- 1Department of Internal Medicine, Third Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- 2Department of Organ Transplantation, the Third Medical Center of Chinese People’s Liberation Army (PLA) General Hospital, Beijing, China
- 3Faculty of Hepatopancreatobiliary Surgery, the First Medical Center of Chinese People’s Liberation Army (PLA) General Hospital, Beijing, China
- 4Department of General Surgery, First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- 5Department of Disease Control and Prevention, Rocket Force Characteristic Medical Center, Beijing, China
Background: Cholangiocarcinoma (CCA) is a malignant tumor originating from bile duct epithelial cells that no obvious clinical symptoms and specific clinical manifestations are shown in the early stage of CCA.
Methods: Propensity score matching (PSM) is a quasi-experimental method in which this study used. Patients were enrolled from Department of General surgery, First Affiliated Hospital of Jinzhou Medical University from March 1, 2010, to December 30, 2019. Totally 170 patients with CCA were enrolled in this study.
Results: We performed a 1:2 PSM study and found that patients with losartan group showed both comparable median OS (overall survival) and TTR (time to recurrence) to those in the patients without losartan group before PSM. However, after matching, patients with losartan group showed favorable median OS and TTR than those in the patients without losartan group. Then we performed Cox proportional hazards models and found that patients with losartan was an independent factor after multivariable analysis for patients with CCA. Furtherly, we sequenced serial cfDNA were performed in 10 patients with losartan and 9 patients without losartan who received adjuvant chemotherapy after tumor resection. These results showed that the treatment of losartan was related with tumor microenvironment and could be potentially useful to combine the immunotherapy for patients with CCA.
Conclusion: In conclusion, this study demonstrated that the treatment of losartan could increase the efficacy of adjuvant chemotherapy and identified as an independent survival predictor for patients with CCA. Moreover, losartan could be potentially useful to combine the immunotherapy for patients with CCA.
Introduction
Cholangiocarcinoma (CCA) is a malignant tumor originating from bile duct epithelial cells that no obvious clinical symptoms and specific clinical manifestations are shown in the early stage of CCA (1, 2). The tumor is usually discovered due to the symptom of biliary tract obstruction in the later stage of the disease, at which point the optimal time for surgery has passed. As a result, the treatment effect and clinical prognosis of CCA are not satisfactory. Patients with surgically resected tumors have a 5-year survival rate of 5%, and the survival time of advanced patients who do not receive surgery is less than 1 year. At present, Cholangiocarcinoma is the second most common hepatic malignancy after hepatocellular carcinoma (HCC), and the overall incidence of cholangiocarcinoma has increased progressively worldwide over the past four decades. cholangiocarcinomas are categorized according to anatomical location as intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA). Each subtype has a distinct epidemiology, biology, prognosis, and strategy for clinical management (3–5).
Surgery is the preferred treatment option for all three disease subtypes, but a minority of patients (approximately 35%) have early-stage disease that is amenable to surgical resection with curative intent. For patients with advanced-stage or unresectable cholangiocarcinoma, the available systemic therapies are of limited effectiveness: the median overall survival with the current standard-of-care chemotherapy regimen (gemcitabine and cisplatin) is <1 year. The combination of gemcitabine and cisplatin is the current first-line chemotherapy for patients with advanced-stage cholangiocarcinoma not amenable to locoregional and surgical options, irrespective of anatomical disease subtype. There are now 2 published randomized studies for iCCA using adjuvant chemotherapy (6–9). The adjuvant standard of care for all CCA subtypes has been established as capecitabine based on the BilCap study and reiterated in the NCCN guidelines,12 and as such patients with CCA treated with capecitabine as adjuvant therapy may anticipate a survival of 51.1 months. The PRODIGE12 study of gemcitabine plus oxaliplatin (GEMOX) in an adjuvant setting was clearly negative; perhaps because it was underpowered for outcome prediction. The iCCA subgroups of BilCap (n = 84) and PRO- DIGE12 (n = 86) had hazard ratios (HRs) of 0.65 (95% CI 0.35–1.18) and 0.718 (95% CI 0.431 to 1.196) respectively, although neither reached statistical significance. It must be emphasized that these post hoc analyses are exploratory rather than conclusive, particularly as the primary endpoint of the PRODIGE12 study was negative (10, 11).
In recent years, studies have suggested that angiotensin II (Ang II) signaling pathway plays an important role in tumor progression. Angiotensin II type 1 receptor (AT1R) overexpression is also associated with poor prognosis, and the Ang II/AT1R axis is closely related to tumor progression (12–14); however, there is also the opposite view. Renin-angiotensin system (RAS) is far more complicated than we think. It has a close relationship with the proliferation, angiogenesis, invasion and metastasis of tumor cells, especially in recent years, some new RAS components have further verified the role of RAS in the development of cancers. Angiotensin II, as the main vasoactive peptide of RAS, plays an important role in the treatment of kidney protection, reducing uric acid, improving insulin resistance, promoting skeletal muscle remodeling, delaying and reversing tissue organ fibrosis, while there is still controversy in tumor treatment. Losartan is an FDA-approved antihypertensive agent that blocks AGTR1 (15–17). Several studies have reported the synergy of losartan with chemotherapy across numerous tumors (15, 18–20). However, no report investigated the effect of losartan on chemotherapy for patients with CCA, which is the overarching objective of this study
In this study, we aimed to compare the survival outcomes related with the effect of losartan in patients CCA after surgery. Furthermore, we sought to assess the synergistic effect of losartan with chemotherapy in patients with CCA.
