- Department of Minimally Invasive Surgery, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of TCM), Guangzhou, China
Lateral neck dissection (LND) is a necessary treatment for thyroid cancer with lateral lymph node metastasis. However, the defect created during open surgery leaves a visible scar on the neck. With advancements in surgical technology, many robotic and endoscopic surgical techniques have been reported as alternatives to open surgery. In this study, we present a case series demonstrating the successful application of a novel hybrid approach for endoscopic LND and a review of different surgical approaches for “scarless” (at the neck) LND. We performed endoscopic LND via a combined chest and transoral approach in 24 patients between January 2021 and March 2022. The surgery was completed successfully in all patients with an average operation time of 298.1 ± 72.9 min. The numbers of positive/retrieved lymph nodes at levels II, III-IV, and VI were 0.7 ± 0.9/8.4 ± 4.1, 3.6 ± 2.7/19.5 ± 6.8, and 4.9 ± 3.9/10.3 ± 4.5, respectively. Complications included transient hypoparathyroidism in 10 patients, transient recurrent laryngeal nerve injury in 1 patient, internal jugular vein (IJN) injury in 1 patient, IJN sacrifice due to cancer invasion in 1 patient, and chyle leak in 1 patient, and no cases of tumor recurrence were observed during follow-up. The present case series indicates that the combined chest and transoral approach is feasible and effective for performing LND. Our review of different approaches for “scarless” (at the neck) LND identified advantages and disadvantages for all techniques. Our novel approach has unique advantages, and thus, it can provide an ideal surgical procedure for specific papillary thyroid carcinoma patients.
1 Introduction
As the incidence of thyroid cancer has continued to rise in recent years, thyroid cancer ranks fifth among the most common malignant tumors in females in 2020 (1). Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, and more than 30% of patients with PTC develop lateral lymph node (LN) metastasis and require lateral neck dissection (LND) (2–6). For this operation, open surgery provides direct exposure to the lateral compartment, thereby enabling surgeons to perform a safe and quick LND. However, the traditional open LND surgery requires the creation of a large incision in the neck that leaves a highly visible scar. This scar on the neck after open LND has been found to have a negative impact on patients’ quality of life (7, 8). To reduce the adverse effect of scarring on the neck appearance, an endoscopic-assisted approach was developed, which allowed the size of the neck incision to be greatly reduced (9). Since then, with technical advancements in minimally invasive surgery, many other approaches for “scarless” (at the neck) LND have been tested over the past decade. Depending on the surgical instruments employed, these techniques can be divided into robotic approaches and endoscopic approaches. According to the different access routes, the robotic approaches are subdivided into the transaxillary approach (TA), retroauricular approach (RA), bilateral axillary breast approach (BABA), transoral approach (TO), and transaxillary and retroauricular approach (TARA), whereas the endoscopic approaches are subdivided into the chest approach and transoral approach. However, all of the reported surgical approaches to date had limitations. For example, the high cost of robotic instruments has made robotic LND difficult to popularize. With the endoscopic LND approaches, due to obstruction by the clavicle, dissection of LNs at levels IV and VI via the chest approach is inherently challenging (10). Additionally, although the superior-to-inferior view of the transoral approach is helpful for LNs dissection at levels IV and VI, it does not permit complete dissection of LNs at level II.
In the present study, we developed and tested a new hybrid endoscopic approach for LND that combines the advantages of the chest approach and the transoral approach and is theoretically more conducive to complete dissection of LNs at levels II-IV and VI. In addition, we reviewed various approaches to “scarless” (at the neck) LND and considered the advantages and disadvantages of each approach. With many techniques available for LND, it is important to determine the most appropriate treatment that matches each patient’s needs.
2 Materials and methods
2.1 Patients
The present case series included all 24 patients who underwent endoscopic LND via the combined chest and transoral approach between January 2021 and March 2022 in our center. The study protocol was approved by the Institutional Review Board for Ethics at the Guangdong Provincial Hospital of Traditional Chinese Medicine. The inclusion criteria for this study were: confirmed diagnosis of PTC with lateral LNs metastasis (level II, III, or IV) and treated by endoscopic LND via the combined chest and transoral approach. The exclusion criteria were: 1) evidence of distant metastasis or metastatic LNs at level I or V; 2) invasion of surrounding tissues; 3) a history of surgery or radiotherapy at the neck; 4) max dimension of the thyroid cancer > 4 cm and 5) body mass index (BMI) > 40 kg/m2.
2.2 Operative procedures
All patients were in supine position with their neck extended when an electromyogram (EMG) tube was used for endotracheal intubation. For all patients, the first prophylactic antibiotic (first-generation cephalosporin, cefazolin sodium 2 g) was administered 30 min before surgery. The first step of the operation was total thyroidectomy and LND via the chest approach. For this step, a parasternal incision was made at the nipple level, and two incisions were made at the 11 o’clock position of the left areola and 1 o’clock position of the right areola. A 10-mm trocar and two 5-mm trocars were inserted through parasternal incision and areola incisions, respectively (Figure 1). High-flow carbon-dioxide gas was insufflated at a pressure of 6 mmHg. A 30° laparoscope was inserted through the 10-mm trocar. After establishment of the initial working space, total thyroidectomy and endoscopic LND were performed in sequence. The detailed procedures for endoscopic LND were described in our previous study (11). It should be mentioned that LNs at level II-IV was routinely dissected. The spinal accessory nerve is used as a landmark to subdivide level II into IIb, the portion above and behind the nerve, and IIa, the portion that lays anteroinferiorly (12). During the operation, we explicitly dissected the spinal accessory nerve and completed dissection of LNs at level IIa and IIb. Next, three other trocars were inserted under the lower lip at the oral vestibular area, including a 10-mm trocar at the midline and two 5-mm trocars at the level of the first premolars (Figure 1). The central compartment was identified by the brachiocephalic trunk artery inferiorly, carotid artery laterally, and a deep layer of deep cervical fascia posteriorly. Careful attention was paid to dissecting and protecting the recurrent laryngeal nerve (RLN) until it descends into the chest. Then the LNs of level IV were observed, especially those between the sternocleidomastoid muscle (SCM) and sternohyoid muscle. Supplementary dissection of LNs at level IV was performed if necessary.
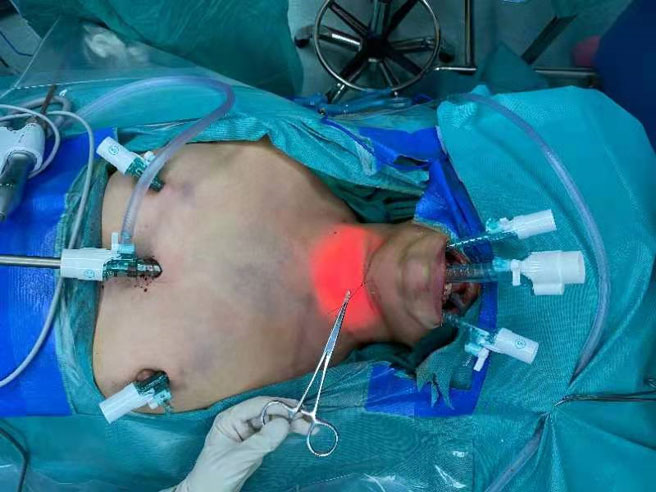
Figure 1 Representative image showing incision and trocar placement for endoscopic LND via the combined chest and transoral approach.
2.3 Postoperative management and follow−up
Patients resumed a normal diet 6 h after surgery, and the second prophylactic antibiotic (cefazolin sodium 2 g) was administered within 24 h after surgery. Once the drainage volume was <30 mL/day, the drainage tubes were removed. The numbers of retrieved and positive LNs, the operation time, and the postoperative hospital stay were recorded. Postoperative follow-up visits were scheduled at 1, 3, and 6 months and then annually thereafter. Hoarseness, cough, vocal cord function, parathyroid hormone level, color and volume of drainage fluid, limb lift restriction, other complications, and tumor recurrence were monitored during follow-up. Limb lift restriction was defined as debilitating shoulder droop and inability to raise the arm above the horizon (12). Hypoparathyroidism was defined when the level of PTH was lower than the lower limit (11 pg/mL) of the normal range of the hormone. The patients with hypoparathyroidism need enteral calcium supplementation. We recommend the use of calcium tablets, combined with calcitriol if necessary, to maintain normal calcium level.
