
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 November 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.989349
 Peng Li1
Peng Li1 Lu Liu2
Lu Liu2 Dong Wang1
Dong Wang1 Ronghua Yang1
Ronghua Yang1 Yunpeng Xuan1
Yunpeng Xuan1 Yudong Han1
Yudong Han1 Jinglong Wang1
Jinglong Wang1 Lijie Guo3
Lijie Guo3 Liwen Zhang3
Liwen Zhang3 Shanshan Zhang3
Shanshan Zhang3 Yongjie Wang1*
Yongjie Wang1*Background: Lung adenocarcinoma (LA) with a micropapillary component (LAMPC) is a histological subtype of lung cancer that has received increasing attention due to its correlation with poor prognosis, and its tendency to recur and metastasize. At present, comprehensive genomic profiles and clinicopathological features for LAMPC remain unclear and require further investigation.
Methods: From September 2009 to October 2020, a total of 465 LAMPC patients were recruited and divided into four groups according to MPC proportions, and the correlations between varying proportions of MPCs and clinicopathological characteristics were analyzed. Twenty-nine (29) LAMPC patients and 89 LA patients without MPC (non-MPC) that had undergone NGS testing were selected for further study The comprehensively analyze genomic variations and the difference between LAMPC and MPC were determined. In addition, Gene alterations of LAMPC between Chinese and Western populations were also compared using cBioPortal data.
Results: A higher proportion of MPCs, associated with higher tumor stage, pleural invasion, and vascular tumor thrombus formation, was determined in LA patients. Compared to non-MPC patients, LAMPC patients were determined to have a lower frequency of single nucleotide variants and a higher frequency of insertion-deletion mutations. Mutations in TP53, CTNNB1, and SMAD4, and ALK rearrangements/fusions were significantly more frequent in LAMPC patients. ERBB2 mutations were only detected in non-MPC patients. Gene mutations in the Wnt pathway were significantly more common in LAMPC patients as compared to non-MPC patients. ALK fusions were more prevalent in younger patients. Patients with KRAS or LBP1B mutations had significantly larger tumor diameters than patients with wild-type KRAS or LBP1B. Patients with KRAS mutations were more likely to develop vascular tumor thrombus. Using the cBioPortal public database, we determined that mutations in EGFR were significantly higher in Chinese patients than in a Memorial Sloan Kettering Cancer Center (MSKCC) Western cohort. ALK fusions were exclusively detected in the Chinese cohort, while mutations in KEAP1 and NOTCH4 were only detected in the MSKCC cohort. Our analysis of signaling pathways revealed that Wnt pathway gene mutations were significantly higher in the Chinese cohort.
Conclusion: LA patients with higher proportions of MPCs were determined to have a higher tumor stage, pleural invasion, and vascular tumor thrombosis formation. We comprehensively analyzed the genomic mutation characteristics of LAMPC patients and identified multiple, novel MPC-related gene alterations and pathway changes. Our data provide further understanding of the nature of the LAMPC and potential drug-targeted gene alterations, which may lead to new therapeutic strategies.
Lung cancer is the leading cause of cancer death in China, and lung adenocarcinoma (LA) is the most prevalent histological type of cancer amongst non-small cell lung cancers, accounting for nearly 50% of worldwide cases (1–3). In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) proposed a new system, in which LA is classified into the following five subtypes according to main histological patterns: lepidic, acinar, papillary, micropapillary, and solid. In 2015, the same classification system was adopted by the World Health Organization (4–6). LA with a micropapillary component (LAMPC), which occurs when a micropapillary pattern accounts for > 1% of an entire tumor, is an established histological subtype of lung cancer. LAMPC is characterized by the presence of tumor cells with floating ring-shaped glandular structures within the alveolar space. Due to its high malignancy and aggressiveness, as well as its poor prognosis and tendency to recur and metastasize, LAMPC has received increasing attention (7–9). However, differences in clinicopathological features and proportions of MPC in LA patients remain unclear.
A multivariate analysis revealed that MPC is an independent risk factor for lower recurrence-free survival and overall survival (OS) (10–12). Currently, surgical resection, chemotherapy, and targeted therapy are the main treatment options for pulmonary micropapillary adenocarcinoma. Patients with LAMPC have increased local recurrence when treated with limited resection (13) and micropapillary predominant LA in stage is benefits from adjuvant chemotherapy (14). Micropapillary-predominant adenocarcinomas respond well to platinum-based chemotherapy and EGFR tyrosine kinase inhibitors (TKI) (15, 16). While micropapillary-predominant adenocarcinomas benefit, to a greater extent, from adjuvant chemotherapy than other histological subtypes, they do not benefit from adjuvant radiotherapy (17). Campos-Parra et al. (18) determined that micropapillary-predominant lung adenocarcinomas respond better to chemotherapy, resulting in a better OS. Another study revealed that LAMPC patients with EGFR mutations treated with EGFR-TKIs had a significantly better post-recurrent survival than patients who did not receive TKI treatment (19).
In this study, we first analyzed correlations between different proportions of MPC and clinicopathological features in LAMPC patients, and then used next generation sequencing (NGS) to comprehensively analyze the genomic characteristics of LAMPC patients. We identified multiple new MPC-related gene alterations and pathway changes which may help us better understand the nature of this cancer subtype and determine potential drug-targeted gene alterations, potentially leading to new therapeutic strategies.
From September 2009 to October 2020, a total of 465 LAMPC patients were recruited from the Department of Thoracic Surgery at the Affiliated Hospital of Qingdao University. The patient cohort included 215 males and 250 females with a median age of 60 years old. Most patients had an early stage tumor and approximately 70% of patients were in Stages I-II. Patients were divided into four groups based on the proportions of MPC. We collected data in order to analyze the correlation between different proportions of MPC and clinicopathological characteristics. To comprehensively analyze genomic variations, 31 LAMPC samples (from 29 LAMPC patients) and additional 89 non-MPC that had undergone NGS testing were selected. Our study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Ethics No.: QYFYKYLL: 999811920), and all patients signed an informed consent form.
Clinicopathological data was collected from 465 patients with LAMPC and included patient gender, age at diagnosis, tumor size, histological subtype, morphological characteristics, clinical stage, pleural invasion, and vascular tumor thrombus invasion and spread through air spaces (STAS). Histological subtypes of LA were defined according to standard criteria provided by the 2011 International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS). TNM staging was based on the 8th Edition of the IASLC.
