- 1The Four School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Key Laboratory of Integrated Oncology and Intelligent Medicine of Zhejiang Province, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
The incidence of hepatocellular carcinoma (HCC) associated with extrahepatic primary malignancy (EHPM) is extremely rare, especially for those that involve primary renal cell carcinoma (PRC). Here we present a case of a 66-year-old male who was diagnosed with HCC complicated with lymph node metastasis at posterior pancreas head and PRC. Biopsy results of the liver and the lymph node confirmed the diagnosis of HCC. The disease progressions of both HCC and PRC are controlled effectively following the initiation of comprehensive therapy including pembrolizumab, lenvatinib, radiotherapy, and transcatheter arterial chemo-embolization (TACE). Ultimately, the patient had successfully access to surgery and complete response (CR) of all the tumors were achieved after surgery.
Introduction
Primary liver cancer is one of the commonest malignancies globally. Among the newly-emerged cases annually, about half occurred in China (1). Hepatocellular carcinoma (HCC) accounted for about 85-90% of primary liver cancer. It is reported that in China, it ranked as high as 4th and 2nd in morbidity and mortality rates among other malignancies and it is also the leading cause of cancer-related death in men aged 60 and below (2, 3). Despite hematogenous dissemination in HCC being relatively common, lymph node metastasis is uncommon when compared to other malignant tumors (such as lung cancer, gastric cancer, and renal cancer), which account for only 1.0% of patients who received operation (4). Furthermore, the presence of lymph node metastasis is considered a manifestation of advanced HCC and usually indicates a worse disease outcome. Surgical resection is the mainstay of treatment for early HCC and possesses a 5-year survival rate of 54.8% (5). However, early symptoms of HCC are often vague and non-specific until the disease progresses into the advanced stage. Also, the majority of HCC cases are usually complicated with hepatitis or cirrhosis, not to mention poorer functional hepatic reserve (FHR) at the late stage. Altogether, diagnosis at a late stage would render the patients non-eligible for radical resection. Therefore, a thorough clinical evaluation and individualized therapy are of utmost importance for HCC patients. We hereby report a case of a male patient who was diagnosed with HCC complicated with lymph node metastasis at posterior pancreas head and PRC.
Case report
A 66-year-old male patient with a history of hepatitis B visited the community hospital in December 2021(baseline) due to yellow discoloration of the skin and the sclera, accompanied with right upper abdominal pain. The abdominal computed tomography (CT) scan revealed a mass in posterior pancreas head adjacent to IVC which suggested a malignant tumor, a hyperdense mass in right kidney, enlarged gallbladder, and cholelithiasis. Ultrasound-guided percutaneous transhepatic gallbladder drainage (PTGD) was performed upon admission but jaundice remained persistent post-operatively. The patient was then transferred to our hospital for further treatment.
On admission, physical examination revealed jaundice of the skin and the sclera accompanied with slight abdominal tenderness over the right hypochondriac region. No rebound tenderness or guarding was felt on palpation of the abdomen. Lab reports were as follow: AFP 9243.33 μg/L, PIVKA-II 897.6 mAU/mL, HBsAg (+), HBeAb (+), HBcAb IgG (+), HBV-DNA 8.81*10^2 IU/mL, Total Bilirubin 167.1 μmol/L, Direct Bilirubin 123.5 μmol/L. The enhanced abdominal CT scan was repeated which confirmed a mass measuring 8.0*4.5 cm at posterior pancreas head which was highly suggestive of malignant tumor. The scan also revealed multiple intrahepatic masses with dimensions ranging 1 to 3 cm, right renal mass which portrayed malignant feature, cholelithiasis, and cholecystitis (Figure 1).