Patients and methods
Patients and treatment schedule
The study was approved by the Ethics Committee of the Institutional Review Board of the Department of General surgery, First Affiliated Hospital of Jinzhou Medical University. Written informed consent was waived owing to the retrospective and deidentified nature of the study. Between March 1, 2010, to December 30, 2019, patients who were pathologically diagnosed with cholangiocarcinoma and treated with curative resection and anti-cancers adjuvant chemotherapy agents as a first-line therapy in First Affiliated Hospital of Jinzhou Medical University were enrolled in this retrospective study. This study was conducted according to the principles of the Declaration of Helsinki. The clinical parameters were collected, including sex, age, stage, presence of liver/brain metastasis, diseases history, previous treatment lines and regimens, history of other treatments and laboratory data.
Next, Postoperative outcomes and treatment included the occurrence of major morbidity (Clavien-Dindo ≥ III) and 30-day mortality. Pathological parameters were collected according to the 8th edition of the AJCC staging system and included tumor stage, tumor size, extent of lymph node involvement, and tumor grading (21, 22).. Follow-up data for all patients were obtained from their most recent medical review, including documented clinical examination and assessment of computed tomography (CT) scans. Patients’ overall survival (OS) was calculated from the date of the index operation to the date of death or last contact. An independent biostatistician managed and maintained the collected data.
Propensity score matching
Propensity score matching (PSM) is a quasi-experimental method in which the researcher uses statistical techniques to construct an artificial control group by matching each treated unit with a non-treated unit of similar characteristics. Using these matches, the researcher can estimate the impact of an intervention. In this study, Firstly, patients in groups with treatment of losartan and without treatment of losartan were paired by the propensity score method referred by Rubin and Rosenbaum and these process were completed by SPSS.22.0 software. The next process is the pairs removed from matching and then the other case was selected like previous step (23, 24).
Identification of somatic alterations in patients
Plasma samples were collected before initial treatment and three months after initiating the DC-CIK. Next generation sequencing was performed on peripheral blood cell-free DNA (cfDNA) by a commercial vendor. Targeted sequencing was performed in 60 plasma cell-free DNA (cfDNA), as well as 30 germ line DNA samples. The target region is about 1.1Mb, which include coding exons and selected introns of 1021 genes. A total of 1021 genes were selected from four sources: 1) known oncogenes and tumor suppressor genes; 2) genes that are targets of agents approved by the FDA or have been assessed in clinical trials; 3) genes implicated in major cancer-related signaling pathways; 4) genes identified in the findings of the TCGA network which covers 12 cancer types. Sequencing libraries were prepared from ctDNA using KAPA DNA Library Preparation Kits (Kapa Biosystems, Inc.), and gDNA sequencing libraries were prepared using the protocols recommended by the Illumina TruSeq DNA Library Preparation Kit. For samples close to the minimum input requirement, additional pre-capture PCR cycles were performed to generate sufficient PCR product for hybridization. Libraries were hybridized to custom-designed biotinylated oligonucleotide probes (Integrated DNA Technology, Coralville, USA) covering target region sequence. DNA sequencing was carried out with the HiSeq3000 Sequencing System (Illumina, San Diego, CA). Somatic SNVs and InDels were detected using the Mutect 2.0 algorithm (https://software.broadinstitute.org/gatk/gatkdocs/current/org_broadinstitute_gatk_tools_walkers_cancer_m2_MuTect2.php) Somatic copy number alterations and structure variations were analyzed using local algorithms.
Statistical methods
Continuous variables were expressed as mean ± SD (standard deviation) and compared using a two-tailed unpaired Student’s t test; categorical variables were compared using χ2 or Fisher analysis. The Greenwood formula was used for the standard deviation. A Cox regression approach was chosen for the evaluation of factors related with overall survival (OS) and time to recurrence (TTR) for patients with CCA (25). Results are reported as odd ratios (OR) and their 95% confidence intervals (CI). A OR > 1 indicated an elevated relation with respect to the reference category. A confidence interval which did not include the value 1 indicated statistical significance at the 5% level. All statistical evaluations were carried out using SPSS software (Statistical Package for the Social Science, version 15.0, SPSS Inc, Chicago, IL) and GraphPad Prism 5 (Version 5.01, GraphPad Software, Inc., USA). A value of p<0.05 was considered to be statistically significant in all the analyses.
