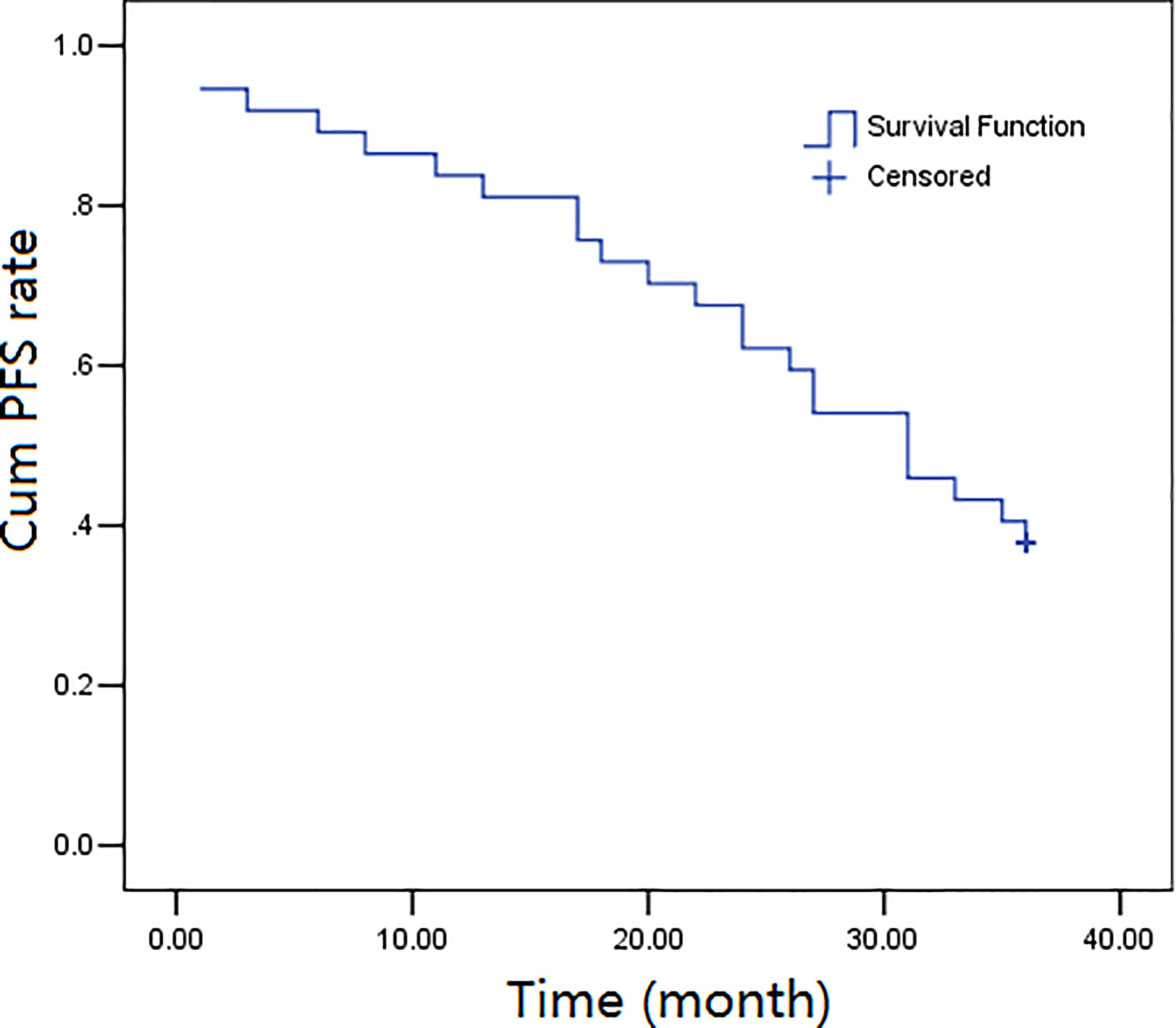
The three-year follow-up (52 months) was completed by all the thirty-seven participants, and the overall survival rates over 1, 2, and 3 years were calculated to be 89.2%, 73.0%, and 45.9%, respectively. The progression-free survival rates were calculated as 83.8%, 62.2%, and 37.8%, over 1, 2, and, 3 years, respectively. Figures 1, 2 depict the OS and PFS survival data.