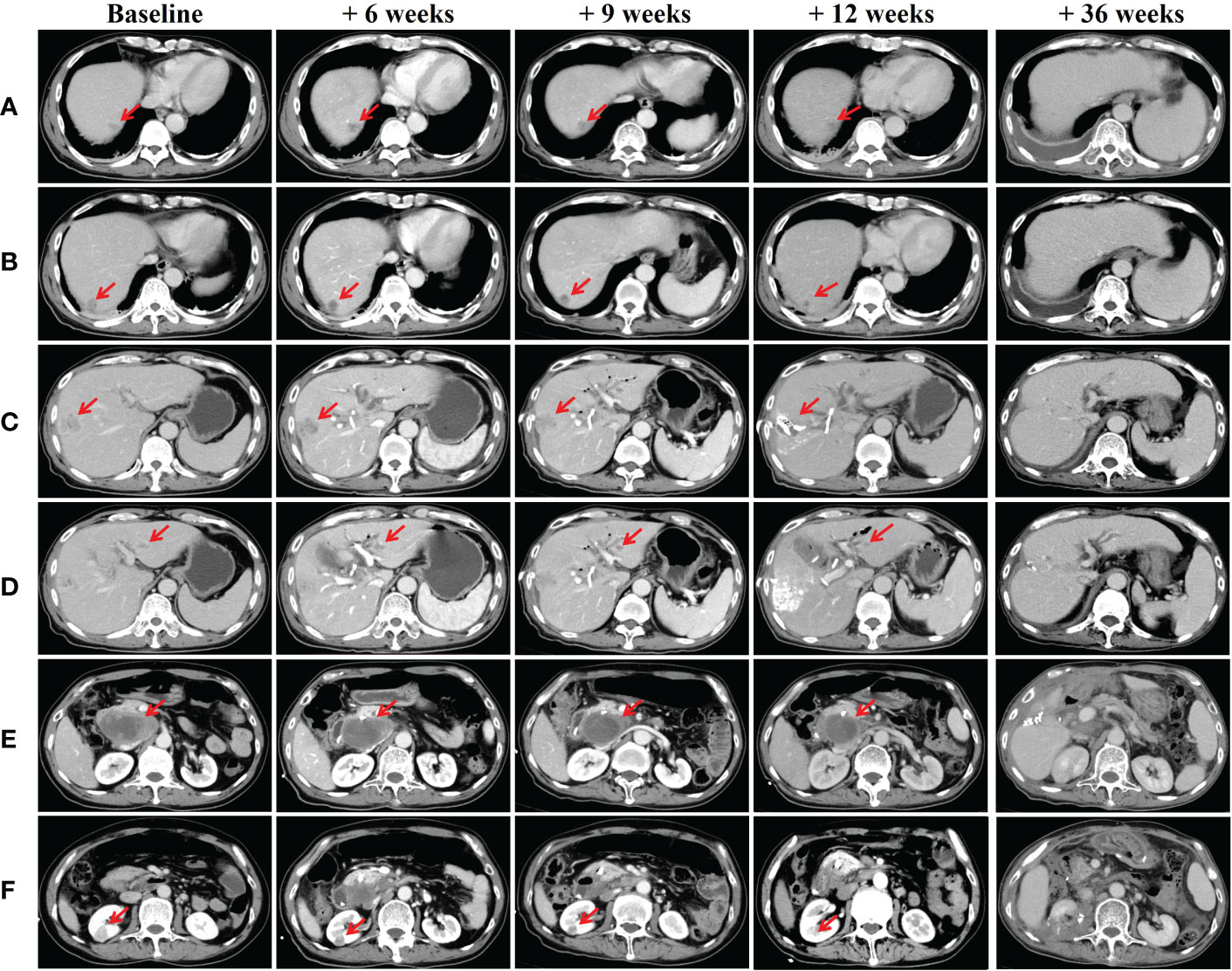
Figure 1 CT scans of the liver, posterior pancreas head and kidneys (A-D) Multiple intrahepatic space-occupying lesions; (E) Mass in posterior pancreas head; (F) Right renal mass. Abdominal CT scans were taken on baseline scan, baseline + 6 weeks (after first session: pembrolizumab and radiotherapy), baseline + 9 weeks (after second session: pembrolizumab plus lenvatinib), baseline + 12 weeks (after third session: pembrolizumab plus TACE) and baseline + 36 weeks (after synchronous partial resection of the liver and partial right nephrectomy).
A multidisciplinary team (MDT) discussion was conducted and diagnosis was narrowed down to (1) posterior pancreas head neoplasm with multiple intrahepatic metastases or primary liver cancer associated with posterior pancreas head metastasis; (2) Primary renal cell carcinoma. To further confirm the diagnosis and alleviate the symptoms, endoscopic retrograde cholangiopancreatography (ERCP)-guided biliary stenting and biopsy were performed in the posterior pancreas head mass. BUS-guided liver mass biopsy was also performed and Pathology test result of the liver indicated a poorly differentiated HCC while the immunohistochemistry test result showed HepPar1 (+), Arg-1 partial (+), GPC-3 (+), AFP (+), CK7 partial (+), CK19 (+), ki-67 (+) 50% (Figure 2). Similarly, pathology test result of posterior pancreas head mass revealed a poorly differentiated carcinoma while the immunohistochemistry result showed HepPar1 (+), Arg-1 slight (+), GPC-3 (+), AFP Partial (+), CK19 (+), ki-67 (+) 50% (Figure 2). Pathology of the right renal mass was unavailable as renal biopsy was strongly denied by both the patient and his family.