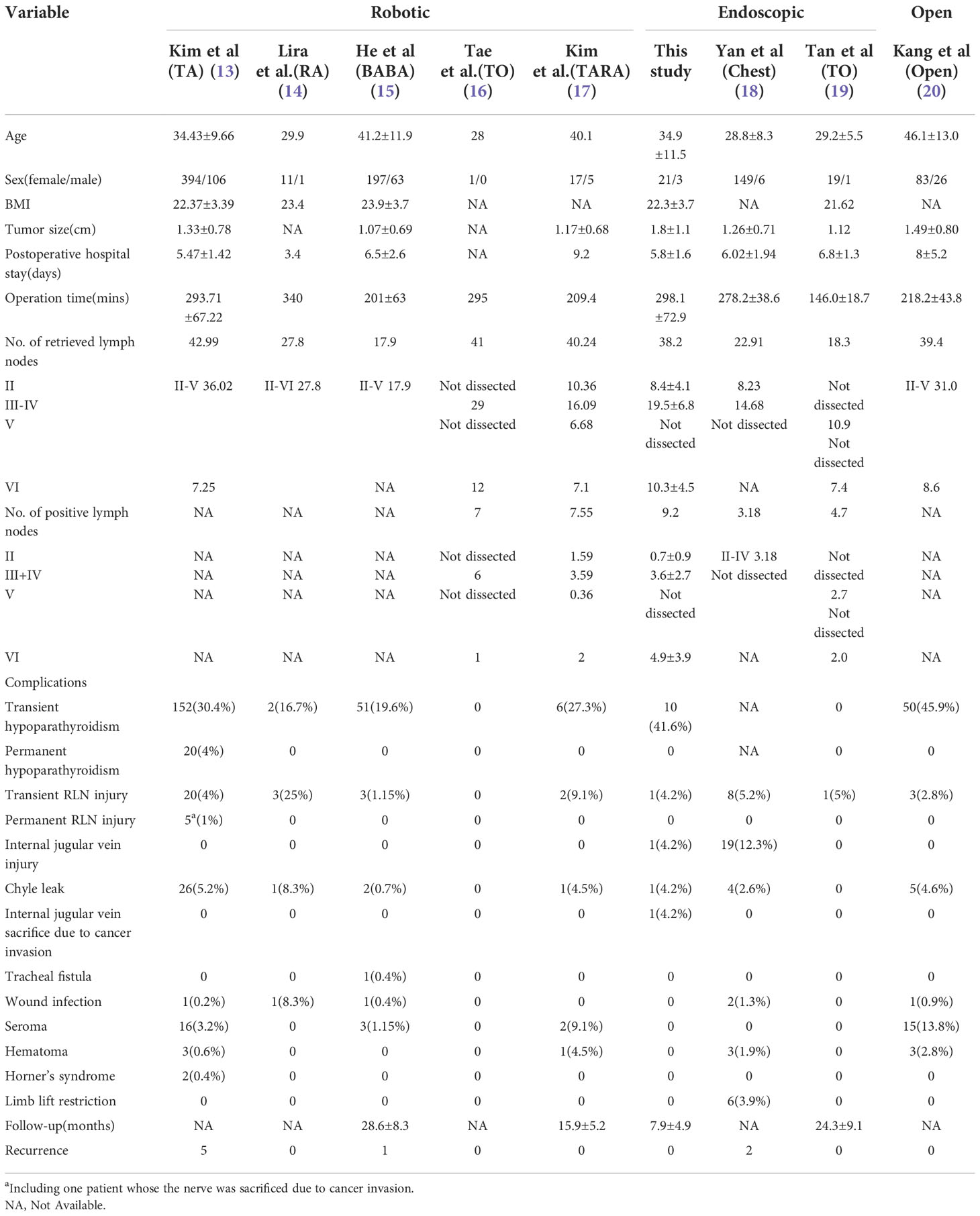
2.4 Literature review
A literature review was performed using the keywords “neck dissection or lateral lymph node dissection” and “endoscopic or robot or robotic” to search titles in the PubMed. Only original English articles involving thyroid cancer patients were selected for review. Because none of the studies were randomized trials and several studies were missing important data, we did not perform an analysis of the combined results from the studies.
3 Results
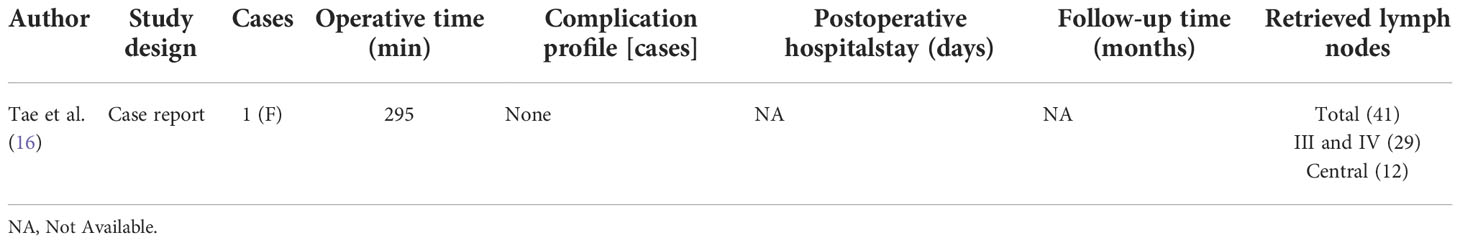
This case series included 21 female patients and 3 male patients with a mean age of 34.9 ± 11.5 years and a mean body mass index (BMI) of 22.3 ± 3.7 kg/m2. In all patients, the combined chest and transoral approach for LND was performed successfully by the same surgeon, and no cases required conversion to open thyroidectomy. The mean operating time which represents - skin to skin was 298.1 ± 72.9 min, and the mean postoperative hospital stay was 5.8 ± 1.6 day. The mean numbers of positive/retrieved LNs from levels II, III-IV and VI were 0.7 ± 0.9/8.4 ± 4.1, 3.6 ± 2.7/19.5 ± 6.8 and 4.9 ± 3.9/10.3 ± 4.5, respectively.
Complications included transient hypoparathyroidism in 10 patients, transient RLN palsy in 1 patient, internal jugular vein (IJN) injury in 1 patient, IJN sacrifice due to cancer invasion in 1 patient, and chyle leak in 1 patient. All cases of transient hypoparathyroidism and RLN palsy resolved spontaneously within 3 months. No patients experienced permanent RLN palsy, permanent hypoparathyroidism, wound infection, limb lift restriction, tracheal fistula, seroma, hematoma or Horner’s syndrome. The mean follow-up period was 7.9 ± 4.9 months, and no cases developed tumor recurrence during follow-up (Table 1).
The characteristics and surgical outcomes of other cohorts reported in previous studies by different investigators also are listed in Table 1 for comparison.
4 Discussion
Neck dissection of a malignancy was first described in 1906, and this surgery requires removal of all lymphatic and non-lymphatic structures between the platysma and the prevertebral fascia in the lateral neck, except for the common carotid artery and vital motor nerves. In 1980, a method for modified radical neck dissection was reported and then accepted as a substitute for classic neck dissection. The modified technique preserves one or more of the following structures: IJV, SCM, and spinal accessory nerve (21). Another method termed selective neck dissection refers to removal of less than all five nodal levels and is directed by the patterns of lymphatic drainage from the primary tumor, with preservation of the IJV, SCM, and spinal accessory nerve (12). Selective neck dissection is the most commonly used neck dissection surgery for the management of lateral neck metastasis of PTC, and an expert consensus in 2017 from China (only published in Chinese) suggested that comprehensive neck dissection of at least nodal levels IIA, III and IV should be performed when indicated to optimize disease control. Although the extent of the surgical dissection is decreased with this method, the required incision for this open surgery was similar in size to that required for traditional neck dissection (20, 22). Studies have documented that the change in neck appearance after open LND can negatively impact the quality of life of patients (7, 8). Therefore, surgeons have attempted to apply minimally invasive techniques to LND for treatment of PTC. As a result of these efforts, today robotic and endoscopic methods for LND as PTC treatment are offered in many hospitals worldwide. Considering the disadvantages of these methods, we developed a novel approach for endoscopic LND, and in this study, recorded the short-term clinical outcomes of patients in order to evaluate the feasibility of our approach. Upon reviewing all previously reported “scarless” (at the neck) LND methods for the treatment of thyroid cancer, we compared the advantages and disadvantages of the various approaches and the novel approach introduced in the present study.
