
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 13 December 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.985457
This article is part of the Research TopicReviews in Molecular and Cellular OncologyView all 47 articles
 Sheyda Khalilian1,2
Sheyda Khalilian1,2 Hamid Abedinlou3
Hamid Abedinlou3 Bashdar Mahmud Hussen4,5
Bashdar Mahmud Hussen4,5 Seyedeh Zahra Hosseini Imani6
Seyedeh Zahra Hosseini Imani6 Soudeh Ghafouri-Fard2*
Soudeh Ghafouri-Fard2*miR-20b is a microRNA with diverse and somehow contradictory roles in the pathogenesis of human disorders, especially cancers. It has been known to be a tumor suppressor in colon cancer, renal cell carcinoma, prostate cancer, osteosarcoma and papillary thyroid cancer. In lung cancer and breast cancers, both tumor suppressor and oncogenic effects have been identified for this miRNA. Finally, in T cell leukemia, hepatocellular carcinoma, esophageal squamous cell carcinoma and cervical and gastric cancers, miR-20b is regarded as an oncogenic miRNA. In several types of cancer, dysregulation of miR-20b has been recognized as a predictive marker for patients’ survival. Dysregulation of miR-20b has also been recognized in Alzheimer’s disease, diabetic retinopathy, myocardial ischemia/infarction, chronic hepatitis B and multiple sclerosis. In the current review, we have summarized the miR-20b targets and related cellular processes. We have also provided a review of participation of this miRNA in different human disorders.
MicroRNAs (miRNAs) have been initially discovered in Caenorhabditis elegans, but further experiments have identified them in most eukaryotes (1–3). These small non-coding RNAs have an average size of 22 nucleotides. The majority of miRNAs are transcribed from DNA sequences into primary miRNA transcripts which are then processed into precursor and mature miRNAs, respectively. In general, 3’ UTR of target transcripts is the main part of interaction between miRNAs and target mRNAs. This type of interaction mainly leads to suppression of expression of target transcripts (4). Yet, miRNAs can also interact with 5′ UTR, coding sequences, and promoters of selected genes (5). In addition, activation of gene expression has been reported to be an uncommon consequence of miRNAs interaction with target mRNAs (6). miRNAs have crucial functions in the regulation of developmental processes as well as pathophysiology of human disorders. The latter is best documented in the carcinogenic processes (7–9). In the context of cancer, several miRNAs have been found to contribute to the regulation of cellular activities leading to carcinogenesis as well as drug resistance (10).
In the current review, we have summarized the impact of a certain miRNA, namely miR-20b-5p in human disorders, especially cancer. miR-20b is encoded by MIR20B gene on Xq26.2. The precursor miRNA has two arms: miR-20b-5p and miR-20b-3p. These miRNAs have been shown to participate in the pathogenesis of a variety of malignant and non-malignant conditions. In the current review, we summarize the role of miR-20b-5p and miR-20b-3p in both conditions and explain its possible therapeutic implications. The reason for selection of miR-20b was its widespread dysregulation in several types of cancers, its tissue-specific effects and its contribution to the etiology of a number of non-malignant conditions which show its important roles in the cellular homeostasis. Moreover, the bioinformatics step revealed association of miR-20b with several important cellular pathways indicating importance of conduction of further research for identification of its roles in different disorders.
First, through a bioinformatics step, we identified miR-20b-5p targets (Figure 1). Based on the results of TargetScan online tool (https://www.targetscan.org/vert_80/), miR-20b-5p is predicted to target a variety of mRNA targets being involved in a wide range of cellular functions.
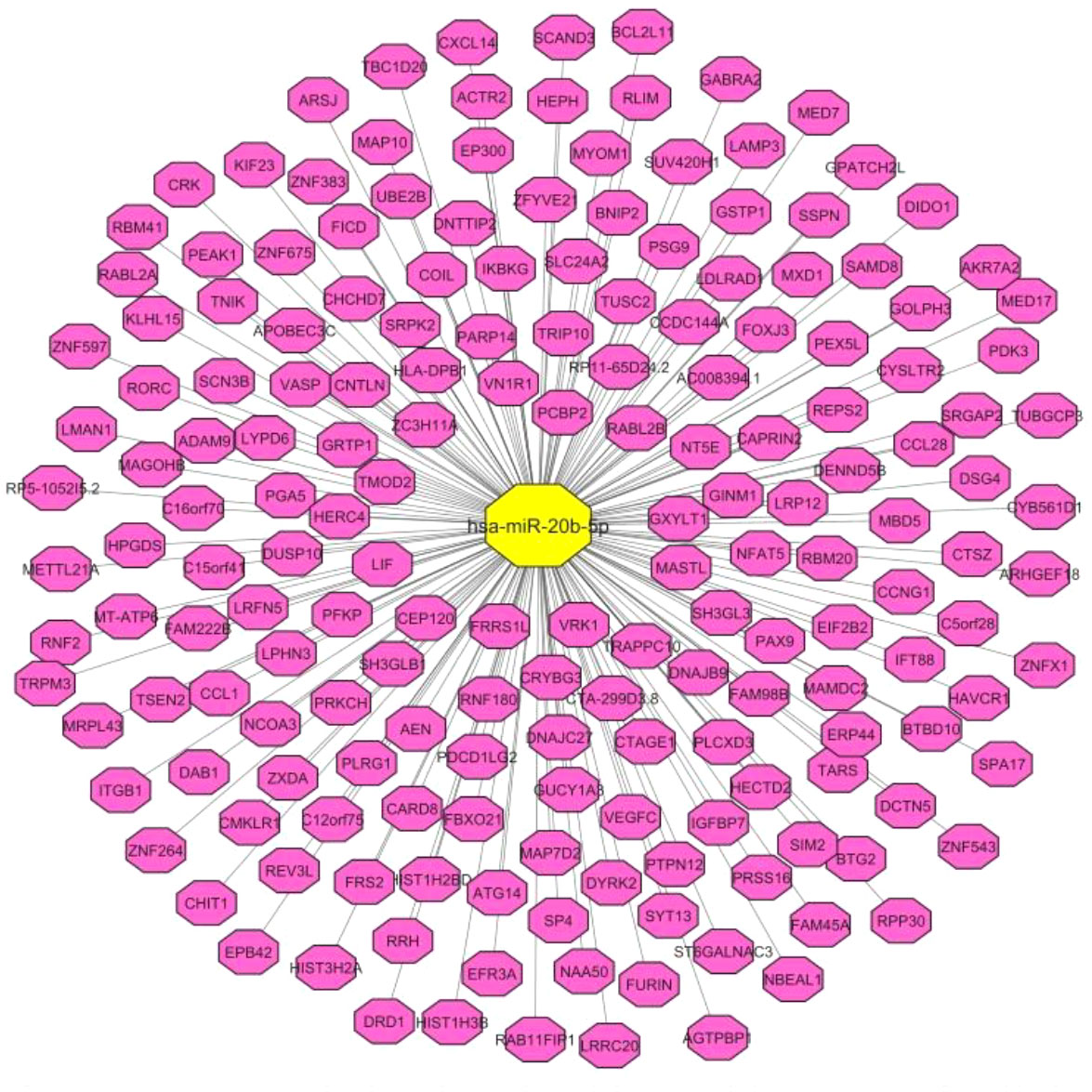
Figure 1 Correlation pairs of miR-20b-5p targets, predicted by TargetScan online tool. The interaction network was constructed by Cytoscape software.