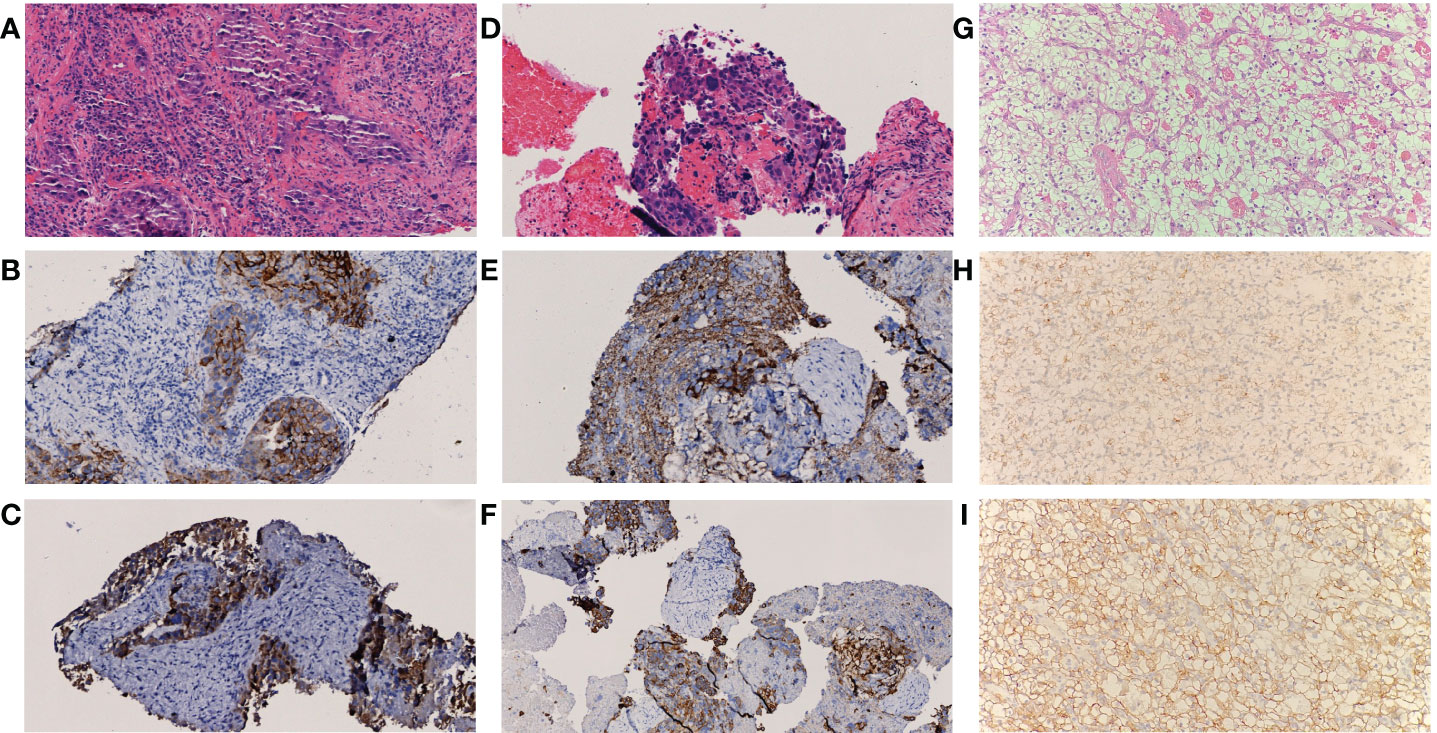
Figure 2 Pathological result of lesions (A-C) Histology of lesions in liver, (D-F) Histology of lesions in posterior pancreas head, (G-I) Histology of lesions in kidney. Both A and C showed atypical cell distributed in sheet manner (H&E staining, 200X); Immunohistochemistry result of samples in (B) GPC-3 (+), (C) CK19 (+), (E) Herpar1 (+) and (F) CK19 (+) indicate tumor cell differentiation of hepatic or hepatoid origin. Histopathological examination in (G) revealed clear-cell renal carcinoma, Furhman grade 2 with maximum diameter of 1.7 cm and negative margine (H&E staining, 200X); Immunohistochemical stain reveals positive marking result in AMACR (H) and CAIX (I), indicate tumor cell differentiation of renal origin.
Our team decided to adhere to the first-line treatment suggested by the National Comprehensive Cancer Network (NCCN) Guidelines for liver cancer since there was no definite target indicated for immunotherapy from the genetic testing. To minimize the effect of obstructive jaundice on PIVKA-II assessment, Vitamin K was supplemented immediately after the alleviation of biliary obstruction. Consequently, PIVKA-II level of the patient was successfully reduced to 64.01 mAU/mL after 10 days of vitamin K prescription which reflected that elevation of PIVKA-II was mainly due to biliary obstruction.