Results
Patient characteristics
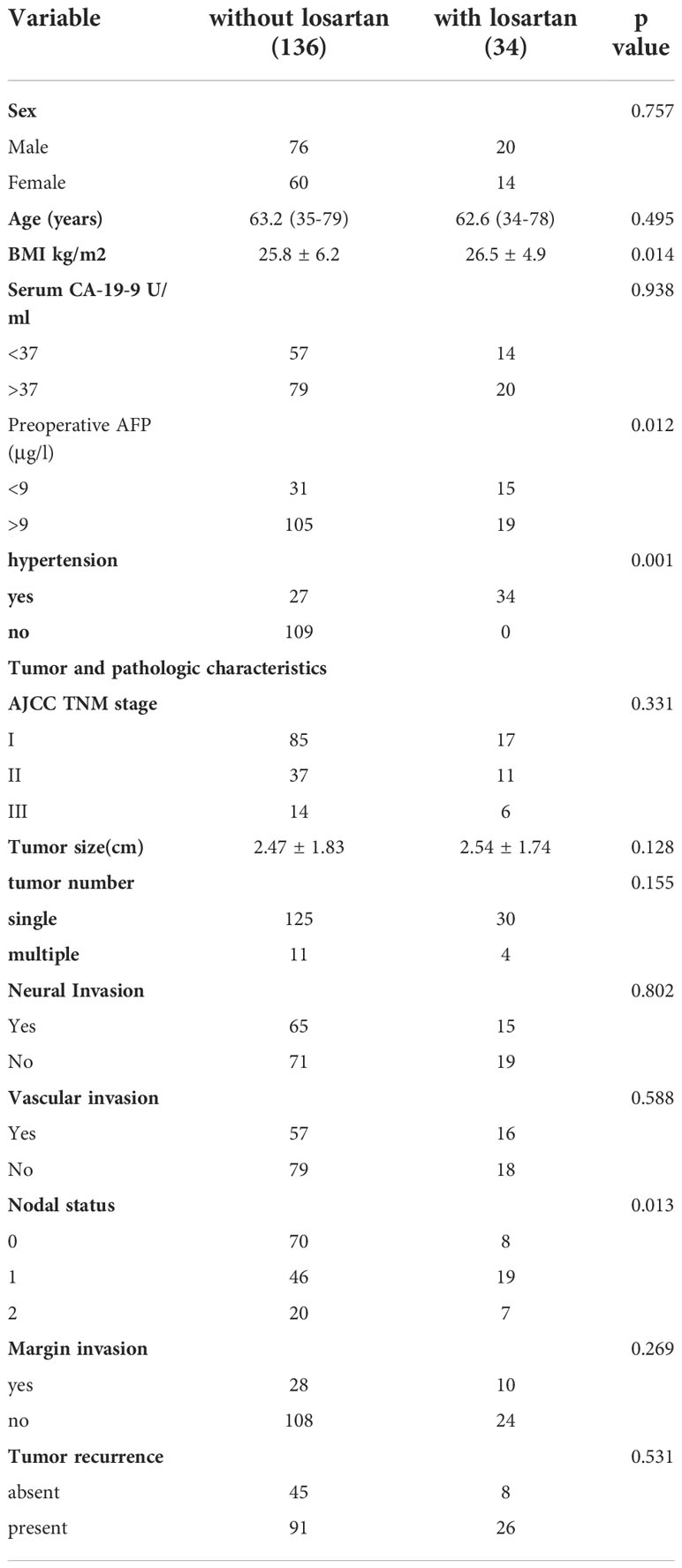
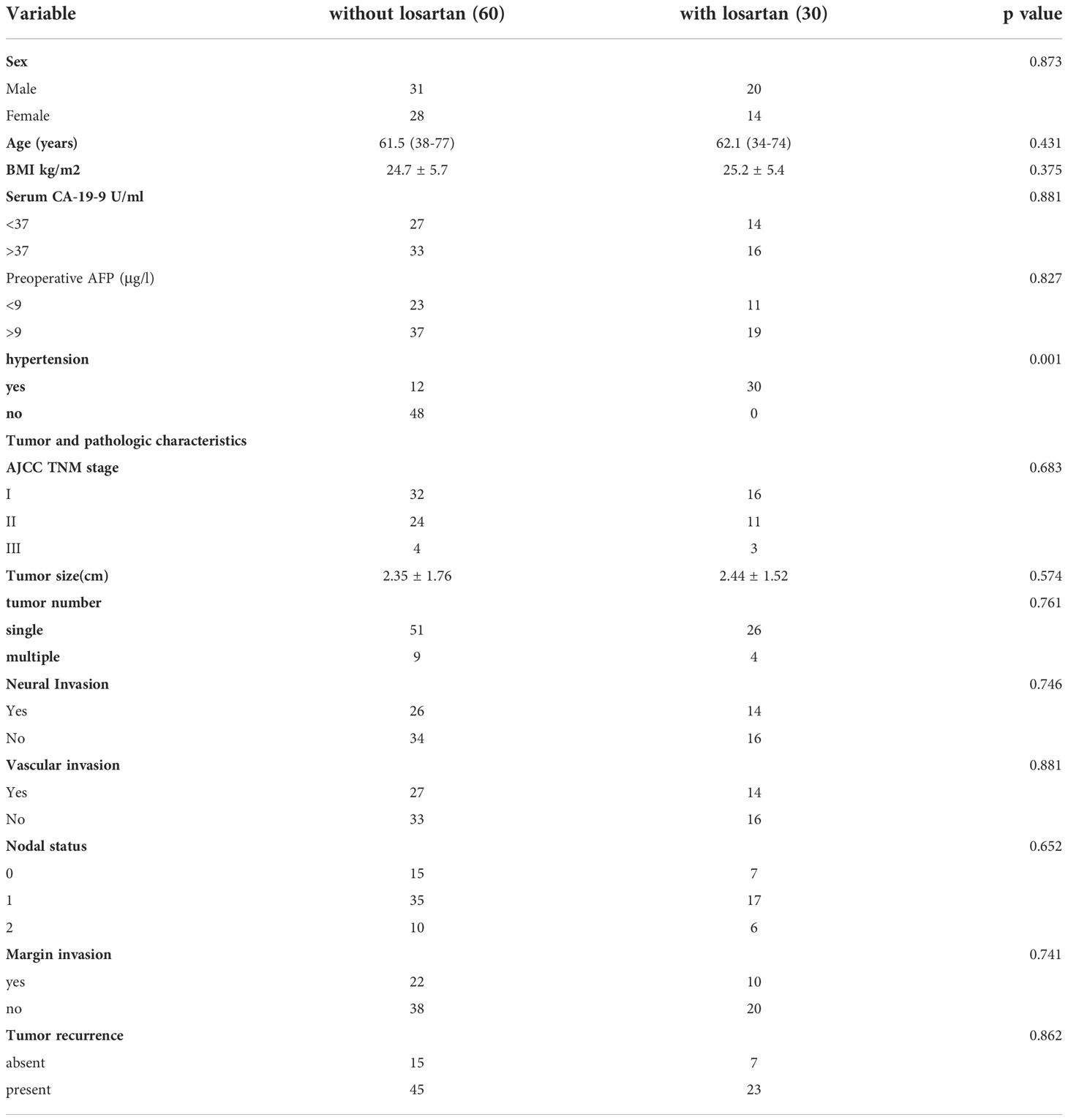
Patients were enrolled from Department of General surgery, First Affiliated Hospital of Jinzhou Medical University from March 1, 2010, to December 30, 2019. Totally 170 patients with CCA were enrolled in this study. After checking all the records in hospital, patients were allocated into two groups: patients with losartan years(n=34) and patients without losartan (n=136). Most patients received long term treatment of losartan because of their cardiovascular diseases and all patients with losartan included in this study at least received treatment of losartan for 4 weeks before and after the adjuvant chemotherapy. Characteristics of all patients are detailed in Table 1. There were significant differences in BMI, Preoperative AFP, hypertension, and the proportion of nodal status between these two groups before PSM. After the propensity score match (we used 1:2 matching since the limited number of patients with losartan), the cohort consisted of 30 patients with losartan and 60 compared patients without losartan. The clinical variables that could be obtained at the time of initial diagnosis and were considered to have influenced the outcomes were used for the 1:2 matching between the two groups. The standardized differences