Formalin-fixed, paraffin-embedded (FFPE) tumor tissue and matched blood samples were collected, and genomic DNA was prepared using a commercial kit (Qiagen, Hilden, Germany). DNA concentrations were measured using Qubit. Patient samples were sequenced using the Illumina (Illumina Inc., CA, USA) NGS platform, with an average sequencing depth of > 50x. Based on the sequencing of at least 420 genes (420−638), a total of 296 genes within the intersection portion of each panel were selected for analysis. Genomic alterations, including single nucleotide variants (SNVs), short and long insertion-deletion variations (indels), copy number variations (CNVs), and gene rearrangements, were included in the analysis.
Statistical analyses were conducted using the R Statistical Software package (R Foundation for Statistical Computing, Vienna, Austria). Data for categorical variables are presented as frequencies and percentages, while data for continuous variables are reported as medians and percentiles. A student t-test or a Wilcoxon rank test were used to compare two continuous data sets. Chi-square tests or Fisher’s exact tests were used to compare two sets of categorical data. A 2-sided p value less than 0.05 was considered statistically significant.
A total of 465 LAMPC patients were divided into four groups according to proportions of MPC. One-hundred and six (106) patients had an MPC below 5% (MPC-1), 163 patients had an MPC of 5-20% (MPC-2), 108 patients had an MPC of 20-50% (MPC-3), and 88 patients had an MPC of at least 50% (MPC-4). We explored the correlation between different proportions of MPC and clinicopathological features, and determined that the proportions of MPC correlated with tumor stage. A higher proportion of MPC correlated with a more advanced stage (p < 0.05) (Table 1 and Figures 1A, B), but did not correlate with N status (p = 0.129) (Table 1 and Figure 1C). The proportion of MPC was significantly correlated with pleural invasion and vascular tumor thrombus formation. In other words, patients with a higher proportion of MPC are more likely to have pleural invasion and vascular tumor thrombus (p < 0.001) (Table 1 and Figures 1D, E). We also assess the correlation between STAS status and MPC, and observed that STAS was found in 214 of 465 (46.0%) cases, and STAS was found to be significant more prevalent in MPC-2 and MPC-3 groups compared to MPC-1 and MPC-4 groups (p <0.001, Table 1 and Figure 1F). Larger tumor diameters were also associated with a higher proportion of MPC (p < 0.001) (Table 1 and Figure 1G). No significant correlation was determined between MPC and gender or age (p > 0.05).

Table 1 Correlation analysis between different micropapillary content and clinicopathologic features.
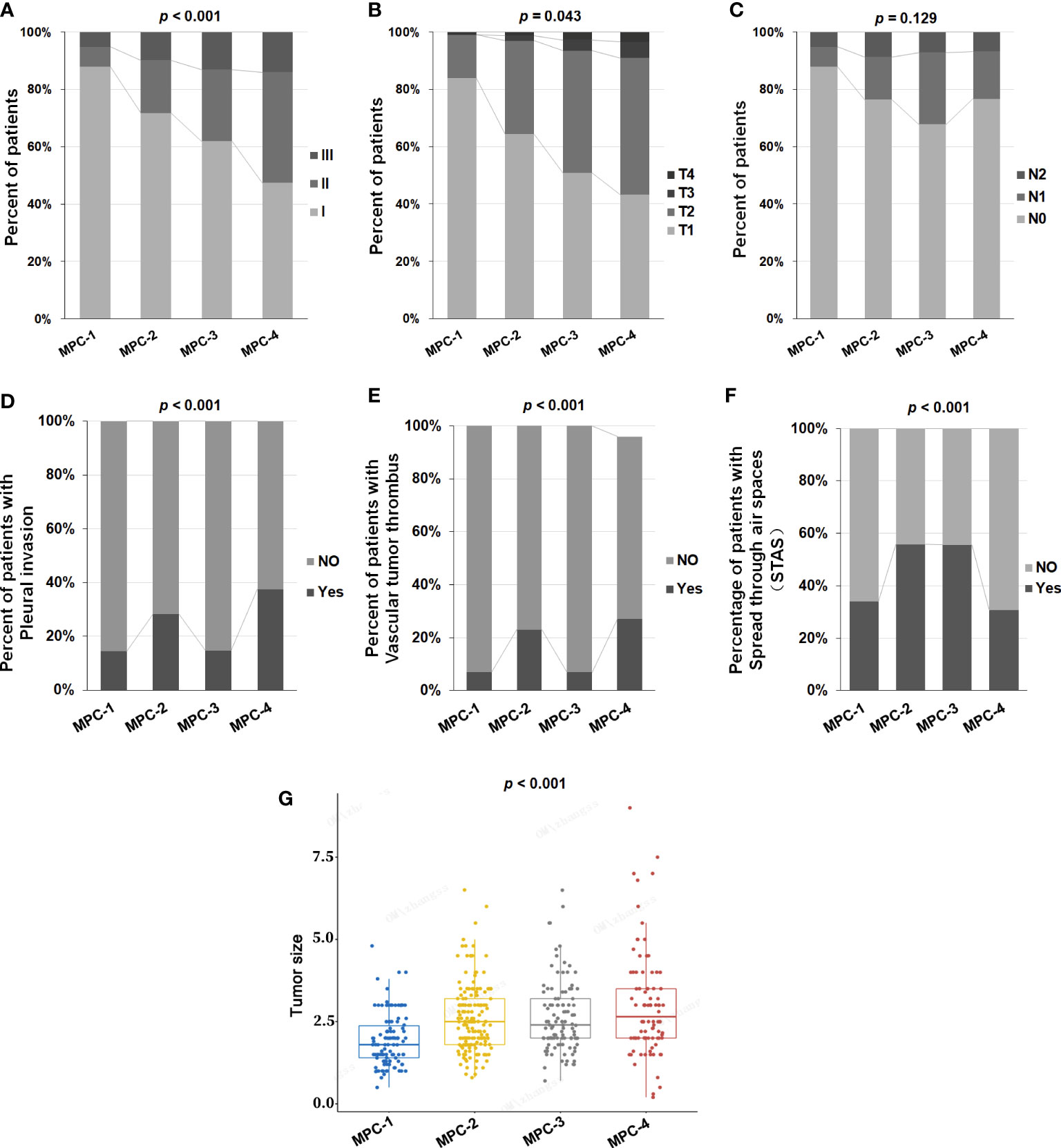
Figure 1 The correlation between different proportions of MPC and clinicopathological features in LAMPC patients. The correlation between different proportions of MPC and (A) stage, (B) T status, (C) N status, (D) pleural invasion, (E) vascular tumor thrombus, (F) spread through air spaces (STAS) and (G) tumor size. p< 0.05 was considered statistically significant, and p < 0.01 was considered more statistically significant.