Then, we identified the biological processes being influenced by this miRNA and its targets (Figure 2). Top target genes of miR-20b-5p are enriched in regulation of biological processes, metabolic processes, cell communication and developmental processes. Cell membrane and nucleus are top cellular components of miR-20b-5p targets. Finally, these targets are mostly implicated in protein binding, ion binding and nucleic acid binding.
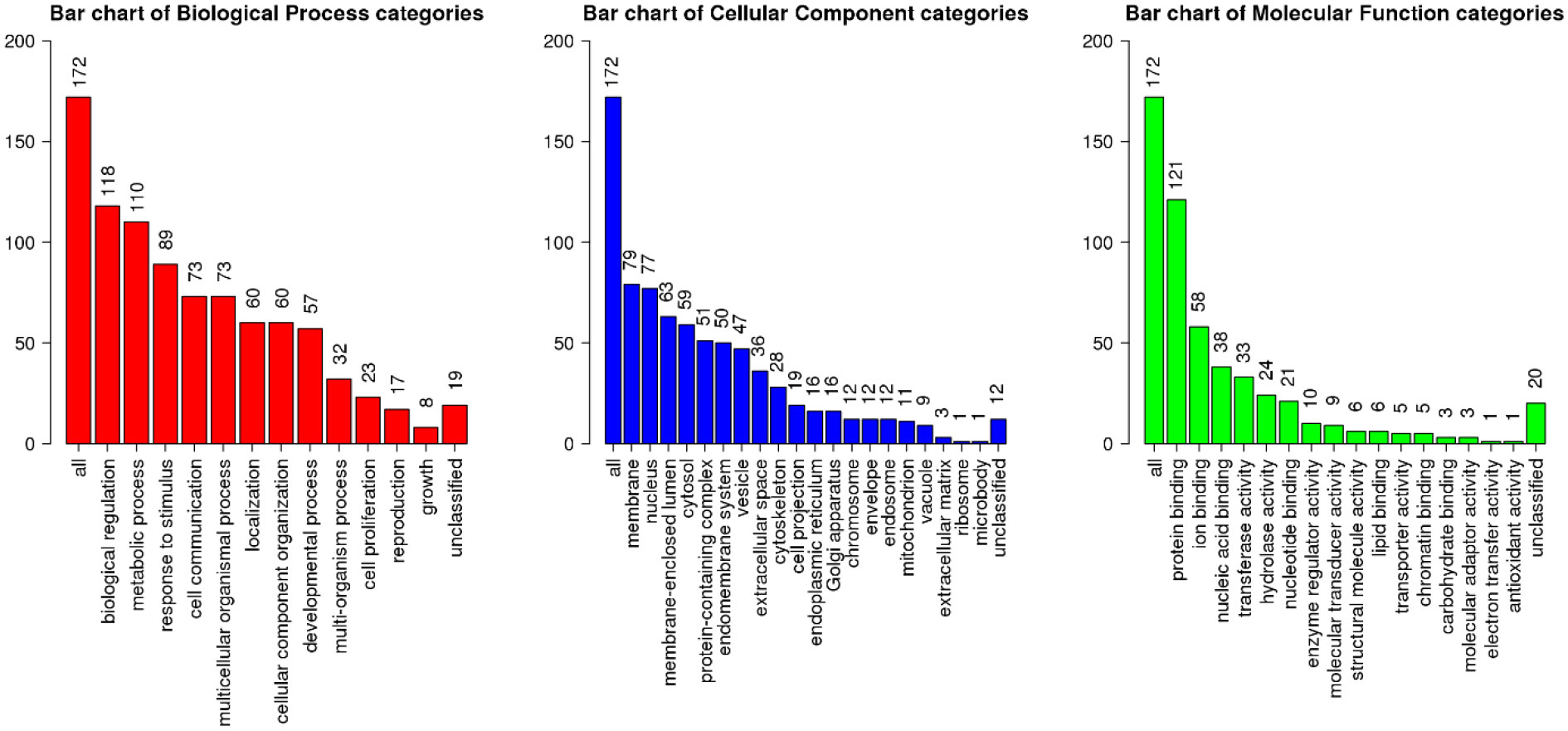
Figure 2 Functional enrichment analysis of top miR-20b-5p target genes, using WebGestalt database. The X-axis displays the name of different GO terms, and the Y-axis demonstrates the count of enriched genes. Besides, the length of the bar is showed according to the number of gene counts. GO, gene ontology.
Several studies have investigated the role of miR-20b-5p in the development or progression of colorectal cancer. Yang et al. have identified a tumor suppressor role for this miRNA in HCT-116 cell line. miR-20b-5p has been shown to inhibit cell cycle transition and reduce their migratory and invasive capacities without affecting cell apoptosis. Mechanistically, miR-20-5p tagets CyclinD1 (CCND1) transcripts in these cells. Up-regulation of miR-20b-5p has led to downregulation of CCND1 levels in HCT-116 cells. CCND1/CDK4/FOXM1 axis has been identified as the main route of participation of miR-20-5p in the pathoetiology of colorectal cancer. Besides, miR-20b-5p could inhibit tumorigenic effects of colorectal cancer cells in Balb/c nude mice (11). Another study in colorectal cancer cells has shown negative correlation between expression levels of miR-20b-5p and MALAT1. Both MALAT1 down-regulation and miR-20b-5p up-regulation could attenuate microsphere formation and self-renewal ability, decrease the proportion of cancer stem cells, and down-regulate levels of stemness-related proteins and those regulating cellular metabolism. The impact of si-MALAT1 and miR-20b-5p-mimic on suppression of tumorigenic abilities of HCT-116 cells has also been confirmed in xenograft model of cancer. Mechanistically, miR-20b-5p targets Oct4 in these cells (12). On the other hand, Ulivi et al. have reported up-regulation of miR-20b-5p circulatory level in metastatic colorectal cancer patients received bevacizumab in correlation with progression free and overall survival (13). Meanwhile, another study has revealed up-regulation of expression of the miR-20b-5p-songing lncRNA COL4A2-AS1 in colorectal cancer tissues and cell lines. In fact, COL4A2-AS1 could promote proliferation, and aerobic glycolysis of colorectal cancer cells via influencing the miR-20b-5p/HIF1A molecular route (14).