after matching were smaller for all background variables compared with those in the unmatched groups and no significant differences were shown between the two groups except for the hypertension status (Table 2).
Survival analysis of patients with different treatments
For all the patients before matching, patients with losartan (15.54 months 95% CI, 111.4‐22.4) group showed a comparable median OS (overall survival) to those in the patients without losartan group (16.57 months 95% CI, 10.2‐21.5) (P =0.302) (Figure 1A). Meanwhile, patients with losartan (11.6 months 95% CI, 4.3‐15.6) group showed no significant difference in median TTR (time to recurrence) compared with those in the patients without losartan group (10.4 months 95% CI, 7.6‐17.8) (P =0.843) (Figure 1B). After matching, patients with losartan (17.8 months 95% CI, 11.5‐23.4) group showed a favorable median OS than those in the patients without losartan group (11.4 months 95% CI, 7.8‐16.5) (P =0.025) (Figure 1C). Moreover, patients with losartan (11.03 months 95% CI, 6.8‐14.3) group showed a longer median TTR (time to recurrence) than those in the patients without losartan group (7.84 months 95% CI, 3.6‐11.7) (P =0.016) (Figure 1D).
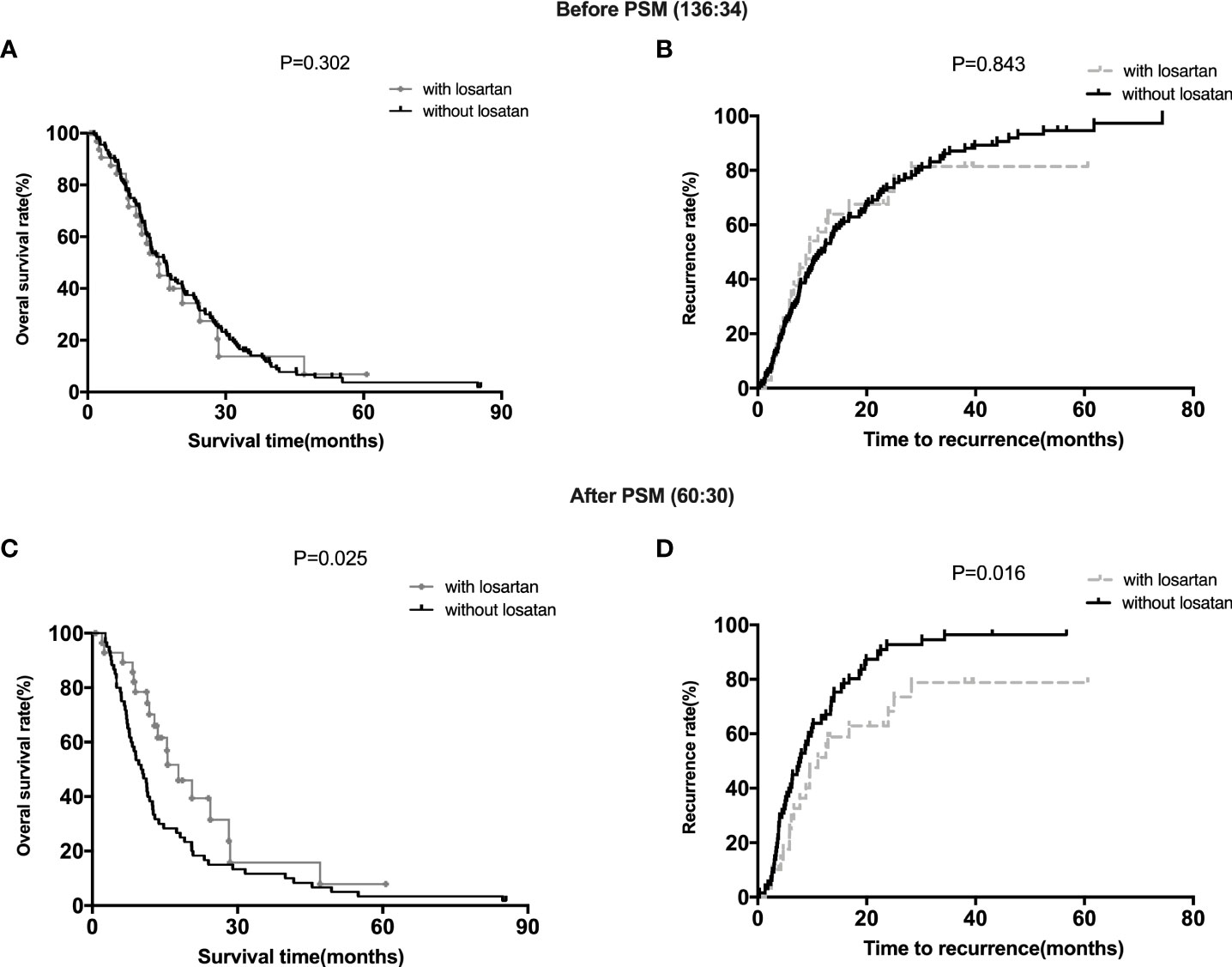