Adverse events
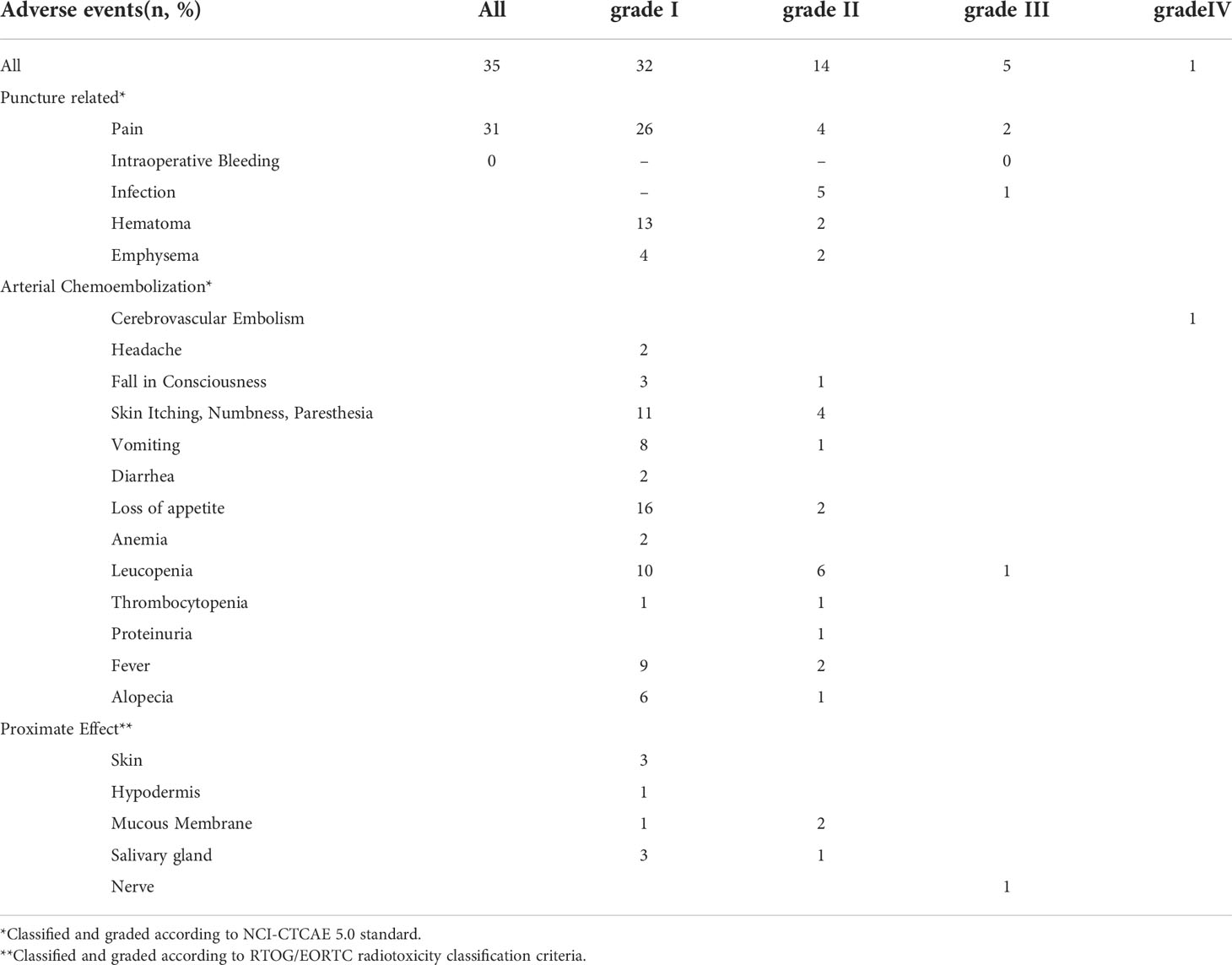
Adverse events occurred in thirty-five patients (94.6%), with grade 3 adverse events in four patients (10.8%), including one case each of puncture point pain, infection, puncture point pain with radioactive brachial plexus injury, and leukopenia. One patient (2.7%) had a grade 4 adverse event, namely cerebral artery embolism, but no grade 5 adverse events were detected (Table 3). Grade 3 and above adverse events were cured after symptomatic treatment, and treatment was discontinued without adverse reactions.