In lung cancer, miR-20b-5p has been identified as a tumor suppressor whose circulating serum exosomal levels could be applied as a diagnostic marker for early stage cancer (15). In this type of cancer, the tumor suppressor role of miR-20b-5p is mainly exerted through regulation of cell cycle transition and enhancement of cell apoptosis. Most notably, down-regulation of this miRNA has been associated with poor prognosis of adenocarcinomas and squamous cell carcinomas of lung (16). On the other hand, another study has reported up-regulation of miR−20b−5p in lung cancer cells. BTG3 has been identified as a target of miR−20b−5p. Up-regulation of miR−20b−5p enhances proliferation and migration of lung cancer cells (17).
Reduction of miR-20b level is also implicated in the progression of breast cancer. Adipose tissue-derived mesenchymal stem cells (ASCs) have been found to enhance breast cancer metastasis. Co-culture of breast cancer cells with these cells has led to enhancement of migration and invasive properties of breast cancer cells. Mechanistically, stem cell factor (SCF) produced by ASCs can reduce miR-20b biogenesis via regulation of c-Kit/MAPK-p38/E2F1 signaling. HIF-1α and VEGFA have been identified as targets of miR-20b. Down-regulation of miR-20b could activate HIF-1α-mediated VEGFA expression. On the other hand, over-expression of miR-20b has abolished epithelial-mesenchymal transition process and lung metastasis of breast cancer cells through suppression of N-cadherin, vimentin and Twist (18) (Figure 3).
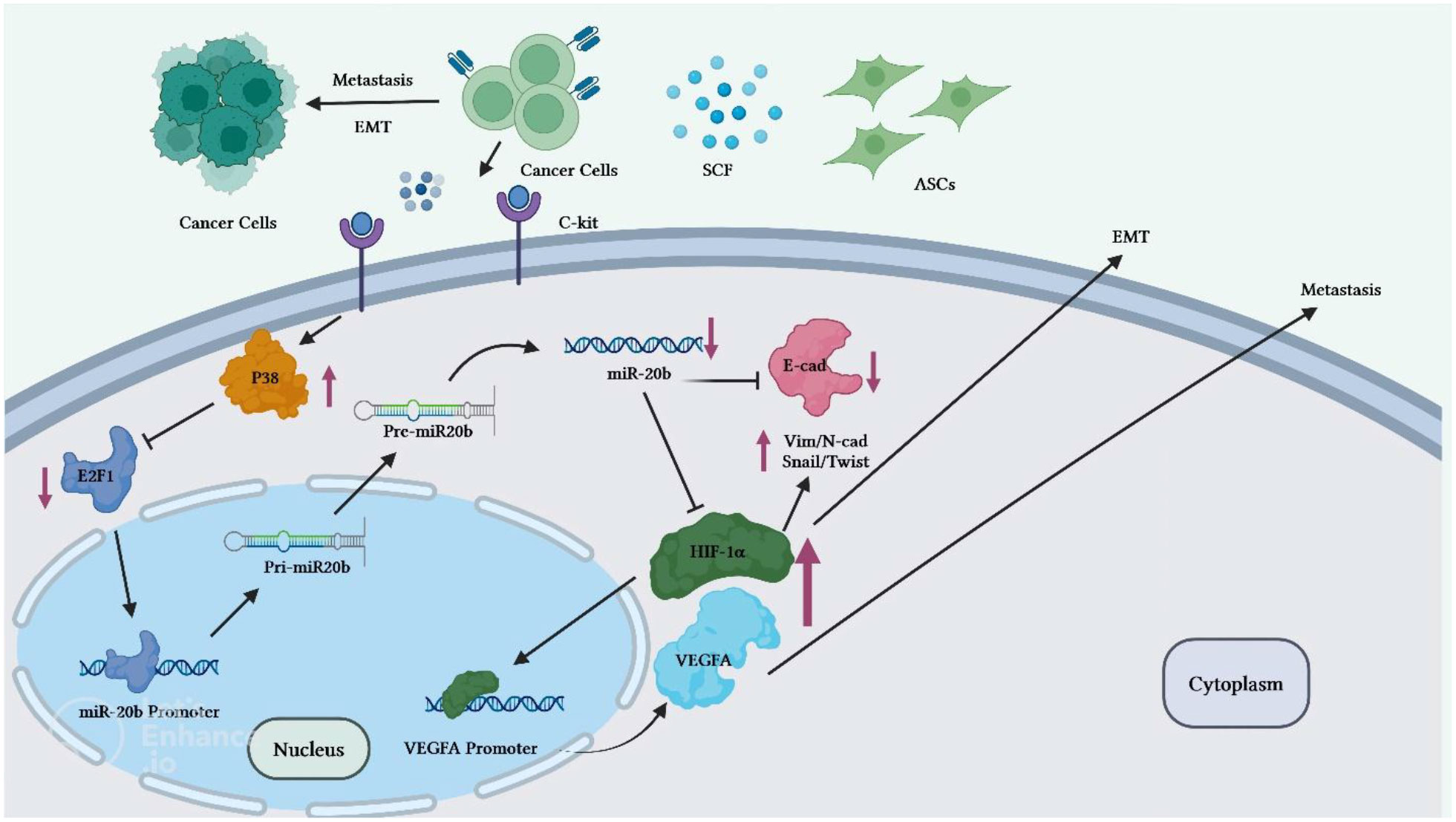
Figure 3 Overview of the potential breast cancer cells ASC-induced metastatic pathways. Through the regulation of miR-20b biogenesis, c-Kit-positive ASCs release high level of SCF to target BC cells. The MAPKp38/E2F1 pathway mediates the SCF-dependent decrease in miR-20b, which enhances activity of HIF-1/VEGFA and promotes EMT and metastasis of BC cells. Activation of the HIF-1α/VEGFA results in the upregulation of the N-cadherin, vimentin, and Twist expressions and decreased expression levels of E-cadherin. Therefore, through miR-20b suppression, ASCs induces metastasis of BC cells in animal models. ASC, Adipose tissue-derived mesenchymal stem cells, BC, Breast cancer cell.