4.1 Literature review of robotic LND techniques
Compare with endoscopic instruments, robotic instruments offer the following advantages: 1) movement with 7 degrees of freedom that is stabilized and not affected by tremor; 2) three-dimensional endoscopic view; 3) automatically controlled traction; and 4) optimized ergonomics (23, 24). The use of robotic instruments for robotic LND provides the following advantages: 1) the ability to achieve more accurate and elaborate dissection facilitates easier identification and preservation of the parathyroid gland, nerves, vessels, and lymphangion, lowering the risks of complications; and 2) improved exposure theoretically allows dissection of LNs at all levels (21). However, compared with endoscopic LND, robotic LND has the following limitations: 1) higher cost; 2) longer operative time; and 3) increased risk of neck pain and stiffness due to separation of the SCM (23). Beyond these general advantages and disadvantages of robotic LND, specific approaches to robotic LND have unique characteristics that can be beneficial in specific cases. Below we review the main robotic LND techniques described in the literature.
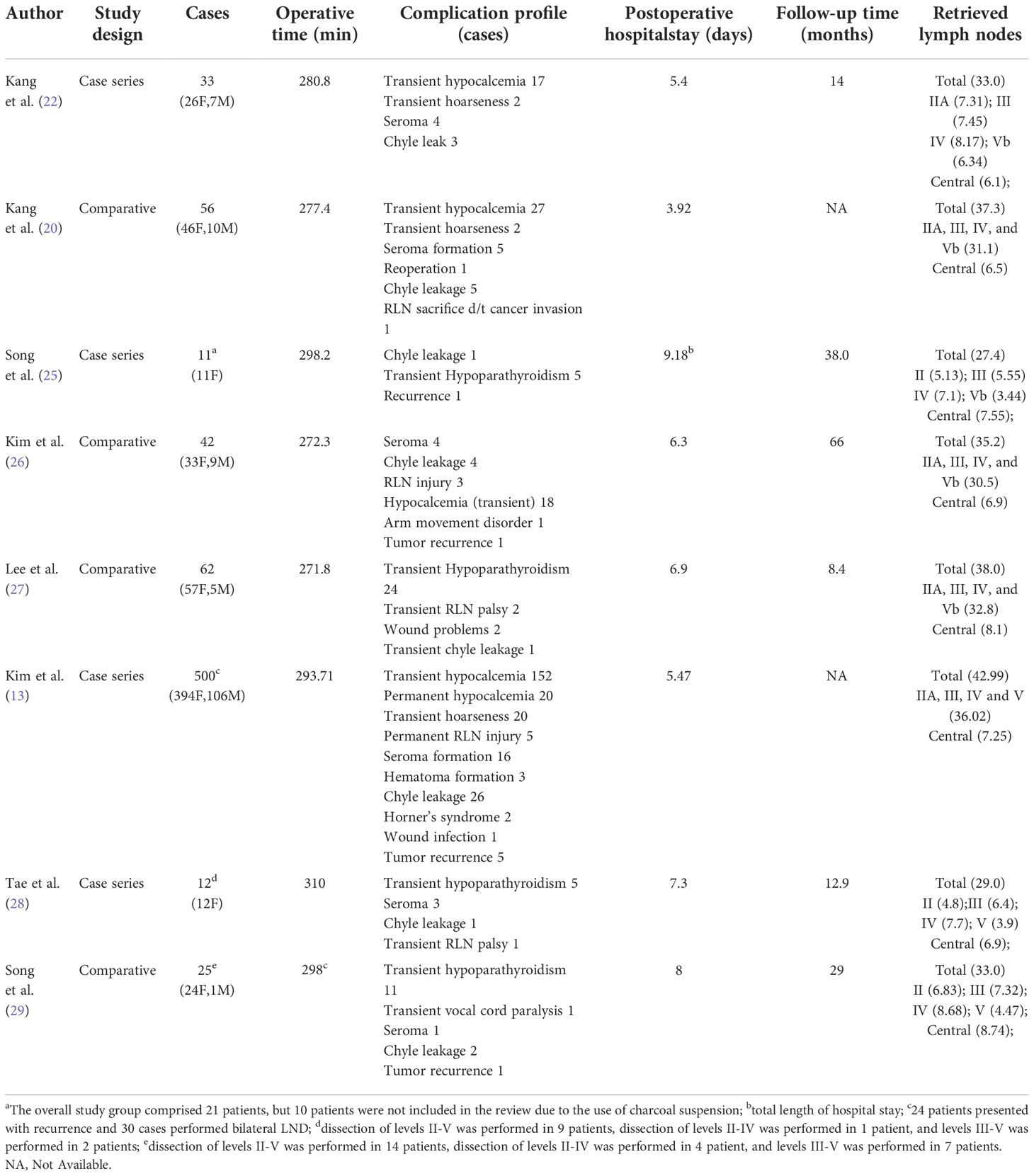
4.1.1 Transaxillary approach (TA)
The TA to robotic LND is also referred to as the gasless unilateral axillary (GUA) or gasless unilateral axillobreast (GUAB) approach. Notably, the transaxillary incision in GUA/GUAB approach was more lateral in the hair-bearing midaxillary line than the incision in the transaxillary approach used by Kang et al, which helped to improve the appearance by hiding the axillary scar completely under the arm (25). Robotic LND via the TA originally required two incisions, including a 7- to 8-cm vertical incision on the axilla and another incision on the breast in the case of GUAB (Figure 2B1). This approach was modified to the GUA approach, which involves only a single axillary incision without the breast incision, and all four robotic arms are inserted through this incision (Figure 2B2). The TA has been the most widely used method of robotic LND, with eight reports on this approach found in the literature, the first of which was published in 2010 (13, 20, 22, 25–29). In most studies, level IIA, III, IV and VB LNs were dissected via the TA. In addition, although LNs at levels IIB and VA are not routinely included in robotic LND, these LNs can also be exposed and dissected via this approach. In a report by Kim et al. (13), a cohort of 500 patients was treated via the TA. The average operating time was 293.71 min, and the average postoperative hospital stay was 5.47 days. Postoperative complications in their study included transient symptomatic hypocalcemia in 152 patients (30.4%), permanent hypocalcemia in 20 patients (4%), transient hoarseness in 20 patients (4%), permanent RLN injury in 5 patients (1%), RLN sacrifice due to cancer invasion in 1 patient (0.2%), seroma in 16 patients (3.2%), hematoma in 3 patients (0.6%), chyle leakage in 26 patients (0.8%), Horner’s syndrome in 2 patients (0.4%), wound infection in 1 patient (0.2%), and tumor recurrence in 5 patients (1%) Table 1). In studies comparing robotic LND approaches to open LND) (20, 26, 27), the reported oncologic and postoperative outcomes did not differ between robotic and open surgeries, and the robotic approaches were found to provide better cosmetic satisfaction among patients than open LND (Table 2).
