- 1Department of Nuclear Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China
- 2Department of General Surgery, Huashan Hospital, Fudan University, Shanghai, China
- 3PET Center, Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Nuclear Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Objective: Fibroblast activation protein (FAP)-targeting radiopharmaceutical based on the FAP-specific inhibitor (FAPI) is considered as a potential alternative agent to FDG for tumor-specific imaging. However, FAP is also expressed in normal adult tissues. The aim of this study was to explore the image features of non-tumoral regions with high uptake of 68Ga-FAPI-04 in positron emission tomography (PET) imaging and to reveal the physiological mechanisms of these regions.
Material: A total of 137 patients who underwent whole-body 68Ga-FAPI-04 PET/MR (n=46) or PET/CT (n=91) were included in this retrospective study. Three experienced nuclear medicine physicians determined the non-tumoral regions according to other imaging modalities (CT, MRI, 18F-FDG PET, or ultrasound), clinical information, or pathological results. The regions of interest (ROIs) were drawn manually, and the maximum standardized uptake value (SUVmax) was measured.
Results: A total of 392 non-tumoral uptake regions were included in this study. The included physiological regions were uterus (n=38), submandibular gland (n=118), nipple (n=37), gingiva (n=65), and esophagus (n=31). The incidence of 68Ga-FAPI-04 uptake in physiological regions was independent of age, the tracer uptakes in the gingiva and esophagus were more common in male patients (p=0.006, 0.009), while that in the nipple was more common in female patients (p < 0.001). The included benign regions were inflammatory lymph node (n =10), pneumonia (n=13), atherosclerosis (n=10), pancreatitis (n=18), osteosclerosis (n=45), and surgical scar (n=7). No significant difference was observed in SUVmax between physiological and benign regions.
Conclusions: A number of organs exhibit physiological uptakes of 68Ga-FAPI-04. Our study showed that regions with high 68Ga-FAPI-04 uptake did not necessarily represent malignancy. Being familiar with physiological and typical benign 68Ga-FAPI-04 uptake regions can be helpful for physicians to interpret images and to make an accurate diagnosis.
Introduction
Cancer-associated fibroblasts (CAFs) and extracellular fibrosis can account for 90% of the total tumor mass (1). Fibroblast activation protein (FAP), a type II membrane-bound glycoprotein of the dipeptidyl peptidase 4 family, is over-expressed in CAFs of many epithelial carcinomas and is involved in a variety of tumor-promoting activities, such as stromal remodeling, angiogenesis, chemotherapy resistance, and immunosuppression (1, 2). Since FAP is expressed at low levels in most normal organs, it is a promising target for imaging and radiation therapy (3). Radiopharmaceuticals targeting FAP have recently been developed based on FAP-specific inhibitors (FAPIs) (4). Among several recently developed tracers targeting FAP, 68Ga-FAPI-04 is regarded as a promising one for having high affinity towards FAP and suitable kinetics (5–7). Without the necessity of fasting in preparation before the scan and an equal or better tumor-to-background ratio compared with 18F-FDG PET scans, 68Ga-FAPI-04 is considered as a potential alternative agent to FDG for tumor-specific imaging (8).
Currently, most FAPI studies are focused on tumor imaging. Besides its high expression in epithelial carcinoma (8), FAP also plays a key role in normal development during embryo-genesis and tissue modeling (9). FAP can also be expressed in normal adult tissues such as active tissue damage, remodeling, inflammation, arthritis, atherosclerotic plaques, and fibrosis (3, 9, 10). Several non-oncology studies on FAPI revealed its unique values in IgG4-related diseases (11). Luo et al. (12) found that compared with 18F-FDG, 68Ga-FAPI-04 was more effective in detecting organs affected by IgG4-related disease. An animal study showed that joint FAPI concentration was correlated with arthritis scores in rats (13). A recent work reported that 68Ga-FAPI-04 focal non-tumoral uptake can occur in fibrous lesions, fibrous hyperplasia, and fibrous activity (14). 68Ga-FAPI-04 could also accumulate in some benign diseases of the bones and joints (15). Recent studies characterized the benign lesions with increased 68Ga-FAPI-04 uptake in PET/CT (16, 17). However, to the best of our knowledge, there are no systematic studies to reveal the pathophysiological mechanisms of non-tumoral 68Ga-FAPI-04 uptake regions. This study aimed to investigate the uptake characteristics in non-tumoral regions using 68Ga-FAPI-04 PET/CT or PET/MR with a relatively large sample size and provide a reference for imaging diagnosis.
Materials and methods
Patients
This retrospective analysis was performed on patients who underwent 68Ga-FAPI-04 PET/CT (Biograph mCT, Siemens Healthineers, Germany; Ingenuity TF, Philips Healthcare, USA; uMI510, United Imaging, China) or 68Ga-FAPI-04 PET/MR (uPMR790 TOF, United Imaging, China) from April 2020 to August 2021. The inclusion criteria were as follows: (i) patients who were able to sign informed consents for examination according to the guidelines of the Clinical Research Ethics Committee; (ii) patients with a predicted survival of more than 6 months. Exclusion criteria were (i) pregnancy, (ii) postmenopausal women with taking hormone replacement or related drugs, and (iii) patients with a predicted survival of <6 months.
Radiopharmaceutical and imaging protocols
Good-manufacturing-practice (GMP)-grade precursors 68Ga-FAPI-04 was synthesized in the Radiochemistry Facility of the PET Center, Huashan Hospital, Fudan University, according to the protocol described previously (18). The radiochemical purity of 68Ga-FAPI-04 was over 95%, and the final product was sterile and pyrogen-free.
Whole-body PET/CT or PET/MR scans were performed 60 min after the injection of 68Ga-FAPI-04 with a dose of 150 ± 35 MBq (4.05 ± 0.95 mCi) from the vertex to the mid-thigh. For PET/CT, a PET scan was acquired after a low-dose CT scan, which was performed at 120 kV and 100–120 valid mAs. Brain PET scanning was performed 5 min/bed, and body PET scanning was performed 3 min/bed. PET/MR was performed with default clinical MRI sequences including T1w and T2w (TE = 2.24 ms, TR = 4.91 ms, flip angle = 10, echo train length = 30, FOV = 549 × 384, matrix = 256 × 329, slice thickness = 2 mm, slice spacing = 2 mm, transverse plane) (18). PET images were reconstructed by ordered subset expectation maximization 3D (OSEM 3D) method with 2 iterations and 20 subsets.
Since different scanners were used in this study, SUV measurements were normalized after data collection. A NEMA IEC body phantom (Data Spectrum Corporation, Durham, NC, USA) with six simulated lesion spheres (diameters: 10, 13, 17, 22, 28, and 37 mm) was applied for SUV normalization with 2, 4, 8, and 16 times the background activity (background activity concentration =2 kBq/ml). A CT scan of the NEMA IEC body phantom was prepared for the attenuation correction of PET/MR. Correlation coefficients were obtained through this phantom study and used to standardize the SUV measurements as previously reported (18, 19).
