- 1Department of General Surgery (Breast and Thyroid Surgery), Shaoxing People’s Hospital, Shaoxing Hospital, Zhejiang University School of Medicine, Shaoxing, Zhejiang, China
- 2Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, Hangzhou, Zhejiang, China
- 3Shaoxing Key Laboratory of Functional Molecular Imaging of Tumor and Interventional Diagnosis and Treatment, Shaoxing, Zhejiang, China
- 4Department of Nephrology, Shaoxing People’s Hospital, Shaoxing Hospital, Zhejiang University School of Medicine, Shaoxing, Zhejiang, China
- 5Medical Research Center Shaoxing People’s Hospital, Shaoxing Hospital, Zhejiang University School of Medicine, Shaoxing, Zhejiang, China
Purpose: Immune checkpoint molecule lymphocyte-activating gene-3 (LAG-3), which is expressed on active lymphocytes, has proven to be associated with immunosuppression and cancer progression in a variety of solid tumors. However, the role of LAG-3+ lymphocytes in human breast cancer (BC) is still not conclusive. We therefore performed a meta-analysis to clarify the role of these cells in prognosis prediction for BC.
Methods: We searched PubMed, Embase, and EBSCO to identify the studies evaluating the association of LAG-3+ lymphocyte infiltration and overall survival (OS) and/or disease-free survival (DFS) in BC patients, then combined extracted data with STATA 12.0.
Results: Eight published studies involving 5,859 BC patients were incorporated into this meta-analysis. We noted that a high number of LAG-3+ tumor-infiltrating lymphocytes were not appreciably associated with OS and DFS in BC patients. Strikingly, in stratified analyses based on the molecular type of BC, LAG-3+ lymphocyte infiltration was remarkably associated with better OS rather than DFS in triple-negative breast cancer (TNBC), whereas it significantly influenced neither OS nor DFS in Her2-positive BC. However, an increased density of these lymphocytes indicated a trend for better OS in Her2-positive BC. In addition, we found that LAG-3+ lymphocyte infiltration was also remarkably associated with prolonged OS in Her2-positive BC patients when they were measured by immunohistochemistry (IHC). In addition, an elevated number of these lymphocytes did not correlate with pathological complete response rate or clinicopathological features including lymph node metastasis.
Conclusion: The infiltration of LAG-3+ lymphocytes ameliorates OS in TNBC and Her2-positive BC, implicating that it is a valuable prognostic biomarker, and applications of anti-LAG-3 antagonists may possibly be not a promising therapeutic strategy for human BC especially for TNBC.
Introduction
Human breast cancer is one of the most common fatal malignancies in women worldwide. The tumor microenvironment (TME) links closely with the initiation, promotion, and progression of breast cancer (BC) via diverse mechanisms such as inducing immune suppression and angiogenesis (1). Immune checkpoint molecules such as programmed cell death 1 (PD-1) and its ligand (PD-L1), cytotoxic T lymphocyte antigen 4 (CTLA-4), have proven to be associated with the formation of the immunosuppressive microenvironment and immune evasion in multiple cancers (2). Currently, immune checkpoint inhibitors such as anti PD-1/PD-L1 antagonists have been utilized in clinical practice, yielding impressive results especially in lung carcinoma (3). However, these inhibitors showed very limited benefit for patients with other solid tumors including BC (4).
Lymphocyte-activating gene-3 (LAG-3) (also known as CD233) is a type I transmembrane protein with structural similarities to CD4. Accumulating evidence has demonstrated that LAG-3 is an inhibitory coreceptor and a new immune checkpoint molecule, which exerts pivotal roles in anti-infection immunity, autoimmunity, and tumor immunity (5). LAG-3 expressed on activated T lymphocytes correlated with T-cell exhaustion, thereby leading to dysfunction of antitumor immunity mediated by effector T cells (6). Recently, the infiltration of LAG-3+lymphocytes has been reported to be associated with tumor progression and poor prognosis in a variety of human cancers such as colorectal cancer (7), renal cell carcinoma (8), head and neck squamous cell carcinoma (HNSCC) (9), and non-small cell lung cancer (10) However, the relationship between LAG-3+ tumor-infiltrating lymphocytes and prognosis in human BC is still not conclusive (11, 12). Hence, it needs further evaluation to clarify the potential of these cells as an effective prognostic biomarker for BC patients.
Herein, we performed this meta-analysis to quantitatively summarize the relationship between LAG-3+ lymphocyte infiltration and clinical outcomes such as overall survival (OS) and disease-free survival (DFS) in BC patients and thereby provided more evidence on the clinical value of these lymphocytes as a prognostic biomarker and LAG-3-targeted therapeutic strategy for BC.