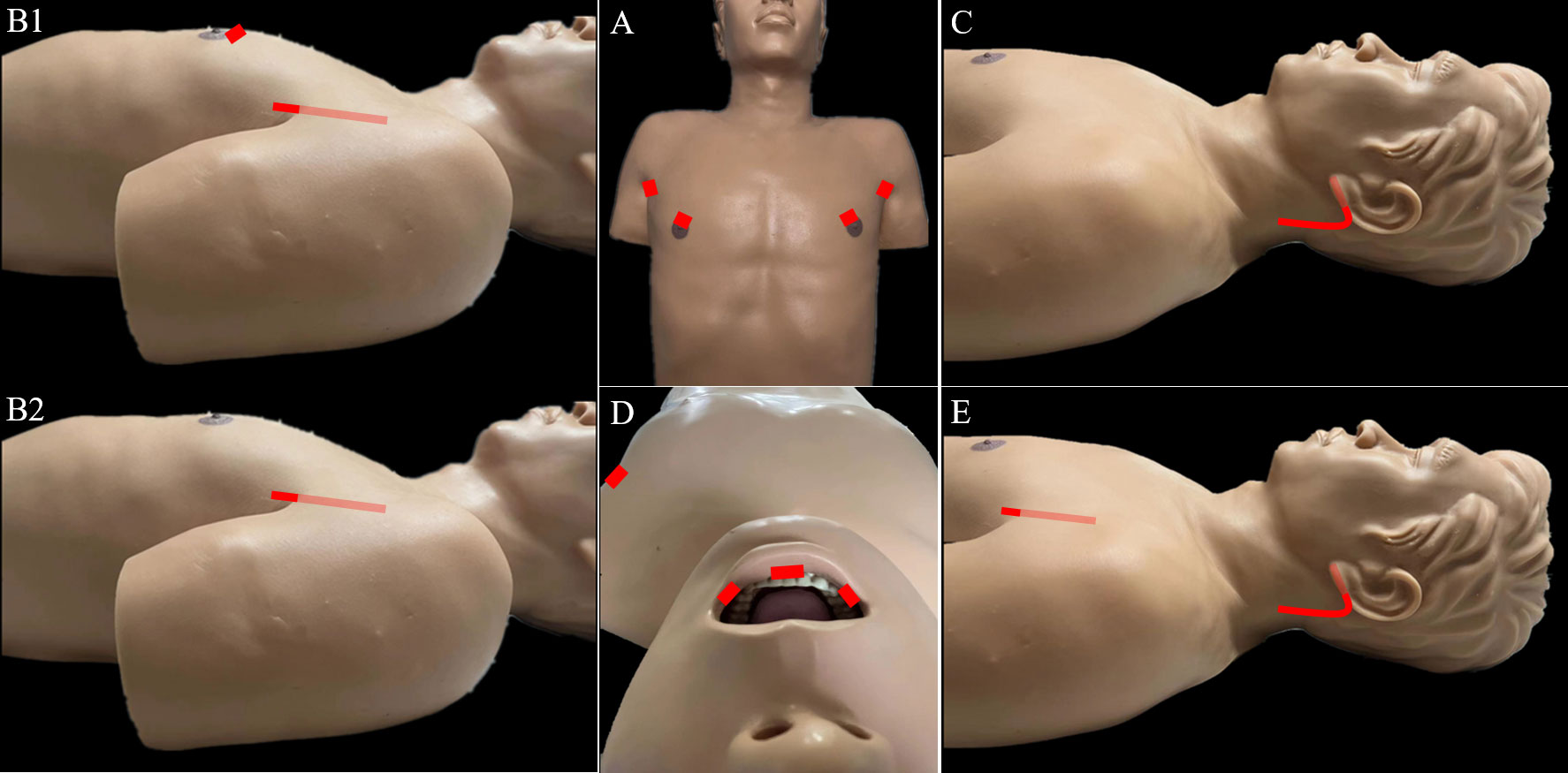
Figure 2 Incisions required for robotic LND via the: (A) bilateral axillary breast approach, (B) transaxillary approach, (C) retroauricular approach, (D) transoral approach, and (E) transaxillary and retroauricular approach.
The reported advantages of the TA include: 1) a view similar to that of the open approach and relatively short operation time; and 2) safety and efficacy well-established by the literature (21, 30). The disadvantages of the TA include: 1) difficulty accessing level II LNs; 2) inability to perform contralateral LND; 3) difficulty of complete removal of level IV LNs due to interference by the clavicle; and 4) possible risks of anterior chest paresthesia and brachial plexus injury (21, 31).
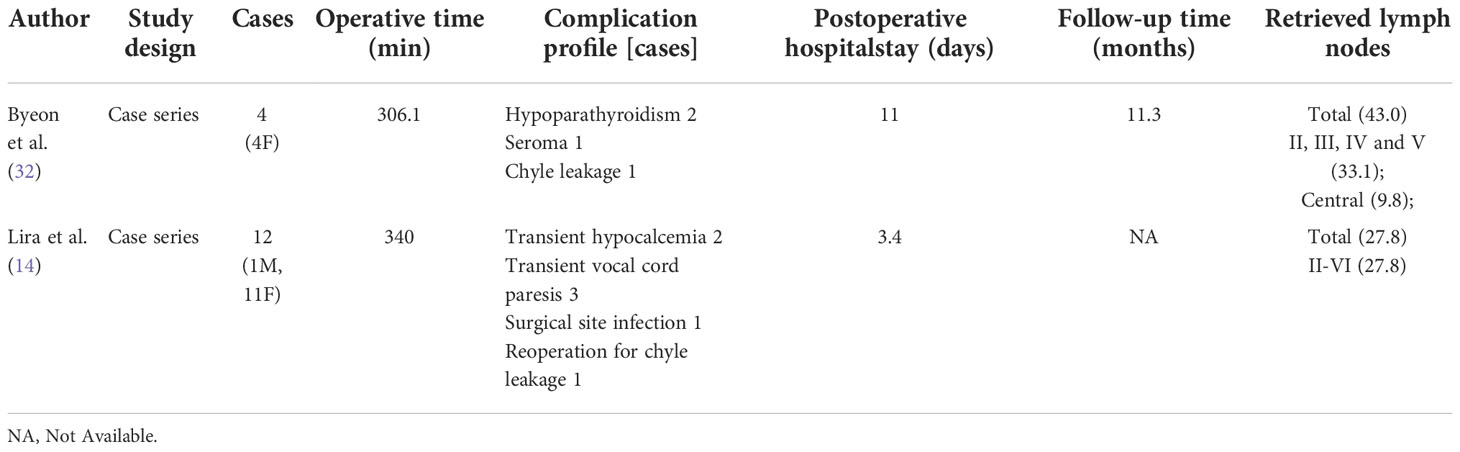
4.1.2 Retroauricular approach (RA)
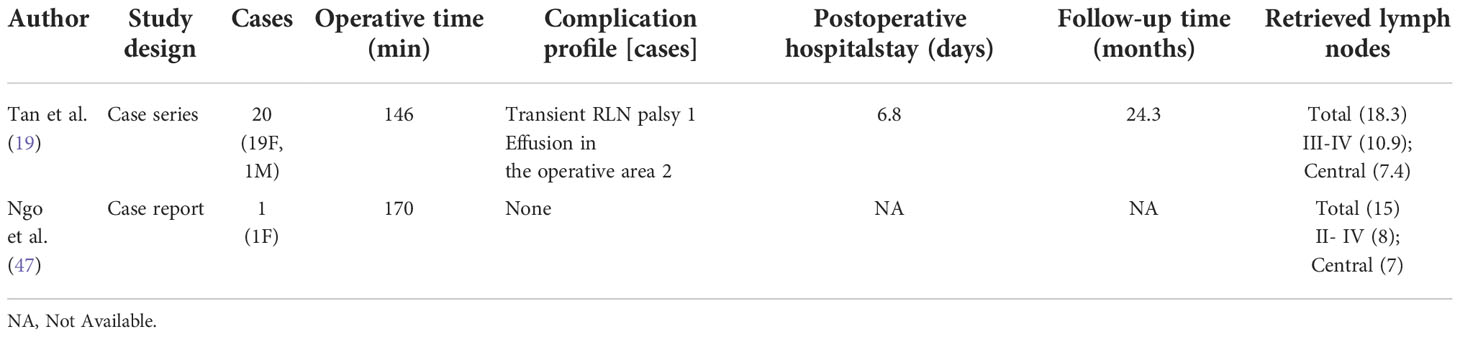
For robotic LND via the RA, an L-shaped incision behind the auricle is needed, and three arms of the da Vinci Surgical System are typically used (Figure 2C). Two case series investigating the RA approach were found in the literature, and the first was published in 2014 (14, 32). In most cases, level II–V LNs were dissected via the RA, and exposure and dissection of the accessory nerve and LNs of levels II and III can be accomplished under direct vision using this approach. In the series of 12 patients reported by Lira et al. (14), complications included postoperative transient hypocalcemia in 2 patients (16.7%), transient vocal cord paresis in 3 patients (25%), surgical site infection in 1 patient (8.3%), and chyle leakage in 1 patient (8.3%) (Table 3) (14).
The noted advantages of the RA include: 1) reduced postoperative discomfort due to a relatively small dissection area; 2) ease of accessing level II; 3) suitability for obese patients; and 4) reduced risk of injury to great vessels, esophagus, and anterior chest sensory nerves (33, 34). The disadvantages of the RA include: 1) risk of injury to greater auricular and marginal mandibular nerves; 2) an opposite operative view compared with the open procedure; and 3) inability to perform contralateral LND (30, 34, 35).