After the patient recovered from jaundice about 44 weeks ago (baseline + 2 weeks) (Total Bilirubin 43.3 μmol/L, Direct Bilirubin 33.2 μmol/L), combined therapy which consisted of pembrolizumab plus lenvatinib (pembrolizumab 200mg Q3W and lenvatinib 8mg QD) was initiated. Subsequently, radiotherapy for the metastatic lymph node posterior pancreas head was started 1 week after the combined therapy (Total radiation dose given was 30 Gy and fractionated into 6 doses of 5Gy each). 40 weeks ago (baseline + 6 weeks), repeated lab test results showed reduced level of AFP and PIVKA-II to 2173.04 μg/L and 63.66 mAU/mL respectively. Follow-up abdominal CT scan revealed multiple intrahepatic lesions which were dimensionally similar to the pre-treatment period. Nevertheless, the mass in the posterior pancreas head showed significant shrinkage and reduced enhancement intensity. The right renal mass was also smaller compared to the previous scan (Figure 1).
Pembrolizumab was administered for the second time about 40 weeks ago (baseline + 6 weeks) and post-therapy follow-up assessments were carried out about 36 weeks ago (baseline + 10 weeks). AFP and PIVKA-II levels were 1096.84 μg/L and 38 mAU/mL respectively. Overall shrinkage in all lesions was observed through CT scan (Figure 1). The third session of therapy which began 35 weeks ago (baseline + 11 weeks) included pembrolizumab and TACE. Post-therapy AFP and PIVKA-II levels were 249 μg/L and 76.28 mAU/mL respectively while other markers were within normal limits. Abdominal CT showed lipiodol deposition, and hepatic pneumatosis at segment VIII of the liver as well as further shrinkage of the aforementioned lesions (Figure 1). Due to the COVID-19 pandemic, the patient underwent the fourth to eighth pembrolizumab therapy at the local hospital. Subsequently, he underwent synchronous partial resection of the liver and partial right nephrectomy about 10 weeks ago (baseline + 36 weeks) with the pathology test result of the renal lesion indicated a clear-cell renal carcinoma while the immunohistochemistry test result showed CK (+), Vim (+), CD10 (+), CAIX (+), AMACR partial (+), PAX8 partial (+), Ki-67 (+) 3% (Figure 2). The posterior pancreas head metastatic lymph node involving pancreas and lower common bile duct was not resected because it was found complete colliquative necrosis and shrinked by 50% during the operation, thus a pancreatoduodenectomy was inevitable for complete resection, but the patient and his family refused to receive this procedure. Afterwards, the patient received the ninth pembrolizumab about 5 weeks ago (baseline + 41 weeks). The latest laboratory results showed a reduction in AFP and PIVKA-II levels to 0.95 μg/L and 21.32 mAU/mL. The latest CT scan showed that all the intrahepatic lesions had disappeared and the posterior pancreas head lymph node metastasis was completely necrosed and shrinked. As such, the patient was considered as complete response (CR) according to RECIST criteria after the comprehensive treatment.