PET/CT and PET/MR imaging review
Three nuclear medicine physicians with 15, 10, and 8 years of experience in interpreting PET/CT and MR imaging determined the physiological and benign tracer uptake regions based on the patients’ clinical data, imaging data (CT, MRI, 18F-FDG PET, or ultrasound), histopathology, and their own experiences in image interpretation. Physiological uptake refers to the slightly elevated uptake of 68Ga-FAPI-04 in generally normal tissues, which usually show no abnormal changes on other imaging modalities (20, 21). Benign uptake refers to inflammation, fibrosis, benign tumors, and other non-malignant tumor regions that may be abnormal on other imaging modalities (16, 22). For any differences in opinion, a consensus was reached by discussion together. The ROI was drawn manually for SUVmax measurement.
Statistical analyses
Shapiro–Wilk normality test was used to analyze the data distribution. Data were expressed as mean ± standard deviation (SD). Data of physiological and benign uptake regions were tested by independent sample T-test. Chi-square test and logistic regression analysis were used to investigate the influence of age and sex on the incidence of physiological uptake regions. Pearson correlation analysis was performed for SUVmax of physiological regions and age. Statistical analysis was performed using SPSS 23.0 statistical software. Two-tailed p < 0.05 was considered statistically significant.
Results
Patient characteristics
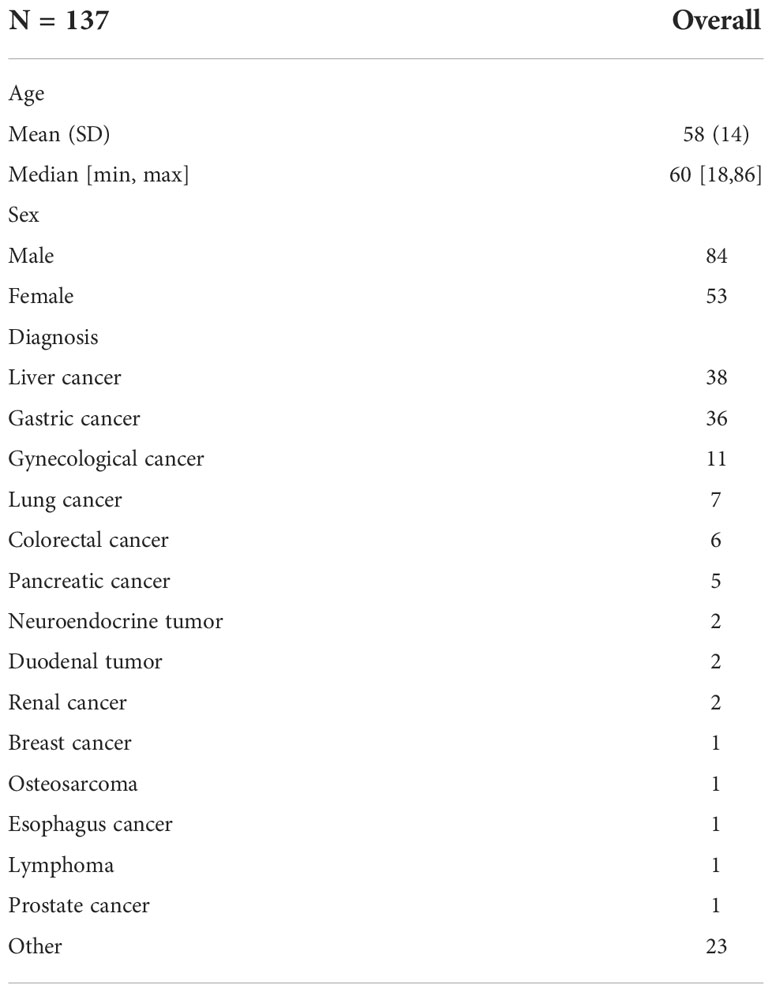