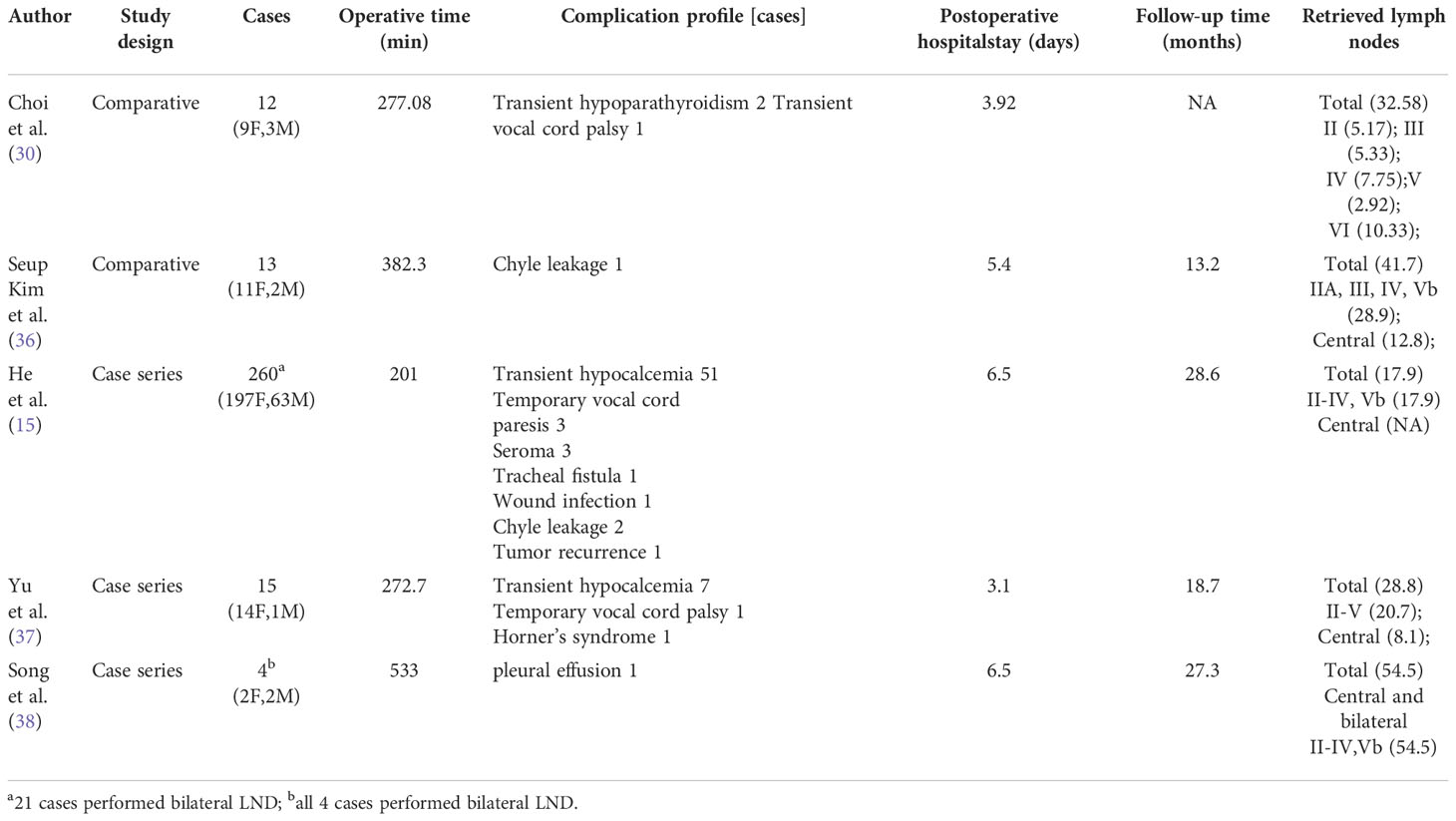
4.1.3 Bilateral axillary breast approach (BABA)
For robotic LND via the BABA, four incisions are needed, including a circumareolar incision in each breast and a vertical incision in each axilla (Figure 2A). The BABA has been a widely used method of robotic LND, with five reports investigating this approach found in the literature, the first of which was published in 2013 (15, 30, 36–38). In most cases, level II-V LNs were dissected via the BABA. Among the cohort of 260 patients studied by He et al. (15), the average operating time for the BABA was 201 min, and the average postoperative hospital stay was 6.5 days. Complications among this cohort included postoperative transient hypocalcemia in 51 patients (19.6%), transient hoarseness in 3 patients (1.2%), seroma in 3 patients (1.2%), chyle leakage in 2 patients (0.8%), tracheal injury in 1 patient (0.4%), wound infection in 1 patient (0.4%) and tumor recurrence in 1 patient (0.4%)(Table 1) . Notably, all 260 patients were extremely satisfied or satisfied with the cosmetic results of the BABA. From their comparative studies, Choi et al. (32) and Kim et al.[ (30, 36) concluded that robotic LND using the BABA is safe and provides oncologic and postoperative outcomes comparable to those of the open surgery (Table 4).
The advantages of the BABA approach include: 1) a symmetric view similar to that obtained with the open approach, and 2) the ability to perform bilateral LND (15, 30). The disadvantages of the BABA include: 1) difficultly performing level IV dissection due to an inferior-to-superior view; 2) difficulty accessing level II; and 3) risk of injury to anterior chest sensory nerves and brachial plexus (30).
4.1.4 Transoral approach (TO)
For robotic LND via the TO, one central incision and two lateral incisions are made in the lower lip at the oral vestibular area, and another incision is made in the axilla for insertion of a third robotic instrument (Figure 2D). The TO has been described in only one case report published in 2020 (16). The operative time was 295 min, and no major postoperative complications occurred. Notably, no LNs of levels II and V were dissected in this case (Table 5).
The apparent advantages of the TO include: 1) excellent access and exposure to the bilateral thyroid lobes, and 2) relative ease of dissection from the inferior side of level IV due to a superior-to-inferior view. The disadvantages of the TO include: 1) risk of mental nerve injury; 2) risk of infection and the need for postoperative antibiotics; 3) inability to completely remove level II and III LNs, making it oncologically unsafe; and 4) an operative view opposite to that of the open procedure (16).
4.1.5 Transaxillary and retroauricular approach (TARA)
For robotic LND via the TARA, in addition to an L-shaped incision behind the auricle, another axillary incision is needed (Figure 2E). The TARA has been reported in one case series and one case report (17, 39), and level II–V LNs were dissected via this approach. Notably, exposure and dissection of the accessory nerve and LNs at levels II, VA, and upper level III can be accomplished under direct vision via the RA incision, and thyroidectomy, central neck dissection, and the dissection of LNs at levels III, VB, and IV can be achieved via the transaxillary approach. Among the 22 patients included in the case series (17), the average operating time was 209.4 min, and the average postoperative hospital stay was 9.2 days. Postoperative complications in this series included transient hypoparathyroidism in 6 patients (27.3%), seroma in 2 patients (9.1%), chyle leakage in 1 patient (4.5%), hematoma in 1 patient (4.5%), earlobe numbness in 6 patients (27.3%), and transient hoarseness in 2 patients (9.1%) (Table 6).
The TARA offers the advantages of both the TA and RA approaches, and dissection of LNs at levels II–V can be achieved via this approach. The disadvantages of the TARA include: 1) the need for more incisions results in greater trauma; 2) risk of injury to the greater auricular nerve, marginal mandibular nerve, anterior chest sensory nerves, and brachial plexus; and 3) inability to perform contralateral LND.
4.2 Literature review of endoscopic LND techniques
Compared with robotic LND, endoscopic LND offers the following advantages: 1) much lower cost of endoscopic devices with the same effectiveness and safety as robotic devices for the treatment of thyroid cancer, and 2) endoscopic devices provide force feedback that robot surgery systems lack. The disadvantages of endoscopic LND include: 1) reduced versatility of the endoscopic devices compared with the robotic devices, which may lead to poor lateral compartment exposure, longer time under anesthesia, and a higher incidence of complications; 2) longitudinal separation of the SCM to expose the lateral compartment; and 3) restricted ability to dissect LNs at level V due to the lateral border of the SCM (11, 18, 23, 24).