Discussion
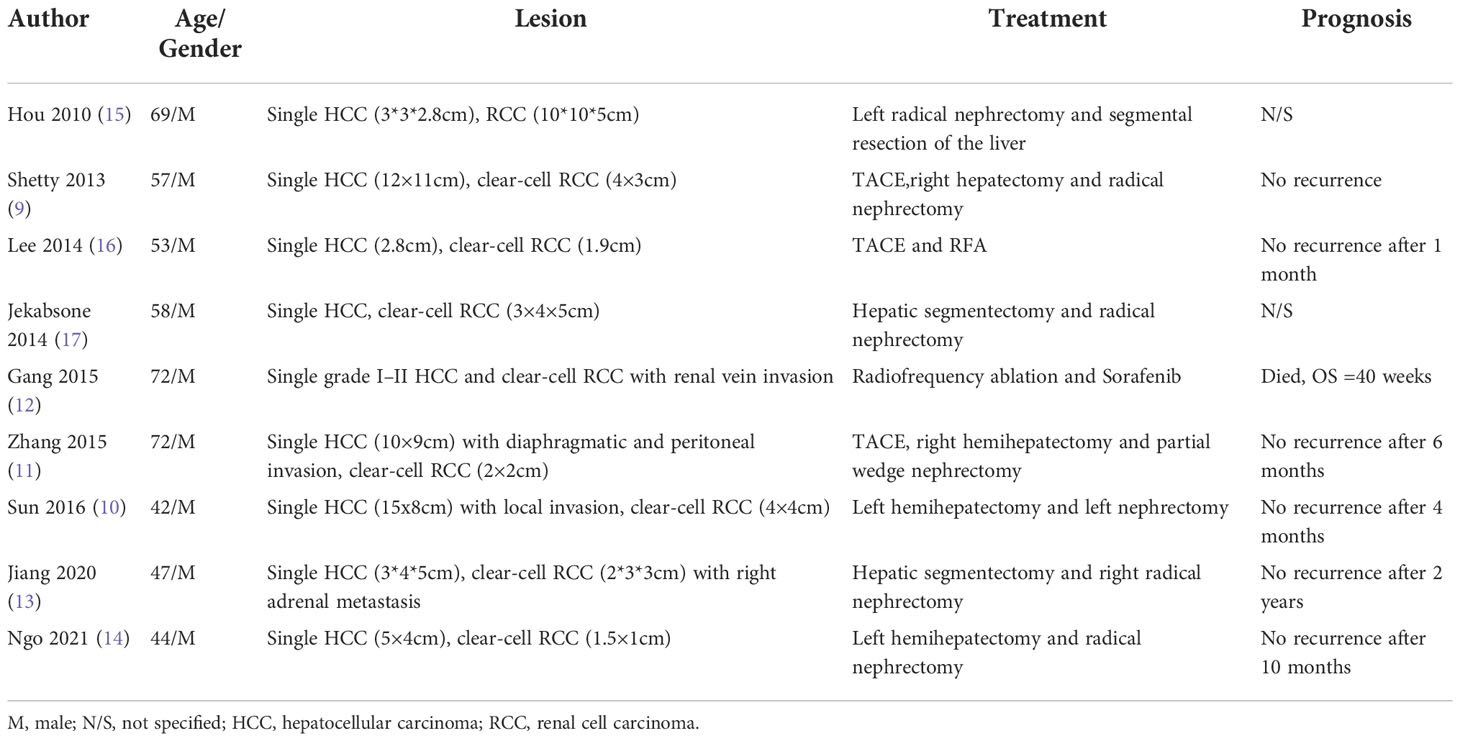
HCC complicated with extrahepatic multiple primary cancer (MPC) is rarely reported, albeit high incidence rate of HCC. With the advancements in early cancer detection, the prevalence of MPC has increased gradually over the past decade. According to previous reports, the incidence rate of MPC ranged between 0.7 and 11.7% (6). The most common extrahepatic tumor site in patients with HCC complicated with MPC may vary from region to region. In Korea for instance, colorectal (30.3%), gastric (27.3%), and breast (12.1%) are the three most common extrahepatic sites involved in MPC patients (7). Whereas, in China, the most common extrahepatic sites refer to lung (15.0%), colorectal (12.5%) and thyroid gland (12.5%) (8). It is even rarer for MPC to occur in the liver and kidney at the same time. To date, only 9 such cases have been reported with none involving HCC complicated with extrahepatic lymph node metastasis (9–17). (Table 1)
Although the pathogenesis of MPC remains unclear, the underlying mechanism was known to be attributed to host-, lifestyle-, environment-, gene- as well as treatment-related factors (18, 19). The risk factors of HCC include viral infection, alcohol intake, obesity, diabetes, and aflatoxin. Among others, Hepatitis C virus infection has been shown to have a significant association with an increased risk of renal cell carcinoma (20, 21).
After an in-depth discussion by the Molecular Tumor Board (MTB), our patient was diagnosed with HCC complicated with lymph node metastasis at posterior pancreas head, combined with PRC. Different from “HCC only” patients, management for second primary cancer should always be taken into careful consideration for HCC-associated MPC patients. As reported by 9 previous cases of HCC-associated PRC, all of them were solitary masses in both organs without lymph node metastasis. Seven out of the 9 cases received surgical treatment while the other 2 received interventional procedures and targeted therapy. Fortunately, all 9 patients achieved satisfactory outcomes after the treatments. However, our patient suffered from multifocal HCC with giant lymph node metastasis in the posterior pancreas head combined with PRC which has rendered the conventional radical resection implausible. Since there was no similar case reported in the past, the management for this patient is considered exploratory.