Patient characteristics are summarized in Table 1. Briefly, 137 patients (84 male and 53 female; age, 58 ± 14 years; range from 18 to 86 years, mostly diagnosed with cancer) were included in this study. Non-tumoral regions were observed in the majority of patients (86.86%). A total of 392 non-tumoral regions were classified as physiological regions (n = 289, SUVmax = 3.62 ± 2.86) or benign regions (n = 103, SUVmax = 3.50 ± 2.25) according to other imaging features, clinical representations, or pathological results. T-test indicated no statistically significant difference between the physiological and benign groups (p = 0.40).
Physiological uptake regions
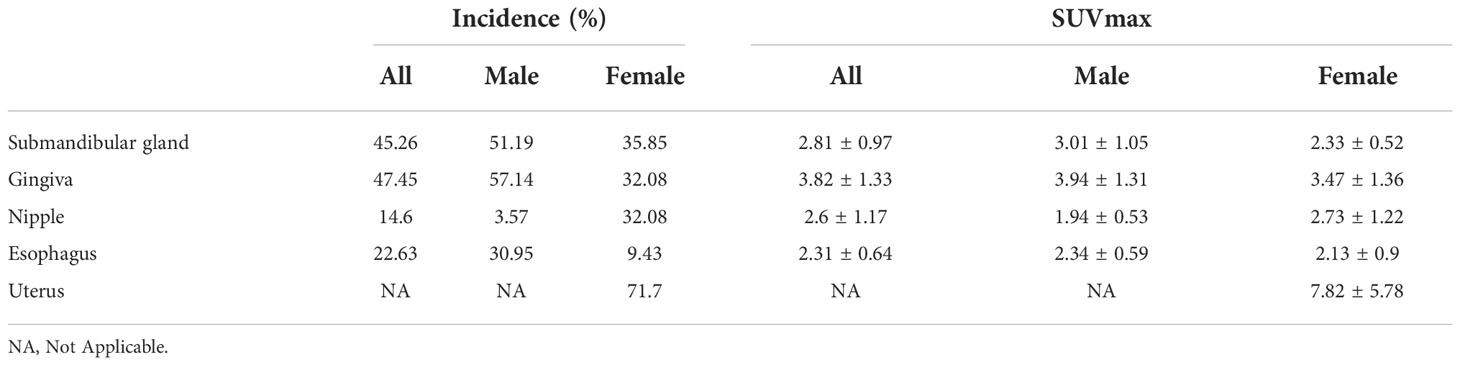
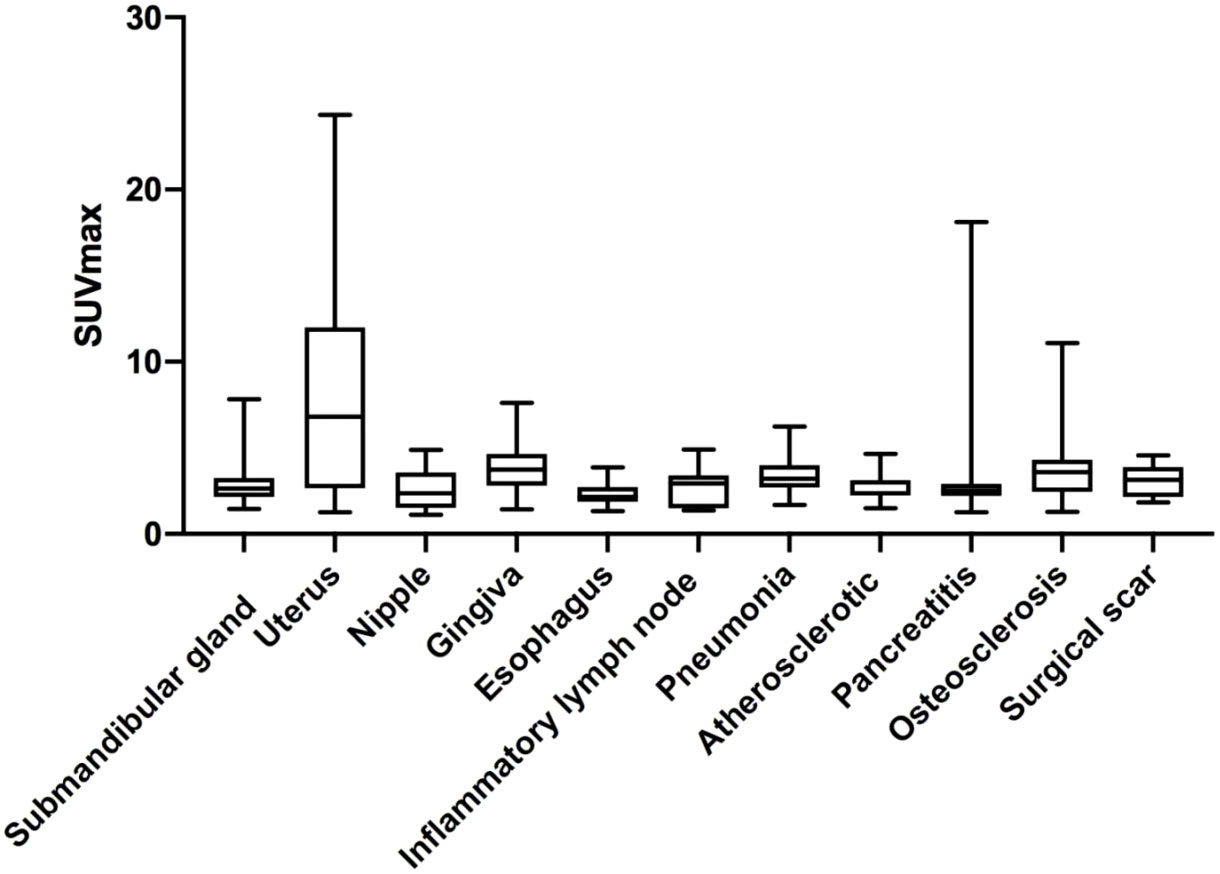
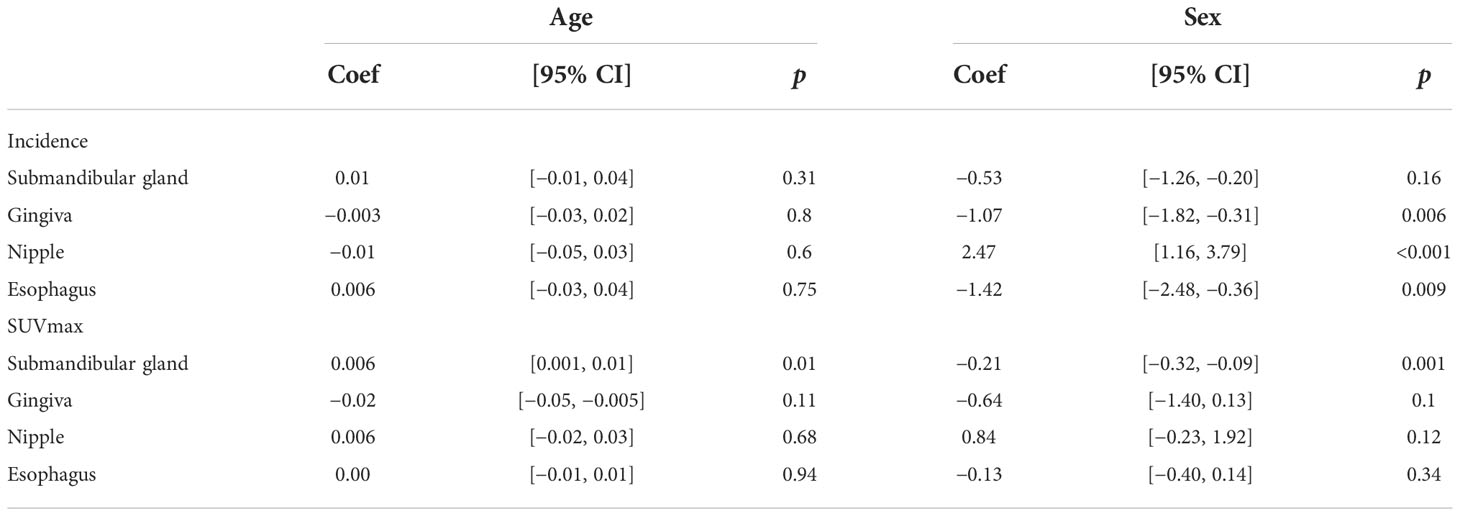
The physiological uptakes are summarized in Table 2 and Figure 1. Elevated 68Ga-FAPI-04 uptakes in the head and neck were primarily observed in the submandibular gland (n = 118, SUVmax range from 1.46 to 7.83) and gingiva (n = 65, SUVmax range from 1.43 to 7.61), while in the chest, elevated uptake was mainly located in the nipple (n = 37, SUVmax range from 1.12 to 4.88) and esophagus (n = 31, SUVmax range from 1.33 to 3.87). Although the incidence of 68Ga-FAPI-04 uptake in the submandibular gland, gingiva, nipple, and esophagus was independent of age (p>0.05), the tracer uptakes in the gingiva and esophagus were more common in male patients (p = 0.006, 0.009), while uptakes in the nipple were more common in female patients (p<0.001) (Table 3). The uptake values of 68Ga-FAPI-04 (SUVmax) in the submandibular gland were positively correlated with age (p = 0.01) and higher in male patients (p = 0.001), while those in other physiological regions were independent of age and sex (all p ≥ 0.05) (Table 3).