Materials and methods
Search strategy
We searched PubMed, Embase, and EBSCO for studies to test the density of LAG-3+ tumor-infiltrating lymphocytes and survival in BC patients from 1980 to 31/05/2022. The keywords adopted for search were (LAG-3 [All Fields] OR CD233 [All Fields]) AND (breast [All Fields] OR mammary [All Fields]) AND (neoplasms [All Fields] OR tumor [All Fields] OR cancer [All Fields] OR carcinoma [All Fields]). A total of 44, 72, and 108 entries were identified in PubMed, Embase, and EBSCO, respectively.
Inclusion and exclusion criteria
Inclusion criteria of the meta-analysis were as follows: studies must have (1) been published as original articles in English; (2) investigated BC patients; and (3) provided hazard ratios (HRs) with 95% confidence interval (CI) or Kaplan–Meier curves of high and low density of LAG-3+ lymphocytes with OS and/or DFS.
We excluded studies that were not published as research articles or full texts including commentaries, case report, letters to editors and conference abstracts, studies that did not provide sufficient data to estimate hazard ratios (HRs), and studies that detected LAG-3+ lymphocytes in peripheral blood or metastatic sites.
Endpoints
In this meta-analysis, we recorded OS as the primary endpoint, whereas DFS was considered as the second endpoint. Individual studies defined cutoffs of LAG-3+ lymphocytes and classified patients into high and low groups.
OS and DFS definition
OS was defined as the time from the date of the first curative operation to the date of the last follow-up, or death from any cause, whereas DFS was the time from the date of the first curative surgery to the date of the first loco-regional or systemic relapse, or death without any type of relapse.
Data extraction
