- 1Department of Urology, Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical College (University), Nanchong, China
- 2Department of Breast Surgery, Guizhou Provincial People’s Hospital, Guiyang, China
- 3Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 4Blood Purification Center of Department of Nephrology, Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical College (University), Nanchong, China
- 5Department of Clinical Laboratory, The People’s Hospital of Gao County, Yibin, China
Objective: To evaluate whether pretreatment albumin−globulin ratio (AGR) can be used as a biomarker for predicting the prognosis of patients with urothelial carcinoma (UC).
Methods: We systematically searched PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Google Scholar and Cochrane Library; the search time was up to May 2022. Stata 16.0 was used for data processing and statistical analysis.
Results: We identified 12 studies with 5,727 patients from 317 unique citations during the meta-analysis. Our results suggested that a low AGR before treatment was significantly associated with poor overall survival (OS) [hazard ratio (HR) = 1.99, 95% confidence interval (CI) = 1.45-2.75, P < 0.001], cancer-specific survival (CSS) [HR=2.01, 95% CI = 1.50-2.69, P < 0.001] and recurrence-free survival (RFS) [HR=1.39, 95% CI = 1.12-1.72, P = 0.002]. Furthermore, we defined different subgroups according to ethnicity, cancer type, cut-off value, sample size and stage. Similar prognostic outcomes for OS and CSS were observed in most subgroups. However, for subgroup of stage, the low pretreatment AGR only predicted the poor survival of patients with non-metastatic UC.
Conclusion: Our meta-analysis revealed that the AGR before treatment could be used as a predictive biomarker to indicate the prognosis of UC patients during clinical practice, especially in patients with non-metastatic UC.
Introduction
Urothelial carcinoma (UC) mainly originates in the bladder, renal pelvis, ureters, and urethra and is the fourth most frequently diagnosed cancer worldwide (1). It has been reported that 90% of UCs occur in the bladder, with bladder cancer (BC) being the most common UC (2). Moreover, according to a World Health Organization (WHO) report, there will be 573,278 new cases and 212,536 deaths BC-related deaths worldwide in 2020. Conversely, upper tract urothelial carcinoma (UTUC) is rare and only accounts for only 5%-10% of all UC patients with a poor prognosis (3). BC can be divided into three subtypes: non-muscle-invasive bladder cancer (NMIBC), muscle-invasive bladder cancer (MIBC), and metastatic BC. Nearly 75% of bladder cancers initially present with NMIBC. Intravesical chemotherapy or intravesical immunotherapy after transurethral resection is the standard treatment for NMIBC (4, 5). In contract, radical cystectomy (RC) with extended pelvic lymphadenectomy is the mainstay of treatment for patients with MIBC (6). However, BC, which has a severe recurrence and progression rate, and an estimated 5-year overall survival (OS) of only 10% to 40%, cannot be entirely overcome by definitive treatment (7). UTUC is characterized by a high degree of malignancy. Radical nephroureterectomy (RNU) with bladder cuffing is the standard treatment for high risk nonmetastatic UTUC patients, but 20%-30% of patients have distant metastasis and a poor survival rate of 5 years after surgery (8, 9). Hence, a valuable prognostic indicator should be identified to predict the survival and recurrence in patients with UC.
In addition to traditional prognostic factors such as tumor TNM stage, grade, lymphovascular invasion (LVI), and node classification, numerous clinical trials have found that smoking history, sex, symptoms and other clinical prognostic factors have also been used to evaluate the prognosis of UC patients (10–12). However, these clinical and pathological factors remain limited in improving outcome predictions for UC patients. Recently, several meta−analysis have identified that preoperative laboratory hematological biomarkers, including C-reactive protein (CRP), hemoglobin, lymphocyte-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), white blood cell count, and De Ritis ratio, may have prognostic value in patients with UC (13, 14).
Albumin and globulin are the main proteins in the serum and closely related to nutritional status and systemic inflammation in cancer patients (15). The albumin-to-globulin ratio (AGR) is a ratio that combines the two indexes (albumin and globulin). Recent studies have revealed that AGR is an independent prognostic factor for several cancers, such as multiple myeloma (16), non-small cell lung cancer (17), and colorectal cancer (18). Furthermore, low AGR is associated with worse survival. However, the use of AGR to evaluate the clinical prognosis of patients with UC remains controversial. Although the published literature shows that UC patients with low AGR have a poorer prognosis, Pradere et al. (19) discovered that pretreatment AGR is not associated with OS or recurrence-free survival (RFS). Accordingly, our study aimed to reveal the prognostic value of pretreatment AGR and to guide our clinical practice.
Materials and methods
Our study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (20).
Literature search
PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), Google Scholar, and Cochrane Library were used to search related published articles that assessed the prognostic value of AGR for UC patients. The time range of the literature search was up to May of 2022. All searches were limited to human studies and no language restrictions were applied.
The search terms were as follows: “urothelial carcinoma”, “urothelial cancer”, “bladder cancer”, “upper tract urothelial carcinoma”, “upper tract urothelial cancer”, “UTUC”, “prognosis”, “prognostic factors”, “survival”, “serum albumin”, “serum globulins”, “albumin to globulin ratio”, “albumin/globulin ratio” and “AGR” as Mesh term or keywords. The above search fields were randomly combined to achieve a complete search. Furthermore, two authors independently performed the searches, and a third author resolved any discrepancies.
Inclusion and exclusion criteria
The included researches should meet the following criteria: (1) patients pathologically diagnosed with UC (including bladder cancer or upper tract urothelial carcinoma); (2) Provision of an exact AGR cut-off value before receiving treatment; (3) hazard ratio (HR) and related 95% confidence intervals (CIs) were provided; (4) corresponding survival outcomes, such as overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), tumor-specific survival (TSS), progression-free survival (PFS), metastasis-free survival (MFS), had been reported; (5) studies with randomized controlled trials (RCTs), case-control studies, or cohort studies.
Studies were excluded based on the following criteria: review articles, repetitive reviews, letters, case reports, studies unrelated to the subject matter, duplicated studies based on the same patients, and studies with no detailed data.
Data extraction and quality assessment
Two researchers separately extracted the relevant data from the included literatures and resolved the disagreements through negotiation or by a senior author. The outcomes of interest for each included study were as follows: first author’s name, country, study design, sample size, intervention, age, cancer type, cut-off value for AGR, follow-up time, and outcome indicators. When univariate and multivariate analyses were conducted in a study, we extracted the multivariate analysis data for follow-up analysis. Based on preliminary search results, the Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included studies (21). According to the evaluation of the three question areas of selection, comparability, and exposure in the scale, a score of more than six stars was considered to indicate high-quality research.