High uptake of 68Ga-FAPI-04 in the uterus was also very common (n=38, mean SUVmax = 7.82 ± 5.78, SUVmax range from 1.26 to 24.33). Increased 68Ga-FAPI-04 uptake in the uterus was observed in 71.70% of female patients and occurred preferentially in premenopausal women (82.14%, p = 0.07). The SUVmax in the uterus did not correlate with the patients’ age (r = −0.11, p = 0.50). When comparing SUVmax in the uterus between premenopausal and postmenopausal groups, no statistically significant difference was observed (SUVmax = 8.40 ± 5.64 vs. 6.93 ± 6.06, p = 0.50).
Benign uptake regions
The benign regions included inflammatory lymph node (n = 10, mean SUVmax = 2.75 ± 1.13, SUVmax range from 1.37 to 4.91), pneumonia (n = 13, mean SUVmax = 3.37 ± 1.22, SUVmax range from 1.68 to 6.24), atherosclerosis (n = 10, mean SUVmax = 2.85 ± 0.84, SUVmax range from 1.49 to 4.65), pancreatitis (n = 18, mean SUVmax = 3.41 ± 3.74, SUVmax range from 1.27 to 18.11), osteosclerosis (n = 45, mean SUVmax = 3.93± 2.22, SUVmax range from 1.28 to 11.09), and surgical scar (n = 7, mean SUVmax = 3.14 ± 0.98, SUVmax range from 1.83 to 4.56). There was no significant difference in SUVmax between these regions (p>0.05).
We found some interesting cases with high 68Ga-FAPI-04 uptakes. A patient with a 30-year history of hepatitis B showed high 68Ga-FAPI-04 uptake in the liver (Figure 2A). High 68Ga-FAPI-04 uptake has also been found in the rectum of a patient with Crohn’s disease (Figure 2B). A man diagnosed with disseminated non-tuberculous mycobacteriosis (tuber colectomy of the left chest wall and CT-guided percutaneous lung puncture biopsy found inflammatory granulomatous lesions; prostate puncture pathology revealed non-specific granulomatous prostatitis; second-generation DNA sequencing results suggested occasional mycobacterium infection) showed lesions throughout the body with high or mild uptake of 68Ga-FAPI-04 (Figure 2C). After anti-infective therapy, the intracranial lesions became smaller.

Figure 2 Interesting cases 68Ga-FAPI-04 imaging. (A) A 65-year-old woman with a history of hepatitis B over 30 years, arrows, cirrhosis of the liver, SUVmax 3.24; (B) a 19-year-old man with a 2-year history of rectal Crohn’s disease, arrows, rectal Crohn’s disease, SUVmax 5.22; (C) a 56-year-old man diagnosed with disease of disseminated non-tuberculous mycobacteriosis. The maximum intensity projection (MIP) image shows various FAPI-avid nodules: brain (SUVmax =2.61), cervical lymph nodes (SUVmax =1.81), upper lobe of right lung (SUVmax = 1.74), subcutaneous nodule on the left chest (SUVmax = 3.52), spleen (SUVmax= 2.03), left kidney (SUVmax = 2.76), and prostate (SUVmax = 4.02).
Discussion
Due to the specific expression of FAP in tumor stromal fibrous tissues, FAP has received increasing attention as a specific marker of CAFs. Meanwhile, activated fibroblasts that undergo extracellular matrix (ECM) remodeling in the tissue due to chronic inflammation, fibrosis, and wound healing can also be observed by FAPI imaging (23–25). In this study, we described the SUVmax of 392 non-tumoral uptake regions in 137 patients who underwent 68Ga-FAPI-04 PET/CT or PET/MR.
Consistent with previous studies (7, 26), physiological uptakes of 68Ga-FAPI-04 were observed in the submandibular gland, nipple, gingiva, and esophagus (Figures 3A–C). In our study, the incidence of 68Ga-FAPI-04 uptakes in the submandibular gland, gingiva, nipple, and esophagus were independent of age. Tracer uptakes in the gingiva and esophagus were more common in male patients, whereas uptake in the nipple was more common in female patients. It indicates that sex may have a more significant effect on physiological expression of FAPI than age. The uptake values of 68Ga-FAPI-04 (SUVmax) in the submandibular gland were positively correlated with age, suggesting that FAP activity in the submandibular gland may be affected by age.
The uptake of 68Ga-FAPI-04 in the uterus was significantly higher compared to other non-tumoral regions in our study (Figure 3C, red arrow). The high uptake in the uterus is considered to stem from the endometrial glandular cells, and its level is significantly lower than that of the malignant component in the uterus (27). Although a recent work suggested that tracer uptake decreases with age (28), in this study, we did not find a significant correlation between the SUVmax of uterus and patient age, in line with a previous study (17) reporting that intense 68Ga-FAPI-04 uptake in the uterus was independent from menopause. High uterine FAP activity might be caused by tissue remodeling and angiogenesis during hormonal periodic changes in regeneration (29).
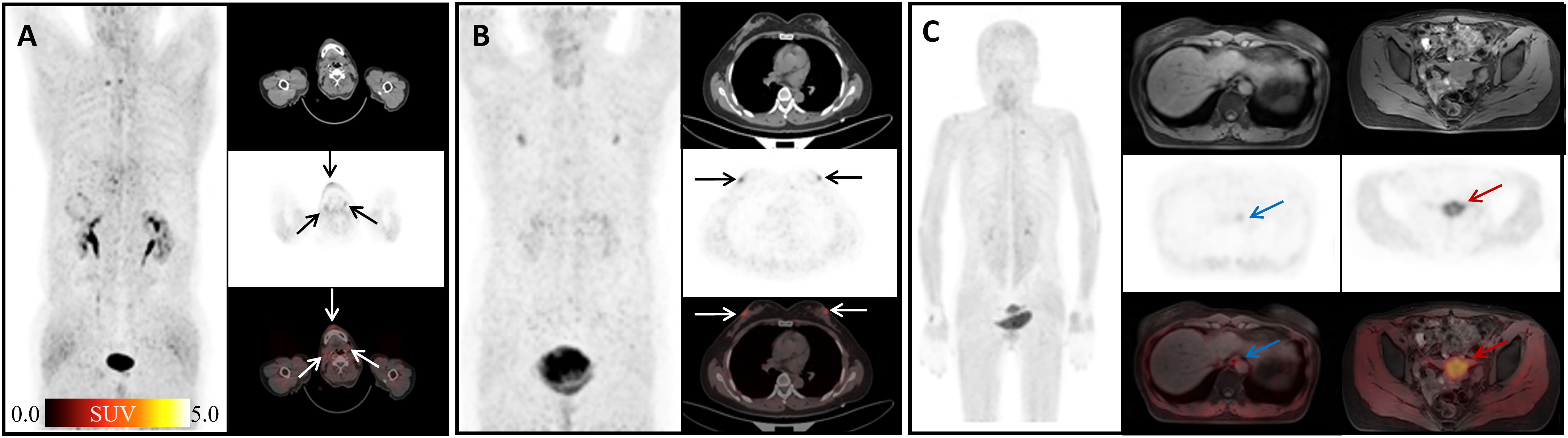
Figure 3 Physiological uptake of 68Ga-FAPI-04. (A) A 60-year-old man with hepatic hilar malignancy after the treatment of transcatheter arterial chemoembolization (TACE); the arrows indicate 68Ga-FAPI-04 uptake in the gingiva and submandibular gland (SUVmax 4.45, 3.79, and 3.64). (B) A 65-year-old woman with microinvasive lung adenocarcinoma 6 months after surgery; arrows show physiological uptake in the nipples with SUVmax 4.88. (C) A 55-year-old woman with signet ring cell carcinoma of stomach 2 months after endoscopic submucosal dissection (ESD); blue arrows show physiological uptake of the esophagus (SUVmax =3.67), and red arrows show uterus (SUVmax=13.94).