4.2.1 Chest approach
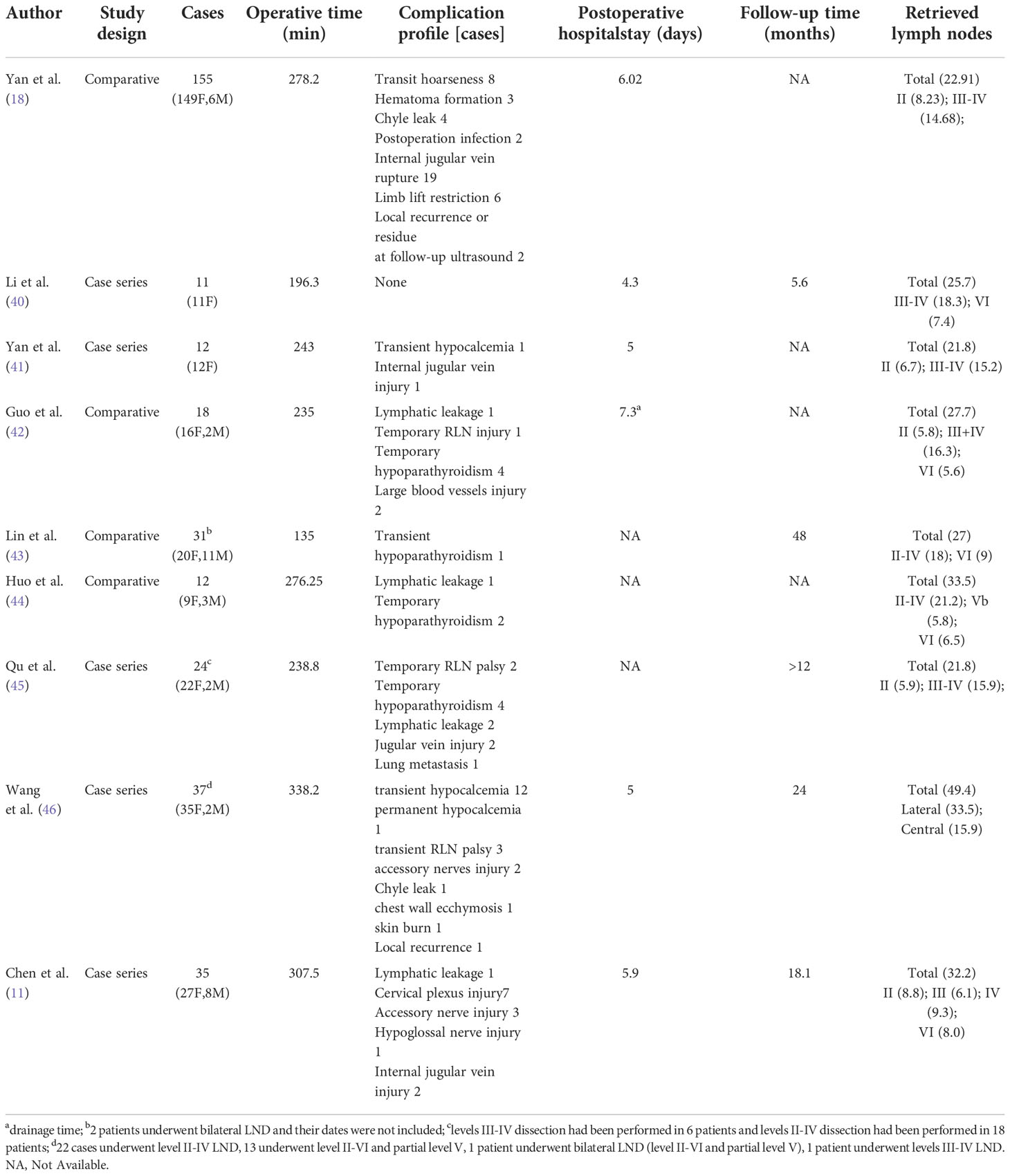
For endoscopic LND via the chest approach, three incisions are made, including a circumareolar incision in each breast and a third incision that can be either a parasternal incision (Figure 3A1) or circumareolar incision (Figure 3A2). The chest approach is the most widely used method of endoscopic LND. We found nine reports on this approach in the literature, the first of which was published in 2011 (11, 18, 40–46). Level II-IV LNs were routinely dissected in studies of endoscopic LND via the chest approach. Yan et al. (18) reported a cohort of 155 patients treated via the chest approach. Among this cohort, the average operating time was 278.2 min, and the average postoperative hospital stay was 6.02 days. Postoperative complications included transient hoarseness in 8 patients (5.2%), hematoma in 3 patients (1.9%), chyle leak in 4 patients (2.6%), infection in 2 patient (1.3%), IJN rupture in 19 patients (12.3%), limb lift restriction in 6 patients (3.9%), and local recurrence or residual tumor tissue in 2 patients (1.3%) (Table 1). In comparative studies of endoscopic LND via the chest approach versus open surgery, the reported oncologic and postoperative outcomes achieved with the chest approach were comparable to those achieved with open surgery (Table 7) (18, 42–44).
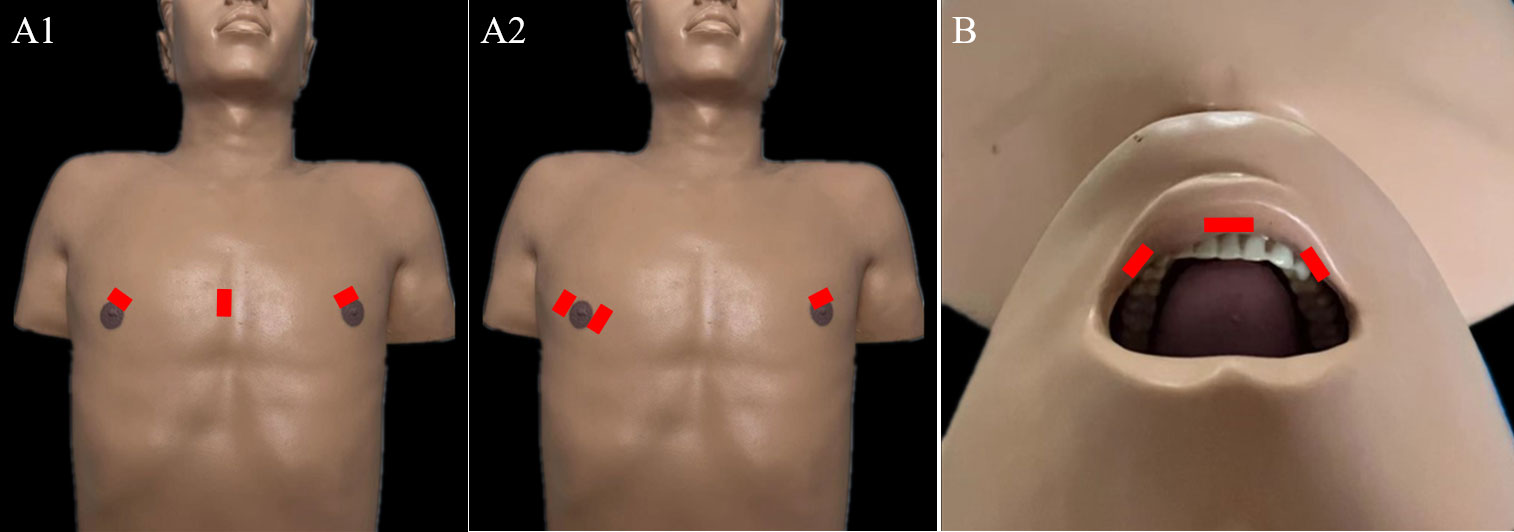
Figure 3 Incisions required for endoscopic LND via the: (A) chest approach and (B) transoral approach.
The advantages of the chest approach for endoscopic LND include: 1) a symmetric view similar to that achieved with the open approach; 2) ability to perform bilateral LND; and 3) relative ease of level II LN dissection due to endoscopic magnification and less space restriction. The disadvantages of the endoscopic chest approach include: 1) restricted access for level IV dissections due to the clavicle, and 2) risk of anterior chest paresthesia.
4.2.2 Transoral approach
For endoscopic LND via the transoral approach, three incisions are needed, including an incision at the midline of the lower lip and two incisions at the level of the first premolars (Figure 3B). Notably, LNs at levels II and V have not been dissected via this approach in the reported studies. The transoral approach is a less reported method of endoscopic LND compared with the chest approach, with only one case series and one case report found in the current literature (19, 47). Tan et al. (19) reported a cohort of 20 patients treated with endoscopic LND via the transoral approach. Among their cohort, the average operating time was 146 min, and the average postoperative hospital stay was 6.8 days. Postoperative complications included transient unilateral RLN palsy in 1 patient (5%) and effusion in the operative field in 2 patients (10%) (Table 8).
The advantages and disadvantages of the transoral approach to endoscopic LND are similar to those of the robotic transoral approach.