Discussion
This research examined the effectiveness and adverse events of treating locally advanced head and neck malignancies using seed implantation in combination with arterial chemoembolization. The thirty-seven patients showed survival rates of 89.2%, 73.0%, and 45.9%, over 1, 2, and 3 years respectively with few serious complications. Adverse events of a higher grade occurred infrequently, with the incidence of grade 3 or higher events calculated to be 16.2%, and only one case of grade four event occurred. There was no serious intraoperative bleeding, which greatly improved the safety of the operation. This was one of the benefits of utilizing I-125 brachytherapy and chemoembolization.
The energy of radioactive seeds is low, and the dose reduces rapidly with distance, which makes it easy to confine the high-dose area to the target lesion, thereby enhancing the anti-tumor effect and minimizing the effects on normal tissues (21). Theoretically, radioactive seed implantation is appropriate for a solitary solid lesion. Since the blood supply arteries for head and neck tumors are relatively fixed, selective intubation can be easily put in place. Interventional local area chemotherapy can enable targeted drug delivery in large doses and high concentrations to lesions, greatly reducing the binding rate of drugs and plasma proteins, and considerably increasing the drug concentration of the lesion. It can suppress tumor cell proliferation, reduce tumor necrosis, cause apoptosis in a significant number of tumor cells and reduce the toxicity and side effects of systemic chemotherapy (22). The embolization of the supplying artery with a gelatin sponge can cause ischemic necrosis and shrinkage in the tumor (23, 24). At the same time, artery embolization before operation can reduce the bleeding caused by injury to the artery during puncture, and reduces the risk of operation.
Brachytherapy is a technique that is considered advantageous for most lip cancers as this kind of therapy helps to retain the salivary glands and other muscular functions (25). Furthermore, it is beneficial to treat most lip cancers (>90%) using brachytherapy as it offers treatment at all stages with a 90% to 95% local control rate as well as providing function retention of the organs and reducing the amount of scarring as a result of the operation. Brachytherapy is an advantageous technique for post-operative treatments in cases of tongue carcinoma when the organ to be treated shows signs of relapse due to risk factors such as perineural involvement, lymphovascular space invasion, and close margins. In such cases, external radiotherapy could cause more damage (xerostomia) and brachytherapy is a much more viable solution (26, 27). The implementation of the correct procedure of implantation of seeds leads to a significantly low and acceptable risk of developing serious complications (soft-tissue or bone necrosis) (<5%) (4).
Despite its numerous advantages brachytherapy is still difficult to attain complete success in the head and neck region as these two regions contain or are adjacent to many important organs (e.g. bones, sinuses, eyes, and major vessels), which may complicate the seed implantation process and lead to a dangerous situation. The practical implementation of the procedure is also difficult to achieve as the clinician’s errors in seed placement may cause more harm. Therefore, it is important to design a detailed preplan to minimize radioactive- and needle-associated damage to normal cells as well as perfectly implement the virtual plan in practice. Although techniques such as ultrasound, CT, MRI, and navigation have helped with reducing human error by guiding needle insertion and seed placement (28, 29), problems still occur. The human error and the limitations of the guiding technologies can be overcome by applying 3D printing templates (30, 31).