Two authors (GM.H. and SM.W.) independently reviewed and extracted information including first author’s name, publication year, number of patients, neoadjuvant therapy (NAT) received or not, time of follow-up, and cutoff value determining a high level of LAG-3+ lymphocytes. OS, DFS, pathological complete response (pCR) rate, and clinicopathological data including lymph node metastasis and tumor differentiation were extracted from the text or tables.
Quality assessment
Two independent authors appraised the quality of included individual studies with the Newcastle–Ottawa Scale (NOS) (13) and achieved consensus for each item with the help of a third author. A score of 6 or above was considered as high quality.
Statistical analysis
We combined relevant data into hazard ratios (HRs) for OS, DFS, and odds ratios (ORs) for pCR rates of NAT and clinicopathological features such as lymph node metastasis with STATA 12.0 according to the random-effect model if statistical heterogeneity was remarkable (14); otherwise, the fixed-effect model was adopted (15, 16). In addition, we also applied sensitivity analysis as well as Begg’s funnel plot and Egger’s test to investigate the influence of each study on the pooled result and potential publication bias, respectively (17). All P values were two-sided, and those below 0.05 were considered as statistical significance.
Results
Search results and description of studies
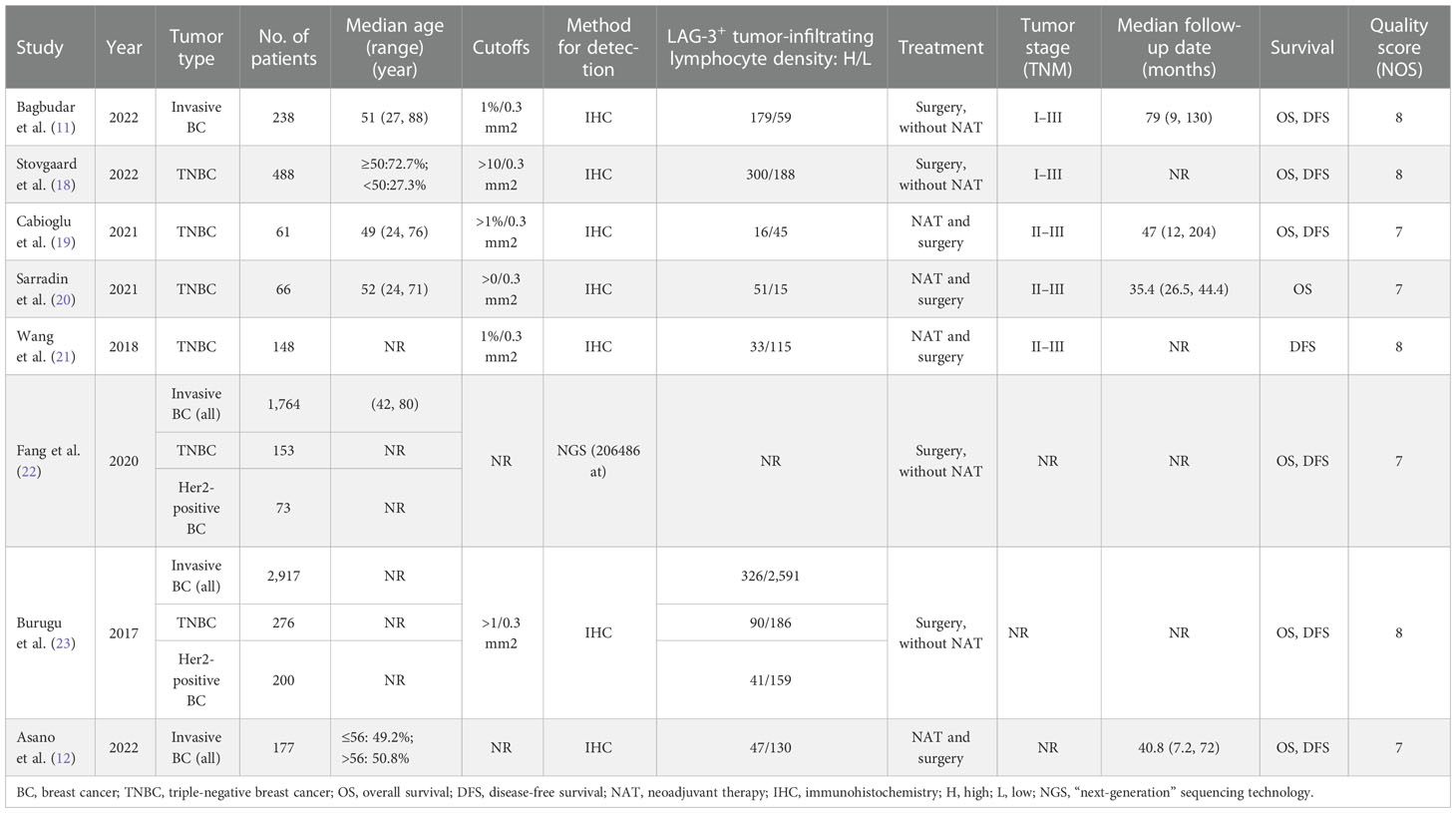
There were 224 records retrieved, and the results are shown in Figure S1. We ultimately included eight studies with 5,859 breast cancer patients for the evaluation of LAG-3+ tumor-infiltrating lymphocytes (11, 12, 18–23) and then appraised all these studies with the Newcastle–Ottawa Scale (NOS). Characteristics of included studies which were in the light of the inclusion criteria and appropriate for data mergence are shown in Table 1 and Table S1.