To further explore the mechanism of malignant progression for tumors caused by MPC, molecular mechanisms related to tumor prognosis, and potential targets for effective treatment in patients with LAMPC, twenty-nine (20) LAMPC and 89 non-MPC patients that had undergone NGS testing were included in this analysis designed to compare differences in genomic characteristics between the LAMPC and non-MPC groups. Clinicopathological features for these 118 LA patients (including age, gender, tumor size, stage, T status, N status, pleural invasion, lymphovascular invasion and STAS) are summarized in Table 2. Higher proportion of LAMPC patients had pleural invasion, lymphovascular invasion and STAS than that patients with non-MPC. The 31 LAMPC samples from 29 MPC patients (two patients had two primary tumor samples) included 5 MPC-1, 13 MPC-2, 10 MPC-3, and 3 MPCs with an unknown proportion. NGS testing was performed for 29 MPC and 89 non-MPC patients. Two-hundred and ninety-six (296) genes were analyzed (Figure 2A). Gene mutations, including SNVs, indels, CNVs, and gene-gene fusions (FUS), were detected. Within the MPC cohort, a total of 159 somatic genes variants were detected. The most predominant variants were SNVs (62.3%, 99/158), CNVs (20.8%, 33/159), fusion/rearrangements (9.4%, 15/159), and indels (7.5%, 12/159). The frequency of SNVs was significantly lower (p = 0.01) in LAMPC patients as compared to non-MPC patients, while indels were more frequent within the LAMPC group than that in the non-MPC group (p < 0.01, Figure 2B). The top frequently altered genes in LAMPC patients were EGFR (61.3%), TP53 (41.9%), ALK (19.4%), CTNNB1 (16.1%), SMAD4 (12.9%), and KRAS (12.9%). The most frequent alterations in non-MPC patients were EGFR (64%), TP53 (22.5%), RBM10 (13.5%), ERBB2 (11.2%), KRAS (7.9%), MDM2 (7%), and TERT (7%). EGFR mutations had comparable pooled mutation rates in the LAMPC and non-MPC groups (61.3% vs. 64.0%). We also determined that mutations in TP53, ALK, CTNNB1, and SMAD4 were significantly enriched in LAMPC patients as compared to non-MPC patients (41.9% vs. 22.5%, p = 0.037; 16.1% vs. 2.2%, p = 0.012; 16.1% vs. 1.1%, p = 0.004; 12.9% vs. 2.2%, p = 0.038, respectively, Figure 2C). These genomic alterations may be potentially correlated with the formation of micropapillary structures. Mutations in KRAS also tended to have a higher frequency rate in LAMPC patients as compared to non-MPC patients (12.9% vs. 7.9%, p = 0.472, Figure 2C), although the rate was not statistically significant. ERBB2 mutations were only detected in non-MPC patients, and RBM10 mutations were detected more frequently in non-MPC than in LAMPC patients (13.5% vs. 6.5%, p = 0.516, Figure 2C).
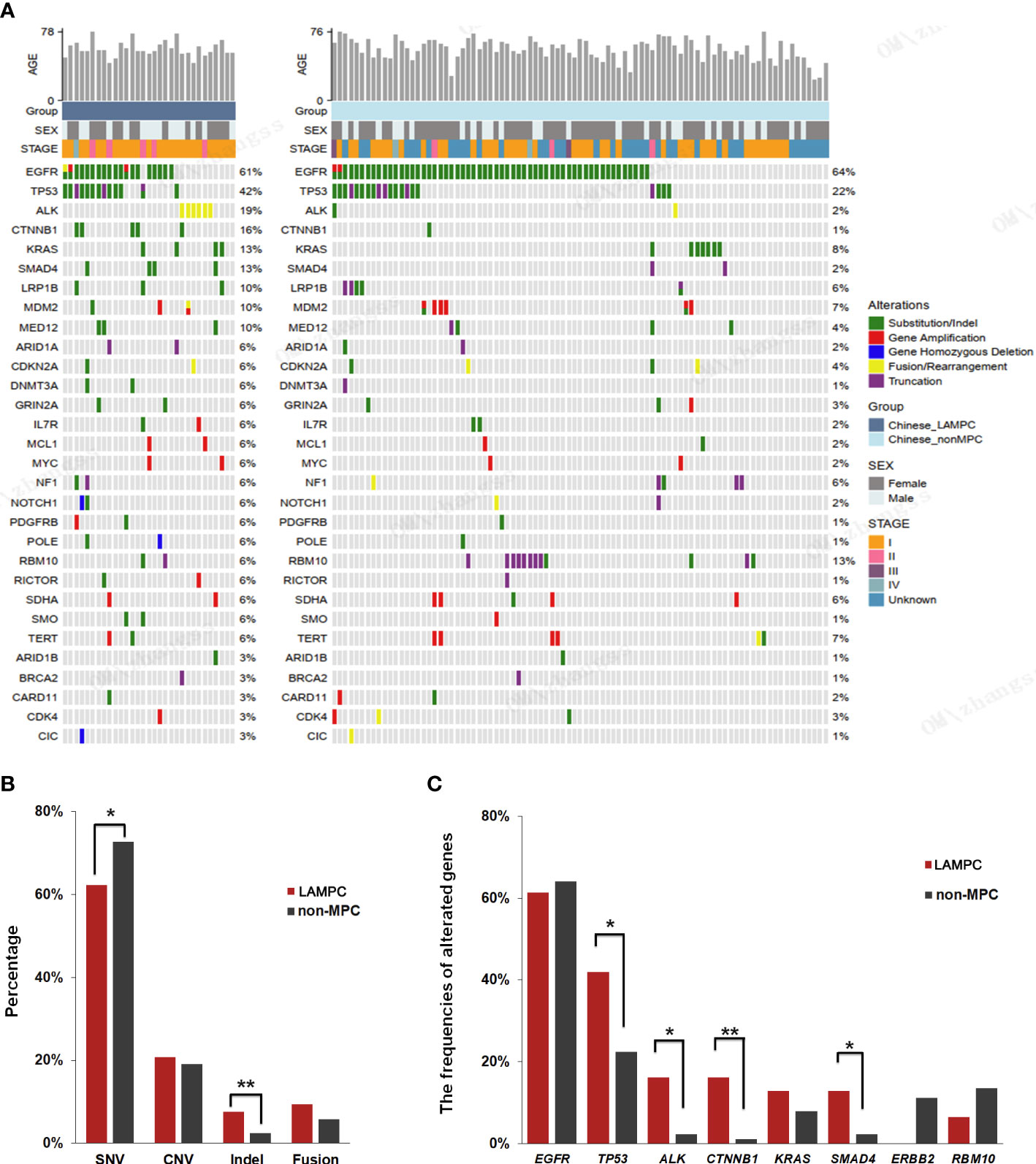
Figure 2 A comparison of genomic alterations between LAMPC and non-MPC patients. (A) A genomic alteration profile of 31 LAMPC samples from 29 patients and 89 non-MPC patients. (B) A comparison of mutation types between the two groups. (C) A comparison of major driver gene mutations between LAMPC and non-MPC groups. *p < 0.05, **p < 0.01.