Due to the complexity of the case, MTB suggested to initiate combined antitumor therapy after the alleviation of biliary obstruction. Also, management of HCC was prioritized over PRC as recommended by urologists and oncologists due to higher malignancy portrayed by HCC. As per the recommendations of the National Comprehensive Cancer Network (NCCN) Guidelines on HCC, pembrolizumab and lenvatinib are preferred as the first-line therapy for advanced-stage liver cancer. And it is possible that the combination of pembrolizumab and lenvatinib can produce a synergistic antitumor effect (22, 23). Apart from that, NCCN Guidelines on PRC recommend the use of pembrolizumab plus lenvatinib as the first-line treatment for late-stage renal cancer (24). As a result of the combined treatment, significant shrinkage was appreciated in all lesions of the patient. (Figure 3)
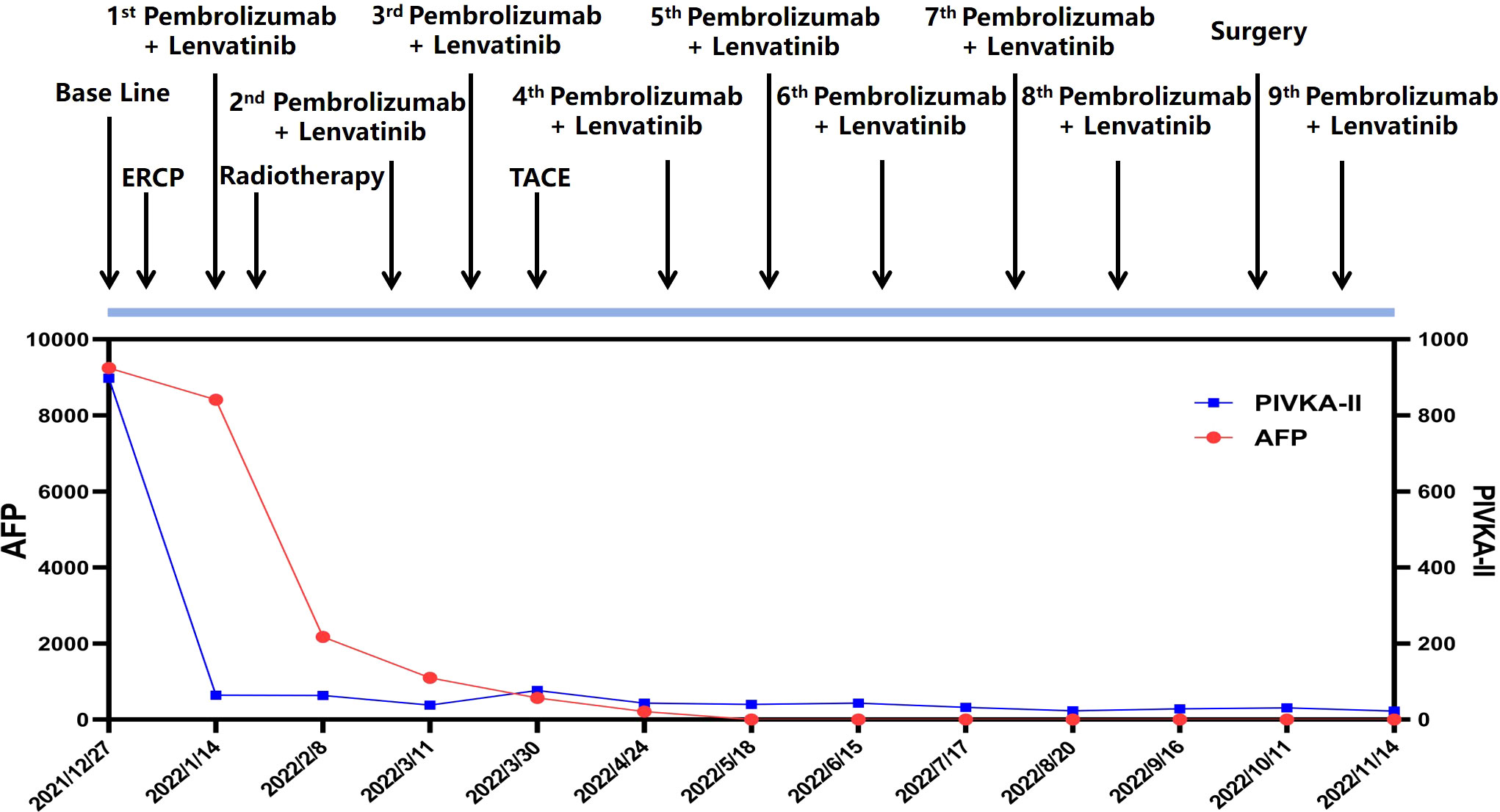
Figure 3 Timeline of the clinical course of the patient and the dynamic changes of tumor marker (AFP and PIVKA-II) along the treatment.
The emergence of stereotactic body radiotherapy (SBRT) has also been playing an important role in preventing radiation-induced liver disease (RILD), the most dreadful complication of conventional radiotherapy (25). It does not only allow precise delivery of high dose radiation to HCC lesion, but also protects the normal liver tissue to ensure optimal liver function. What’s more, synergistic effect of SBRT can be observed in targeted therapy and immunotherapy by multiple mechanisms. Generally, repeated radiotherapy would in turn causes radioresistance in the tumor (26, 27). In this regard, targeted therapies are employed to modulate tumor cells and angiogenesis as well as promote ceramide-mediated cell apoptosis to create a radiosensitive microenvironment (28, 29). The combination of SBRT and PD-1 inhibitor can optimise antitumor immunity. SBRT promotes the release of tumor-associated antigens (TAAs) to overcome the immune evasion and resistance to PD-1 inhibitors (30, 31). Notably, SBRT can reduce the tumor burden by both direct killing and indirect immune effect that can further improve the therapeutic effect of PD-1 inhibitors (32).