The timing of non-platinum-based chemotherapy and radiotherapy was examined in a study by UKHAN1 (32) to determine its impact on the effectiveness of the treatment. The study sought to ascertain whether the clinical results were affected by administering chemotherapy simultaneously with radiotherapy or as maintenance therapy, or both. The results depicted the advantage of two courses of concurrent non-platinum chemoradiotherapy treatment according to the reduced recurrence and mortality rate in patients who had not undergone previous surgery. Even after a long follow-up, there was no increase in the mortality and recurrence rate. From this study, it can be inferred that it may be best to perform arterial chemotherapy at the same time as the implantation of radioactive particles. In addition, a large-scale meta-analysis of randomized clinical trial and individual patient data comparing 16 different modalities for treatment of advanced head and neck cancer found that hyperfractionated radiotherapy with concomitant chemotherapy showed the best hazard ratio of 0.63. It is important to have randomized controlled trials of the present treatment modality to inform its comparative outcomes versus alternative clinical regimes (33). The present study is an observational study that has shown good safety and efficacy of I-125 brachytherapy combined with pre-operative transarterial chemoembolization. These data highlight the need for clinical trials in this domain.
The blood supplying arteries of tumors are generally abundant, and bleeding occurs easily when the tumor is punctured. Tumor hemorrhage can be complicated easily with infection, which not only increases the pain and economic burden of patients, but also spreads to the whole body and may lead to the death of patients with septic shock. Injury of large vessels causes large and rapid bleeding, leading to hemorrhagic shock and death. Heavy bleeding from the head and neck could lead to death by asphyxiation. Preoperative arterial embolization effectively prevented the occurrence of this situation (34). There were no bleeding events requiring medical intervention in this study.
Previous studies have shown that the incidence of short-term and long-term complications of head and neck tumor surgery with seed implantation is low (35, 36). The majority of adverse events associated with particle implantation therapy in this study were mild, only one patient progressed from preoperative grade II to III during the follow-up, and the patient had preoperative tumor invasion of brachial plexus accompanied by paresthesia and weak lifting power (grade 4 muscle strength). Three months after the operation, the tumor almost disappeared, but the neurological symptoms were aggravated, the paresthesia was more obvious than before, and the weakness was aggravated (muscle strength grade 3) while the nerve injury was upgraded from preoperative grade 2 to grade 3 (CTC 4.0 classification). However, the possibility of irreversible nerve injury before surgery and gradual aggravation after surgery due to tumor invasion cannot be completely ruled out.
In this study, the greatest risk of interventional surgery was cerebral infarction caused by internal carotid or vertebrobasilar artery system embolism. The only case of Grade 4 adverse event occurred due to this. This patient was a young woman, diagnosed with poorly differentiated nasopharyngeal squamous cell carcinoma. The nasopharyngeal tumor was uncontrolled after 3 months of radiotherapy so she was treated with I-125 brachytherapy by preoperative chemoembolization. After the operation, she was diagnosed with left eyeball abduction disorder, left central facial paralysis, decreased grip strength of left upper limb, lucidity, lethargy, and left limb paralysis and coma occurred the next day. The blood supply disturbance of the right middle cerebral artery was clinically diagnosed as caused by arterial embolism during interventional therapy. After dehydration, thrombolysis, vascular dilation, hormone therapy, microcirculation improvement, and intracranial pressure reduction, the patient returned to normal one week after the operation. To avoid this situation, angiography should be done before the injection of the embolic agent to make sure that there is no “dangerous anastomosis” and vasospasm before embolization. The embolization should be carried out after the super selection of target vessels, and complete embolization should not be forced. If blood flow is significantly slowed, the operation should be stopped. During embolization, the embolization should be strictly prevented from flowing back into the cervical or vertebrobasilar artery system (37, 38). In addition, pre and post-procedure oral hygiene management for prevention of complications is essential in brachytherapy for head and neck cancers (39). Furthermore, greater research regarding high-dose brachytherapy (HDR-BRT) is essential as increasing evidence shows its good clinical performance when used alone or as boost therapy (40). Moreover, as brachytherapy can be used as a definitive modality, boost modality and as salvage therapy, the clinical efficacy of the current protocol in these different investigations should be examined (41).