In addition, the genomic alterations were also analyzed in MSKCC_MPC and MSKCC_non-MPC patients (Table 3). The top frequently altered genes in MSKCC_MPC patients were TP53 (42.9%), KRAS (34.3%), EGFR (17.1%), KEAP1 (17.1%), NOTCH4 (17.1%) and STK11 (17.1%). The most frequent alterations in MSKCC_non-MPC patients were KRAS (38.7%), TP53 (36.2%), EGFR (31.4%), RBM10 (17.4%), STK11 (14.9%) and KEAP1 (11.0%). High-frequency mutant genes were generally consistent between the MSKCC_MPC and MSKCC_non-MPC patients. We found that NOTCH4 mutation was significantly higher in the MSKCC_MPC group than in the MSKCC_non-MPC group (17.1% vs 3.6%, p = 0.003, Table 4).
Further enrichment using a signaling pathway analysis were performed in chinese_MPC patients and Memorial Sloan Kettering Cancer Center (MSKCC)_MPC patients. For chinese_MPC patients, 77.4% (24/31) harbored genomic alterations for the RTK/RAS/MAPK pathway, 22.6% (7/31) had cell-cycle pathway alterations, 38.7% (12/31) had Wnt pathway alterations, and 25.8% (8/31) had PI3K/AKT/mTOR pathway alterations (Figures 3A, B). The frequency of Wnt signaling pathway gene mutations was significantly higher in LAMPC patients as compared to non-MPC patients (38.7% vs. 13.5%, p = 0.002, Figure 3B). These results demonstrated that the Wnt alterations in signaling pathway may be involved in the regulatory mechanism of MPC formation.
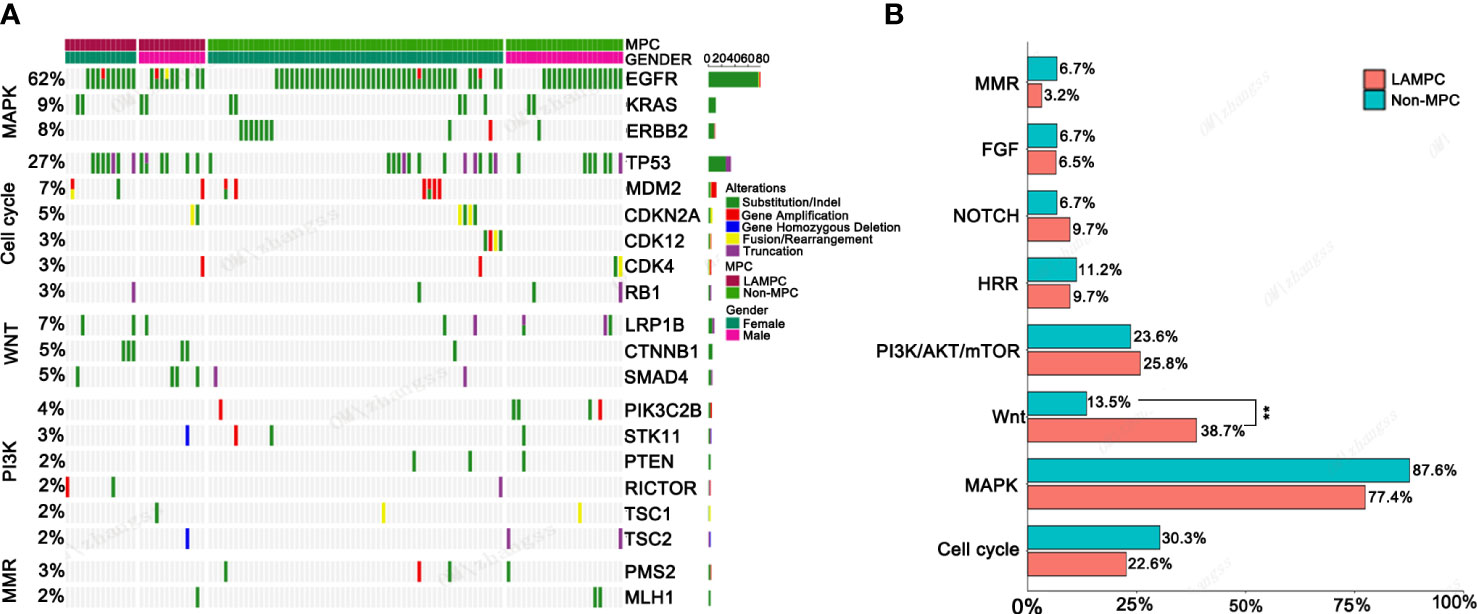
Figure 3 The genomic mutations involved in important signaling pathways in LAMPC and non-MPC patients. (A) The genomic alteration profile of pathways between LAMPC and non-MPC patients. (B) A comparison of the frequency of pathway changes between LAMPC and non-MPC patients. **p < 0.01.
