
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 October 2022
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.984817
Introduction: As neoadjuvant chemotherapy (NCT) has been successfully introduced in gastric cancer (GC), more biomarkers are needed to evaluate the efficacy. Cancer-associated fibroblasts (CAFs) is associated with chemoresistance and prognosis. Three biomarkers, CD10, fibroblast activation protein-α (FAP) and G-protein-coupled receptor 77 (GPR77), have been proved to express in CAFs. However, their predictive values for efficacy of NCT and prognosis in gastric cancer is unknown.
Methods: Totally, specimens of 171 locally advanced gastric cancer patients who underwent NCT and D2 radical gastrectomy and matched preoperative biopsy specimens were retrospectively analyzed. Tumor regression grade (TRG) is reevaluated according to Mandard TRG. Expressions of CD10, FAP and GPR77 in CAFs before NCT (pre-) and after NCT (post-) were evaluated by immunohistochemistry. Survival curves on overall survival (OS) were obtained by Kaplan-Meier method, and differences were analyzed by log-rank test. Associations between categorical variables were explored by chi-square test or Fisher’s exact method. Univariable and multivariate analyses were performed by logistic regression model and Cox proportional hazard regression model.
Results: High expressions of post-CD10, post-FAP, post-GPR77 and pre-CD10 were related to worse TRG (all p<0.05). In multivariable analysis, post- and pre-FAP were independent predictive factors to TRG (p<0.010). Post-CD10 (p=0.032) and post-FAP (p=0.013) were related to OS in univariable analysis, but none of biomarkers were independent prognostic factors in multivariable analysis.
Conclusions: Expressions of CD10, FAP and GPR77 in CAFs were related to chemoresistance and overall survival, and these biomarkers have predictive values for tumor regression and prognosis in locally advanced gastric cancer patients.
As neoadjuvant chemotherapy (NCT) followed by surgery has become a recommended treatment plan for locally advanced gastric cancer (GC) (1), the evaluation of the effectiveness of NCT is becoming increasingly important. Pathologically, TNM system (2) and tumor regression grade (TRG) (3) were widely used to evaluate the efficacy of NCT qualitatively and quantitatively. However, these two methods rely heavily on the subjective judgment of pathologists, which means subjective bias is inevitable. Therefore, more objective evaluation indicators are needed. On this point, the expressions of biomarkers are promising.
Cancer-associated fibroblasts (CAFs), one of the primary stromal cell types in the tumor stroma, play an important role in chemoresistance and tumor progression (4), by secreting cytokines, chemokines and exosomes (5, 6), remodeling the extracellular matrix (ECM) (7), facilitating angiogenesis, suppressing antitumor immune responses, and promoting resistance to therapy (8). Many biomarkers have been used to identify CAFs, and different biomarkers showed various functions.
Fibroblast-activation protein (FAP), a type II integral membrane protein that belongs to the membrane-bound serine protease family, has been used as a specific marker of activated CAFs (9). FAP could promote cell proliferation and migration by various processes, such as producing ECM (10), increasing growth factors (11) facilitating angiogenesis (12) and regulating antitumor immune response (13). FAP could also regulate drug sensitivity by interacting with membrane proteins (14), or increasing the expression of chemokines (15). FAP showed a prognostic value in many cancers, and a high expression of FAP tended to predict a poor prognosis (16–18). However, whether FAP has predictive values on efficacy of NCT and prognosis in locally advanced GC patients who underwent NCT is still unknown.
CD10, a cell surface zinc-dependent metalloprotease that could regulate the biological activities of various peptide substrates (19), has been proved to be correlated with tumor progression and aggressiveness in many cancers such as melanoma (20), colorectal cancer (21) and breast cancer (22). G-protein-coupled receptor 77 (GPR77, also named C5aR2 and C5L2), one of the C5a receptors, is a powerful regulator of immune function. Activation of GPR77 could regulate cytokine production including IL-6 and TNF-α, which are important for tumor promotion and progression (23). CD10 and GPR77 have been recently proved to express on a CAFs subset, and correlate with cancer formation, chemoresistance and poor survival in breast and lung cancer patients (8). However, the expression of these two biomarkers in CAFs in gastric cancer and their predictive values are still unknown.
In this study, we assessed the expressions of FAP, CD10 and GPR77 in CAFs of gastric cancer samples after and before treatment, and evaluated their predictive values on the efficacy of NCT and prognosis in locally advanced gastric cancer patients.