Statistical analysis
In this study, Stata 16 (StataCorp LP, University City, Texas, USA) was used for the statistical analysis. Multivariate HR and corresponding 95% CI were extracted from each study, and data were synthesized to assess the prognostic value of AGR on UC patients survival. The heterogeneity between the included studies was verified using Cochranes Q test and I2 test. Regarding the heterogeneity test results, the random-effects model was used when heterogeneity was present (I2 ≥ 50% or p < 0.1). Otherwise, fixed-effects models were used for the analyses. I2 > 50% indicated significant heterogeneity between studies. We performed subgroup analyses based on ethnicity, cancer type, cutoff value, sample size and stage to evaluate the heterogeneity. Sensitivity analysis, involving the removal of each individual study, was also used to assess the robustness of our survival outcomes. Additionally, Begg’s test was performed to explore potential publication bias. value of P less than 0.05 was considered statistically significant.
Results
Description of studies and quality assessment
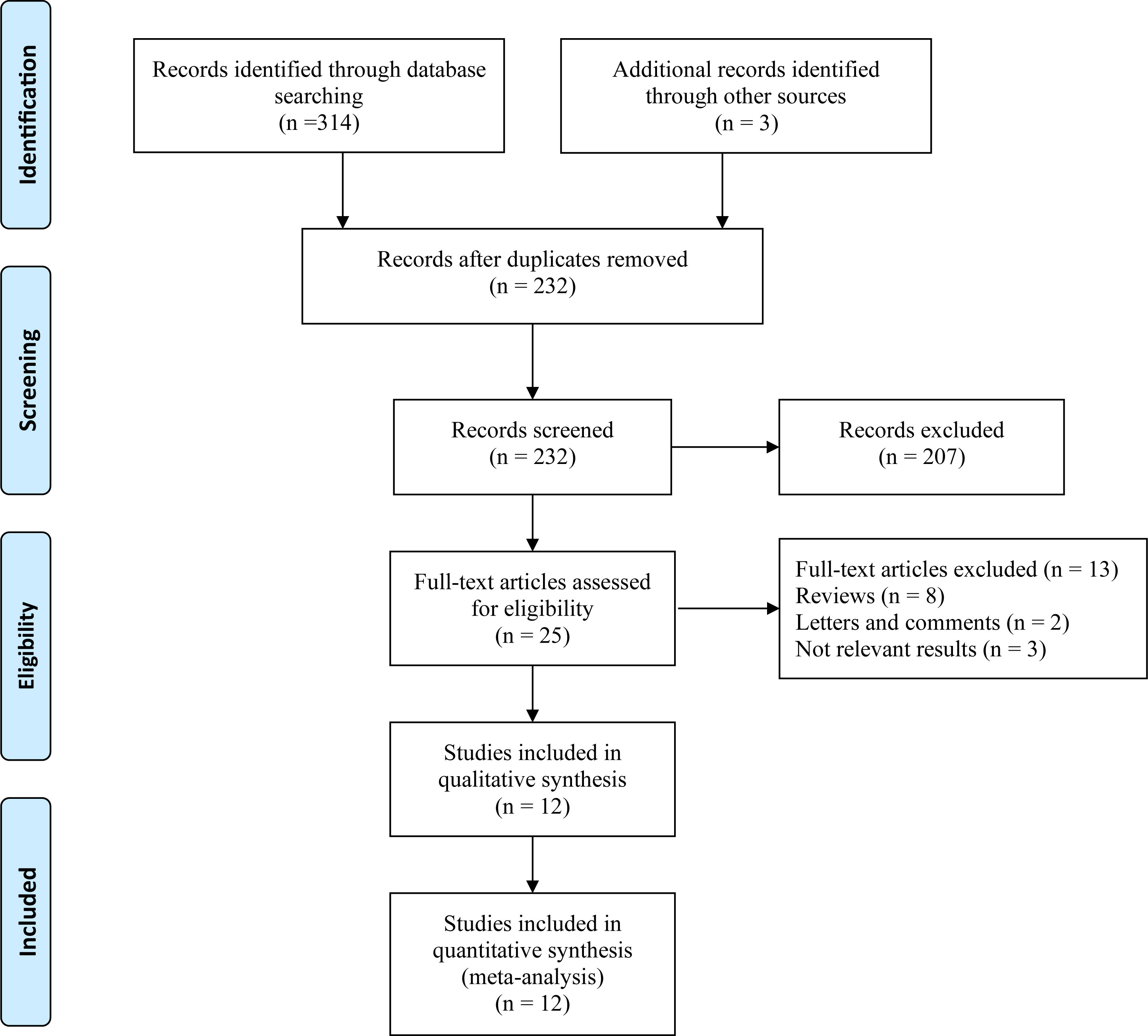
In total, 317 articles were retrieved from the databases and manually searched. After removing the relevant repeated studies, 232 studies remained. Subsequently, 207 articles were excluded from the analysis of research topics and abstracts. Full-text analysis was carried out for the remaining 25 eligible studies; relevant data could not be extracted from eight reviews, two letters and comments, and three articles. Finally, 12 articles with 5,727 patients were included in this study for further analysis (Figure 1).
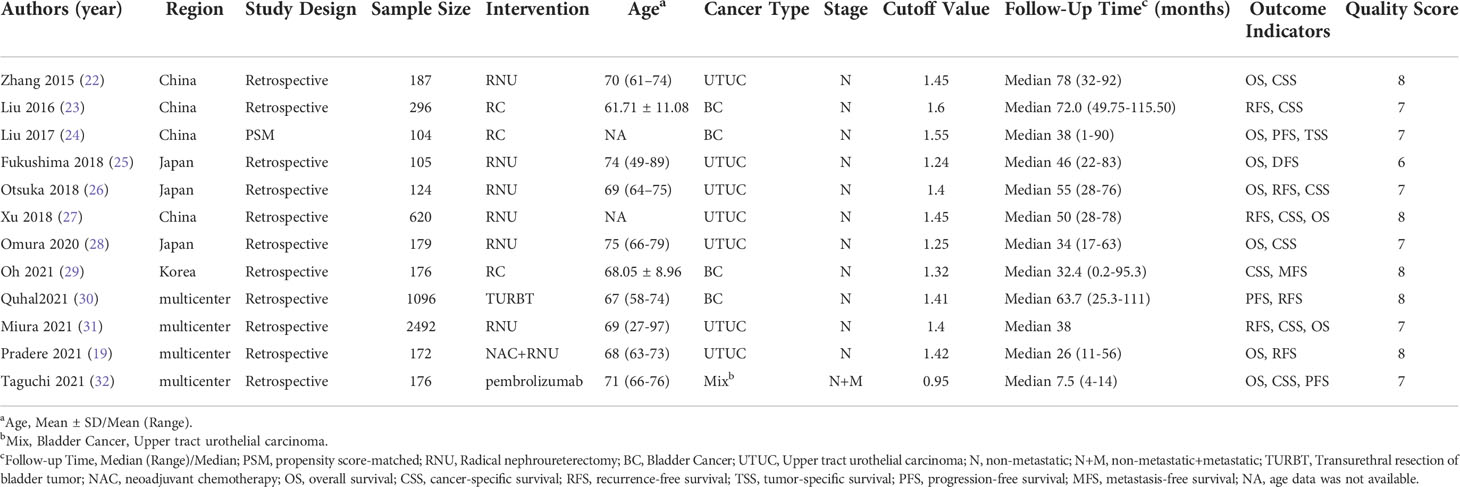
The baseline data are presented in Table 1. Eleven retrospective articles and one propensity-matched scoring study were included. All the studies have focused on bladder urothelial cancer and upper tract urothelial carcinoma. The included literatures were single-center or multicenter studies, which were published between 2015 and 2021. The cutoff values for the AGR ranged from 0.95 to 1.55. The mean follow-up time ranged from 7.5 to 78 months. Additionally, all included studies with a NOS score of 6 or higher were regarded as high-quality studies (Table 1).