We also analyzed the correlation between major driver genes and clinicopathological features, including age, gender, tumor size, nerve membrane invasion, pleural invasion, and vascular tumor thrombus, in LAMPC patients. The results indicated that ALK fusion was significant in younger patients as compared to elderly patients (median: 52.7 vs. 60.1, p = 0.035, Figure 4A and Table 5). KRAS and LBP1B mutations were significantly associated with tumor size, and patients with KRAS or LBP1B mutations had significantly larger tumor diameters as compared to patients with wild-type (median: 3.1 vs. 1.8 cm, p = 0.025; median:4.0 vs 2.0 cm, p = 0.013, respectively Figures 4B, C, and Table 5). Vascular tumor thrombus was additionally found in two of four LAMPC patients with KRAS mutations, but not in patients with wild-type KRAS, suggesting that LAMPC patients with KRAS mutations are more likely to develop vascular tumor thrombus (p = 0.0129, Figure 4D and Table 5).
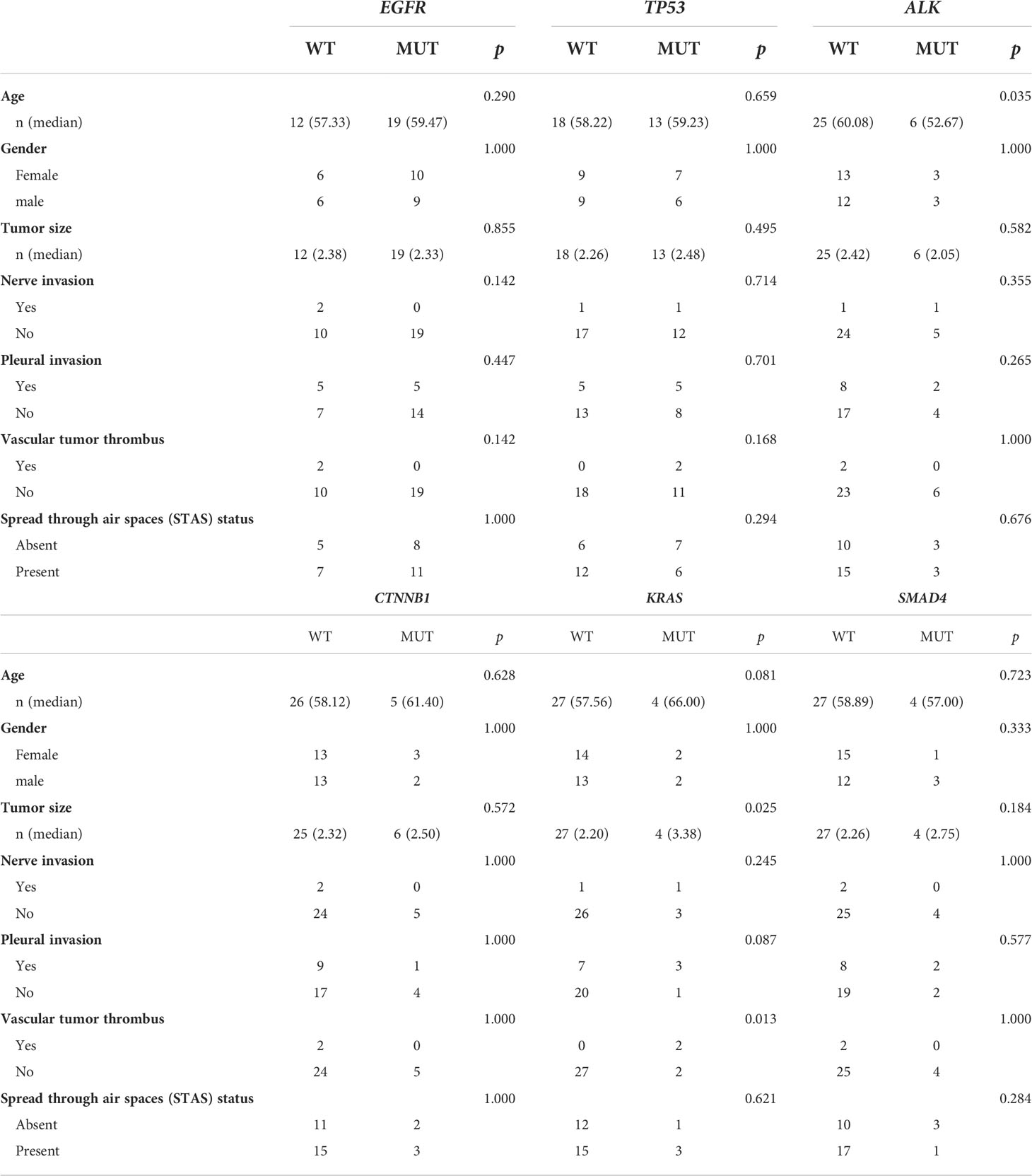
Table 5 Correlation analysis between major driver gene variants and clinical characteristics in patients with MPC.