Li et al. have reported the presence of numerous GC-rich binding motifs for EGR1 in the promoter of miR-20b. Expression of this miRNA has been found to be increased by ionizing radiation and its over-expression has been correlated with expression levels of EGR1 in a certain breast cancer cell line. Expression of miR-20b has been decreased by siRNA-mediated silencing of EGR1. Inhibition of miR-20b expression has suppressed proliferation and migration of breast cancer cells and resulted in G0/G1 and S phase arrest. This miRNA has been found to target several tumor suppressor genes, including PTEN and BRCA1. Thus, inhibition of miR-20b has led to enhancement of PTEN and BRCA1 expression. Besides, miR-20b expression has been correlated with expression levels of EGR1 in breast cancer tissues (19). Figure 4 shows transcriptional activation of miR-20b by EGR1.
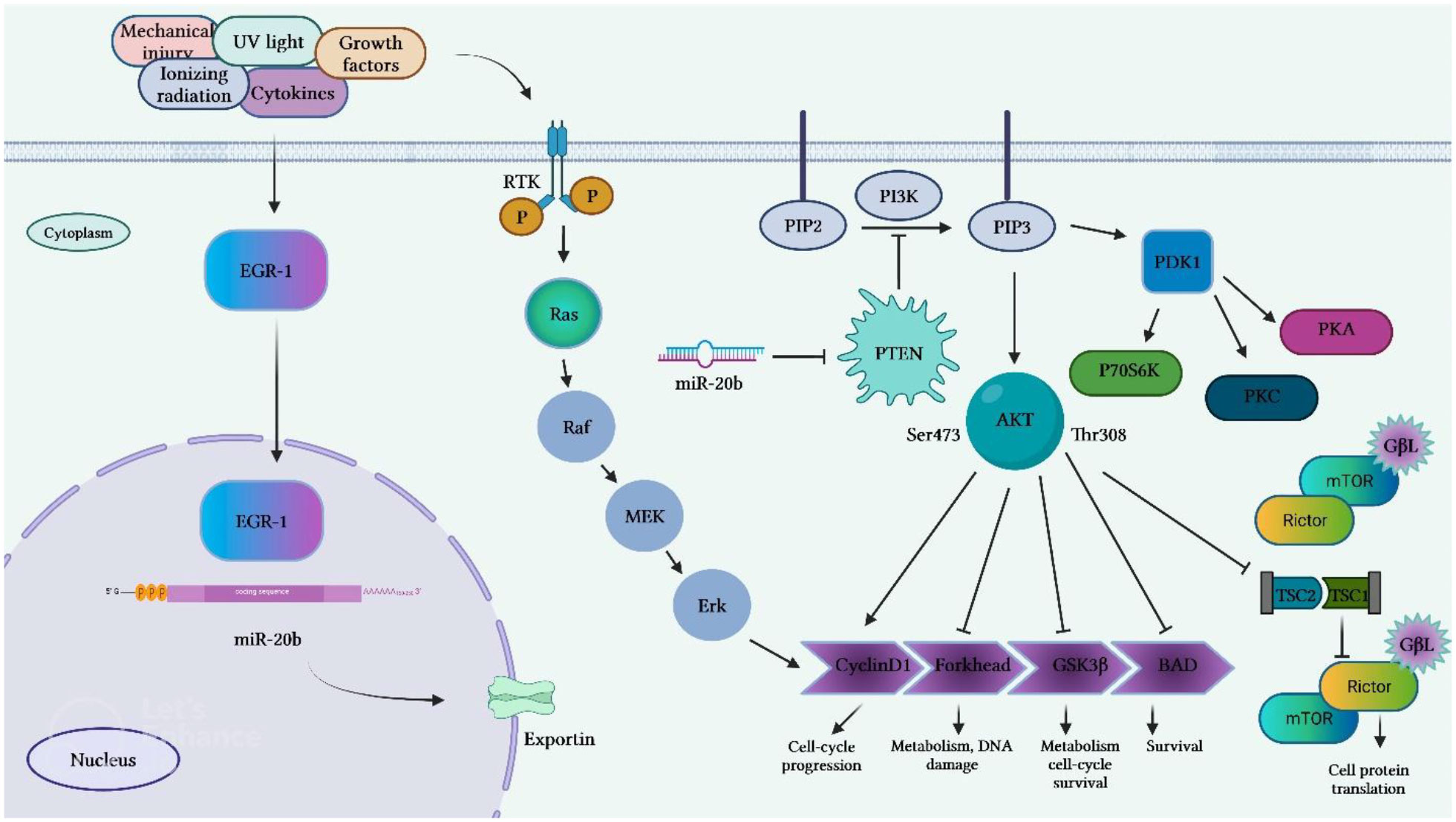
Figure 4 Transcriptional activation of miR-20b by EGR1 that leads to targeting PTEN. EGR1 is an important factor in transcription of miR-20b. EGR1 is triggered by numerous extracellular stimuli, including growth hormones, cytokines, ionizing radiation, ultraviolet light, and mechanical damage. After activation, EGR1 enters the nucleus and binds to the miR-20b promoter, resulting in the transcription of miR-20b. The mature miR-20b in collaboration with other constituents of RISC, which detects and binds to PTEN mRNAs induces suppression of PTEN expression, thus promoting cell proliferation and migration.
miR-20b has been found to be down-regulated in papillary thyroid carcinoma tissues compared with paratumoral tissues. Notably, down-regulation of miR-20b has been associated with metastasis to cervical lymph nodes and TNM staging. Over-expression of miR-20b has suppressed viability, migration, and invasive properties of malignant cells and inhibited MAPK/ERK signaling via targeting SOS1 and ERK2. Besides, over-expression of miR-20b has suppressed growth of papillary thyroid carcinoma cells in vivo (20). Figure 5 shows miR-20b- induced regulation of MAPK/ERK pathway in this type of cancer.