Association of AGR with OS
Nine studies (19, 22, 24–28, 31, 32) reported an association between pretreatment AGR and OS in UC patients. Owing to the heterogeneity test outcome (I2 = 76%, p < 0.001), we used a random-effects model. Our meta-analysis revealed that compared with the high AGR group, the low AGR group had inferior OS, and the difference between the two groups was statistically significant [HR = 1.99, 95% CI (1.45-2.75), p < 0.001, Figure 2].
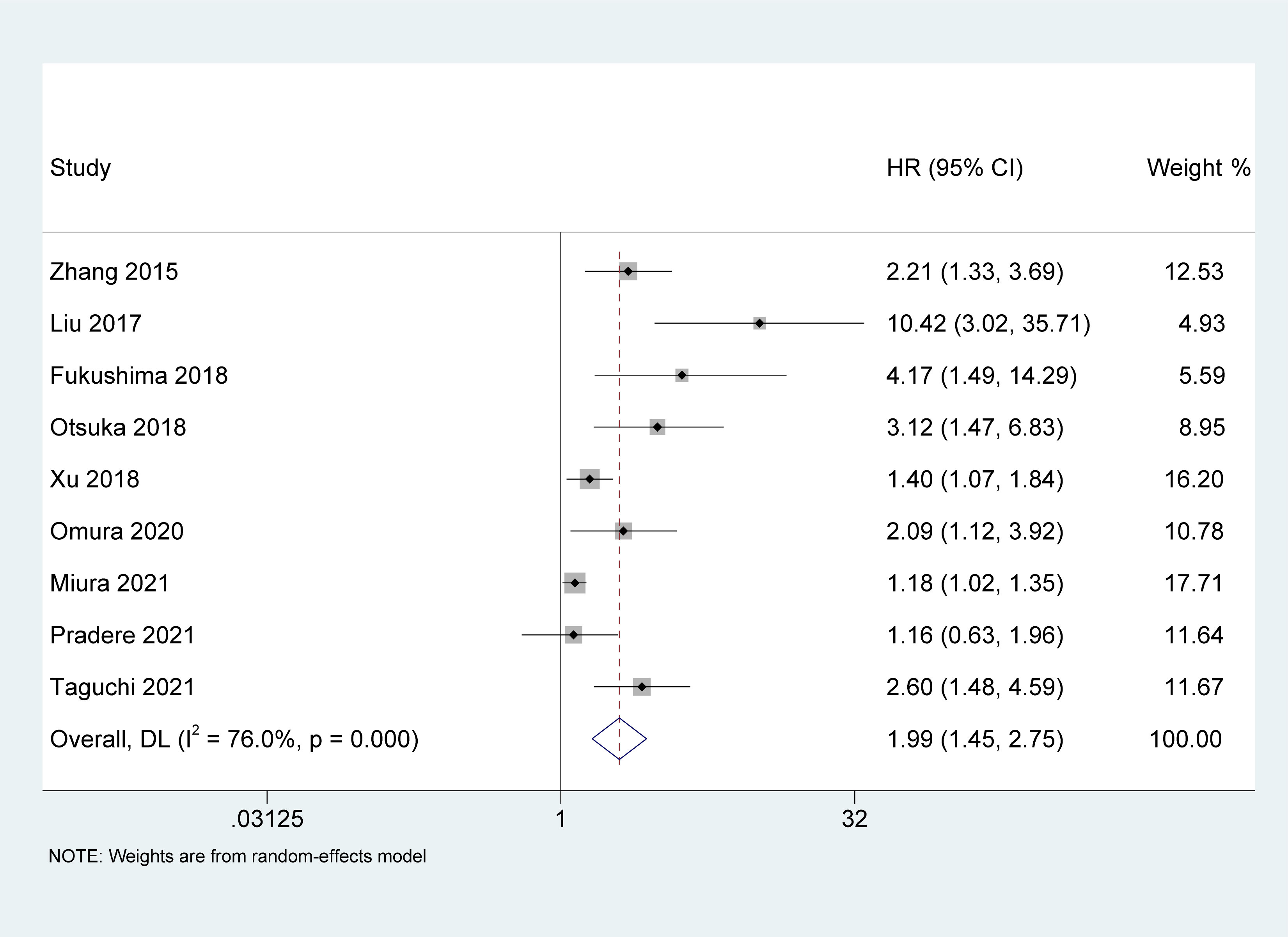
Figure 2 Forest plot and meta-analysis of the association between overall survival and albumin to globulin ratio.
Association of AGR with CSS
Study reports from eight studies (22, 23, 26–29, 31, 32), with 4,250 patients enrolled, indicated the prognostic value of AGR in patients with UC on CSS. The pooled results indicated that the lower pretreatment AGR was correlated with poorer CSS [HR = 2.01, 95% CI (1.50-2.69), P < 0.001, Figure 3], with a high heterogeneity (I2 = 61.7%, p = 0.011).