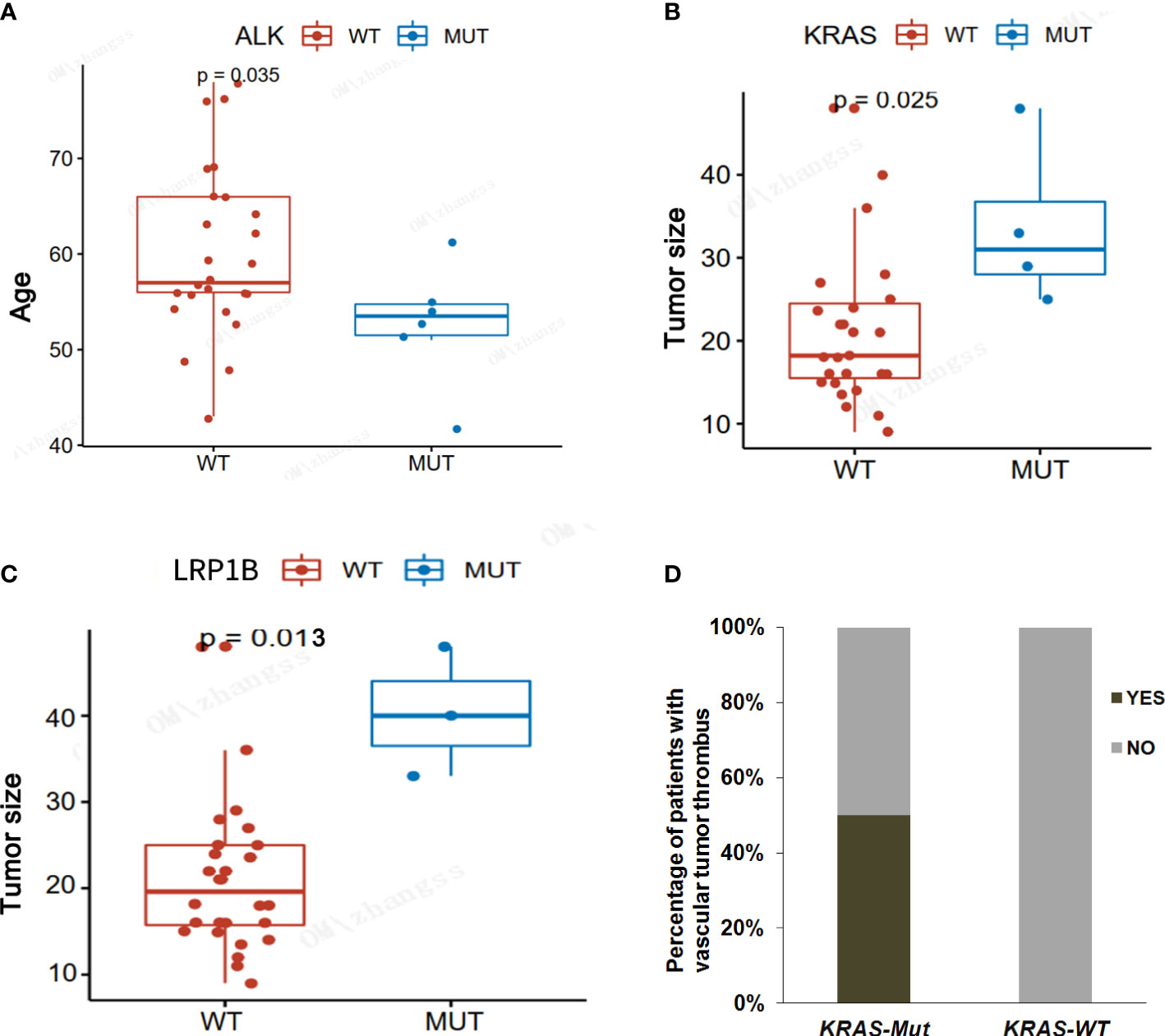
Figure 4 Correlation analyses between major driver gene alterations and clinicopathological characteristics in LAMPC patients. (A) A correlation analysis between ALK mutation and age. (B) A correlation analysis between KRAS mutation and tumor size. (C) A correlation analysis between LRBP1 mutation and tumor size. (D) A correlation analysis between KRAS mutation and vascular tumor thrombus. p< 0.05 was considered statistically significant, and p < 0.01 was considered more statistically significant.
We analyzed targeted sequencing data generated from 35 MPC patients in a MSKCC cohort using the cBioPortal public database. Patients in this group included 12 males and 23 females. Seventy-seven percent (77.1%) of patients had an early stage tumor (Stage I-III). For MSKCC_MPC patients, 74.3% patients harbored genomic alterations for the MAPK pathway, and 42.9% patients harbored genomic alterations for the PI3K/AKT/mTOR pathway. Particularly, 37.1% and 25.7% MSKCC_MPC patients with alterations respectively observed in HRR and NOTCH pathway, which was significantly higher than that in MSKCC_nonMPC patients (37.1% vs. 21.5%, p = 0.037; 25.7% vs 10.5%, p = 0.012; Figure 5A). There are still differences in the pathways in which the genetic mutations are located between the Chinese_MPC and MSKCC_MPC cohorts. A partial gene analysis, based on the intersection of panel data, indicated that in the MSKCC cohort, the most frequently mutated genes were: TP53 (42.9%), KRAS (34.3%), NOTCH4 (17.1%), STK11 (17.1%), KEAP1 (17.1%), and EGFR (17.1%). A comparative analysis revealed that EGFR mutations were significantly higher in the Chinese cohort as compared to the MSKCC cohort (61.3% vs. 17.1%, p = 0.0003). ALK fusions were exclusively detected in the Chinese cohort, and mutations in KEAP1 and NOTCH4 were only detected in the MSKCC cohort (Figure 5B). A major signaling pathway analysis revealed that HRR-related pathway alterations were significantly higher in the MSKCC cohort as compared to the Chinese cohort (34.3% vs. 9.7%, p = 0.0207), while the frequency of Wnt pathway gene mutations was significantly higher in the Chinese cohort as compared to the MSKCC cohort (38.7% vs. 11.4%, p = 0.0196) (Figure 5C).

Figure 5 A comparison of gene mutations in the Chinese LAMPC and MSKCC LAMPC cohorts. (A) A comparison of the frequency of pathway changes between MSKCC_MPC and MSKCC_non-MPC patients. A comparison of frequencies for (B) main driver gene alterations and (C) pathways changes in the Chinese LAMPC and MSKCC LAMPC cohorts. *p < 0.05, **p < 0.01.
Micropapillary adenocarcinoma is one of the most aggressive histologic subtypes of lung adenocarcinoma. Patients with LAMPC have a high risk of early recurrence after surgery, and the risk for recurrence persisted over long term. Although the incidence of MPC-predominant LA is low, nearly half of LA patients have a minor proportion of MPC, which, for these patients, may contribute to a poor prognosis. Therefore, to better understand the biology of this subtype of LA and to develop effective treatments, a comprehensive analysis of genetic alterations for LAMPC is necessary.
Several previous studies have determined that the presence of at least a 5% MPC is inversely associated with survival (21, 22). Patients with sub-centimeter LA, with a ≥ 5% MPC, treated with a wedge resection, had a higher risk of recurrence (23). However, a MPC < 5% has also been determined to have a significant impact on OS (22). At present, the extent to which the percentage of MPC affects tumor progression is unclear. In our study, we determined that LA patients with a higher proportion of MPC had a higher tumor stage, pleural invasion, and vascular tumor thrombus formation. Our results are in-line with previous reports which indicate that a higher proportion of MPC is associated with a high degree of cancer aggressiveness, advanced stage, a high maximum standardized uptake value and distant metastasis, and a poor prognosis, even if MPC is not predominant (24, 25). In past studies, the presence of MPC, lymph node metastasis, pleural invasion, and gender have been shown to be associated with a poor prognosis (26).These researches suggested the increase in micropapillae component is a possible reflection of tumor progression secondary to accumulation of molecular alterations, and relate to the degree of tumor malignancy.