Conclusion
In conclusion, 125I brachytherapy combined with preoperative arterial chemoembolization is a safe and feasible treatment for patients with locally advanced head and neck tumors. These patients achieved good rates of local control and overall survival with fewer serious adverse events. However, prospective randomized controlled trials are still needed to confirm the conclusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The project was approved by the Ethics Committee of Ganzhou People’s Hospital, Ganzhou 341000, Jiangxi, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MZ conceptualized the research idea, carried out data analysis, led the paper writing, supervised the project, and edited the manuscript. JZ and BH performed the data analysis. LH, SS, HZ, CChe, and CChu were responsible for study design and data collection. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Science and Technology Program of Jiangxi Provincial Health and Welfare Committee (Grant No.: 202212514), Key Research and Development Program of Ganzhou City Science and Technology Bureau (Grant No.: 2020-275), Doctoral Research Initiation Project of Ganzhou People’s Hospital (Grant No.: Bsqd2020002), and Shandong Province Social Science Program Research Project (Grant No.: 20CTQJ07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. (2021) 398(10318):2289–99. doi: 10.1016/S0140-6736(21)01550-6
3. Baliga S, Kabarriti R, Ohri N, Haynes-Lewis H, Yaparpalvi R, Kalnicki S, et al. Stereotactic body radiotherapy for recurrent head and neck cancer: A critical review. Head Neck. (2017) 39(3):595–601. doi: 10.1002/hed.24633
4. Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guerin F, et al. Brachytherapy: An overview for clinicians. CA Cancer J Clin (2019) 69(5):386–401. doi: 10.3322/caac.21578
5. Kovacs G, Martinez-Monge R, Budrukkar A, Guinot GL, Johansson B, Strnad V, et al. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update - improvement by cross-sectional imaging-based treatment planning and stepping source technology. Radiother Oncol (2017) 122(2):248–54. doi: 10.1016/j.radonc.2016.10.008
6. Takacsi-Nagy Z, Martinez-Mongue R, Mazeron JJ, Anker CJ, Harrison LB. American Brachytherapy society task group report: Combined external beam irradiation and interstitial brachytherapy for the base of tongue tumors and other head and neck sites in the era of new technologies. Brachytherapy. (2017) 16(1):44–58. doi: 10.1016/j.brachy.2016.07.005
7. Yu LJ, Na M, Wang JJ, Jiang P, Yuan HSh, Liu C, et al. CT-guided iodine-125 seed permanent implantation for recurrent head and neck cancers. Radiat Oncol (2010) 5(1):68. doi: 10.1186/1748-717X-5-68
8. Ji Z, Jiang Y, Tian S, Guo F, Peng R, Xu F, et al. The effectiveness and prognostic factors of CT-guided radioactive I-125 seed implantation for the treatment of recurrent head and neck cancer after external beam radiation therapy. Int J Radiat Oncol Biol Phys (2019) 103(3):638–45. doi: 10.1016/j.ijrobp.2018.10.034
9. Chen Y, Jiang Y, Ji Z, Jiang P, Xu F, Zhang Y, et al. Efficacy and safety of CT-guided (125)I seed implantation as a salvage treatment for locally recurrent head and neck soft tissue sarcoma after surgery and external beam radiotherapy: A 12-year study at a single institution. Brachytherapy. (2020) 19(1):81–9. doi: 10.1016/j.brachy.2019.09.006
10. Kee DL, Gal J, Falk AT, Schiappa R, Chand ME, Gautier M, et al. Brachytherapy versus external beam radiotherapy boost for prostate cancer: Systematic review with meta-analysis of randomized trials. Cancer Treat Rev (2018) 70:265–71. doi: 10.1016/j.ctrv.2018.10.004