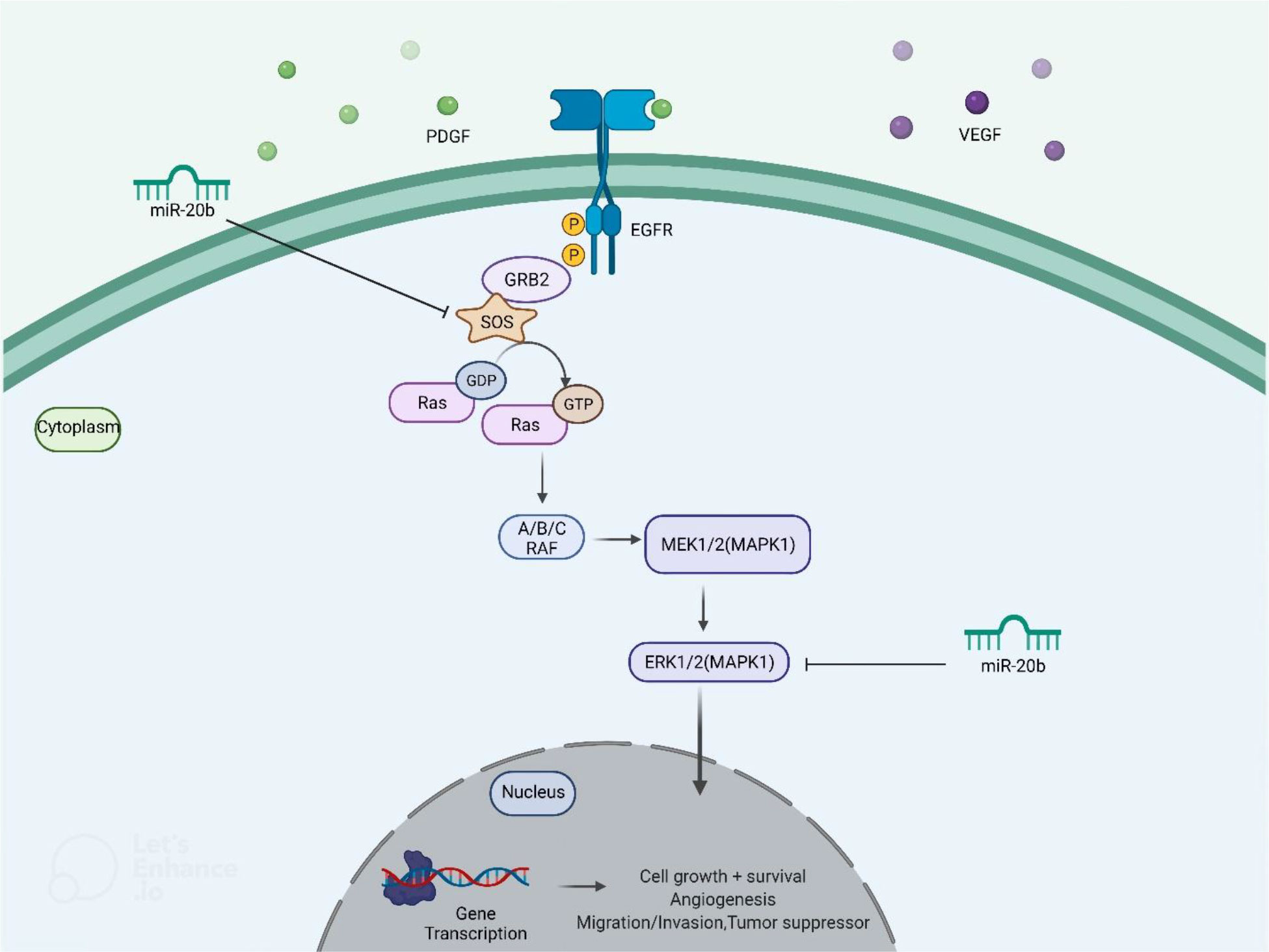
Figure 5 miR-20b- induced regulation of MAPK/ERK pathway. Through targeting SOS1 and ERK2, ectopic overexpression of miR-20b suppresses MAPK/ERK signaling cascade, leading to a reduction in cell initiation, progression, and metastasis.
The impact of miR-20b in the pathogenesis of different types of cancers is shown in Table 1.
Diagnostic role of miR-20b has been assesses in lung, esophageal and renal cancers (Table 2). Expression levels of this miRNA in peripheral blood or serum samples be used as diagnostic marker for these types of cancers with area under ROC curve values ranging from 0.78 (22) to 0.85 (26) in renal and esophageal cancers, respectively.
Expression of miR-20-5p has been shown to be speedily and stably decreased upon oxidative stress. H2O2 could hamper G1/S transition and suppress DNA synthesis. Up-regulation of miR-20b-5p could rescue cells from growth arrest. miR-20b-5p could regulate expressions of p21, CCND1 and E2F1. Oxidative stress could decrease expression of E2F1, in line with obstruction of G1/S transition and suppression of DNA synthesis and proliferation. The underlying mechanism includes autoregulatory feedback between miRNAs and E2F1 and E2F1 response to repair oxidative stress-associated DNA damage (38). Since oxidative stress is involved in the pathoetiology of several human disorders, particularly age-related pathologies, miR-20-5p represents an important biomarker and target for treatment of these conditions.
Figure 6 shows miR-20b dependent and independent regulation of E2F1 levels and G1/S transition.

Figure 6 miR-20b-5p dependent and independent regulation of E2F1 levels and G1/S transition. The pathways I, II, and III as miRNA-dependent, and IV as miRNA-independent methods are proposed to modulate E2F1 levels under oxidative stress. In the pathways I and II, the lower expression of miRNAs leads to an increase in the p21 expression, and CCND1/2 and CDK4/6, respectively. In the pathway II, miRNA and E2F1 have an auto-regulatory feedback effect on each other. Finally, in the pathway IV, E2F1 is recruited to DNA break sites as a result of oxidative stress causing DNA damage responses. Overall, E2F1 is downregulated, the G1/S transition and DNA synthesis are inhibited, cell proliferation is repressed, leading to cell senescence. The proposed scheme is more fully described in the text.