For advanced HCC, TACE is often regarded as the first-line treatment (33–35), and TACE combined with lenvatinib has been shown to significantly improve the treatment efficacy and prolong overall survival (36). Therefore, TACE was also included in our management strategy to achieve a better survival benefit.
In conclusion, we report for the first case of multifocal HCC with giant lymph node metastasis in posterior pancreas head accompanied with PRC. His tumor biomarkers’ level were significantly reduced as well as the tumor sizes after comprehensive treatment including pembrolizumab plus lenvatinib, radiotherapy and TACE. Ultimately, the patient had successfully access to surgery and complete response of all the tumors were achieved. Pembrolizumab plus lenvatinib remains the maintenance therapy in the follow-up. Since there were no precedent cases and treatment guidelines for this type of MPC, we hope to provide a reference for diagnosis and treatment of similar patients in the future through our experience in this case.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC and ZZ performed the literature search and drafted the manuscript. XX designed and supervised the study. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the patient in this report and his family.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology (2019) 156(2):477–91.e471. doi: 10.1053/j.gastro.2018.08.065
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW, Zeng HM, et al. Report of cancer incidence and mortality in China, 2014. Zhonghua Zhong Liu Za Zhi (2018) 40(1):5–13. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002
4. Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, et al. Report of the 20th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res (2020) 50(1):15–46. doi: 10.1111/hepr.13438
5. Holzner ML, Tabrizian P, Parvin-Nejad FP, Fei K, Gunasekaran G, Rocha C, et al. Resection of mixed hepatocellular-cholangiocarcinoma, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma. Liver Transpl (2020) 26(7):888–98. doi: 10.1002/lt.25786
6. Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol (2003) 26(1):79–83. doi: 10.1097/00000421-200302000-00015
7. Hong S, Jeong SH, Lee SS, Chung JW, Yang SW, Chung SM, et al. Prevalence and outcomes of extrahepatic primary malignancy associated with hepatocellular carcinoma in a Korean population. BMC Cancer (2015) 15:146. doi: 10.1186/s12885-015-1169-1
8. Xu W, Liao W, Ge P, Ren J, Xu H, Yang H, et al. Multiple primary malignancies in patients with hepatocellular carcinoma: A largest series with 26-year follow-up. Med (Baltimore) (2016) 95(17):e3491. doi: 10.1097/MD.0000000000003491
9. Shetty GS, Bhalla P, Desai SM, Wagle PK, Mehta HS. Synchronous hepatocellular carcinoma with renal cell carcinoma: a case report and review of literature of multiple synchronous primary malignancies. Indian J Surg (2013) 75(Suppl 1):290–2. doi: 10.1007/s12262-012-0684-4
10. Sun JJ, Yang TB, Yang YH, Liu WF, Song JX. Synchronous double primary malignancies of the liver and kidney: A case report. Oncol Lett (2016) 11(3):2057–60. doi: 10.3892/ol.2016.4194
11. Zhang W, Wang Q, Jiang YX, Lu Q, Yu WJ, Liu Y, et al. Simultaneous double primary clear cell carcinomas of liver and kidney: a case report and review of literature. Int J Clin Exp Pathol (2015) 8(1):995–9.
12. Gang G, Hongkai Y, Xu Z. Sorafenib combined with radiofrequency ablation in the treatment of a patient with renal cell carcinoma plus primary hepatocellular carcinoma. J Cancer Res Ther (2015) 11(4):1026. doi: 10.4103/0973-1482.150405
13. Jiang H, Zhao S, Li G. Simultaneous renal clear cell carcinoma and primary clear cell carcinoma of the liver: A case report. Med (Baltimore) (2020) 99(47):e23263. doi: 10.1097/MD.0000000000023263
14. Ngo DHA, Le Trong B, Le Dinh D, Le Dinh K, Pham Anh V, Nguyen Van M, et al. Synchronous renal cell carcinoma and hepatocellular carcinoma. Res Rep Urol (2021) 13:251–6. doi: 10.2147/RRU.S307541
15. Hou TC, Wu CC, Yang CR, Wang J. Synchronous renal cell carcinoma and clear cell hepatocellular carcinoma mimicking metastatic disease. Pathol Res Pract (2010) 206(5):342–5. doi: 10.1016/j.prp.2009.06.008
16. Lee YS, Kim JH, Yoon HY, Choe WH, Kwon SY, Lee CH. A synchronous hepatocellular carcinoma and renal cell carcinoma treated with radio-frequency ablation. Clin Mol Hepatol (2014) 20(3):306–9. doi: 10.3350/cmh.2014.20.3.306
17. Jekabsone S, Bogdanova T, Abolins A, Vanags A, Gardovskis J, Strumfa I. Synchronous renal and hepatocellular carcinomas. Acta Chirurgica Latviensis (2015) 14(2):40–2. doi: 10.1515/chilat-2015-0008
18. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: Challenges and approaches, a review. ESMO Open (2017) 2(2):e000172. doi: 10.1136/esmoopen-2017-000172