11. Akiyama H, Pesznyák C, Béla D, Ferenczi Ö, Major T, Polgár C, et al. Image guided high-dose-rate brachytherapy versus volumetric modulated arc therapy for head and neck cancer: A comparative analysis of dosimetry for target volume and organs at risk. Radiol Oncol (2018) 52(4):461–7. doi: 10.2478/raon-2018-0042
12. Chen YF, Lo YC, Lin WC, Lin CH, Chiang HJ, Chen JF, et al. Transarterial embolization for control of bleeding in patients with head and neck cancer. Otolaryngology–Head Neck Surgery. (2010) 142(1):90–4. doi: 10.1016/j.otohns.2009.09.031
13. Chatani S, Sato Y, Murata S, Hasegawa T, Tsukii R, Nagasawa K, et al. Transarterial embolization for bleeding in patients with head and neck cancer: who benefits? Laryngoscope (2021) 131(11):E2777–83. doi: 10.1002/lary.29611
14. Hong WK, Bromer R. Chemotherapy in head and neck cancer. N Engl J Med (1983) 308(2):75–9. doi: 10.1056/NEJM198301133080204
15. Bi Y, Shi X, Zhang W, Lu L, Han X, Ren J, et al. Feasibility of drug-eluting embolics chemoembolization for the management of recurrent/advanced head and neck cancer. J Vasc Interv Radiol (2022) 33(8):949–55. doi: 10.1016/j.jvir.2022.05.002
16. Gupta AK, Purkayastha S, Bodhey NK, Kapilamoorthy TR, Kesavadas C. Preoperative embolization of hypervascular head and neck tumors. Australas Radiol (2007) 51(5):446–52. doi: 10.1111/j.1440-1673.2007.01869.x
17. Zhang M, Chu C, Huang L, et al. CT-MR image fusion for post-implant dosimetry analysis in brain tumor seed implantation - a preliminary study. Dis Markers (2022) 2022:6310262. doi: 10.1155/2022/6310262
18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 11). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
19. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C
20. National Cancer Institute. Common terminology criteria for adverse events (CTCAE). U.S. Department OF Health and Human Services (2017) Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
21. Mobit P, Badragan I. An evaluation of the AAPM-TG43 dosimetry protocol for I-125 brachytherapy seed. Phys Med Biol (2004) 49:3161–70. doi: 10.1088/0031-9155/49/14/010
22. Aigner KR, Selak E, Aigner K. Short-term intra-arterial infusion chemotherapy for head and neck cancer patients maintaining quality of life. J Cancer Res Clin Oncol (2019) 145(1):261–8. doi: 10.1007/s00432-018-2784-4
23. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. NCCN clinical practice guidelines in oncology head and neck cancers. J Natl Compr Canc Netw (2020) 18(7):873–98. doi: 10.6004/jnccn.2020.0031..
24. Nibu K-I, Hayashi R, Asakage T, Ojiri H, Kimata Y, Kodaira , et al. Japanese Clinical practice guidelines for head and neck cancer. Auris Nasus Larynx (2017) 44(4):375–80. . doi: 10.1016/j.anl.2017.02.004
25. Kovacs G, Martinez–Monge R, Budrukkar A, Guinot JL, Johansson B, Strnad V, et al. GECESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update - improvement by cross-sectional imaging-based treatment planning and stepping source technology. Radiother Oncol (2017) 122(2):248254. doi: 10.1016/j.radonc.2016.10.008
26. Chargari C, Van Limbergen E, Mahantshetty U, Deutsch E, Haie-Meder C. Radiobiology of brachytherapy: The historical view based on linear quadratic model and perspectives for optimization. Cancer Radiother. (2018) 22:312–8. doi: 10.1016/j.canrad.2017.11.011
27. Petereit DG, Frank SJ, Viswanathan AN, Erickson B, Eifel P, Nguyen PL, et al. Brachytherapy: Where has it gone? J Clin Oncol (2015) 33(9):980–2. doi: 10.1200/JCO.2014.59.8128
28. Dolezel M, Odrazka K, Zizka J, Vanasek J, Kohlova T, Kroulik , et al. MRI-Based preplanning using CT and MRI data fusion in patients with cervical cancer treated with 3D-based brachytherapy: Feasibility and accuracy study. Int J Radiat Oncol Biol Phys (2012) 84(1):146–52. doi: 10.1016/j.ijrobp.2011.11.003