Meta-analyses
OS
As for the association between high density of LAG-3+ tumor-infiltrating lymphocytes and OS in BC, we included seven studies with 5,711 patients for data combination. The pooled data indicated that an elevated density of LAG-3+ lymphocytes within tumor had no considerable prognostic effect on OS (HR = 0.93, 95% CI 0.83 to 1.06, P = 0.276) in all BC patients (Figure 1).
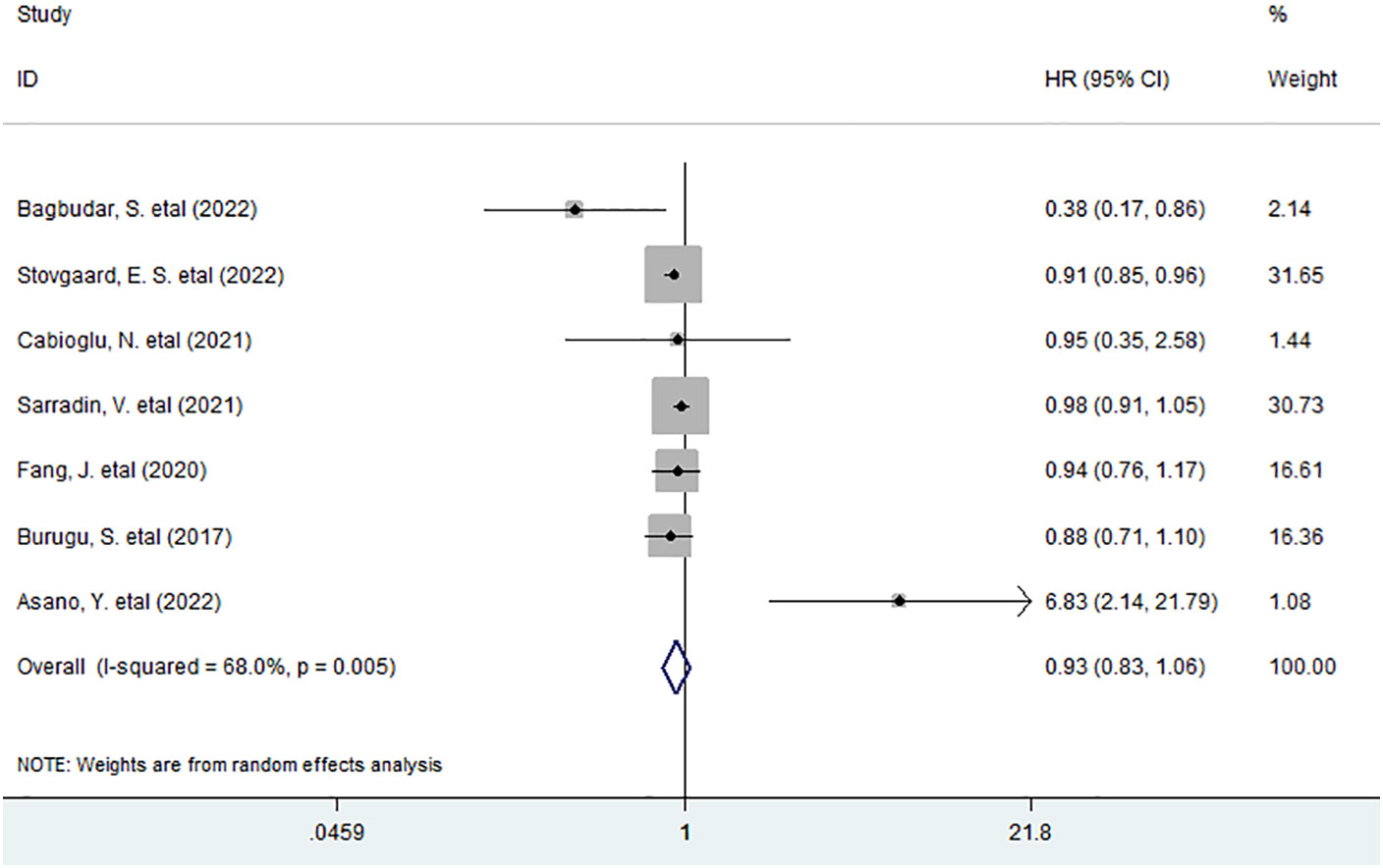
Figure 1 Forest plots describing HR of the association between LAG-3+ tumor-infiltrating lymphocytes and OS in breast cancer patients.
In stratified analyses based on molecular type of BC such as triple-negative BC (TNBC), Her2-positive BC, and luminal BC, as shown in Figure 2, six studies with 1,105 TNBC patients were pooled into the subgroup analysis. The results showed that LAG-3+ lymphocyte infiltration was appreciably associated with improved OS in TNBC patients (HR = 0.84, 95% CI 0.72 to 0.98, P = 0.031), whereas increased density of LAG-3+ tumor-infiltrating lymphocytes indicated a trend for better OS but was not significant in Her2-positive BC patients (HR = 0.69, 95% CI 0.32 to 1.49, P = 0.345).
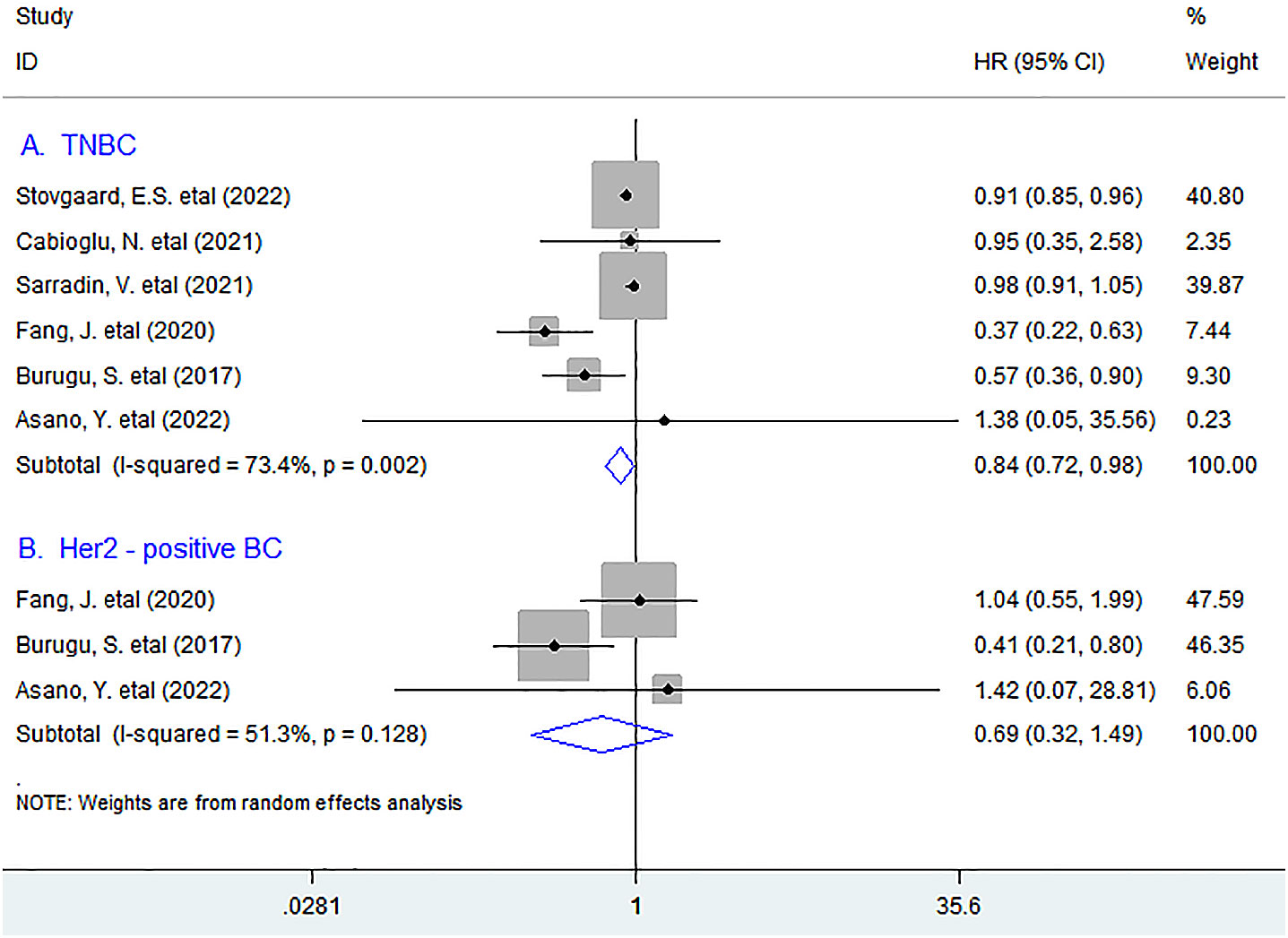
Figure 2 Stratified analyses describing HRs of the association between LAG-3+ tumor-infiltrating lymphocytes and OS in TNBC and Her2-positive breast cancer. TNBC, triple-negative breast cancer.
In addition, we further performed subgroup analyses according to the detection methods for LAG-3+ lymphocytes in TNBC and Her2-positive BC and noted that LAG-3+ lymphocyte infiltration was remarkably associated with prolonged OS in Her2-positive BC (HR = 0.43, 95% CI 0.23 to 0.83, P = 0.012), whereas it only showed a trend for improved OS in TNBC (HR = 0.93, 95% CI 0.85 to 1.01, P = 0.094) patients when such subset was measured by immunohistochemistry (IHC) (Figure 3).