FAP can be induced by fibrosis foci during pulmonary fibrosis in ongoing tissue remodeling (30). In this study, elevated uptake of 68Ga-FAPI-04 was found in 13 pneumonia lesions (mean SUVmax = 3.37) (Figures 4A, B), yet still lower than that in lung cancer lesions (SUVmax>12) according to the literature (31). Although 68Ga-FAPI-04 PET/CT is inferior to 18F-FDG PET/CT in detecting lymph nodes involved in IgG4-related diseases (12), Schmidkonz et al. reported high uptake of FAPI in lymph nodes infiltrated by a fibrotic process and decreased FAPI uptake in those after anti-fibrosis therapy (11). Inflammatory lymph nodes in our study also showed high uptake of 68Ga-FAPI-04 (Figure 4C), and SUVmax was lower than that of fibrotic lymph nodes reported before (11). Mixed type of proliferative and fibrotic lymph nodes in our study may have led to such results.
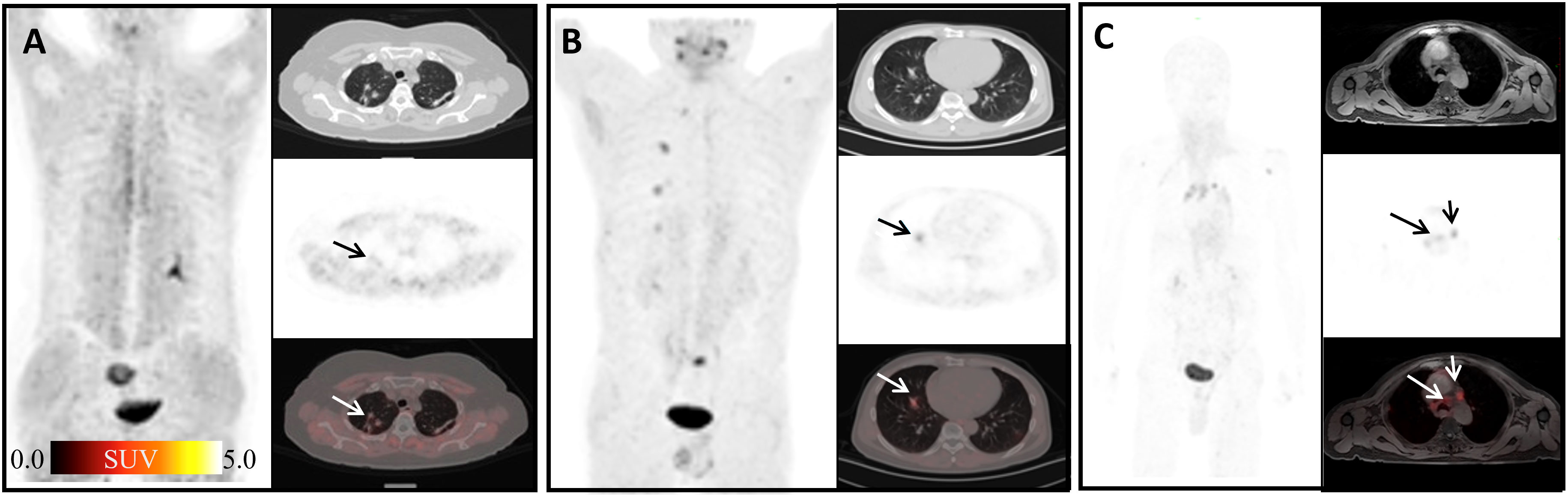
Figure 4 Physiological uptake of 68Ga-FAPI-04. (A) A 64-year-old female patient with gastric cancer 1 year after surgery; the arrows show organized pneumonia in the upper lobes of right lung with SUVmax of 1.76. (B) A 60-year-old male patient after liver cancer surgery; the arrows indicate the inflammatory lesion in the middle lobe of the right lung with SUVmax 3.24. (C) A 50-year-old male patient with weight loss of 10 kg in recent 6 months; arrows show mediastinal inflammatory lymph nodes with SUVmax of 4.91.
FAP has recently been proposed as an inflammation-induced protease involved in the formation of vulnerable plaques (32). It has been reported that FAP expression was enhanced in the human atherosclerotic vessel and increased upon plaque progression (33). In our study, atherosclerotic plaques showed slightly high uptake of 68Ga-FAPI-04 with mean SUVmax = 2.85 (Figure 5A). Forty-five joints in our study showed high 68Ga-FAPI-04 uptake (Figures 5B, C). In a study of the biological distribution of FAPI in cancer patients, mild low-grade uptake in the knee and shoulder was observed in three patients with no clinical symptoms of arthritis (34). FAP expression has been observed in synovial tissue samples of rheumatoid arthritis (35). In osteoarthritis, higher levels of FAP expression on the surface of the cartilage and on chondrocyte membranes were detected by Milner et al. (36). Terry Sy and his colleagues found that In-28H1 (anti-FAP antibody) radionuclide imaging could be used to evaluate the treatment response to etanercept in arthritic mice (13). Therefore, 68Ga-FAPI-04 might present a potential therapeutic target of arthritis, and 68Ga-FAPI-04 imaging has potential value in diagnosis and therapeutic efficacy evaluation in the future.