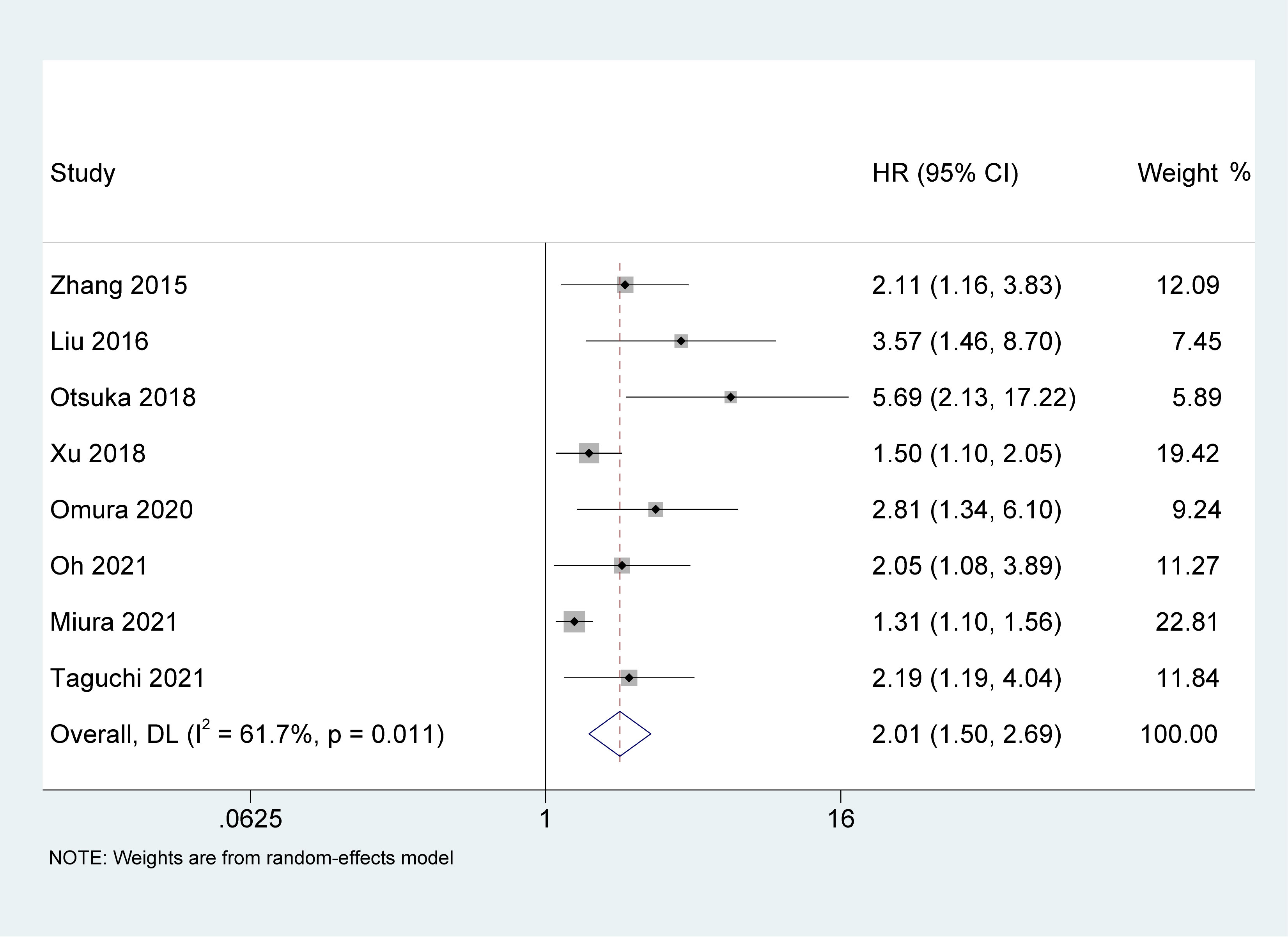
Figure 3 Forest plot and meta-analysis of the association between cancer-specific survival and albumin to globulin ratio.
Association of AGR with RFS
Six studies (19, 23, 26, 27, 30, 31) with 4,628 patients recorded the impact of pretreatment AGR on RFS. Because of the high heterogeneity between the studies (I2 = 61.2%, p = 0.024), a random-effects model was used. The results of the meta-analysis demonstrated that pretreatment AGR was an independent risk factor for poor RFS in patients with UC [HR = 1.39, 95% CI (1.12-1.72), P = 0.002, Figure 4].
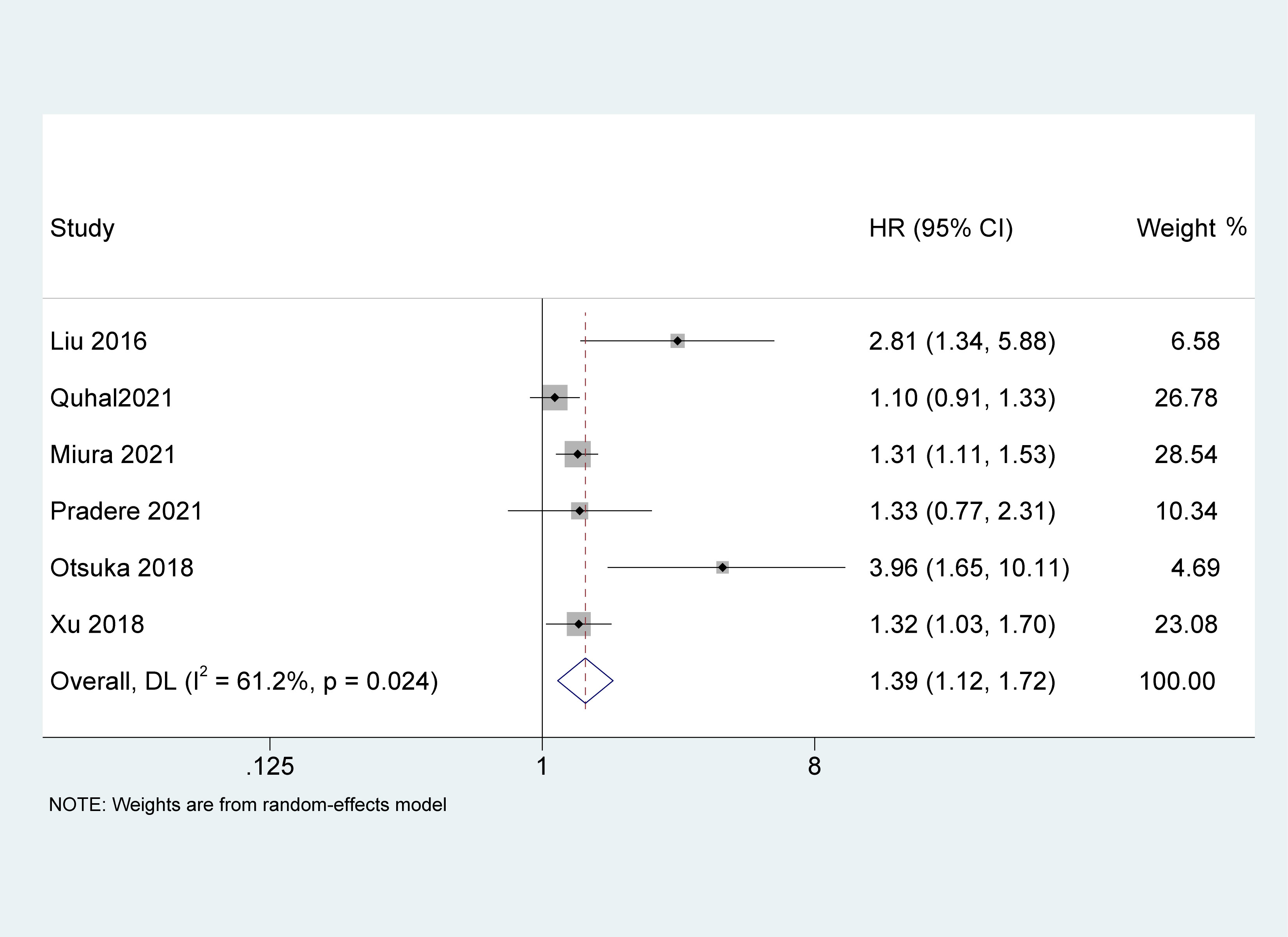
Figure 4 Forest plot and meta-analysis of the association between recurrence-free survival and albumin to globulin ratio.