Different study groups have assessed impact of miR-20b dysregulation in non-malignant conditions (Table 3). For instance, Wang et al. have reported participation of hsa-miR20b-5p in Alzheimer’s disease pathogenesis through regulation of Aβ. They have demonstrated alterations in miR-20b-5p level in association with progression of Alzheimer’s disease in three brain regions. miR-20b-5p could affect calcium homeostasis, neurite outgrowth, and branch points in human neurons in vitro. Notably, rs13897515 polymorphism of the MIR20B gene has been found to be associated with difference in Aβ1-42 levels in the CSF. Although miR-20b-5p has been shown to downregulate APP, it has been paradoxically associated with susceptibility to Alzheimer’s disease (39). Another study has shown that miR-20b-5p, via targeting RhoC gene, could disturb progression of Alzheimer’s’ disease by regulating the cell apoptosis, and cell viability (40).
miR-20b-5p is also involved in the pathogenesis of diabetic complications. Suppression of circulating exosomal miR-20b-5p has been found to accelerate diabetic wound repair (41). Another study has shown that down-regulation of the miR-20b-5p-sponging circular RNA circDMNT3B participates in the diabetic-associated dysfunction of retinal vessels (42). Moreover, inhibition of circulating exosomal miR‐20b‐5p could restore Wnt9b signals and reverse diabetes‐associated defects in wound healing (43). Therefore, this miRNA is a putative target for amelioration of diabetic complications.
miR-20b has been found to exert diverse and somehow contradictory roles in the pathogenesis of human disorders, especially cancers. It has been known to be a tumor suppressor in colorectal cancer (11), renal cell carcinoma (22), prostate cancer (24), osteosarcoma (32) and papillary thyroid carcinoma (20). In lung cancer and breast cancers, both tumor suppressor and oncogenic effects have been identified for this miRNA. Finally, in T cell leukemia (29), hepatocellular carcinoma (30), esophageal squamous cell carcinoma (37) and cervical (35) and gastric (36) cancers, miR-20b is regarded as an oncogenic miRNA. Such different roles of miR-20b might be related to tissue-specific targets of this miRNA. Based on our in silico approach, we demonstrated enrichment of miR-20b-5p targets in regulation of biological processes, metabolic processes, cell communication and developmental processes. Notably, these targets are mostly implicated in protein binding, ion binding and nucleic acid binding. Different levels of targets that are involved in binding with biomolecules in each tissue might affect the specific functions of miR-20b in these tissues.
Moreover, the precursor miRNA has two arms, namely -5p and -3p. Depending on the tissue or cell types, both arms might exert functional roles. Different functions of these arms might explain the tissue-specific roles of this miRNA.
In several types of cancer, dysregulation of miR-20b has been recognized as a predictive marker for patients’ survival. Although application of miR-20b as a diagnostic marker has only been investigated in lung, esophageal and renal cancers, the obtained results have been promising since serum/blood levels of this miRNA could separate cancer patients from healthy controls with acceptable diagnostic power. This finding potentiates this miRNA as a tool in non-invasive diagnostic approaches. Since this miRNA is dysregulated in several cancers, identification of abnormal levels of this miRNA does not point to a certain diagnosis. Instead it can be used for follow-up of patients after conduction of anti-cancer therapies for early diagnosis of cancer recurrence or metastasis.
Dysregulation of miR-20b has also been detected in Alzheimer’s disease, diabetic retinopathy, myocardial ischemia/infarction, chronic hepatitis B and multiple sclerosis. Wnt/β-catenin, AKT, NF-κB-p65, NLRP3/caspase-1/IL-1β and NF-kB/MAPK have been identified as the most important pathways being influenced by miR-20b in these conditions. Therefore, miR-20b-modulating therapies might affect course of these disorders through modulation of activity of these signaling pathways.
miR-20b has also been found to be sponged by a number of lncRNAs such as MALAT1, WWOX-AS1, PART1, DUXAP8 and COL4A2-AS1 as well as the circular RNA CircHIPK3. Thus, dysregulation of these non-coding RNAs might be regarded as a possible mechansim for alterations in the expression levels of miR-20b. Other putative mechanisms such as alterations in the epigenetic marks in the promoter of MIR20b should be assessed in future studies.
Taken together, miR-20b is a promising diagnostic marker in cancer and a putative therapeutic target in diverse human disorders. Future studies are needed to evaluate the efficacy of miR-20b-targeting strategies in animal and cell models of different disorders. In addition, the importance of this miRNA in modulation of response of cancer patients to chemotherapy, radiotherapy and targeted therapies should be assessed in future studies.
Conceptualization, study design, investigation, validation of the collected papers, designing the tables and figures, review and editing were performed by SK; Figure preparation and completing the tables were performed by HA; Data collection and completing the tables were performed by SI; Draft manuscript preparation, revision and supervision were performed by SGF. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in c. elegans. Cell (1993) 75(5):855–62. doi: 10.1016/0092-8674(93)90530-4
2. Lee RC, Feinbaum RL, Ambros V. The c. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. cell (1993) 75(5):843–54. doi: 10.1016/0092-8674(93)90529-y
3. MacFarlane L-A, Murphy P R. MicroRNA: biogenesis, function and role in cancer. Curr Genomics (2010) 11(7):537–61. doi: 10.2174/138920210793175895
4. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol (2014) 15(8):509–24. doi: 10.1038/nrm3838
5. Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the seed supports microRNA targeting specificity. Mol Cell (2016) 64(2):320–33. doi: 10.1016/j.molcel.2016.09.004
6. Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev: RNA (2012) 3(3):311–30. doi: 10.1002/wrna.121
7. Fathi M, Ghafouri-Fard S, Abak A, Taheri M. Emerging roles of miRNAs in the development of pancreatic cancer. BioMed Pharmacother (2021) 141:111914. doi: 10.1016/j.biopha.2021.111914
8. Ghafouri-Fard S, Shirvani-Farsani Z, Taheri M. The role of microRNAs in the pathogenesis of thyroid cancer. Noncoding RNA Res (2020) 5(3):88–98. doi: 10.1016/j.ncrna.2020.06.001
9. Hussen BM, Abdullah ST, Rasul MF, Salihi A, Ghafouri-Fard S, Hidayat HJ, et al. MicroRNAs: Important players in breast cancer angiogenesis and therapeutic targets. Front Mol Biosci (2021) 8. doi: 10.3389/fmolb.2021.764025
10. Tripathi SK, Pandey K, Panda M, Biswal BK. Chapter 11 - emerging roles of microRNAs as a regulator in the progression of lung cancer and their implications in its diagnosis and therapy. In: Mallick B, editor. AGO-driven non-coding RNAs. Academic Press (2019). p. 293–318.