Figure 3 Stratified analyses describing HRs of the association between LAG-3+ tumor-infiltrating lymphocytes and OS in TNBC and Her2-positive breast cancer according to the detection methods. TNBC, triple-negative breast cancer; IHC, immunohistochemistry; NGS, “Next-generation” sequencing technology.
DFS
Seven studies with the IHC method involving 5,793 patients investigated the association between LAG-3+ tumor-infiltrating lymphocytes and DFS, and the meta-analysis exhibited that LAG-3+ lymphocyte infiltration was not associated with DFS in all BC patients (HR = 0.91, 95% CI 0.74 to 1.11, P = 0.323) (Figure 4).
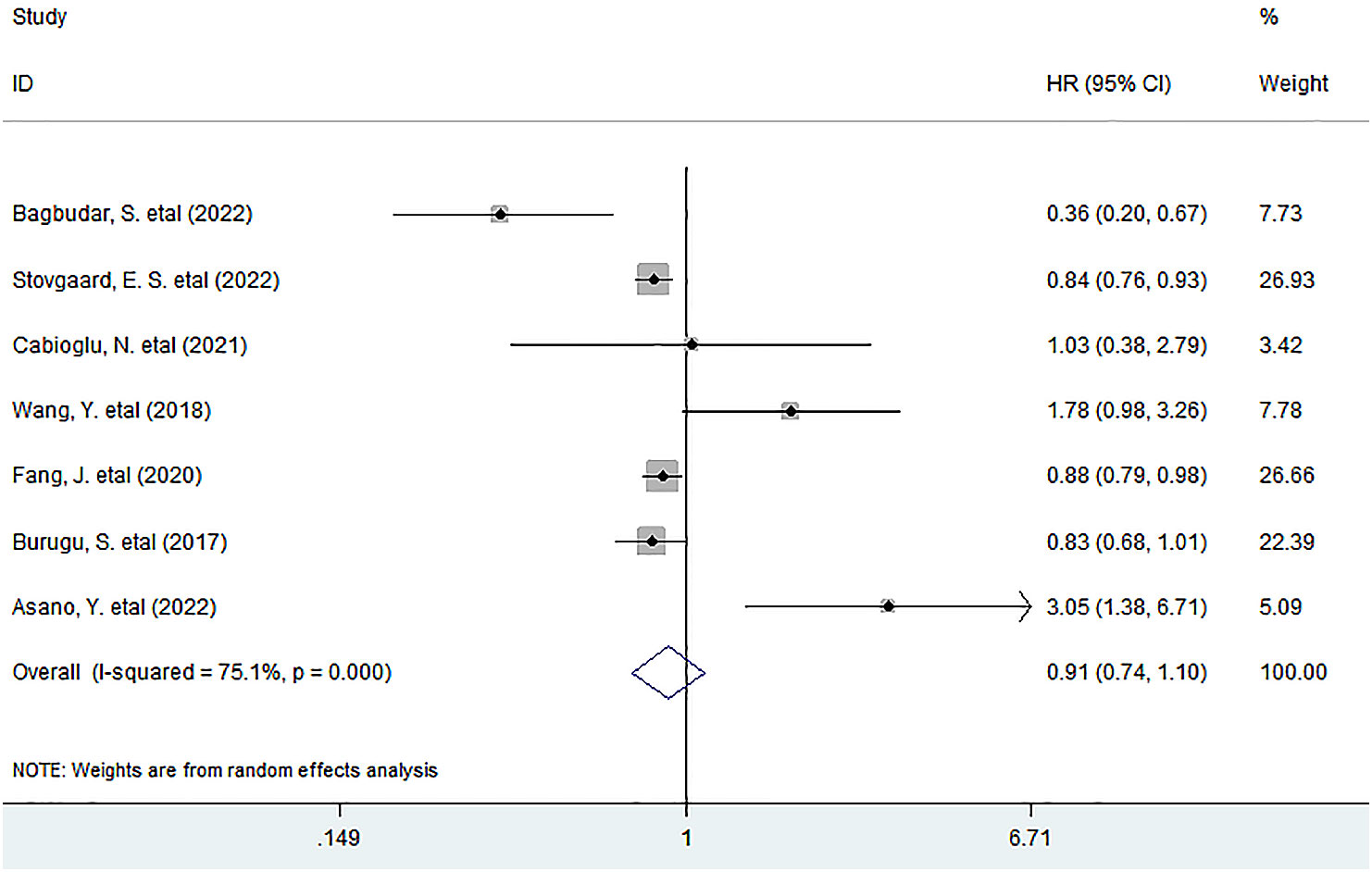
Figure 4 Forest plots describing HR of the association between LAG-3+ tumor-infiltrating lymphocytes and DFS in breast cancer patients.
In stratified analyses, we found that there was no significant association between the infiltration of LAG-3+lymphocytes and DFS in TNBC (HR = 1.06, 95% CI 0.66 to 1.70, P = 0.802). A similar result was observed between LAG-3+ lymphocyte infiltration and DFS in Her2-positive BC patients (HR = 1.72, 95% CI 0.08 to 38.05, P = 0.731) (Figure 5).

Figure 5 Stratified analyses describing HRs of the association between the LAG-3+ tumor-infiltrating lymphocytes and DFS in TNBC and Her2-positive breast cancer. TNBC, triple-negative breast cancer.