19. Copur MS, Manapuram S. Multiple primary tumors over a lifetime. Oncol (Williston Park) (2019) 33(7):629384.
20. Ma Y, Huang Z, Jian Z, Wei X. The association between hepatitis c virus infection and renal cell cancer, prostate cancer, and bladder cancer: A systematic review and meta-analysis. Sci Rep (2021) 11(1):10833. doi: 10.1038/s41598-021-90404-2
21. Wu D, Hu S, Chen G, Chen L, Liu J, Chen W, et al. Association of hepatitis c infection and risk of kidney cancer: A systematic review and meta-analysis of observational studies. J Viral Hepat (2021) 28(2):226–35. doi: 10.1111/jvh.13434
22. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One (2019) 14(2):e0212513. doi: 10.1371/journal.pone.0212513
23. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci (2018) 109(12):3993–4002. doi: 10.1111/cas.13806
24. Motzer RJ, Jonasch E, Michaelson MD, Nandagopal L, Gore JL, George S, et al. NCCN guidelines insights: Kidney cancer, version 2.2020. J Natl Compr Canc Netw (2019) 17(11):1278–85. doi: 10.6004/jnccn.2019.0054
25. Koay EJ, Owen D, Das P. Radiation-induced liver disease and modern radiotherapy. Semin Radiat Oncol (2018) 28(4):321–31. doi: 10.1016/j.semradonc.2018.06.007
26. Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and predicting radiation response. Semin Radiat Oncol (2015) 25(4):260–72. doi: 10.1016/j.semradonc.2015.05.004
27. Gil Marques F, Poli E, Malaquias J, Carvalho T, Portelo A, Ramires A, et al. Low doses of ionizing radiation activate endothelial cells and induce angiogenesis in peritumoral tissues. Radiother Oncol (2019) 141:256–61. doi: 10.1016/j.radonc.2019.06.035
28. Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: Exploring mechanisms of interaction. Clin Cancer Res (2003) 9(6):1957–71. doi: 10.1159/000070622
29. Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene (2003) 22(37):5897–906. doi: 10.1038/sj.onc.1206702
30. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat Rev Clin Oncol (2016) 13(8):516–24. doi: 10.1038/nrclinonc.2016.30
31. Yuan Z, Fromm A, Ahmed KA, Grass GD, Yang GQ, Oliver DE, et al. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-Negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol (2017) 12(9):e135–6. doi: 10.1016/j.jtho.2017.04.029
32. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-Cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature (2017) 545(7652):60–5. doi: 10.1038/nature22079
33. Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan society of hepatology 2013 update (3rd JSH-HCC guidelines). Hepatol Res (2015) 45(2):123–7. doi: 10.1111/hepr.12464
34. Chan SL, Mo FK, Johnson PJ, Liem GS, Chan TC, Poon MC, et al. Prospective validation of the Chinese university prognostic index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol (2011) 26(2):340–7. doi: 10.1111/j.1440-1746.2010.06329.x
35. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology (2014) 146(7):1691–1700 e1693. doi: 10.1053/j.gastro.2014.02.032
Keywords: hepatocellular carcinoma, primary renal carcinoma, lymph node metastasis, targeted therapy, immunotherapy, transcatheter arterial chemo-embolization
Citation: Chen J, Zhou Z, Chen W, Khan AA, Liu Z, Wang K, Yang F and Xu X (2022) Hepatocellular carcinoma complicated with huge posterior pancreas head lymph node metastasis and primary renal carcinoma: A case report. Front. Oncol. 12:989172. doi: 10.3389/fonc.2022.989172
Received: 08 July 2022; Accepted: 24 November 2022;
Published: 08 December 2022.
Edited by:
Jiang Chen, Zhejiang University, ChinaReviewed by:
Chen Hu, Xiamen University, ChinaVincenzo Lizzi, Universitaria Ospedali Riuniti di Foggia, Italy
Copyright © 2022 Chen, Zhou, Chen, Khan, Liu, Wang, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Xu, emp4dUB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jun Chen1,2,3†
Jun Chen1,2,3† Zhiyi Zhou
Zhiyi Zhou Abid Ali Khan
Abid Ali Khan Zhikun Liu
Zhikun Liu Fan Yang
Fan Yang Xiao Xu
Xiao Xu