Surgical resection, chemotherapy, and targeted therapy are currently chief options for the treatment of LAMPC. At present, in patients with small size LAMPC, lobectomy is the best option for a potential cure (13). However, following the complete resection of Stage I LA, the presence of MPC is still associated with a poor prognosis (26).Comprehensive genomic profiles aid in our understanding of the MPC molecular mechanisms that lead to a poor prognosis, further helping us discover potential therapeutic targets aimed at providing more accurate individualized treatment for LAMPC. However, at present, only a few, incomplete studies containing a genomic comparison of LAMPC versus non-MPC exist. In such studies, EGFR mutations have been determined to be strongly associated with MPC-predominant subtypes, with prevalence rates of EGFR, KRAS, and PIK3CA mutations in LAMPC, respectively, at 76.0%, 6.0%, and 2.0% (27). In the same study, no BRAF, NRAS, ALK, PDGFRA, or other mutations were elucidated. EGFR mutations have also been determined to be more frequent in MPC-predominant LA patients than in non-MPC patients (28). We determined that the frequency of SNVs was significantly lower in LAMPC patients, whereas the frequency of indels was higher in LAMPC patients as compared to non-MPC patients. In our study, the most common mutated genes in LAMPC patients were EGFR (61.3%), TP53 (41.9%), ALK (19.4%), CTNNB1 (16.1%), SMAD4 (12.9%), and KRAS (12.9%). TP53, CTNNB1, and SMAD4 mutations, as well as ALK rearrangements/fusions, were significantly higher in LAMPC patients. ERBB2 mutations were only detected in non-MPC patients. Our results are in contrast to a recent study by Zhang et al. (29), who determined similar mutation profiles and no significant differences in genomic alterations between LAMPC and non-MPC patients. We additionally found that ALK fusion was more frequent in younger patients, and that patients harboring mutations in KRAS or LBP1B had significantly larger tumor diameters as compared to patients with wild-type KRAS, suggesting that KRAS or LBP1B alterations are associated with LAMPC tumor progression. TTF1, MTOR, BAI3, and CDKN2A have been determined to be the most common mutated genes in Asian and European LAMPC patient cohorts (20). Mutations of EGFR, KRAS, and BRAF in LA, with at least a 75% MPC within a Western population, were investigated and 73% of patients were determined to harbor mutually exclusive mutations in one of these three genes (30). A Japanese study revealed that EGFR mutations are present in 40.1% of LAMPC (31). In our study, which employed the cBioPortal public database, EGFR mutations were determined to be significantly higher in the Chinese cohort as compared to the MSKCC cohort, ALK fusions were exclusively detected in the Chinese cohort, and KEAP1 and NOTCH4 mutations were only detected in the MSKCC_MPC cohort,and we suspect that NOTCH4 gene mutations may be a characteristic mutation unique to the MPC cohort of Western populations. Given the aggressive nature of LAMPC, our findings have implications for developing clinical therapeutic strategies to target LAMPC.
Our signaling pathway analysis revealed that in LAMPC the RTK/RAS/MAPK pathway, the cell-cycle pathway, the Wnt pathway, and the PI3K/AKT/mTOR pathway are altered. Wnt signaling pathway gene mutations were found to be significantly higher in LAMPC patients as compared to the non-MPC patients. The frequency of Wnt pathway gene mutations was also determined to be significantly higher in the Chinese cohort. Wnt signaling is key in regulating development and stemness, and has also been closely associated with cancer (32). Mutated Wnt pathway components can cause multiple growth-related pathologies and are frequent drivers in human cancers (33). For example, a past study revealed that WNT/β-catenin pathway activation via WNT1 overexpression and AXIN1 downregulation correlates with cadherin-catenin complex disruption and increased lymph node involvement in micropapillary-predominant LA (34). Such results suggest that the Wnt signaling pathway may be involved in regulatory mechanisms associated with MPC formation, and that it may play a role in the development of LAMPC.
In conclusion, we demonstrated that LA patients with a higher proportion of MPC tend to have a higher tumor stage, and are prone to pleural invasion and vascular tumor thrombosis formation, implying a potential link between MPC content and tumor malignancy. We comprehensively analyzed the characteristics of genomic alterations in LAMPC patients, and identified multiple new gene alterations and pathway changes associated with LAMPC. Our results provide more information regarding the nature of this cancer subtype and may aid future innovations related to drug-targeted gene alterations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Ethics No.: QYFYKYLL: 999811920), and all patients signed an informed consent form. The patients/participants provided their written informed consent to participate in this study.
Project development: YW, PL, LL, and DW. Data collection: RY, YX, YH and JW. Data analysis: PL, LZ and SZ. Manuscript writing/editing: YW, PL, LL, DW, RY, YX, YH, JW and LG. All authors contributed to the article and approved the submitted version.
This study was supported by funding from Wu Jieping Medical Foundation (320.6750.2021-01-4).