11. Yang H, Lin J, Jiang J, Ji J, Wang C, Zhang J. miR-20b-5p functions as tumor suppressor microRNA by targeting cyclinD1 in colon cancer. Cell Cycle (2020) 19(21):2939–54. doi: 10.1080/15384101.2020.1829824
12. Tang D, Yang Z, Long F, Luo L, Yang B, Zhu R, et al. Long noncoding RNA MALAT1 mediates stem cell-like properties in human colorectal cancer cells by regulating miR-20b-5p/Oct4 axis. J Cell Physiol (2019) 234(11):20816–28. doi: 10.1002/jcp.28687
13. Ulivi P, Canale M, Passardi A, Marisi G, Valgiusti M, Frassineti GL, et al. Circulating plasma levels of miR-20b, miR-29b and miR-155 as predictors of bevacizumab efficacy in patients with metastatic colorectal cancer. Int J Mol Sci (2018) 19(1):307. doi: 10.3390/ijms19010307
14. Yu Z, Wang Y, Deng J, Liu D, Zhang L, Shao H, et al. Long non-coding RNA COL4A2-AS1 facilitates cell proliferation and glycolysis of colorectal cancer cells via miR-20b-5p/hypoxia inducible factor 1 alpha subunit axis. Bioengineered (2021) 12(1):6251–63. doi: 10.1080/21655979.2021.1969833
15. Zhang Z-J, Song X-G, Xie L, Wang K-Y, Tang Y-Y, Yu M-, et al. Circulating serum exosomal miR-20b-5p and miR-3187-5p as efficient diagnostic biomarkers for early-stage non-small cell lung cancer. Exp Biol Med (2020) 245(16):1428–36. doi: 10.1177/1535370220945987
16. Arora S, Singh P, Rahmani AH, Almatroodi SA, Dohare R, Syed MA. Unravelling the role of miR-20b-5p, CCNB1, HMGA2 and E2F7 in development and progression of non-small cell lung cancer (NSCLC). Biology (2020) 9(8):201. doi: 10.3390/biology9080201
17. Peng L, Li S, Li Y, Wan M, Fang X, Zhao Y, et al. Regulation of BTG3 by microRNA−20b−5p in non−small cell lung cancer. Oncol Lett (2019) 18(1):137–44. doi: 10.3892/ol.2019.10333
18. Xu H, Li W, Luo S, Yuan J, Hao L. Adipose derived stem cells promote tumor metastasis in breast cancer cells by stem cell factor inhibition of miR20b. Cell Signalling (2019) 62:109350. doi: 10.1016/j.cellsig.2019.109350
19. Li D, Ilnytskyy Y, Kovalchuk A, Khachigian LM, Bronson RT, Wang B, et al. Crucial role for early growth response-1 in the transcriptional regulation of miR-20b in breast cancer. Oncotarget (2013) 4(9):1373–87. doi: 10.18632/oncotarget.1165
20. Hong S, Yu S, Li J, Yin Y, Liu Y, Zhang Q, et al. MiR-20b displays tumor-suppressor functions in papillary thyroid carcinoma by regulating the MAPK/ERK signaling pathway. Thyroid (2016) 26(12):1733–43. doi: 10.1089/thy.2015.0578
21. Hua R, Zhang Y, Yan X, Tang D, Li X, Ni Q, et al. Syndecan-2, negatively regulated by miR-20b-5p, contributes to 5-fluorouracil resistance of colorectal cancer cells via the JNK/ERK signaling pathway. Acta Biochim Biophys Sin (2021) 53(11):1547–57. doi: 10.1093/abbs/gmab124
22. Huang G, Li H, Wang J, Peng X, Liu K, Zhao L, et al. Combination of tumor suppressor miR-20b-5p, miR-30a-5p, and miR-196a-5p as a serum diagnostic panel for renal cell carcinoma. Pathology-Res Practice (2020) 216(11):153152. doi: 10.1016/j.prp.2020.153152
23. Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinf (2016) 14(1):42–54. doi: 10.1016/j.gpb.2015.09.006
24. Qi J-C, Yang Z, Zhang Y-P, Lu B-S, Yin Y-W, Liu K-L, et al. miR-20b-5p, TGFBR2, and E2F1 form a regulatory loop to participate in epithelial to mesenchymal transition in prostate cancer. Front Oncol (2020) 9:1535. doi: 10.3389/fonc.2019.01535
25. Xia L, Li F, Qiu J, Feng Z, Xu Z, Chen Z, et al. Oncogenic miR-20b-5p contributes to malignant behaviors of breast cancer stem cells by bidirectionally regulating CCND1 and E2F1. BMC Cancer (2020) 20(1):1–13. doi: 10.1186/s12885-020-07395-y
26. Yu J, Chen S, Niu Y, Liu M, Zhang J, Yang Z, et al. Functional significance and therapeutic potential of miRNA-20b-5p in esophageal squamous cell carcinoma. Mol Therapy-Nucleic Acids (2020) 21:315–31. doi: 10.1016/j.omtn.2020.05.015
27. Papageorgiou SG, Kontos CK, Tsiakanikas P, Stavroulaki G, Bouchla A, Vasilatou D, et al. Elevated miR-20b-5p expression in peripheral blood mononuclear cells: A novel, independent molecular biomarker of favorable prognosis in chronic lymphocytic leukemia. Leukemia Res (2018) 70:1–7. doi: 10.1016/j.leukres.2018.04.014
28. Pantazis T-L, Giotakis AI, Karamagkiolas S, Giotakis I, Konstantoulakis M, Liakea A, et al. Low expression of miR-20b-5p indicates favorable prognosis in laryngeal squamous cell carcinoma, especially in patients with non-infiltrated regional lymph nodes. Am J Otolaryngol (2020) 41(5):102563. doi: 10.1016/j.amjoto.2020.102563
29. Drobna M, Szarzyńska B, Jaksik R, Sędek Ł, Kuchmiy A, Taghon T, et al. Hsa-miR-20b-5p and hsa-miR-363-3p affect expression of PTEN and BIM tumor suppressor genes and modulate survival of T-ALL cells in vitro. Cells (2020) 9(5):1137. doi: 10.3390/cells9051137
30. Xu D, Liu X, Wu J, Wang Y, Zhou K, Chen W, et al. LncRNA WWOX-AS1 sponges miR-20b-5p in hepatocellular carcinoma and represses its progression by upregulating WWOX. Cancer Biol Ther (2020) 21(10):927–36. doi: 10.1080/15384047.2020.1806689
31. Li Z, Wu L, Tan W, Zhang K, Lin Q, Zhu J, et al. MiR-20b-5p promotes hepatocellular carcinoma cell proliferation, migration and invasion by down-regulating CPEB3. Ann Hepatol (2021) 23:100345. doi: 10.1016/j.aohep.2021.100345
32. Pan Z, Mo F, Liu H, Zeng J, Huang K, Huang S, et al. LncRNA prostate androgen-regulated transcript 1 (PART 1) functions as an oncogene in osteosarcoma via sponging miR-20b-5p to upregulate BAMBI. Ann Trans Med (2021) 9(6):488. doi: 10.21037/atm-21-658
33. Wang L, Adiga A, Venkatramanan S, Chen J, Lewis B, Marathe M. “Examining deep learning models with multiple data sources for COVID-19 forecasting”, in 2020 IEEE international conference on big data (Big Data). IEEE (2020). p. 3846–55.