4.2.3 Combined chest and transoral approach
The endoscopic LND approach reported in the present study seeks to combine the advantages of the chest and transoral approaches to endoscopic LND. With this new approach, dissection of LNs at levels II-IV was first performed via the chest approach. Then supplementary dissection of the inferior side of level IV was performed via the transoral approach. As a result, this approach afforded completely unrestricted dissection of LNs at levels II-IV via endoscopic surgery. The disadvantages of our combined approach include: 1) greater trauma due to the requirement of more incisions, and 2) potential risks of prolonged operation time, mental nerve injury, infection, and anterior chest paresthesia. However, among the 24 patients in the present study, the hospital stay was comparable with that in a study of the chest approach for endoscopic LND (48) Table 1) which suggests that the addition of the small incision in the lower lip does not significantly increase the patient’s trauma. The operating time for the combined chest and transoral approach in this study was slightly longer than that for the chest approach, and this is attributed to the fact that once the initial operating space is established via the chest approach, no additional time is required for flap separation via the transoral approach. In addition, no patients developed with mental nerve injury or infection. Consistently, in previous studies of the transoral endoscopic thyroidectomy vestibular approach (TOETVA) technique, postoperative infection was rare, and the risk of mental nerve injury was low (49–51). Notably, the number of central LNs retrieved using our approach was greater than that achieved in the study by Kim et al. (13), and the number of lateral LNs retrieved via our combined chest and transoral approach was greater than that achieved via the chest approach (48) (Table 1). These advantages in LN dissection may be due to the benefits of the transoral approach for dissection of central LNs and supplementary LN dissection from the inferior side of level IV.
4.3 Limitations of the present study
Some limitations of our study should be acknowledged. First, this was a retrospective study and lacked of control group. The safety of the combined chest and transoral approach for endoscopic LND should be evaluated in prospective randomized controlled trials in the future. Additionally, the mean follow-up period in our cohort was only 7.9 ± 4.9 months, which is too short to draw conclusions on the effectiveness of our approach for the treatment of PTC.
5 Conclusions
Many different approaches for “scarless” (at the neck) LND have been reported, and each approach has a unique set of advantages and disadvantages. Surgeons need to be familiar with the advantages and disadvantages of each approach in order to recommend appropriate approaches for patients according to the individual characteristics of patients and tumors as well as the experience of the surgeon. The combined chest and transoral approach introduced in this study offers advantages of both the chest approach and transoral approach, potentially allowing more extensive dissection of LNs and achieving better oncological results. Once the safety of this approach is confirmed in a larger cohort, this novel approach may provide the most ideal surgical method for select patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board for Ethics at the Guangdong Provincial Hospital of Traditional Chinese Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Z-XC, J-BC, F-SP, and YQ designed the concept of this study. Z-HL, X-BZ, B-YC, W-WZ, and YC collected the datasets. Z-XC, J-BC, and F-SP analyzed the data and wrote the manuscript. Z-XC and YQ revised the manuscript. All authors have read and approved the manuscript.
Acknowledgments
We thank Medjaden Inc. for scientific editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Hu D, Zhou J, He W, Peng J, Cao Y, Ren H, et al. Risk factors of lateral lymph node metastasis in cN0 papillary thyroid carcinoma. World J Surg Oncol (2018) 16:30. doi: 10.1186/s12957-018-1336-3
3. Cracchiolo JR, Wong RJ. Management of the lateral neck in well differentiated thyroid cancer. Eur J Surg Oncol (2018) 44:332–7. doi: 10.1016/j.ejso.2017.06.004
4. Ducoudray R, Tresallet C, Godiris-Petit G, Tissier F, Leenhardt L, Menegaux F. Prophylactic lymph node dissection in papillary thyroid carcinoma: is there a place for lateral neck dissection? World J Surg (2013) 37:1584–91. doi: 10.1007/s00268-013-2020-y
5. Mulla MG, Knoefel WT, Gilbert J, McGregor A, Schulte KM. Lateral cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the lateral compartment. Clin Endocrinol (Oxf) (2012) 77:126–31. doi: 10.1111/j.1365-2265.2012.04336.x
6. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg (2005) 29:917–20. doi: 10.1007/s00268-005-7789-x
7. Inoue H, Nibu K, Saito M, Otsuki N, Ishida H, Onitsuka T, et al. Quality of life after neck dissection. Arch Otolaryngol Head Neck Surg (2006) 132:662–6. doi: 10.1001/archotol.132.6.662
8. Shah S, Har-El G, Rosenfeld RM. Short-term and long-term quality of life after neck dissection. Head Neck (2001) 23:954–61. doi: 10.1002/hed.1138
9. Miccoli P, Berti P, Raffaelli M, Conte M, Materazzi G, Galleri D. Minimally invasive video-assisted thyroidectomy. Am J Surg (2001) 181:567–70. doi: 10.1016/S0002-9610(01)00625-0
10. Zhang Z, Sun B, Ouyang H, Cong R, Xia F, Li X. Endoscopic lateral neck dissection: A new frontier in endoscopic thyroid surgery. Front Endocrinol (Lausanne) (2021) 12:796984. doi: 10.3389/fendo.2021.796984
11. Chen ZX, Song YM, Chen JB, Zhang XB, Lin ZH, Cai BY, et al. Qin's seven steps for endoscopic selective lateral neck dissection via the chest approach in patients with papillary thyroid cancer: experience of 35 cases. Surg Endosc (2022) 36:2524–31. doi: 10.1007/s00464-021-08540-9
12. Stack BC Jr., Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, American Thyroid association surgical affairs c, et al American Thyroid association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid (2012) 22:501–8. doi: 10.1089/thy.2011.0312
13. Kim JK, Lee CR, Kang SW, Jeong JJ, Nam KH, Chung WY. Robotic transaxillary lateral neck dissection for thyroid cancer: learning experience from 500 cases. Surg Endosc (2022) 36:2436–44. doi: 10.1007/s00464-021-08526-7
14. Lira RB, Chulam TC, Kowalski LP. Variations and results of retroauricular robotic thyroid surgery associated or not with neck dissection. Gland Surg (2018) 7:S42–52. doi: 10.21037/gs.2018.03.04
15. He Q, Zhu J, Zhuang D, Fan Z, Zheng L, Zhou P, et al. Robotic lateral cervical lymph node dissection via bilateral axillo-breast approach for papillary thyroid carcinoma: a single-center experience of 260 cases. J Robot Surg (2020) 14:317–23. doi: 10.1007/s11701-019-00986-3
16. Tae K, Kim KH. Transoral robotic selective neck dissection for papillary thyroid carcinoma: Dissection of levels III and IV. Head Neck (2020) 42:3084–8. doi: 10.1002/hed.26379
17. Kim WS, Koh YW, Byeon HK, Park YM, Chung HJ, Kim ES, et al. Robot-assisted neck dissection via a transaxillary and retroauricular approach versus a conventional transcervical approach in papillary thyroid cancer with cervical lymph node metastases. J Laparoendosc Adv Surg Tech A (2014) 24:367–72. doi: 10.1089/lap.2013.0296
18. Yan HC, Xiang C, Wang Y, Wang P. Scarless endoscopic thyroidectomy (SET) lateral neck dissection for papillary thyroid carcinoma through breast approach: 10 years of experience. Surg Endosc (2020) 35 (2021), 3540–3546. doi: 10.1007/s00464-020-07814-y
19. Tan Y, Guo B, Deng X, Ding Z, Wu B, Niu Y, et al. Transoral endoscopic selective lateral neck dissection for papillary thyroid carcinoma: a pilot study. Surg Endosc (2020) 34:5274–82. doi: 10.1007/s00464-019-07314-8
20. Kang SW, Lee SH, Park JH, Jeong JS, Park S, Lee CR, et al. A comparative study of the surgical outcomes of robotic and conventional open modified radical neck dissection for papillary thyroid carcinoma with lateral neck node metastasis. Surg Endosc (2012) 26:3251–7. doi: 10.1007/s00464-012-2333-1