All patients with gastric cancer between January 2010 and June 2018 at our institute were reviewed. Patients fulfilled the following inclusion criteria were included (1): pathologically confirmed gastric adenocarcinoma; (2) locally advanced gastric cancer (8th American Joint Committee on Cancer [AJCC] clinical stage: cT2N1M0-T4N3M0, II-III); (3) underwent NCT with or without postoperative therapy; (4) received curative gastrectomy surgery; and (5) specimens before and after treatment were available. Patients with following exclusion criteria were excluded: (1) underwent preoperative radiotherapy; (2) suffering from gastric remnant cancer or other malignant tumors; (3) incomplete information on staging or therapy; or (4) insufficient slices or blocks to evaluate biomarkers. After selected, 171 patients met the inclusion criteria of our study. For NCT therapy, 133 (77.8%) patients underwent SOX, 28 (16.4%) patients underwent FOLFOX and 10 (5.8%) patients underwent XELOX.
All slides and blocks indicating surgical specimens (post-treatment) and diagnostic specimens (pre-treatment) were retrieved from the biospecimen library of our hospital and were separately reviewed by two experienced gastrointestinal pathologists (Y.Z. and D.L.). TNM stage was reevaluated according to the eighth edition of the AJCC cancer staging guideline. Histological regression grade of the primary tumor was assessed according to the Mandard system: TRG 1 (complete fibrosis with no evidence of residual tumor, i.e., complete regression), TRG 2 (fibrosis with rare tumor cells), TRG 3 (fibrosis and residual tumor with a dominance of fibrosis), TRG 4 (fibrosis and residual tumor with a dominance of tumor), and TRG 5 (extensive residual tumor without evidence of regression). When disagreement appeared between pathologists, an agreement would be reached by joint rereview and discussion through a multihead microscope. Other extracted histopathological characteristics were reconfirmed during the evaluation process.
CAFs biomarkers including CD10, FAP and GPR77 were assessed by immunohistochemical staining method. Immunohistochemical staining method in this study was based on and modified from the method in Su et al. article (8). Briefly, specimens were incubated with specific primary antibodies (for CD10, a mouse monoclonal primary antibody (Ready-to-use; Maxim, Fujian, China) incubated at room temperature for an hour; for FAP, a rabbit monoclonal primary antibody (1:800 dilution; Boster, California, USA) and GPR77, a rabbit polyclonal primary antibody (1:400 dilution; Abcam, Shanghai, China): overnight at 4°C).
For every biomarker, the result of immunohistochemistry was evaluated by the total point, which was equal to the score of the proportion of positive area multiplied by the score of the intensity of the staining. The score of the proportion of positive area was defined as score 0-4, score 0: <1% positive area; score 1: 1%-25% positive area; score 2: 25%-49% positive area; score 3: 50%-74% positive area; and score 4: 75%-100% positive area. The intensity of the staining was defined as score 1-3: score 1: slight, light brown linear or granular staining on the cell membrane or cytoplasm; score 2: moderate, brown linear or granular staining on the cell membrane and cytoplasm; and score 3: strong, dark brown linear or granular staining on the cell membrane and cytoplasm. The total point was divided into 4 groups: 0: 0 point; 1+: 1-2 points; 2+: 3-4 points; and 3+: >4 points. For all biomarkers, the results of immunohistochemistry were divided into negative (total score 0 and 1+) and positive (total score 2+ and 3+). FAP was found to be expressed in cytoplasm of CAFs, while CD10 and GPR77 was found to be expressed in cytoplasm and cell membrane of CAFs.
Relationships among categorical variables were investigated by the chi-square test or Fisher’s exact test. Logistic regression analysis was used to explore the factors associated with pathological response. Cox regression analysis was used to assess the risk factors on overall survival (OS), and factors with p-value < 0.05 were included in the multivariable analysis. Because of collinearity between ypN and ypTNM, ypTNM was not included in multivariable analyses. Survival curves for OS were obtained using the Kaplan-Meier method, and the log-rank test was used to compare differences. All patients were followed up every 3 months during the first 2 years, every 6 months for the following 3 years and annually thereafter. OS was the time from initial treatment to death from any cause or last date of follow-up. Data were proceeded by SPSS ver. 25.0 (IBM Corp., Armonk, NY) and R 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria).
The examples of Mandard TRG are shown in Figure 1. Totally, 797 slides indicating surgical specimens were reviewed. The median number of reviewed slides was 4, with an interquartile range from 3 to 5. After revaluation, the number of patients was 10, 48, 57, 50 and 6 in the group of TRG 1-5, respectively. There was no significant difference in survival between TRG 1 and TRG 2 (p=0.374), so these two categories were classified into the responder group. Similarly, no significant difference was found among TRG 3, TRG 4 and TRG 5 (p=0.560), so these two categories were classified into the non-responder group. The survival curves of Mandard TRG were shown in Supplementary Figure 1.