Subgroup analysis
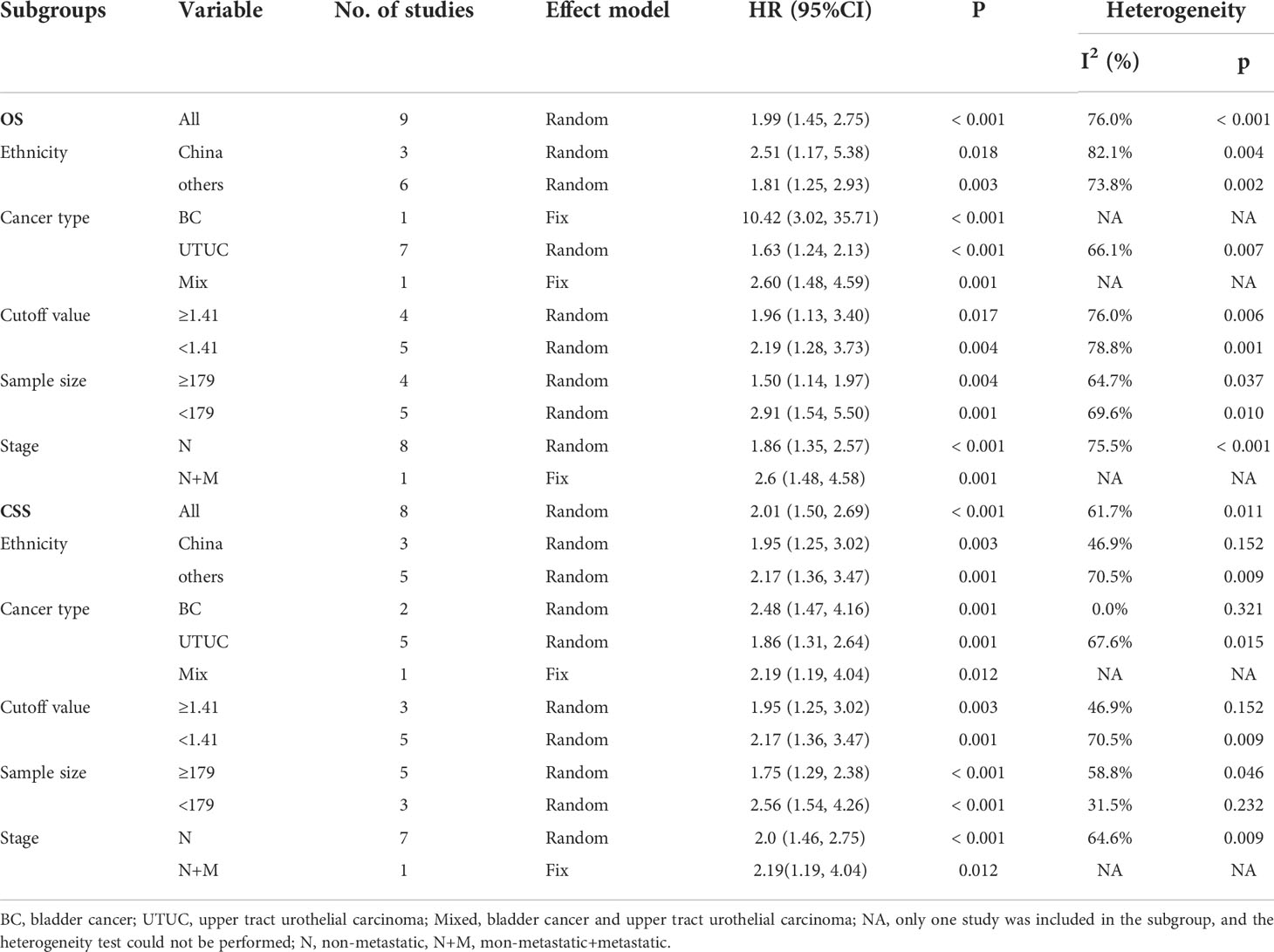
We conducted a subgroup analysis for OS and CSS with limited studies, and stratified by ethnicity, cancer type, cutoff value, and sample size (Table 2). Stratified according to the ethnicity, in the Chinese population, the low pretreatment AGR was positively correlated with poor OS [HR = 2.51, 95% CI (1.17, 5.38), P = 0.018] and CSS [HR=1.95, 95%CI (1.25, 3.02), P=0.003]. Similarly, in other regional subgroups, we found that a low AGR was an independent risk factor for OS [HR = 1.81, 95% CI (1.25, 2.93), P = 0.003] and CSS [HR = 2.17, 95% CI (1.36, 3.47), P=0.001]. However, low AGR was not related to RFS in the Chinese population. Subgroup analyses stratified by cancer type, our results showed that the low AGR predicted a worse OS [HR = 1.63, 95% CI (1.24, 2.13), p < 0.001] and CSS [HR = 1.86, 95% CI (1.31, 2.64), P = 0.001] in the UTUC group. The same results were observed in BC group [OS: HR = 10.42, 95% CI (3.02, 35.71), p < 0.001; CSS: HR=2.48, 95%CI (1.47, 4.16), P=0.001] and mix group [OS: HR = 2.60, 95% CI (1.48, 4.59), P = 0.001; CSS: HR = 2.19, 95% CI (1.19, 4.04), P = 0.001] with limited articles. For subgroup of cut-off value, the meta-analysis demonstrated that in the cut-off value <1.41 [OS: HR = 2.19, 95% CI (1.28, 3.73), P = 0.004; CSS: HR = 2.17, 95% CI (1.36, 3.47), P = 0.001] and cut-off value ≥1.41 [OS: HR = 1.89, 95% CI (1.13, 3.16), P = 0.017; CSS: HR = 1.95, 95% CI (1.25, 3.02), P = 0.003], all the above results indicated that the low pretreatment AGR was related to poor OS and CSS. Stratified by sample size, lower OS and CSS were more likely to be related to low AGR, either in the sample size ≥179 group [OS: HR = 1.50, 95% CI (1.14, 1.97), P = 0.005; CSS: HR = 1.75, 95% CI (1.29, 2.38), p < 0.001] or sample size <179 [OS: HR = 2.90, 95% CI (1.46, 5.73), P = 0.001; CSS: HR = 2.56, 95% CI (1.54, 4.26), p < 0.001]. In addition, stratified by stage, due to limited data, we only observed that low AGR was associated with a poor OS [HR = 1.86, 95% CI (1.35,2.57), p < 0.001] and CSS [HR = 1.81, 95% CI (1.46, 2.75), p < 0.001] in patients with non-metastatic UC.