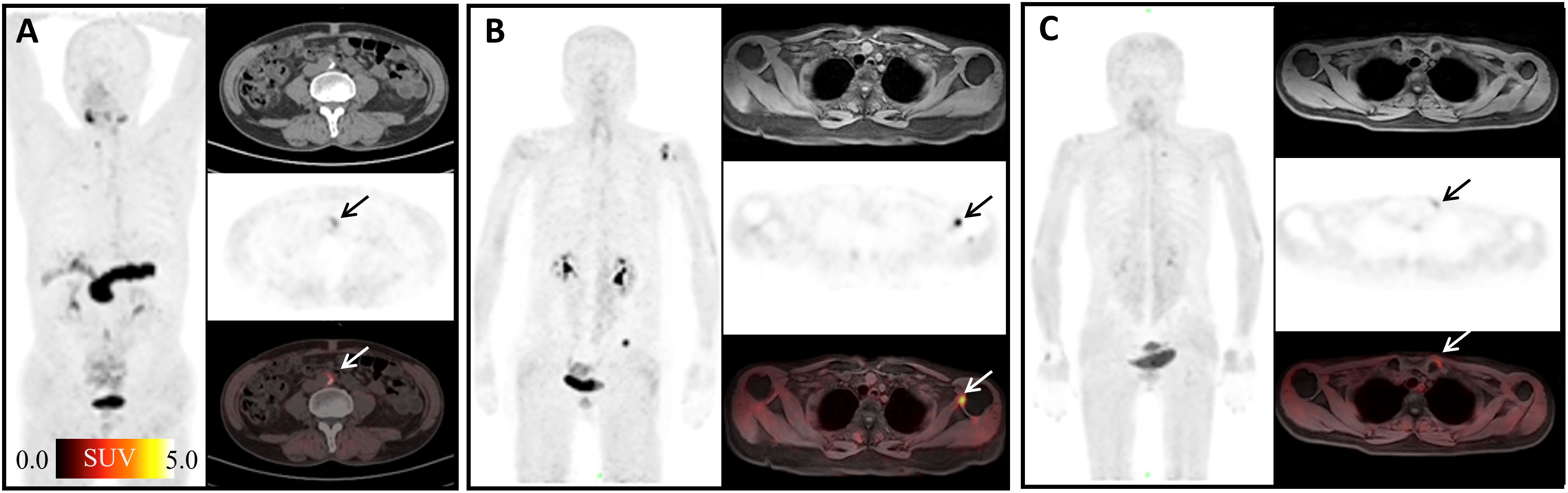
Figure 5 Physiological uptake of 68Ga-FAPI-04. (A) A 72-year-old man with duodenal papillary tumor; arrows show atherosclerosis of the abdominal aorta with SUVmax =4.65. (B) A 70-year-old woman presented with adenocarcinoma at the descending colon–sigmoid junction; arrows show left shoulder arthritis with SUVmax =11.09. (C) A 55-year-old woman with signet ring cell carcinoma of stomach 2 months after ESD; arrows show left sternoclavicular arthritis with SUVmax = 8.21.
It has been reported that 68Ga-FAPI-04 could show focal high uptake in pancreatic fibrous lesions, fibroplasia, or fibrotic activity (14). Our study also found non-tumoral high uptake in pancreas caused by inflammation (mean SUVmax = 2.55) (Figures 6A, B). Seven surgical scars in our study showed high uptake of 68Ga-FAPI-04 (mean SUVmax = 3.34) (Figures 6C, D). Keloid is a fibroproliferative reticular dermal disorder characterized by inflammation, increased deposition of ECM protein, and invasion of the surrounding healthy skin (37). FAP expression is observed in keloid (37) and in the physiological process of wound healing (38). Consistent with its role in fibrosis, FAP has been found to be expressed in fibroblasts and hepatic stellate cells (HSCs) activated in cirrhosis but not in normal human livers (39, 40). Crohn’s disease is a chronic inflammatory bowel disease in which myofibroblasts play a key role in the process of fibrosis. It is worth mentioning that the myofibroblasts isolated from a colon specimen of a patient with stenosis were FAP positive. Tumor necrosis factor (TNF) and transforming growth factor (TGF) can further induce the expression of FAP (41). The systemic non-tuberculous mycobacterium granuloma case suggests that 68Ga-FAPI-04 PET can be used as an effective imaging tool to detect the degree of infection and evaluate the therapeutic effect.
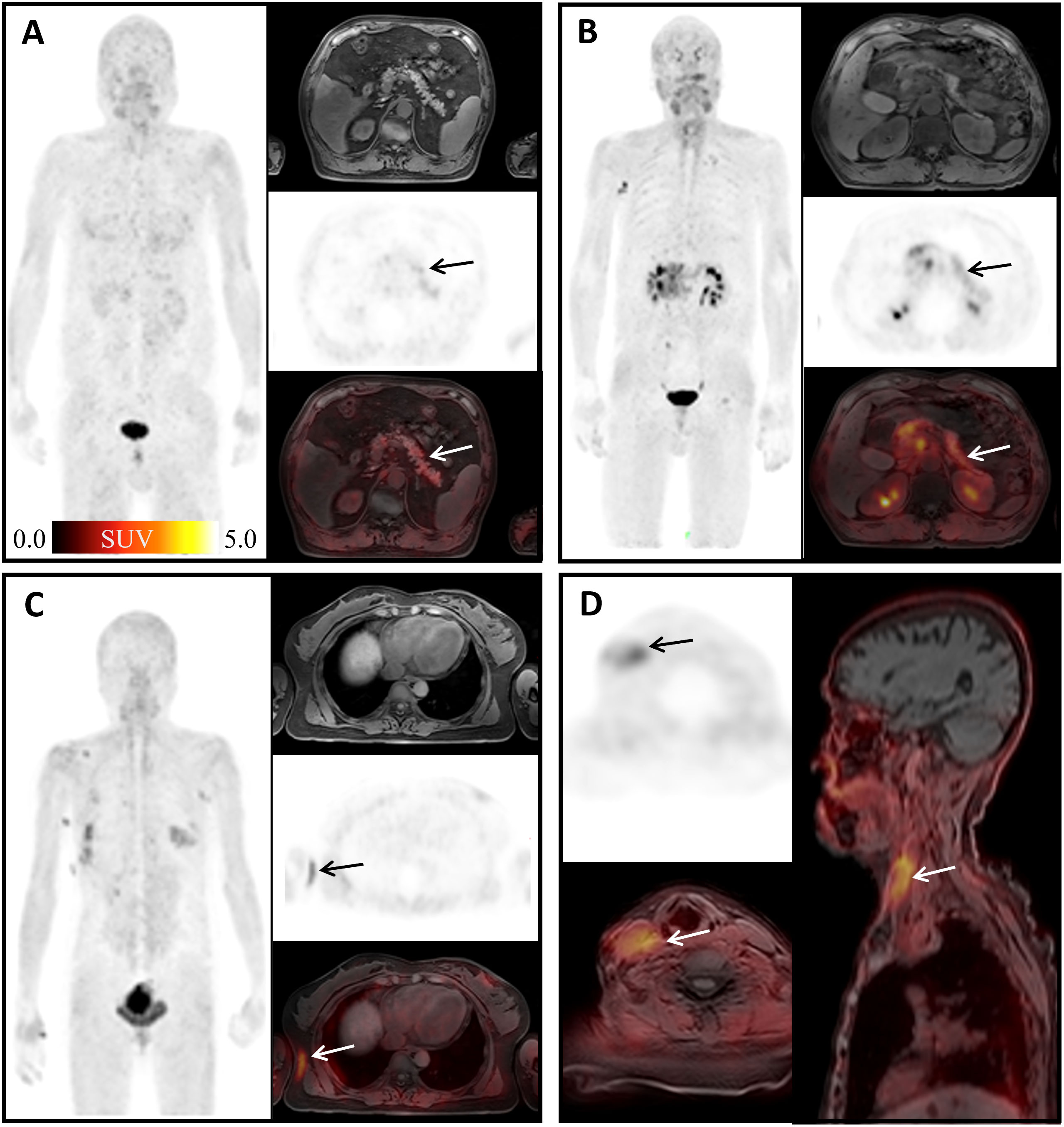
Figure 6 Physiological uptake of 68Ga-FAPI-04. (A) A 67-year-old man with mucinous adenocarcinoma of the right lung 1 year after surgery; arrows show pancreatic diffuse inflammatory uptake, SUVmax =4.66. (B) A 63-year-old man diagnosed with duodenal malignancy; arrows show obstructive pancreatitis, SUVmax = 18.11. (C) A 49-year-old woman, 3 months after surgery for early microinfiltrating adenocarcinoma of the right middle lobe and 2 months after surgery for left breast fibroma; arrows show surgical scar on the right chest with SUVmax =3.89. (D) An 83-year-old woman diagnosed with gastric cancer 6 months ago; arrows show deep vein catheterization area of the right neck with SUVmax =4.56.