21. Kang SW, Kim MJ, Chung WY. Gasless, transaxillary robotic neck dissection: the technique and evidence. Gland Surg (2018) 7:466–72. doi: 10.21037/gs.2017.09.09
22. Kang SW, Lee SH, Ryu HR, Lee KY, Jeong JJ, Nam KH, et al. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery (2010) 148:1214–21. doi: 10.1016/j.surg.2010.09.016
23. Pena Gonzalez JA, Pascual Queralt M, Salvador Bayarri JT, Rosales Bordes A, Palou Redorta J, Villavicencio Mavrich H. [Evolution of open versus laparoscopic/robotic surgery: 10 years of changes in urology]. Actas Urol Esp (2010) 34:223–31. doi: 10.1016/s2173-5786(10)70053-6
24. Herron DM, Marohn M, Group S-MRSC. A consensus document on robotic surgery. Surg Endosc (2008) 22:313–25. doi: 10.1007/s00464-007-9727-5
25. Song CM, Ji YB, Sung ES, Kim DS, Koo HR, Tae K. Comparison of robotic versus conventional selective neck dissection and total thyroidectomy for papillary thyroid carcinoma. Otolaryngol Head Neck Surg (2016) 154:1005–13. doi: 10.1177/0194599816638084
26. Kim MJ, Lee J, Lee SG, Choi JB, Kim TH, Ban EJ, et al. Transaxillary robotic modified radical neck dissection: a 5-year assessment of operative and oncologic outcomes. Surg Endosc (2017) 31:1599–606. doi: 10.1007/s00464-016-5146-9
27. Lee J, Kwon IS, Bae EH, Chung WY. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J Clin Endocrinol Metab (2013) 98:2701–8. doi: 10.1210/jc.2013-1583
28. Tae K, Ji YB, Song CM, Min HJ, Lee SH, Kim DS. Robotic lateral neck dissection by a gasless unilateral axillobreast approach for differentiated thyroid carcinoma: our early experience. Surg Laparosc Endosc Percutan Tech (2014) 24:e128–132. doi: 10.1097/SLE.0b013e3182a4bfa1
29. Song CM, Park JS, Park W, Ji YB, Cho SH, Tae K. Feasibility of charcoal tattooing for localization of metastatic lymph nodes in robotic selective neck dissection for papillary thyroid carcinoma. Ann Surg Oncol (2015) 22 Suppl 3:S669–675. doi: 10.1245/s10434-015-4860-1
30. Choi YS, Hong YT, Yi JW. Initial experience with robotic modified radical neck dissection using the da Vinci xi system through the bilateral axillo-breast approach. Clin Exp Otorhinolaryngol (2021) 14:137–44. doi: 10.21053/ceo.2020.01585
31. Chang EHE, Kim HY, Koh YW, Chung WY. Overview of robotic thyroidectomy. Gland Surg (2017) 6:218–28. doi: 10.21037/gs.2017.03.18
32. Byeon HK, Holsinger FC, Tufano RP, Chung HJ, Kim WS, Koh YW, et al. Robotic total thyroidectomy with modified radical neck dissection via unilateral retroauricular approach. Ann Surg Oncol (2014) 21:3872–5. doi: 10.1245/s10434-014-3896-y
33. Berber E, Bernet V, Fahey TJ, Kebebew E, Shaha A, Stack BC Jr., American Thyroid association surgical affairs c, et alAmerican Thyroid association statement on remote-access thyroid surgery. Thyroid (2016) 26:331–7. doi: 10.1089/thy.2015.0407
34. Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. clinical feasibility and safety. Laryngoscope (2011) 121:1636–41. doi: 10.1002/lary.21832
35. Sung ES, Ji YB, Song CM, Yun BR, Chung WS, Tae K. Robotic thyroidectomy: Comparison of a postauricular facelift approach with a gasless unilateral axillary approach. Otolaryngol Head Neck Surg (2016) 154:997–1004. doi: 10.1177/0194599816636366
36. Seup Kim B, Kang KH, Park SJ. Robotic modified radical neck dissection by bilateral axillary breast approach for papillary thyroid carcinoma with lateral neck metastasis. Head Neck (2015) 37:37–45. doi: 10.1002/hed.23545
37. Yu HW, Chai YJ, Kim SJ, Choi JY, Lee KE. Robotic-assisted modified radical neck dissection using a bilateral axillo-breast approach (robotic BABA MRND) for papillary thyroid carcinoma with lateral lymph node metastasis. Surg Endosc (2018) 32:2322–7. doi: 10.1007/s00464-017-5927-9
38. Song RY, Sohn HJ, Paek SH, Kang KH. The first report of robotic bilateral modified radical neck dissection through the bilateral axillo-breast approach for papillary thyroid carcinoma with bilateral lateral neck metastasis. Surg Laparosc Endosc Percutan Tech (2020) 30:e18–22. doi: 10.1097/SLE.0000000000000590
39. Byeon HK, Ban MJ, Lee JM, Ha JG, Kim ES, Koh YW, et al. Robot-assisted sistrunk's operation, total thyroidectomy, and neck dissection via a transaxillary and retroauricular (TARA) approach in papillary carcinoma arising in thyroglossal duct cyst and thyroid gland. Ann Surg Oncol (2012) 19:4259–61. doi: 10.1245/s10434-012-2674-y
40. Li Z, Wang P, Wang Y, Xu S, Cao L, Que R, et al. Endoscopic lateral neck dissection via breast approach for papillary thyroid carcinoma: a preliminary report. Surg Endosc (2011) 25:890–6. doi: 10.1007/s00464-010-1292-7
41. Yan H, Wang Y, Wang P, Xie Q, Zhao Q. "Scarless" (in the neck) endoscopic thyroidectomy (SET) with ipsilateral levels II, III, and IV dissection via breast approach for papillary thyroid carcinoma: a preliminary report. Surg Endosc (2015) 29:2158–63. doi: 10.1007/s00464-014-3911-1
42. Guo Y, Qu R, Huo J, Wang C, Hu X, Chen C, et al. Technique for endoscopic thyroidectomy with selective lateral neck dissection via a chest-breast approach. Surg Endosc (2019) 33:1334–41. doi: 10.1007/s00464-018-06608-7
43. Lin P, Liang F, Cai Q, Han P, Chen R, Xiao Z, et al. Comparative study of gasless endoscopic selective lateral neck dissection via the anterior chest approach versus conventional open surgery for papillary thyroid carcinoma. Surg Endosc (2020) 35 (2021), 693–701. doi: 10.1007/s00464-020-07434-6
44. Huo J, Guo Y, Hu X, Chen X, Liu W, Luo L, et al. Endoscopic thyroidectomy with level vb dissection Via a chest-breast approach: Technical updates for selective lateral neck dissection. Surg Laparosc Endosc Percutan Tech (2021) 31:342–5. doi: 10.1097/SLE.0000000000000887
45. Qu R, Hu X, Guo Y, Zhou X, Huo J, Chen Y, et al. Endoscopic lateral neck dissection (IIAAnd IV) using a breast approach: Outcomes from a series of the first 24 cases. Surg Laparosc Endosc Percutan Tech (2020) 31:66–70. doi: 10.1097/SLE.0000000000000849
46. Wang B, Weng YJ, Wang SS, Zhao WX, Yan SY, Zhang LY, et al. Feasibility and safety of needle-assisted endoscopic thyroidectomy with lateral neck dissection for papillary thyroid carcinoma: a preliminary experience. Head Neck (2019) 41:2367–75. doi: 10.1002/hed.25705
47. Ngo DQ, Tran TD, Le DT, Ngo QX, Van Le Q. Transoral endoscopic modified radical neck dissection for papillary thyroid carcinoma. Ann Surg Oncol (2021) 28:2766. doi: 10.1245/s10434-020-09466-7
48. Yan HC, Xiang C, Wang Y, Wang P. Scarless endoscopic thyroidectomy (SET) lateral neck dissection for papillary thyroid carcinoma through breast approach: 10 years of experience. Surg Endosc (2021) 35:3540–6. doi: 10.1007/s00464-020-07814-y
49. Anuwong A, Ketwong K, Jitpratoom P, Sasanakietkul T, Duh QY. Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg (2018) 153:21–7. doi: 10.1001/jamasurg.2017.3366
50. Nguyen HX, Nguyen HX, Nguyen TTP, Van Le Q. Transoral endoscopic thyroidectomy by vestibular approach in Viet nam: surgical outcomes and long-term follow-up. Surg Endosc (2021) 36 (2022), 4248–4254. doi: 10.1007/s00464-021-08759-6
Keywords: lateral neck dissection, scarless, thyroid cancer, robotic, endoscopic
Citation: Chen Z-X, Chen J-B, Pang F-S, Lin Z-H, Zhang X-B, Cai B-Y, Zheng W-W, Cao Y and Qin Y (2022) A novel hybrid approach for “Scarless” (at the neck) lateral neck dissection for papillary thyroid carcinoma: A case series and literature review. Front. Oncol. 12:985761. doi: 10.3389/fonc.2022.985761
Received: 04 July 2022; Accepted: 21 November 2022;
Published: 09 December 2022.
Edited by:
Giuseppe Mercante, Humanitas University, ItalyReviewed by:
Pornpeera Jitpratoom, Police General Hospital, ThailandAllen Lee Feng, Massachusetts Eye & Ear Infirmary and Harvard Medical School, United States
Copyright © 2022 Chen, Chen, Pang, Lin, Zhang, Cai, Zheng, Cao and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Qin, Z3pxaW55b3VAMTYzLmNvbQ==; Zhen-Xin Chen, Y2hlbnpoeG5AMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhen-Xin Chen†*
Zhen-Xin Chen†* You Qin
You Qin