Sensitivity analysis
Because of the high heterogeneity of some parameters, we performed a sensitivity analysis for the pooled HRs of OS and CSS. No significant change in the pooled HR was observed according to the leave-one-out test. Hence, we believe that our results are reliable (Figure S1).
Publication bias
Begg’s test was performed to assess the publication bias in this study. Visual examination of the funnel plot and statistical analysis revealed potential publication bias was exist for OS (p = 0.048) and CSS (p = 0.004). Subsequently, a test of trim and fill in STATA using the “metatrim” command was adopted to further investigate the publication bias; furthermore, there were four potentially missing studies according to the filled funnel plot. However, our pooled results basically did not change after adding four unpublished studies (Figure S2).
Discussion
UC is a highly invasive tumor with a high mortality rate. Even in cases where patients received radical surgery, 20%-30% of them still had a recurrence and distant metastasis that seriously affected their quality of life (33–35). As common predictive indicators for UC, including tumor stage, grade and lymph node status, it is difficult to accurately predict the prognosis of patients before treatment alone (13). In recent years, researchers have developed nomograms to predict the survival outcomes in a variety of urinary cancer patients. Zhang et al. (36) developed a nomogram including the clinicopathological features, AGR, C-reactive protein/albumin ratio (CAR), and neutrophil-lymphocyte ratio (NLR) to predict OS and PFS after radical surgery for bladder cancer. Chen et al. used the data of T stage, AGR, NLR, and monocyte to lymphocyte ratio (MLR) to construct a nomogram and use it to evaluate the survival outcome of clear cell renal cell carcinoma (ccRCC) patients (37). Although the nomogram provides an accurate prediction, we need to incorporate multiple factors that will increase the financial burden of patients. Additionally, some pathological indicators need to be obtained by surgery, which will bring trauma to the patients. Therefore, identifying a noninvasive, inexpensive and easily accessible prognostic biomarker is of great significance in guiding the individual treatment of patients with UC.
Recently, studies have shown that AGR can be used as an economic and practical marker to evaluate the therapeutic effect and prognosis of different cancers (15–17, 38). Similarly, for UC patients, most studies identified that AGR before treatment was an independent prognostic factor in multivariate analysis (22–29, 31, 32, 39). However, the prognostic value of AGR remains controversial in some clinical studies (19, 30). Although the previous two meta-analyses reported that low AGR had poor survival (13, 40), the reliability of their research conclusions was insufficient because only two studies were included. Thus, it is necessary to use the published literatures to further clarify the value of pretreatment AGR in the clinical diagnosis and prognosis of patients with UC.
In the current study, we performed a meta-analysis on 12 articles involving 5,727 patients to explore the prognostic effect of AGR in UC patients. According to the pooled analysis results, this conclusion was in line with most of the included studies, which suggested that pretreatment AGR was an independent predictor of survival outcomes. With a decrease of AGR, patients with UC had worse OS, CSS and RFS outcomes. Subgroup analyses of OS and CSS by race, cancer type, cutoff, or sample size also showed that low AGR was significantly associated with poorer OS and CSS in all UC patients. However, for tumor stage, limited data was used to demonstrated that low AGR was an independent prognostic marker for patients with non-metastatic UC.
It is worth noting that sensitivity analysis confirmed that the pooled results were stable. However, from the Cochrane’s Q test and I2 test, we found that moderate to extreme inter-study heterogeneity for survival outcomes among the included studies. Hence, a random effects model was used to minimize the impact of heterogeneity on the overall effects. This discrepancy might be because the population of participants used for the study was limited to Asia. Moreover, there were different treatment regimens and subsequent treatment approaches.
Albumin and globulins are the two most abundant proteins in human blood plasma and can be easily and cost-effectively measured. As the major serum protein, albumin plays an vital role in carrying out antioxidant activities, maintaining colloidal osmotic pressure, binding and transporting hormones, cations and fatty acids, as well as in maintaining capillary membrane stability (41, 42). Similarly, the albumin level either reflects the body nutritional status or represents the systemic inflammation (43). Overall, the inflammatory response to cancer is strongly linked to its prognosis and can be used to predict the clinical course (44, 45). Inflammation plays a pivotal step in cancer occurrence and development. In an inflammatory environment, tumor cells release various inflammatory mediators, such as tumor necrosis factor (TNF), interleukin-1 and interleukin-6, and increase vascular permeability by damaging vascular endothelial cells. In contrast, malnutrition and inflammatory cytokines can inhibit the production of albumin, resulting in low serum albumin concentration, which could influence cell proliferation and weaken human immune defense mechanisms (33, 43). Globulin contains several immune-related proteins, such as C-reactive protein, complement components, fibrinogen and serum amyloid A, which are involved in regulating immunity and inflammation (27, 32). Chronic inflammation can affect not only the tumor growth but also angiogenesis and cancer migration (46). Previous studies have demonstrated that pre-treatment albumin and globulin are two potentially valuable elements related to the prognosis and can define the risk stratification of cancer patients (33, 47, 48).
Unfortunately, when albumin and globulin are used alone as an evaluation indices, unstable results may occur and easily interfered with external confounding factors. Consequently, we speculate that AGR combines two independent prognostic parameters, which have a higher predictive value than serum albumin or globulin levels alone. Several clinical trials have reported that AGR could be as a potential prognostic biomarker and that the decrease in AGR was correlates significantly with tumor stage and grade in different human cancers, including urinary system cancers (16, 31, 49, 50). Previous meta-analyses have also clarified the prognostic value of the AGR in various solid tumors. A meta-analysis conducted by Lv et al. (40) discovered that decreased AGR resulted in worse OS and PFS, and increased cancer recurrence or progression in many malignancies. In colorectal cancer, Ma et al. (51) provided evidence that low pretreatment AGR was related poor OS (HR=2.07, P < 0.01) and DFS/PFS (HR=2.10, P = 0.01), and advanced clinicopathological features, including age, tumor size, node metastasis stage, tumor depth. Additionally, many multicenter studies successively explored the correlation between the AGR and UC in recent years. A multicenter research team performed a retrospective study involving 2492 patients with non-metastatic UTUC receiving radical nephroureterectomy (RNU) and demonstrated that lower preoperative AGR is associated with locally advanced disease and worse clinical outcomes (31). In Pradere’s study of patients with UTUC undergoing neoadjuvant platin-based chemotherapy and RNU, the patients with a low pretreatment AGR had a markedly shorter OS and RFS than those with a high AGR group (19). In 2021, the finding of Taguchi and his colleagues proved that the AGR before treatment could assist in predicting the survival of UC patients treated with pembrolizumab (32). According to the current analysis, our results further revealed that low pretreatment AGR provides an accurate prognostic efficiency for OS, CSS, and RFS in UC patients. Therefore, we recommend that AGR could act as an efficient prognostic indicator for UC.