We next investigated whether LAG-3+ tumor-infiltrating lymphocytes correlated with the pCR rate of NAT or clinicopathological features such as lymph node metastasis and primary tumor stage of BC. We observed that LAG-3+ lymphocyte infiltration was neither noticeably associated with pCR rate (OR = 1.06, 95% CI 0.18 to 6.21, P = 0.945) in TNBC nor with primary tumor stage (T1/T2) (OR = 1.02, 95% CI 0.54 to 1.91, P = 0.957), lymph node metastasis (OR = 1.19, 95% CI 0.89 to 1.58, P = 0.243), or tumor differentiation (OR = 0.68, 95% CI 0.20 to 2.38, P = 0.552) of all BC patients (Figure S2).
Sensitivity analysis
Sensitivity analysis demonstrated that each included research had no impact on the overall result for OS or DFS. (Figure S3).
Publication bias
Funnel plot and Egger’s test indicated that no significant publication bias existed between high density of LAG-3+ tumor-infiltrating lymphocytes and OS (P =0.764) or DFS (P =0.548) in BC patients.
Discussion
In preclinical studies, LAG-3 expressing on tumor-infiltrating T cells could confer them immunosuppressive property and promote tumor progression in the TME, thereby predicting worse prognosis. However, in this study, we unexpectedly found that there was no significant association between high density of LAG-3+ tumor-infiltrating lymphocytes and survival including OS and DFS in BC patients. Strikingly, in subgroup analyses based on molecular type of BC, LAG-3+ lymphocyte infiltration was appreciably associated with better OS rather than DFS in TNBC, whereas it did not affect OS or DFS significantly in Her2-positive BC. However, LAG-3+ lymphocyte infiltration was remarkably associated with prolonged OS in Her2-positive BC rather than in TNBC patients when they were measured by IHC. In addition, an elevated number of these lymphocytes did not correlate with pCR rate after NAT or clinicopathological features. These findings suggested that LAG-3+ tumor-infiltrating lymphocytes might not promote but impede tumor progression in human BC.