Figure 1 Survival Analysis of Patients with different treatments. (A, B): the OS and TTR for patients with or without losartan before PSM; (C, D): the OS and TTR for patients with or without losartan after PSM.
Predictors associated with clinical outcomes
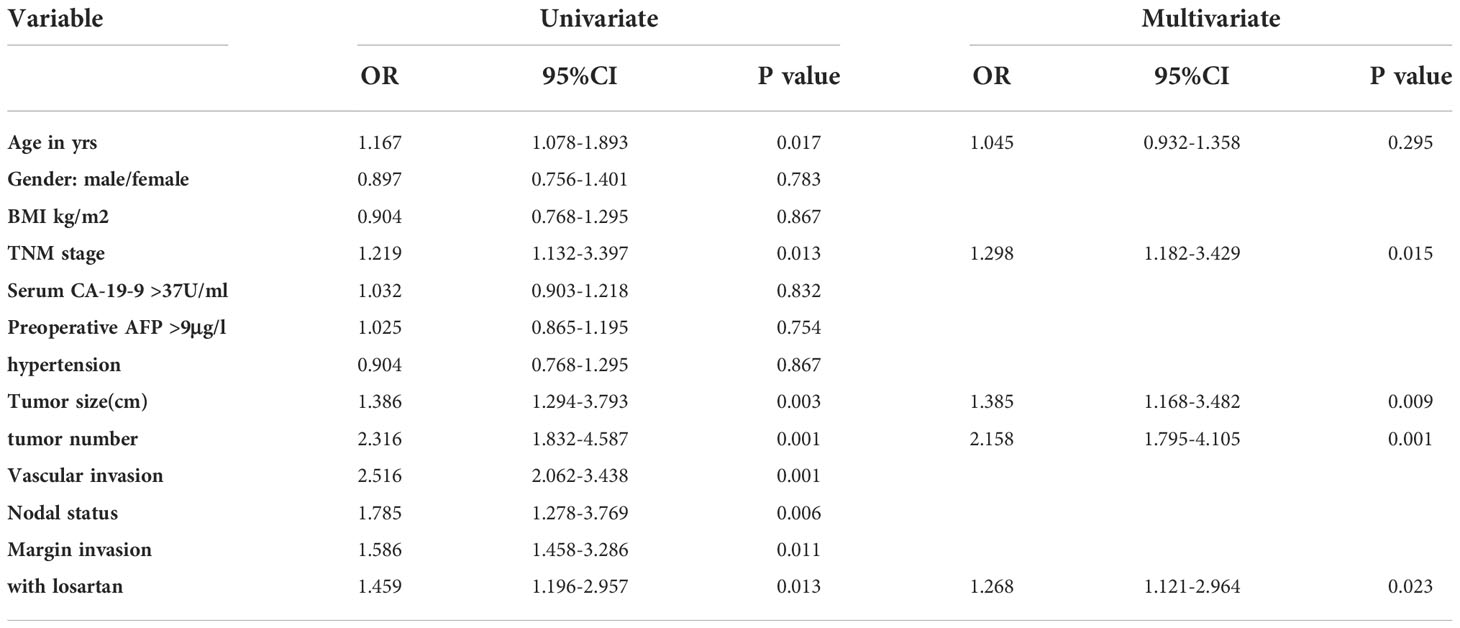
Cox proportional hazards models were then used to quantify the prognostic factors associated with survival in patients with CCA. We performed and Cox proportional hazards analysis both before and after the PSM analysis. The results of univariate analysis after PSM were shown in Table 3. After univariate analysis, A multivariable analysis was performed to assess the factors that demonstrated significant effects in univariate analysis. After adjusting for competing risk factors, TNM stage (HR:2.325, 95% CI:1.561-4.475, P =0.001), tumor size (HR:1.832, 95% CI:1.341-3.142, P =0.008), tumor number (HR:2.042, 95% CI:1.516-3.394, P =0.003), positive lymph nodes(HR:1.709, 95% CI:1.185-3.165, P =0.014) and margin invasion (HR:1.385, 95% CI:1.184-2.953, P =0.021) were independent factors associated with overall survival before PSM. However, after PSM, TNM stage (HR:1.298, 95% CI:1.182-3.429, P =0.015), tumor size (HR:1.385, 95% CI:1.168-3.482, P =0.009), tumor size (HR:2.158, 95% CI:1.795-4.105, P =0.001) and patients with losartan (HR:1.268, 95% CI:1.121-2.964, P =0.023) were independent factors after multivariable analysis (Table 3).