While our study contributes important evidence around the prognostic value of AGR, it has some limitations. Firstly, most of the included studies had a relatively small sample size. Secondly, since all studies were retrospective in design and most of the population came from Asia, the evidence obtained was limited. Thirdly, different treatment strategies may introduce bias in the results. Fourthly, the cutoff values of pretreatment AGR was inconsistent in different studies, which also led to bias in our results. Lastly, since other clinically relevant pathological data, such as tumor stage and grade were not available, further subgroup analysis could not be performed.
Conclusions
According to our analysis, this meta-analysis reveals that the pretreatment AGR is an independent prognostic indicator for patients with UC, especially for non-metastatic UC patients. Low AGR is related to worse OS, CSS and RFS. However, future studies with larger sample sizes and randomized controlled trials are needed to confirm this conclusion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
They conceived and designed the experiments: LT and XY. Analysed the data: ZX, XF, JL, and JW. Contributed reagents/materials/analysis: JL, CN, YX, and HW. Wrote the manuscript: ZX and XF. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.992118/full#supplementary-material
Supplementary Figure 1 | Forest plot for sensitivity analysis. (A) overall survival and (B) cancer-specific survival.
Supplementary Figure 2 | Begg’s test for (A) overall survival and (B) cancer-specific survival; Trim and fill method for (C) overall survival and (D) cancer-specific survival.
References
1. Bellmunt J, Valderrama BP, Puente J, Grande E, Bolós MV, Lainez N, et al. Recent therapeutic advances in urothelial carcinoma: A paradigm shift in disease management. Crit Rev Oncol/Hematol (2022) 174:103683. doi: 10.1016/j.critrevonc.2022.103683
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol (2013) 189:1214–21. doi: 10.1016/j.juro.2012.05.079
4. Zhuo Z, Song Z, Ma Z, Zhang Y, Xu G, Chen G. Chlorophyllin e6−mediated photodynamic therapy inhibits proliferation and induces apoptosis in human bladder cancer cells. Oncol Rep (2019) 41:2181–93. doi: 10.3892/or.2019.7013
5. Pak S, Kim SY, Kim SH, Joung JY, Park WS, Chung J, et al. Association between antibiotic treatment and the efficacy of intravesical BCG therapy in patients with high-risk non-muscle invasive bladder cancer. Front Oncol (2021) 11:570077. doi: 10.3389/fonc.2021.570077
6. Gakis G, Black PC, Bochner BH, Boorjian SA, Stenzl A, Thalmann GN, et al. Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol (2017) 71:545–57. doi: 10.1016/j.eururo.2016.09.035
7. Zaghloul MS, Christodouleas JP, Smith A, Abdallah A, William H, Khaled HM, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: A randomized phase 2 trial. JAMA Surg (2018) 153:e174591. doi: 10.1001/jamasurg.2017.4591
8. Suh J, Jung JH, Jeong CW, Kwak C, Kim HH, Ku JH. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: A systemic review and meta-analysis. Front Oncol (2019) 9:1365. doi: 10.3389/fonc.2019.01365
9. Hu X, Dou WC, Shao YX, Liu JB, Xiong SC, Yang WX, et al. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2019) 45:747–54. doi: 10.1016/j.ejso.2019.03.003
10. Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol (2013) 189:1662–9. doi: 10.1016/j.juro.2012.10.057
11. Wang Y, Zhang HX, Zhang H, He HY. Clinicopathological characteristics and prognosis of young patients with upper tract urethelial carcinoma. Zhonghua bing li xue za zhi = Chin J Pathol (2021) 50:90–6. doi: 10.3760/cma.j.cn112151-20200714-00556.
12. Wang Y, He HY. Clinicopathologic characteristics and prognosis of upper tract urothelial carcinoma: an analysis of 368 radical nephroureterectomy specimens. Zhonghua bing li xue za zhi = Chin J Pathol (2016) 45:681–6. doi: 10.3760/cma.j.issn.0529-5807.2016.10.003
13. Mori K, Janisch F, Mostafaei H, Lysenko I, Kimura S, Egawa S, et al. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: A systematic review and meta-analysis. Urologic Oncol (2020) 38:315–33. doi: 10.1016/j.urolonc.2020.01.015
14. Mori K, Miura N, Mostafaei H, Quhal F, Motlagh RS, Lysenko I, et al. Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta-analysis. Int J Clin Oncol (2020) 25:1459–74. doi: 10.1007/s10147-020-01690-1
15. Chung JW, Park DJ, Chun SY, Choi SH, Lee JN, Kim BS, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci Rep (2020) 10:11999. doi: 10.1038/s41598-020-68975-3
16. Cai Y, Zhao Y, Dai Q, Xu M, Xu X, Xia W. Prognostic value of the albumin-globulin ratio and albumin-globulin score in patients with multiple myeloma. J Int Med Res (2021) 49:300060521997736. doi: 10.1177/0300060521997736
17. Guo X, Shao J, Zhai B, Zou Q, Yan J, Gu H, et al. Relationship and prognostic significance between preoperative serum albumin to globulin ratio and CT features of non-small cell lung cancer. Eur J Radiol (2020) 128:109039. doi: 10.1016/j.ejrad.2020.109039
18. Quan L, Jiang X, Jia X, Cheng F. Prognostic value of the albumin-to-Globulin ratio in patients with colorectal cancer: A meta-analysis. Nutr Cancer (2022) 27:1–11. doi: 10.1080/01635581.2022.2076890
19. Pradere B, D'Andrea D, Schuettfort VM, Foerster B, Quhal F, Mori K, et al. Pre-therapy serum albumin-to-globulin ratio in patients treated with neoadjuvant chemotherapy and radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol (2021) 39:2567–77. doi: 10.1007/s00345-020-03479-3
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.) (2021) 372:n71. doi: 10.1136/bmj.n71
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
22. Zhang B, Yu W, Zhou LQ, He ZS, Shen C, He Q, et al. Prognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinoma. PloS One (2015) 10:e0144961. doi: 10.1371/journal.pone.0144961
23. Liu J, Dai Y, Zhou F, Long Z, Li Y, Liu B, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urologic Oncol (2016) 34:484.e481–484.e488. doi: 10.1016/j.urolonc.2016.05.024
24. Liu Z, Huang H, Li S, Yu W, Li W, Jin J, et al. The prognostic value of preoperative serum albumin-globulin ratio for high-grade bladder urothelial carcinoma treated with radical cystectomy: A propensity score-matched analysis. J Cancer Res Ther (2017) 13:837–43. doi: 10.4103/jcrt.JCRT_237_17