LG, SZ and LZ are employees of OrigiMed.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68 (6):394–424. doi: 10.3322/caac.21492
2. Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer (2014) 84(1):13–22. doi: 10.1016/j.lungcan.2014.01.009
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International association for the study of lung Cancer/American thoracic Society/European respiratory society: international multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc Am Thorac Soc (2011) 8(5):381–5. doi: 10.1513/pats.201107-042ST
5. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol (2015) 10(9):1240–2. doi: 10.1097/JTO.0000000000000663
6. Ci B, Yang DM, Cai L, Yang L, Girard L, Fujimoto J, et al. Molecular differences across invasive lung adenocarcinoma morphological subgroups. Trans Lung Cancer Res (2020) 9(4):1029–40. doi: 10.21037/tlcr-19-321
7. Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, et al. Predictive value of the international association for the study of lung cancer/American thoracic Society/European respiratory society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol (2014) 32(22):2357–64. doi: 10.1200/JCO.2013.50.1049
8. Mansuet-Lupo A, Bobbio A, Blons H, Becht E, Ouakrim H, Didelot A, et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: The experience of a French cohort. Chest (2014) 146(3):633–43. doi: 10.1378/chest.13-2499
9. Sonoda D, Kamizaki K, Matsuo Y, Aruga K, Mikubo M, Yamashita K, et al. Characterization of morphological alterations in micropapillary adenocarcinoma of the lung using an established cell line. Oncol Rep (2022) 47(1):19. doi: 10.3892/or.2021.8230
10. Ohe M, Yokose T, Sakuma Y, Miyagi Y, Okamoto N, Osanai S, et al. Stromal micropapillary component as a novel unfavorable prognostic factor of lung adenocarcinoma. Diagn Pathol (2012) 7:3. doi: 10.1186/1746-1596-7-3
11. Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: Clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg (2014) 147(3):921–28.e922. doi: 10.1016/j.jtcvs.2013.09.045
12. Li C, Shen Y, Hu F, Chu T, Yang X, Shao J, et al. Micropapillary pattern is associated with the development of brain metastases and the reduction of survival time in EGFR-mutation lung adenocarcinoma patients with surgery. Lung Cancer (2020) 141:72–7. doi: 10.1016/j.lungcan.2020.01.007
13. Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Institute (2013) 105(16):1212–20. doi: 10.1093/jnci/djt166
14. Wang C, Yang J, Lu M. Micropapillary predominant lung adenocarcinoma in stage IA benefits from adjuvant chemotherapy. Ann Surg Oncol (2020) 27(6):2051–60. doi: 10.1245/s10434-019-08113-0
15. Campos-Parra AD, Aviles A, Contreras-Reyes S, Rojas-Marin CE, Sanchez-Reyes R, Borbolla-Escoboza RJ, et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur Respir J (2014) 43(5):1439–47. doi: 10.1183/09031936.00138813
16. Sumiyoshi S, Yoshizawa A, Sonobe M, Kobayashi M, Fujimoto M, Tsuruyama T, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer (2013) 81(1):53–9. doi: 10.1016/j.lungcan.2013.04.003
17. Cao Y, Zhu LZ, Jiang MJ, Yuan Y. Clinical impacts of a micropapillary pattern in lung adenocarcinoma: a review. OncoTargets Ther (2016) 9:149–58. doi: 10.2147/OTT.S94747
18. Hung JJ, Jeng WJ, Chou TY, Hsu WH, Wu KJ, Huang BS, et al. Prognostic value of the new international association for the study of lung Cancer/American thoracic Society/European respiratory society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg (2013) 258(6):1079–86. doi: 10.1097/SLA.0b013e31828920c0
19. Zhang Y, Wang R, Cai D, Li Y, Pan Y, Hu H, et al. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol (2014) 9(12):1772–8. doi: 10.1097/JTO.0000000000000341
20. Lessard F, Morin F, Ivanchuk S, Langlois F, Stefanovsky V, Rutka J, et al. The ARF tumor suppressor controls ribosome biogenesis by regulating the RNA polymerase I transcription factor TTF-I. Mol Cell (2010) 38(4):539–50. doi: 10.1016/j.molcel.2010.03.015
21. Yanagawa N, Shiono S, Abiko M, Katahira M, Osakabe M, Ogata SY. The clinical impact of solid and micropapillary patterns in resected lung adenocarcinoma. J Thorac Oncol (2016) 11(11):1976–83. doi: 10.1016/j.jtho.2016.06.014
22. Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol (2016) 23(6):2099–105. doi: 10.1245/s10434-015-5043-9
23. Yao J, Zhu E, Li M, Liu J, Zhang L, Ke H, et al. Prognostic impact of micropapillary component in patients with node-negative subcentimeter lung adenocarcinoma: A Chinese cohort study. Thorac Cancer (2020) 11(12):3566–75. doi: 10.1111/1759-7714.13702
24. Wang W, Hu Z, Zhao J, Huang Y, Rao S, Yang J, et al. Both the presence of a micropapillary component and the micropapillary predominant subtype predict poor prognosis after lung adenocarcinoma resection: A meta-analysis. J Cardiothorac Surg (2020) 15(1):154. doi: 10.1186/s13019-020-01199-8
25. Wang K, Xue M, Qiu J, Liu L, Wang Y, Li R, et al. Genomics analysis and nomogram risk prediction of occult lymph node metastasis in non-predominant micropapillary component of lung adenocarcinoma measuring </= 3 cm. Front Oncol (2022) 12:945997. doi: 10.3389/fonc.2022.945997
26. Watanabe K, Sakamaki K, Ito H, Yokose T, Yamada K, Nakayama H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardio-Thoracic Surg (2020) 58(5):1010–8. doi: 10.1093/ejcts/ezaa138
27. Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol (2013) 30(3):645. doi: 10.1007/s12032-013-0645-1
28. Zhang J, Sun J, Zhang Z, Wang A, Liang X, Lu J, et al. Driver mutation profiles and clinicopathological correlation in pulmonary adenocarcinoma with a micropapillary component. Hum Pathol (2019) 85:242–50. doi: 10.1016/j.humpath.2018.11.008
29. Zhang S, Xu Y, Zhao P, Bao H, Wang X, Liu R, et al. Integrated analysis of genomic and immunological features in lung adenocarcinoma with micropapillary component. Front Oncol (2021) 11:652193. doi: 10.3389/fonc.2021.652193
30. De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol (2009) 131(5):694–700. doi: 10.1309/AJCPBS85VJEOBPDO
31. Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac (2013) 8(1):52–61. doi: 10.1097/JTO.0b013e3182769aa8
32. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene (2017) 36(11):1461–73. doi: 10.1038/onc.2016.304
33. Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer (2021) 21(1):5–21. doi: 10.1038/s41568-020-00307-z
34. Zhu L, Yang S, Zheng L, Zhang G, Cheng G. WNT/beta-catenin pathway activation via Wnt1 overexpression and Axin1 downregulation correlates with cadherin-catenin complex disruption and increased lymph node involvement in micropapillary-predominant lung adenocarcinoma. J Thorac Dis (2020) 12(10):5906–15. doi: 10.21037/jtd-20-1495
Keywords: lung adenocarcinoma, micropapillary component, next-generation sequencing, clinicopathological features, prognosis
Citation: Li P, Liu L, Wang D, Yang R, Xuan Y, Han Y, Wang J, Guo L, Zhang L, Zhang S and Wang Y (2022) Genomic and clinicopathological features of lung adenocarcinomas with micropapillary component. Front. Oncol. 12:989349. doi: 10.3389/fonc.2022.989349
Received: 08 July 2022; Accepted: 24 October 2022;
Published: 15 November 2022.
Edited by:
Helmut H. Popper, Medical University of Graz, AustriaCopyright © 2022 Li, Liu, Wang, Yang, Xuan, Han, Wang, Guo, Zhang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Wang, d2FuZ3lvbmdqaWVAcWR1aG9zcGl0YWwuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.