29. Krempien R, Hoppe H, Kahrs L, Daeuber S, Schorr O, Eggers G, et al. Projector-based augmented reality for intuitive intraoperative guidance in image-guided 3D interstitial brachytherapy. Int J Radiat Oncol Biol Phys (2008) 70(3):944–52. doi: 10.1016/j.ijrobp.2007.10.048
30. Huang M, Zhang J, Zheng L, Zheng L, Liu SM, Yu GY., et al. Accuracy evaluation of a 3D-printed individual template for needle guidance in head and neck brachytherapy. J Radiat Res (2016) 57(6):662–7. doi: 10.1093/jrr/rrw033
31. Qiu B, Jiang Y, Ji Z, Sun H, Fan J, Li W, et al. The accuracy of individualized 3D-printing template-assisted I125 radioactive seed implantation for recurrent/metastatic head and neck cancer. Front Oncol (2021) 11:664996. doi: 10.3389/fonc.2021.664996
32. Tobias JS, Monson K, Gupta N, Macdougall H, Glaholm J, Hutchison , et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK head and neck (UKHAN1) trial. Lancet Oncol (2009) 10(9):872–6. doi: 10.1016/S1470-2045(09)70306-7
33. Petit C, Lacas B, Pignon JP, Le QT, Grégoire V, Grau C, et al. Chemotherapy and radiotherapy in locally advanced head and neck cancer: An individual patient data network meta-analysis. Lancet Oncol (2021) 22:727–36. doi: 10.1016/S1470-2045(21)00076-0
34. Rangel-Castilla L, Shah AH, Klucznik RP, Diaz OM. Preoperative onyx embolization of hypervascular head, neck, and spinal tumors: Experience with 100 consecutive cases from a single tertiary center. J neurointerv Surg (2014) 6(1):51–6. doi: 10.1136/neurintsurg-2012-010542
35. Jiang Y, Ji Z, Guo F, Peng R, Sun H, Fan J, et al. Side effects of CT-guided implantation of 125I seeds for recurrent malignant tumors of the head and neck assisted by 3D printing non-co-planar template. Radiat Oncol (2018) 13(1):18. doi: 10.1186/s13014-018-0959-4
36. Jiang Y, Zhen P, Dai J, Li Y, Liu S, Xu J, et al. Long-term safety and efficacy of CT-guided I125 radioactive seed implantation as a salvage therapy for recurrent head and neck squamous carcinoma: A multicenter retrospective study. Front Oncol (2021) 11:645077. doi: 10.3389/fonc.2021.645077
37. De Marini P, Greget M, Boatta E, Jahn C, Enescu I, Garnon J, et al. Safety and technical efficacy of preoperative embolization of head and neck paragangliomas: A 10-year mono-centric experience and systematic review. Clin Imaging (2021) 80:292–9. doi: 10.1016/j.clinimag.2021.08.014
38. Duffis EJ, Gandhi CD, Prestigiacomo CJ, Abruzzo T, Albuquerque F, Bulsara KR, et al. Head, neck, and brain tumor embolization guidelines. J neurointerv Surg (2012) 4(4):251–5. doi: 10.1136/neurintsurg-2012-010350
39. Mallick S, Goura KR. Brachytherapy in head and neck cancers. In: In practical radiation oncology. Singapore: Springer (2020). p. 117–20.
40. Bhalavat R, Chandra M, Pareek V, Nellore L, George K, Nandakumar P, et al. High-dose-rate interstitial brachytherapy in head and neck cancer: do we need a look back into a forgotten art–a single institute experience. J Contemp Brachytherapy. (2017) 9(2):124–31. doi: 10.5114/jcb.2017.67147
Keywords: brachytherapy, chemoembolization, head and neck cancer, intervention, efficacy
Citation: Zhang M, Zhang J, Hu B, Huang L, Song S, Zhu H, Chen C and Chu C (2022) The efficacy and safety of 125I brachytherapy combined with pre-operative transarterial chemoembolization in patients with locally advanced head and neck cancer. Front. Oncol. 12:992399. doi: 10.3389/fonc.2022.992399
Received: 12 July 2022; Accepted: 23 August 2022;
Published: 14 September 2022.
Edited by:
Zhijie Xu, Xiangya Hospital, Central South University, ChinaReviewed by:
Min Wang, Beijing Hospital, Peking University, ChinaLisha Jiang, Sichuan University, China
Copyright © 2022 Zhang, Zhang, Hu, Huang, Song, Zhu, Chen and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Menglong Zhang, bWVuZ2xvbmd6aGFuZ0AxNjMuY29t
 Menglong Zhang
Menglong Zhang Jian Zhang1
Jian Zhang1