Genome alterations in ctDNA
We have found that sequenced serial cfDNA were performed in 10 patients with losartan and 9 patients without losartan who received adjuvant chemotherapy after tumor resection. About 10Gb and 2Gb sequencing data were generated for each ctDNA sample and gDNA sample, respectively. The average coverage of depth was 1323-fold (706–2094) for ctDNA samples and then somatic SNVs and Indels were identified based on these sequencing data. We aimed to determine the associations of the alterations of ctDNA with losartan responses to adjuvant chemotherapy after surgery. Among these patients divided into with losartan and without losartan groups, the comparison of all gene mutations and the frequencies of these genes pre-and post-chemotherapy were showed in Figure 2A, B. Among the 10 patients who received losartan combined with chemotherapy, there were 10 gene mutations including NTRK3, DDR1, STAT3, CDH1, PTEN etc (Figure 2A) before treatments and there were 14 gene mutations after treatment. Moreover, the frequency of the ctDNA mutations were significantly increased after the combined treatments for patients with CCA. However, Among the 9 patients who received chemotherapy without losartan, there were 8 gene mutations (Figure 2B) before treatments and there were 8 gene mutations after treatment. Meanwhile, the frequency of the ctDNA mutations showed no significant difference between these 2 groups.
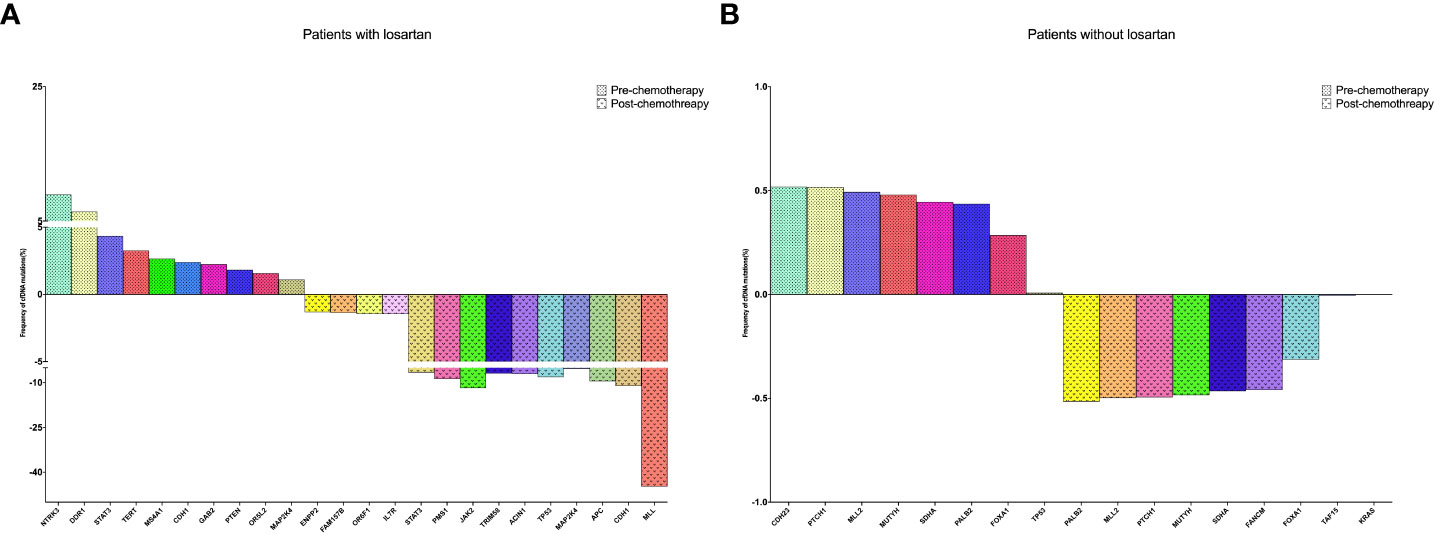
Figure 2 Sequenced serial cfDNA were performed in 10 patients with losartan and 9 patients without losartan who received adjuvant chemotherapy after tumor resection. (A) The changing of CfDNA in patients received losartan; (B) The changing of CfDNA in patients without losartan.
Discussion
Surgical resection is the only potentially curative treatment for patients with cholangiocarcinoma. For both perihilar cholangiocarcinoma (pCCA) and intrahepatic cholangiocarcinoma (iCCA), 5‐year overall survival of about 30% has been reported in large series. Most patients with cholangiocarcinoma are ineligible for surgical resection because of metastatic or locally advanced disease at the time of presentation. For patients received surgical resection or not, the incidence of CCA patients receiving adjuvant chemotherapy is reported to be as high as 46.6%, although cholangiocarcinoma is less sensitive to chemotherapeutics than other cancers. Fluoropyrimidine‐ and gemcitabine‐based chemotherapy regimens are widely used and were confirmed to prolong OS in recent meta‐analyses (26, 27).