TNBC is usually characterized by an aggressive phenotype, associated with poor prognosis. Current approaches are limited to chemotherapy due to the lack of specific therapeutic targets (24). Previous studies have shown that patients with TNBC would be more likely to achieve pCR after NAT, owing to an abundance of TILs in TME, and high expression of LAG-3 in tumor tissue correlated with more tumor-infiltrating CD8+T cells in TNBC (21), suggesting that an elevated level of LAG-3 before treatment is associated with a high pCR rate in TNBC. However, in this study, we noted that LAG-3+ lymphocyte infiltration did not notably improve the pCR rate in TNBC after NAT, which might attribute to the heterogeneous composition of the TME. In addition, detection methods for LAG-3+ lymphocytes appeared to influence their prognostic effect in TNBC and Her2-positive BC, and further investigation with much more studies should be performed to verify these results.
Prior data have suggested that the presence of an active immune system exerted key roles in preventing long-term recurrence in early-stage disease, contributing to the maintenance of cancer cells in a dormant status, although the underlying mechanism has not been clarified (25). In addition, multiple studies have demonstrated that the infiltration of memory T lymphocytes and CD8+ T cells was predictive of better prognosis in human BC (26, 27); thus, the infiltration of LAG-3+ lymphocytes that were positively correlated with the high density of CD8+T cells in the TME of TNBC (21) would reflect an active host antitumor immunity, thereby improving survival.
There was a paradoxical phenomenon that an elevated expression of immune checkpoint molecules such as CTLA-4 and PD-L1 and improved survival of cancer patients (28, 29), as these molecules could induce an immunosuppressive microenvironment and promote immune evasion and tumor progression. However, current data also suggested that the presence of immune checkpoint molecules expressing TILs might in fact indicate that there was a developing cancer-immune interaction, which was described as an inflamed tumor such as TNBC (30) and usually implicates better prognosis.
There were some limitations in this meta-analysis. The cutoff values were inconsistent between included studies; in addition, morphometric analyses for LAG-3+ lymphocytes used in individual included studies were not consistent, which might cause statistical heterogeneity. Finally, there was only one or two studies included in some subgroup analyses.
In conclusion, an elevated density of LAG-3+ tumor-infiltrating lymphocytes ameliorates OS in TNBC, implicating that it is a valuable prognostic biomarker, and applications of anti-LAG-3 antagonists may possibly not be a promising therapeutic strategy for human BC especially for TNBC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
GMH conceived of the study, participated in its design, extracted data, performed the statistical analysis, and drafted the manuscript; SMW participated in data extraction; SXW and QND participated in data curation. LMH participated in the design of the study. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 82173080, GMH) and Zhejiang Provincial Natural Science Foundation of China (Grant No. LY23H160002, GMH). This work was partly granted from High-level Talent Training Project in Health of Zhejiang Province (GMH).
Acknowledgments
We thank all the members of the departments who helped in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.986903/full#supplementary-material
Abbreviations
TNBC, triple-negative breast cancer; OS, overall survival; DFS, disease-free survival; HR, hazard ratio; OR, odds ratios; Cl, confidence interval; LAG-3, lymphocyte-activating gene-3; pCR, pathological complete response; TME, tumor microenvironment; NR, not reported; NAT, neoadjuvant therapy; IHC, immunohistochemistry; NGS, “next-generation” sequencing technology.
References
1. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat Rev Immunol (2011) 11(10):702–11. doi: 10.1038/nri3064
2. Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol (2021) 12:731798. doi: 10.3389/fphar.2021.731798
3. Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in non-small cell lung cancer: Facts and hopes. Clin Cancer Res (2019) 25(15):4592–602. doi: 10.1158/1078-0432.Ccr-18-1538
4. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
5. Ruffo E, Wu RC, Bruno TC, Workman CJ, Vignali DAA. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin Immunol (2019) 42:101305. doi: 10.1016/j.smim.2019.101305
6. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. (2016) 44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001
7. Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol (2014) 31(8):82. doi: 10.1007/s12032-014-0082-9