Similar to findings of FDG, our study showed that regions with high 68Ga-FAPI-04 uptake did not necessarily represent malignancy. A previous FAPI study analyzed SUVmax of 28 different types of tumors (31) and reported that although 68Ga-FAPI-04 uptake was higher in malignant lesions than in benign lesions and physiological uptake regions, there was still some overlap. There was no statistically significant difference in SUVmax between the benign uptake regions and the physiological regions. This suggests that SUVmax cannot be used as a differential diagnostic index of physiological and benign uptake regions.
There were some limitations in our study. Since this study was retrospective, pathological verification of the lesions was challenging. Most of the diagnosis were based on the clinical history and the experience of the reviewers and with reference to other imaging modalities (CT, MRI, ultrasound, etc.), similar to previous studies. Although our sample size was relatively large, it was not possible to cover all non-tumor uptake regions. There is still a need to accumulate more cases in order to summarize the features of non-malignant lesion uptake in 68Ga-FAPI-04 as a way to improve the accuracy of diagnosis.
Conclusions
This study evaluated the SUVmax of 68Ga-FAPI-04 in non-tumoral uptake regions with a relatively large sample population and elaborated the possible pathophysiological mechanisms of these non-tumoral uptake regions. The results indicated that quite a few tissues exhibit physiological uptake of 68Ga-FAPI-04. Gender has a more significant effect on physiological expression of 68Ga-FAPI-04 than age. No statistical differences in 68Ga-FAPI-04 uptake were found between benign and physiological high uptake regions. Our study showed that regions with high 68Ga-FAPI-04 uptake did not necessarily represent malignancy, and therefore, being familiar with physiological 68Ga-FAPI-04 uptake and the uptake of typical benign lesions can be helpful for physicians to interpret images and diagnose disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NQ participated in its design and coordination and drafted the manuscript. HW conducted statistical processing on the data. HYW and SR reviewed the images. ZY and XC contributed to data collection. YG, FX, FH, and JZ provided critical review and substantially revised the manuscript. All authors read and approved the final manuscript.
Funding
The study was partially supported by the National Natural Science Foundation of China (81871388), Project of Science and Technology Commission of Shanghai Municipality (19DZ1930703).
Acknowledgments
We would like to thank Qiaoyi Xue (Central Research Institute, UIH Group, Shanghai, China) for her linguistic assistance and her substantial revision during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jansen K, Heirbaut L, Cheng JD, Joossens J, Ryabtsova O, Cos P, et al. Selective inhibitors of fibroblast activation protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold. ACS Med Chem Lett (2013) 4(5):491–6. doi: 10.1021/ml300410d
2. Poplawski SE, Lai JH, Li Y, Jin Z, Liu Y, Wu W, et al. Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase. J Med Chem (2013) 56(9):3467–77. doi: 10.1021/jm400351a
3. Pure E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene (2018) 37(32):4343–57. doi: 10.1038/s41388-018-0275-3
4. Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med (2018) 59(9):1423–9. doi: 10.2967/jnumed.118.210435
5. Giesel FL, Adeberg S, Syed M, Lindner T, Jimenez-Franco LD, Mavriopoulou E, et al. FAPI-74 PET/CT using either (18)F-AlF or cold-kit (68)Ga labeling: Biodistribution, radiation dosimetry, and tumor delineation in lung cancer patients. J Nucl Med (2021) 62(2):201–7. doi: 10.2967/jnumed.120.245084
6. Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med (2018) 59(9):1415–22. doi: 10.2967/jnumed.118.210443
7. Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. (68)Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med (2019) 60(3):386–92. doi: 10.2967/jnumed.118.215913
8. Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, et al. Expression of the fibroblast activation protein during mouse embryo development. Int J Dev Biol (2001) 45(2):445–7.
9. Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: Differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U.S.A. (1988) 85(9):3110–4. doi: 10.1073/pnas.85.9.3110
10. Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging (2020) 13(9):e010628. doi: 10.1161/CIRCIMAGING.120.010628
11. Schmidkonz C, Rauber S, Atzinger A, Agarwal R, Gotz TI, Soare A, et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann Rheum Dis (2020) 79(11):1485–91. doi: 10.1136/annrheumdis-2020-217408
12. Luo Y, Pan Q, Yang H, Peng L, Zhang W, Li F. Fibroblast activation protein-targeted PET/CT with (68)Ga-FAPI for imaging IgG4-related disease: Comparison to (18)F-FDG PET/CT. J Nucl Med (2021) 62(2):266–71. doi: 10.2967/jnumed.120.244723
13. Terry SY, Koenders MI, Franssen GM, Nayak TK, Freimoser-Grundschober A, Klein C, et al. Monitoring therapy response of experimental arthritis with radiolabeled tracers targeting fibroblasts, macrophages, or integrin Alphavbeta3. J Nucl Med (2016) 57(3):467–72. doi: 10.2967/jnumed.115.162628
14. Zhang X, Song W, Qin C, Liu F, Lan X. Non-malignant findings of focal (68)Ga-FAPI-04 uptake in pancreas. Eur J Nucl Med Mol Imaging (2021) 28(8):2635–41. doi: 10.1007/s00259-021-05194-6
15. Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, et al. Increased uptake of (68)Ga-DOTA-FAPI-04 in bones and joints: Metastases and beyond. Eur J Nucl Med Mol Imaging (2021) 49(2):709–20. doi: 10.1007/s00259-021-05472-3
16. Zheng S, Lin R, Chen S, Zheng J, Lin Z, Zhang Y, et al. Characterization of the benign lesions with increased (68)Ga-FAPI-04 uptake in PET/CT. Ann Nucl Med (2021) 35(12):1312–20. doi: 10.1007/s12149-021-01673-w
17. Gundogan C, Guzel Y, Can C, Kaplan I, Komek H. FAPI-04 uptake in healthy tissues of cancer patients in (68)Ga-FAPI-04 PET/CT imaging. Contrast Media Mol Imaging (2021) 2021:9750080. doi: 10.1155/2021/9750080
18. Jiang D, Chen X, You Z, Wang H, Zhang X, Li X, et al. Comparison of [(68) Ga]Ga-FAPI-04 and [(18)F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: a bicentric retrospective study. Eur J Nucl Med Mol Imaging (2021) 49(2):732–42. doi: 10.1007/s00259-021-05441-w
19. Wang Q, Li YM, Li Y, Hua FC, Wang QS, Zhang XL, et al. (18)F-FDGPET/CT in fever of unknown origin and inflammation of unknown origin: a Chinese multi-center study. Eur J Nucl Med Mol Imaging (2019) 46(1):159–65. doi: 10.1007/s00259-018-4121-1
20. Kou Y, Jiang X, Yao Y, Shen J, Jiang X, Chen S, et al. Physiological tracer distribution and benign lesion incidental uptake of Al18F-NOTA-FAPI-04 on PET/CT imaging. Nucl Med Commun (2022) 43(7):847–54. doi: 10.1097/MNM.0000000000001563
21. Mu X, Huang X, Li M, Sun W, Fu W. Comparison of physiological uptake of normal tissues in patients with cancer using 18F-FAPI-04 and 18F-FAPI-42 PET/CT. Front Nucl Med (2022) 2:927843. doi: 10.3389/fnume.2022.927843
22. Dabir M, Novruzov E, Mattes-György K, Beu M, Dendl K, Antke C, et al. Distinguishing benign and malignant findings on [68 ga]-FAPI PET/CT based on quantitative SUV measurements. Mol Imaging Biol (2022) 1–10. doi: 10.1007/s11307-022-01759-5
23. Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol (1994) 124(4):401–4. doi: 10.1083/jcb.124.4.401
24. Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery (2002) 131(2):129–34. doi: 10.1067/msy.2002.119192
25. Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer (1988) 41(5):707–12. doi: 10.1002/ijc.2910410512
26. Keane FM, Yao TW, Seelk S, Gall MG, Chowdhury S, Poplawski SE, et al. Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs. FEBS Open Bio (2013) 4:43–54. doi: 10.1016/j.fob.2013.12.001
27. Dolznig H, Schweifer N, Puri C, Kraut N, Rettig WJ, Kerjaschki D, et al. Characterization of cancer stroma markers: in silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun (2005) 5:10.
28. Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D, et al. Pitfalls and common findings in (68)Ga-FAPI-PET - a pictorial analysis. J Nucl Med (2021) 63(6):890–6. doi: 10.2967/jnumed.121.262808
29. Zhang X, Song W, Qin C, Song Y, Liu F, Hu F, et al. Uterine uptake of 68Ga-FAPI-04 in uterine pathology and physiology. Clin Nucl Med (2022) 47(1):7–13. doi: 10.1097/rlu.0000000000003968
30. Acharya PS, Zukas A, Chandan V, Katzenstein AL, Pure E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol (2006) 37(3):352–60. doi: 10.1016/j.humpath.2005.11.020
31. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J Nucl Med (2019) 60(6):801–5. doi: 10.2967/jnumed.119.227967
32. Meletta R, Muller Herde A, Chiotellis A, Isa M, Rancic Z, Borel N, et al. Evaluation of the radiolabeled boronic acid-based FAP inhibitor MIP-1232 for atherosclerotic plaque imaging. Molecules (2015) 20(2):2081–99. doi: 10.3390/molecules20022081
33. Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast aactivation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur Heart J (2011) 32(21):2713–22. doi: 10.1093/eurheartj/ehq519
34. Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, et al. A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res (2003) 9(5):1639–47.
35. Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther (2006) 8(6):R171. doi: 10.1186/ar2080
36. Milner JM, Kevorkian L, Young DA, Jones D, Wait R, Donell ST, et al. Fibroblast activation protein alpha is expressed by chondrocytes following a pro-inflammatory stimulus and is elevated in osteoarthritis. Arthritis Res Ther (2006) 8(1):R23. doi: 10.1186/ar1877
37. Dienus K, Bayat A, Gilmore BF, Seifert O. Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: Implications for development of a novel treatment option. Arch Dermatol Res (2010) 302(10):725–31. doi: 10.1007/s00403-010-1084-x
38. Gao MQ, Kim BG, Kang S, Choi YP, Park H, Kang KS, et al. Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial-mesenchymal transition-like state in breast cancer cells in vitro. J Cell Sci (2010) 123(Pt 20):3507–14. doi: 10.1242/jcs.072900
39. Rettig WJ, Su SL, Fortunato SR, Scanlan MJ, Raj BK, Garin-Chesa P, et al. Fibroblast activation protein: Purification, epitope mapping and induction by growth factors. Int J Cancer (1994) 58(3):385–92. doi: 10.1002/ijc.2910580314
40. Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Muller E, et al. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology (1999) 29(6):1768–78. doi: 10.1002/hep.510290631
Keywords: 68 Ga-FAPI-04, SUV, physiological uptake, benign uptake, multicenter retrospective study
Citation: Qi N, Wang H, Wang H, Ren S, You Z, Chen X, Guan Y, Xie F, Hua F and Zhao J (2022) Non-tumoral uptake of 68Ga-FAPI-04 PET: A retrospective study. Front. Oncol. 12:989595. doi: 10.3389/fonc.2022.989595
Received: 08 July 2022; Accepted: 18 November 2022;
Published: 01 December 2022.
Edited by:
Sridhar Nimmagadda, Johns Hopkins University, United StatesReviewed by:
Vetri Sudar Jayaprakasam, Memorial Sloan Kettering Cancer Center, United StatesMin Yang, Jiangsu Institute of Nuclear Medicine, China
Copyright © 2022 Qi, Wang, Wang, Ren, You, Chen, Guan, Xie, Hua and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhao, cGV0Y2VudGVyQDEyNi5jb20=; Fengchun Hua, aHVhZmNAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Na Qi
Na Qi Hao Wang
Hao Wang Haiyan Wang1†
Haiyan Wang1† Shuhua Ren
Shuhua Ren Yihui Guan
Yihui Guan Fang Xie
Fang Xie Fengchun Hua
Fengchun Hua Jun Zhao
Jun Zhao