25. Fukushima H, Kobayashi M, Kawano K, Morimoto S. Prognostic value of Albumin/Globulin ratio in patients with upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Anticancer Res (2018) 38:2329–34. doi: 10.21873/anticanres.12478
26. Otsuka M, Kamasako T, Uemura T, Takeshita N, Shinozaki T, Kobayashi M, et al. Prognostic role of the preoperative serum albumin : globulin ratio after radical nephroureterectomy for upper tract urothelial carcinoma. Int J Urol Off J Japanese Urological Assoc (2018) 25:871–8. doi: 10.1111/iju.13767
27. Xu H, Tan P, Ai J, Huang Y, Lin T, Yang L, et al. Prognostic impact of preoperative albumin-globulin ratio on oncologic outcomes in upper tract urothelial carcinoma treated with radical nephroureterectomy. Clin Genitourinary Cancer (2018) 16:e1059–68. doi: 10.1016/j.clgc.2018.06.003
28. Omura S, Taguchi S, Miyagawa S, Matsumoto R, Samejima M, Ninomiya N, et al. Prognostic significance of the albumin-to-globulin ratio for upper tract urothelial carcinoma. BMC Urol (2020) 20:133. doi: 10.1186/s12894-020-00700-8
29. Oh JS, Park DJ, Byeon KH, Ha YS, Kim TH, Yoo ES, et al. Decrease of preoperative serum albumin-to-Globulin ratio as a prognostic indicator after radical cystectomy in patients with urothelial bladder cancer. Urol J (2021) 18:66–73. doi: 10.22037/uj.v16i7.6350
30. Quhal F, Pradere B, Laukhtina E, Sari Motlagh R, Mostafaei H, Mori K, et al. Prognostic value of albumin to globulin ratio in non-muscle-invasive bladder cancer. World J Urol (2021) 39:3345–52. doi: 10.1007/s00345-020-03586-1
31. Miura N, Mori K, Laukhtina E, Schuettfort VM, Abufaraj M, Teoh JYC, et al. Prognostic value of the preoperative albumin-globulin ratio in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy: results from a large multicenter international collaboration. Japanese J Clin Oncol (2021) 51:1149–57. doi: 10.1093/jjco/hyab023
32. Taguchi S, Kawai T, Nakagawa T, Nakamura Y, Kamei J, Obinata D, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep (2021) 11:15623. doi: 10.1038/s41598-021-95061-z
33. Liu J, Wang F, Li S, Huang W, Jia Y, Wei C. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep (2018) 38:1–13. doi: 10.1042/BSR20180214
34. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
35. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol (2021) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
36. Zhang W, Yang F, Kadier A, Chen Y, Yu Y, Zhang J, et al. Development of nomograms related to inflammatory biomarkers to estimate the prognosis of bladder cancer after radical cystectomy. Ann Trans Med (2021) 9:1440. doi: 10.21037/atm-21-4097
37. Chen Z, Shao Y, Yao H, Zhuang Q, Wang K, Xing Z, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget (2017) 8:48291–302. doi: 10.18632/oncotarget.15162
38. Chung JW, Ha YS, Kim SW, Park SC, Kang TW, Jeong YB, et al. The prognostic value of the pretreatment serum albumin to globulin ratio for predicting adverse pathology in patients undergoing radical prostatectomy for prostate cancer. Invest Clin Urol (2021) 62:545–52. doi: 10.4111/icu.20210105
39. Niwa N, Matsumoto K, Ide H, Nagata H, Oya M. Prognostic value of pretreatment albumin-to-Globulin ratio in patients with non-Muscle-Invasive bladder cancer. Clin Genitourinary Cancer (2018) 16:e655–61. doi: 10.1016/j.clgc.2017.12.013
40. Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clinica Chimica acta; Int J Clin Chem (2018) 476:81–91. doi: 10.1016/j.cca.2017.11.019
41. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J (2010) 9:69. doi: 10.1186/1475-2891-9-69
42. Li H, Chen C, Li ZM, Yang Y, Xing CQ, Li Y, et al. Specific interaction with human serum albumin reduces ginsenoside cytotoxicity in human umbilical vein endothelial cells. Front Pharmacol (2020) 11:498. doi: 10.3389/fphar.2020.00498
43. He X, Guo S, Chen D, Yang G, Chen X, Zhang Y, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer (2017) 8:258–65. doi: 10.7150/jca.16525
44. Karimi S, Vyas MV, Gonen L, Tabasinejad R, Ostrom QT, Barnholtz-Sloan J, et al. Prognostic significance of preoperative neutrophilia on recurrence-free survival in meningioma. Neuro-oncology (2017) 19:1503–10. doi: 10.1093/neuonc/nox089
45. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol (2017) 3:827–31. doi: 10.1001/jamaoncol.2016.3834
46. Wang J, Yang ZR, Dong WG, Zhang JX, Guo XF, Song J, et al. Cooperative inhibitory effect of sinomenine combined with 5-fluorouracil on esophageal carcinoma. World J Gastroenterol (2013) 19:8292–300. doi: 10.3748/wjg.v19.i45.8292
47. Yang H, Wang K, Liang Z, Guo S, Zhang P, Xu Y, et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin Otolaryngol Off J ENT-UK ; Off J Netherlands Soc Oto-Rhino-Laryngology Cervico-Facial Surg (2020) 45:167–76. doi: 10.1111/coa.13454
48. Li J, Wang Y, Wu Y, Li J, Che G. Prognostic value of pretreatment albumin to globulin ratio in lung cancer: A meta-analysis. Nutr Cancer (2021) 73:75–82. doi: 10.1080/01635581.2020.1737155
49. Takata N, Miyagawa M, Matsuda T, Takakado M, Okada T, Kawaguchi N, et al. Usefulness of albumin-globulin ratio as a clinical prognostic factor in patients with thyroid cancer treated with radioiodine. Ann Nucl Med (2021) 35:1015–21. doi: 10.1007/s12149-021-01635-2
50. Guo HW, Yuan TZ, Chen JX, Zheng Y. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: A meta-analysis. PloS One (2018) 13:e0189839. doi: 10.1371/journal.pone.0189839
Keywords: bladder cancer, upper tract urothelial carcinoma, urothelial cancer, albumin−globulin ratio, prognosis
Citation: Xia Z, Fu X, Li J, Wu J, Niu C, Xu Y, Wang H, Yuan X and Tang L (2022) Prognostic value of pretreatment serum albumin−globulin ratio in urothelial carcinoma: A systematic review and meta-analysis. Front. Oncol. 12:992118. doi: 10.3389/fonc.2022.992118
Received: 12 July 2022; Accepted: 01 August 2022;
Published: 16 August 2022.
Edited by:
Ming Yin, The Ohio State University, United StatesReviewed by:
Octavian Sabin Tataru, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaXiaochen Zhou, The First Affiliated Hospital of Nanchang University, China
Copyright © 2022 Xia, Fu, Li, Wu, Niu, Xu, Wang, Yuan and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinzhu Yuan, eXVhbnhpbnpodUAxMjYuY29t; Lingtong Tang, MzY0Njg2MTQ5QHFxLmNvbQ==
†These authors have contributed equally to this work
 Zhongyou Xia
Zhongyou Xia Xueqin Fu2†
Xueqin Fu2† Jinze Li
Jinze Li