8. Giraldo NA, Becht E, Pagès F, Skliris G, Verkarre V, Vano Y, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res (2015) 21(13):3031–40. doi: 10.1158/1078-0432.Ccr-14-2926
9. Deng WW, Mao L, Yu GT, Bu LL, Ma SR, Liu B, et al. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology. (2016) 5(11):e1239005. doi: 10.1080/2162402x.2016.1239005
10. He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2017) 12(5):814–23. doi: 10.1016/j.jtho.2017.01.019
11. Bagbudar S, Karanlik H, Cabioglu N, Bayram A, Ibis K, Aydin E, et al. Prognostic implications of immune infiltrates in the breast cancer microenvironment: The role of expressions of CTLA-4, PD-1, and LAG-3. Appl immunohistochemistry Mol morphology AIMM / Off Publ Soc Appl Immunohistochemistry. (2022) 30(2):99–107. doi: 10.1097/pai.0000000000000978
12. Asano Y, Kashiwagi S, Takada K, Ishihara S, Goto W, Morisaki T, et al. Clinical significance of expression of immunoadjuvant molecules (LAG-3, TIM-3, OX-40) in neoadjuvant chemotherapy for breast cancer. Anticancer Res (2022) 42(1):125–36. doi: 10.21873/anticanres.15466
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
15. Kuritz SJ, Landis JR, Koch GG. A general overview of mantel-haenszel methods: Applications and recent developments. Annu Rev Public Health (1988) 9:123–60. doi: 10.1146/annurev.pu.09.050188.001011
16. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin trials. (2007) 28(2):105–14. doi: 10.1016/j.cct.2006.04.004
17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
18. Stovgaard ES, Kümler I, List-Jensen K, Roslind A, Christensen IJ, Høgdall E, et al. Prognostic and clinicopathologic associations of LAG-3 expression in triple-negative breast cancer. Appl immunohistochemistry Mol morphology AIMM / Off Publ Soc Appl Immunohistochemistry. (2022) 30(1):62–71. doi: 10.1097/pai.0000000000000954
19. Cabioglu N, Onder S, Oner G, Karatay H, Tukenmez M, Muslumanoglu M, et al. TIM3 expression on TILs is associated with poor response to neoadjuvant chemotherapy in patients with locally advanced triple-negative breast cancer. BMC Cancer. (2021) 21(1):357. doi: 10.1186/s12885-021-08054-6
20. Sarradin V, Lusque A, Filleron T, Dalenc F, Franchet C. Immune microenvironment changes induced by neoadjuvant chemotherapy in triple-negative breast cancers: The MIMOSA-1 study. Breast Cancer Res BCR. (2021) 23(1):61. doi: 10.1186/s13058-021-01437-4
21. Wang Y, Dong T, Xuan Q, Zhao H, Qin L, Zhang Q. Lymphocyte-activation gene-3 expression and prognostic value in neoadjuvant-treated triple-negative breast cancer. J Breast cancer. (2018) 21(2):124–33. doi: 10.4048/jbc.2018.21.2.124
22. Fang J, Chen F, Liu D, Gu F, Chen Z, Wang Y. Prognostic value of immune checkpoint molecules in breast cancer. Bioscience Rep (2020) 40(7). doi: 10.1042/bsr20201054
23. Burugu S, Gao D, Leung S, Chia SK, Nielsen TO. LAG-3+ tumor infiltrating lymphocytes in breast cancer: Clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol Off J Eur Soc Med Oncol / ESMO. (2017) 28(12):2977–84. doi: 10.1093/annonc/mdx557
24. Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer–current status and future directions. Ann Oncol Off J Eur Soc Med Oncol / ESMO. (2009) 20(12):1913–27. doi: 10.1093/annonc/mdp492
25. Linde N, Fluegen G, Aguirre-Ghiso JA. The relationship between dormant cancer cells and their microenvironment. Adv Cancer Res (2016) 132:45–71. doi: 10.1016/bs.acr.2016.07.002
26. Ahmadvand S, Faghih Z, Montazer M, Safaei A, Mokhtari M, Jafari P, et al. Importance of CD45RO+ tumor-infiltrating lymphocytes in post-operative survival of breast cancer patients. Cell Oncol (2019) 42(3):343–56. doi: 10.1007/s13402-019-00430-6
27. Peng GL, Li L, Guo YW, Yu P, Yin XJ, Wang S, et al. CD8(+) cytotoxic and FoxP3(+) regulatory T lymphocytes serve as prognostic factors in breast cancer. Am J Trans Res (2019) 11(8):5039–53.
28. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab investigation; J Tech Methods pathology. (2014) 94(1):107–16. doi: 10.1038/labinvest.2013.130
29. Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer immunology immunotherapy CII. (2015) 64(7):853–60. doi: 10.1007/s00262-015-1696-2
Keywords: LAG-3+tumor-infiltrating lymphocytes, tumor microenvironment, triple-negative breast cancer, favorable prognosis, meta-analysis
Citation: Hu G, Wang S, Wang S, Ding Q and Huang L (2023) LAG-3+ tumor-infiltrating lymphocytes ameliorates overall survival in triple-negative breast cancer patients. Front. Oncol. 12:986903. doi: 10.3389/fonc.2022.986903
Received: 05 July 2022; Accepted: 06 December 2022;
Published: 24 January 2023.
Edited by:
Xiaosong Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Mohamed Alorabi, Ain Shams University, EgyptZheng Wang, Shanghai Jiao Tong University, China
Copyright © 2023 Hu, Wang, Wang, Ding and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoming Hu, aGdtcGxqQDEyNi5jb20=; Liming Huang, c2hhb3hpbmdobG1AMTI2LmNvbQ==
 Guoming Hu
Guoming Hu Shimin Wang4
Shimin Wang4