In addition to its important role in diseases such as hypertension, the RAS system also contributes to the formation of a tumor microenvironment and promotes immunosuppression, etc. Ang II is one of the most active components in RAS. Overexpression of AGTR1 is typically associated with more aggressive tumor features and worse outcomes (28, 29). Additionally, RAS antagonists, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) may suppress tumor progression in a variety of human or mouse cancer, including lung cancer and pancreatic cancer (30–32), and losartan is the world’s first non-peptide AT1R antagonist for clinical use. More interestingly, AngII inhibits the infiltration of intratumoral T lymphocytes and the accumulation of immunosuppressive cell types (M2-like macrophages, myeloid-derived suppressor cells and T regulatory cells). Generally, the AngII/AGTR1 axis is considered to promote the formation of immunosuppressive microenvironments and favor tumor growth (33, 34). However, there are also conflicting reports suggesting potential tumor type specific differences (19, 35, 36). Therefore, we first screened patients with CCA from the clinical database of Department of General surgery, First Affiliated Hospital of Jinzhou Medical University, and then divided them into patients with losartan and patients without losartan combined with the treatment of adjuvant chemotherapy. We performed a 1:2 PSM study and found that patients with losartan group showed both comparable median OS (overall survival) and TTR (time to recurrence) to those in the patients without losartan group before PSM. However, after matching, patients with losartan group showed favorable median OS and TTR than those in the patients without losartan group. Then we performed Cox proportional hazards models and found that patients with losartan was an independent factor after multivariable analysis for patients with CCA. Furtherly, we sequenced serial cfDNA were performed in 10 patients with losartan and 9 patients without losartan who received adjuvant chemotherapy after tumor resection. These results showed that the treatment of losartan was related with tumor microenvironment and could be potentially useful to combine the immunotherapy for patients with CCA.
The present study has several limitations. First, this is a retrospective study in which selection biases are unavoidable, despite attempts to minimize these using large, independent, cohorts of consecutive patients. secondly, the potential mechanism of losartan combined with chemotherapy regimens as well as use of radiation therapy should be further explored.
In conclusion, this study demonstrated that the treatment of losartan could increase the efficacy of adjuvant chemotherapy and identified as an independent survival predictor for patients with CCA. Moreover, losartan could be potentially useful to combine the immunotherapy for patients with CCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceived and designed the experiments: QL, ZC, TW; Performed the experiments:BL, XW, X-YG, TY, QL; Statistical analysis: QL, ZC, TW; Wrote the paper: QL, WW. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int (2019) 39 Suppl 1(Suppl Suppl 1):143–55. doi: 10.1111/liv.14089
2. El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am (2019) 28(4):587–99. doi: 10.1016/j.soc.2019.06.002
3. Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev (2009) 35(4):322–7. doi: 10.1016/j.ctrv.2008.11.009
4. Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol (2015) 33(24):2617–22. doi: 10.1200/jco.2014.60.2219
5. Howell M, Valle JW. The role of adjuvant chemotherapy and radiotherapy for cholangiocarcinoma. Best Pract Res Clin Gastroenterol (2015) 29(2):333–43. doi: 10.1016/j.bpg.2015.03.001
6. Kim YS, Hwang IG, Park SE, Go SI, Kang JH, Park I, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol (2016) 77(5):979–85. doi: 10.1007/s00280-016-3014-x
7. Chun YS, Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control (2017) 24(3):1073274817729241. doi: 10.1177/1073274817729241
8. Nassour I, Mokdad AA, Porembka MR, Choti MA, Polanco PM, Mansour JC, et al. Adjuvant therapy is associated with improved survival in resected perihilar cholangiocarcinoma: A propensity matched study. Ann Surg Oncol (2018) 25(5):1193–201. doi: 10.1245/s10434-018-6388-7
9. Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PloS One (2020) 15(2):e0229292. doi: 10.1371/journal.pone.0229292
10. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72(2):353–63. doi: 10.1016/j.jhep.2019.10.009
11. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72(2):364–77. doi: 10.1016/j.jhep.2019.11.020
12. Namazi S, Daneshian A, Mohammadianpanah M, Jafari P, Ardeshir-Rouhani-Fard S, Nasirabadi S. The impact of renin-angiotensin system, angiotensin І converting enzyme (insertion/deletion), and angiotensin ІІ type 1 receptor (A1166C) polymorphisms on breast cancer survival in Iran. Gene (2013) 532(1):125–31. doi: 10.1016/j.gene.2013.09.020
13. Park YA, Choi CH, Do IG, Song SY, Lee JK, Cho YJ, et al. Dual targeting of angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian carcinoma. Gynecol Oncol (2014) 135(1):108–17. doi: 10.1016/j.ygyno.2014.06.031
14. Pringle KG, Delforce SJ, Wang Y, Ashton KA, Proietto A, Otton G, et al. Renin-angiotensin system gene polymorphisms and endometrial cancer. Endocr Connect (2016) 5(3):128–35. doi: 10.1530/ec-15-0112
15. Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D, et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U.S.A. (2019) 116(6):2210–9. doi: 10.1073/pnas.1818357116
16. Wu L, Vasilijic S, Sun Y, Chen J, Landegger LD, Zhang Y, et al. Losartan prevents tumor-induced hearing loss and augments radiation efficacy in NF2 schwannoma rodent models. Sci Transl Med (2021) 13(602):4816. doi: 10.1126/scitranslmed.abd4816
17. Regan DP, Chow L, Das S, Haines L, Palmer E, Kurihara JN, et al. Losartan blocks osteosarcoma-elicited monocyte recruitment, and combined with the kinase inhibitor toceranib, exerts significant clinical benefit in canine metastatic osteosarcoma. Clin Cancer Res (2022) 28(4):662–76. doi: 10.1158/1078-0432.Ccr-21-2105
18. Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol (2019) 5(7):1020–7. doi: 10.1001/jamaoncol.2019.0892
19. Mainetti LE, Rico MJ, Kaufman CD, Grillo MC, Guercetti J, Baglioni MV, et al. Losartan improves the therapeutic effect of metronomic cyclophosphamide in triple negative mammary cancer models. Oncotarget (2020) 11(32):3048–60. doi: 10.18632/oncotarget.27694
20. Shen Y, Wang X, Lu J, Salfenmoser M, Wirsik NM, Schleussner N, et al. Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell (2020) 37(6):800–817.e7. doi: 10.1016/j.ccell.2020.05.005
21. Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, et al. Proposed modification of the 8th edition of the AJCC staging system for pancreatic ductal adenocarcinoma. Ann Surg (2019) 269(5):944–50. doi: 10.1097/sla.0000000000002668
22. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol (2018) 25(4):845–7. doi: 10.1245/s10434-017-6025-x
23. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics (1996) 52(1):249–64. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B
24. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med (1998) 17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
25. Frost HR, Andrew AS, Karagas MR, Moore JH. A screening-testing approach for detecting gene-environment interactions using sequential penalized and unpenalized multiple logistic regression. Pac Symp Biocomput (2015) 12:183–94. doi: 10.1002/cam4.2925
26. Wang L, Deng M, Ke Q, Lou J, Zheng S, Bi X, et al. Postoperative adjuvant therapy following radical resection for intrahepatic cholangiocarcinoma: A multicenter retrospective study. Cancer Med (2020) 9(8):2674–85. doi: 10.1002/cam4.2925
27. Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun (2021) 27:100354. doi: 10.1016/j.ctarc.2021.100354
28. Bernardi S, Zennaro C, Palmisano S, Velkoska E, Sabato N, Toffoli B, et al. Characterization and significance of ACE2 and mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst (2012) 13(1):202–9. doi: 10.1177/1470320311426023
29. Volonte D, Sedorovitz M, Cespedes VE, Beecher ML, Galbiati F. Cell autonomous angiotensin II signaling controls the pleiotropic functions of oncogenic K-ras. J Biol Chem (2021) 296:100242. doi: 10.1074/jbc.RA120.015188
30. Kasi A, Allen J, Mehta K, Dandawate P, Saha S, Bossmann S, et al. Association of losartan with outcomes in metastatic pancreatic cancer patients treated with chemotherapy. J Clin Transl Res (2021) 7(2):257–62. doi: 10.1186/s13014-021-01775-9
31. Li W, Li S, Chen IX, Liu Y, Ramjiawan RR, Leung CH, et al. Combining losartan with radiotherapy increases tumor control and inhibits lung metastases from a HER2/neu-positive orthotopic breast cancer model. Radiat Oncol (2021) 16(1):48. doi: 10.1186/s13014-021-01775-9
32. Takagi H, Kaji K, Nishimura N, Ishida K, Ogawa H, Takaya H, et al. The angiotensin II receptor blocker losartan sensitizes human liver cancer cells to lenvatinib-mediated cytostatic and angiostatic effects. Cells (2021) 10(3):575. doi: 10.3390/cells10030575
33. Singh A, Srivastava N, Amit S, Prasad SN, Misra MP, Ateeq B. Association of AGTR1 (A1166C) and ACE (I/D) polymorphisms with breast cancer risk in north Indian population. Transl Oncol (2018) 11(2):233–42. doi: 10.1016/j.tranon.2017.12.007
34. Panza S, Malivindi R, Caruso A, Russo U, Giordano F, Győrffy B, et al. Novel insights into the antagonistic effects of losartan against angiotensin II/AGTR1 signaling in glioblastoma cells. Cancers (Basel) (2021) 13(18):4555. doi: 10.3390/cancers13184555
35. Hashemzehi M, Naghibzadeh N, Asgharzadeh F, Mostafapour A, Hassanian SM, Ferns GA, et al. The therapeutic potential of losartan in lung metastasis of colorectal cancer. Excli J (2020) 19:927–35. doi: 10.17179/excli2020-2093
Keywords: Cholangio carcinoma, prognosis, PSM, losartan, chemotherapy
Citation: Li Q, Chang Z, Wang T, Liu B, Wang X, Ge X-Y, Yang T, Liu Q and Wang W (2022) Synergy of Losartan and chemotherapy for patients with cholangiocarcinoma: A propensity score-matched analysis. Front. Oncol. 12:989080. doi: 10.3389/fonc.2022.989080
Received: 08 July 2022; Accepted: 21 September 2022;
Published: 23 November 2022.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Guoliang Qiao, Massachusetts General Hospital and Harvard Medical School, United StatesFeng Shen, Second Military Medical University, China
Copyright © 2022 Li, Chang, Wang, Liu, Wang, Ge, Yang, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2FuZ3dlaWx5eXlAZ21haWwuY29t; Qu Liu, bGVvbmxpdTUyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Qing Li1†
Qing Li1† Wei Wang
Wei Wang