34. Pang R, Yang S. lncRNA DUXAP8 inhibits papillary thyroid carcinoma cell apoptosis via sponging the miR−20b−5p/SOS1 axis. Oncol Rep (2021) 45(5):1–10. doi: 10.3892/or.2021.8015
35. Szekerczés T, Galamb Á, Varga N, Benczik M, Kocsis A, Schlachter K, et al. Increased miR-20b level in high grade cervical intraepithelial neoplasia. Pathol Oncol Res (2020) 26(4):2633–40. doi: 10.1007/s12253-020-00852-w
36. Streleckienė G. Hsa-miR-20b-5p, hsa-miR-451a-5p and hsa-miR-1468-5p role in gastric cancer pathogenesis. (2017).
37. Chen D, Su H, Li Y, Wu X, Li Y, Wei C, et al. miR-20b and miR-125a promote tumorigenesis in radioresistant esophageal carcinoma cells. Aging (Albany NY) (2021) 13(7):9566. doi: 10.18632/aging.202690
38. Tai L, Huang C-J, Choo KB, Cheong SK, Kamarul T. Oxidative stress down-regulates MiR-20b-5p, MiR-106a-5p and E2F1 expression to suppress the G1/S transition of the cell cycle in multipotent stromal cells. Int J Med Sci (2020) 17(4):457–70. doi: 10.7150/ijms.38832
39. Wang R, Chopra N, Nho K, Maloney B, Obukhov AG, Nelson PT, et al. Human microRNA (miR-20b-5p) modulates alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences alzheimer’s biomarkers. Mol Psychiatry (2022), 27(2): 1256–73.
40. Tian Z, Dong Q, Wu T, Guo J. MicroRNA-20b-5p aggravates neuronal apoptosis induced by β-amyloid via down-regulation of ras homolog family member c in alzheimer’s disease. Neurosci Lett (2021) 742:135542. doi: 10.1016/j.neulet.2020.135542
41. Chen K, Yu T, Wang X. Inhibition of circulating exosomal miRNA-20b-5p accelerates diabetic wound repair. Int J Nanomed (2021) 16:371. doi: 10.2147/IJN.S287875
42. Zhu K, Hu X, Chen H, Li F, Yin N, Liu A-L, et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine (2019) 49:341–53. doi: 10.1016/j.ebiom.2019.10.004
43. Xiong Y, Chen L, Yan C, Zhou W, Endo Y, Liu J, et al. Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small (2020) 16(3):1904044. doi: 10.1002/smll.201904044
44. Katayama M, Wiklander OP, Fritz T, Caidahl K, El-Andaloussi S, Zierath JR, et al. Circulating exosomal miR-20b-5p is elevated in type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes (2019) 68(3):515–26. doi: 10.2337/db18-0470
45. Qiu Z, Wang Y, Liu W, Li C, Zhao R, Long X, et al. CircHIPK3 regulates the autophagy and apoptosis of hypoxia/reoxygenation-stimulated cardiomyocytes via the miR-20b-5p/ATG7 axis. Cell Death Discov (2021) 7(1):1–12. doi: 10.1038/s41420-021-00448-6
46. Zhou Z, Chen S, Tian Z, Deng S, Yi X, Yang S, et al. miR-20b-5p attenuates hypoxia-induced apoptosis in cardiomyocytes via the HIF-1 α/NF-κ b pathway. Acta Biochim Biophys Sin (2020) 52(9):927–34. doi: 10.1093/abbs/gmaa056
47. Li J-Z, Ye L-H, Wang D-H, Zhang H-C, Li T-Y, Liu Z-Q, et al. The identify role and molecular mechanism of the MALAT1/hsa-mir-20b-5p/TXNIP axis in liver inflammation caused by CHB in patients with chronic HBV infection complicated with NAFLD. Virus Res (2021) 298:198405. doi: 10.1016/j.virusres.2021.198405
48. Zhen W, Hui D, Wenying S, Yulong S. MicroRNA-20b-5p regulates propofol-preconditioning-induced inhibition of autophagy in hypoxia-and-reoxygenation-stimulated endothelial cells. J Biosci (2020) 45(1):1–9. doi: 10.1007/s12038-020-9998-8
49. Tang B, Bao N, He G, Wang J. Long noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7 axis in hepatic ischemia/reperfusion injury. Gene (2019) 686:56–62. doi: 10.1016/j.gene.2018.10.059
50. Lou J, Wang Y, Zhang Z, Qiu W. MiR-20b inhibits mycobacterium tuberculosis induced inflammation in the lung of mice through targeting NLRP3. Exp Cell Res (2017) 358(2):120–8. doi: 10.1016/j.yexcr.2017.06.007
Keywords: miRNA, miR-20b-5p, cancer, expression, biomarker
Citation: Khalilian S, Abedinlou H, Hussen BM, Imani SZH and Ghafouri-Fard S (2022) The emerging role of miR-20b in human cancer and other disorders: Pathophysiology and therapeutic implications. Front. Oncol. 12:985457. doi: 10.3389/fonc.2022.985457
Received: 03 July 2022; Accepted: 16 November 2022;
Published: 13 December 2022.
Edited by:
Feng Wang, Affiliated Hospital of Nantong University, ChinaReviewed by:
Qiang Guo, Hubei University of Medicine, ChinaCopyright © 2022 Khalilian, Abedinlou, Hussen, Imani and Ghafouri-Fard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soudeh Ghafouri-Fard, cy5naGFmb3VyaWZhcmRAc2JtdS5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.