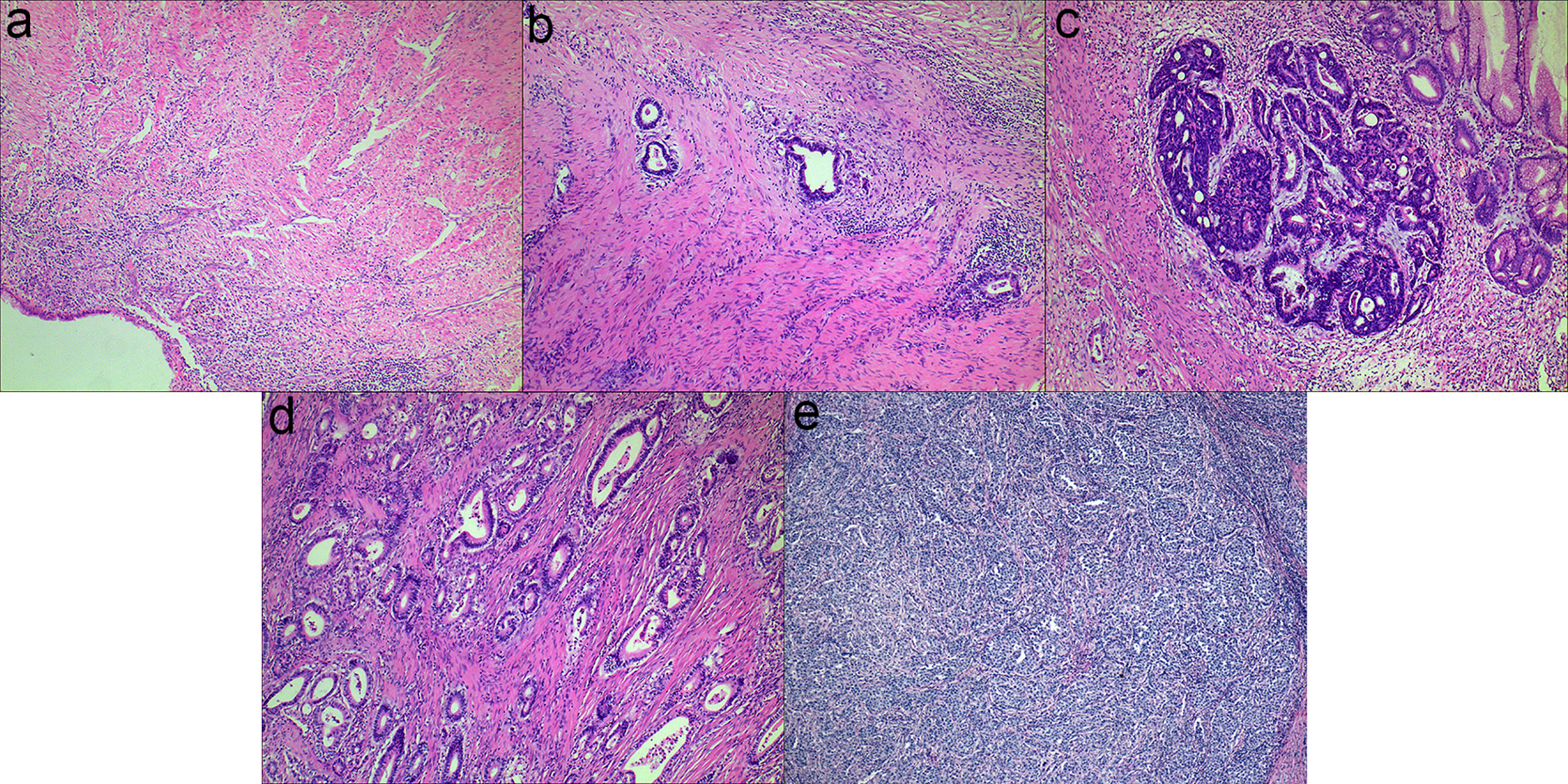
Figure 1 Examples of Mandard TRG (a-e): (A) TRG 1, complete tumor regression; (B) TRG 2, rare residual tumor; (C) TRG 3, more residual tumor but less than fibrosis; (D) TRG 4, residual tumor with signs of regression; (E) TRG 5, residual tumor without regression.
Morphologically, CAFs are a group of spindle-shaped cells surrounding the tumor cells. In 171 specimens after treatment, in CAFs, 31 cases were positive for CD10, with a positive rate of 18.1%; 68 cases were positive for FAP, with a positive rate of 39.8%; 21 cases were positive for GPR77, with a positive rate of 12.3%.
Among 171 specimens before treatment, in CAFs, 26 (15.2%) cases were positive for CD10, 34 cases (19.9%) were positive for FAP, and 15 cases (8.8%) were positive for GPR77. Examples of expression of biomarkers were shown in Figure 2.
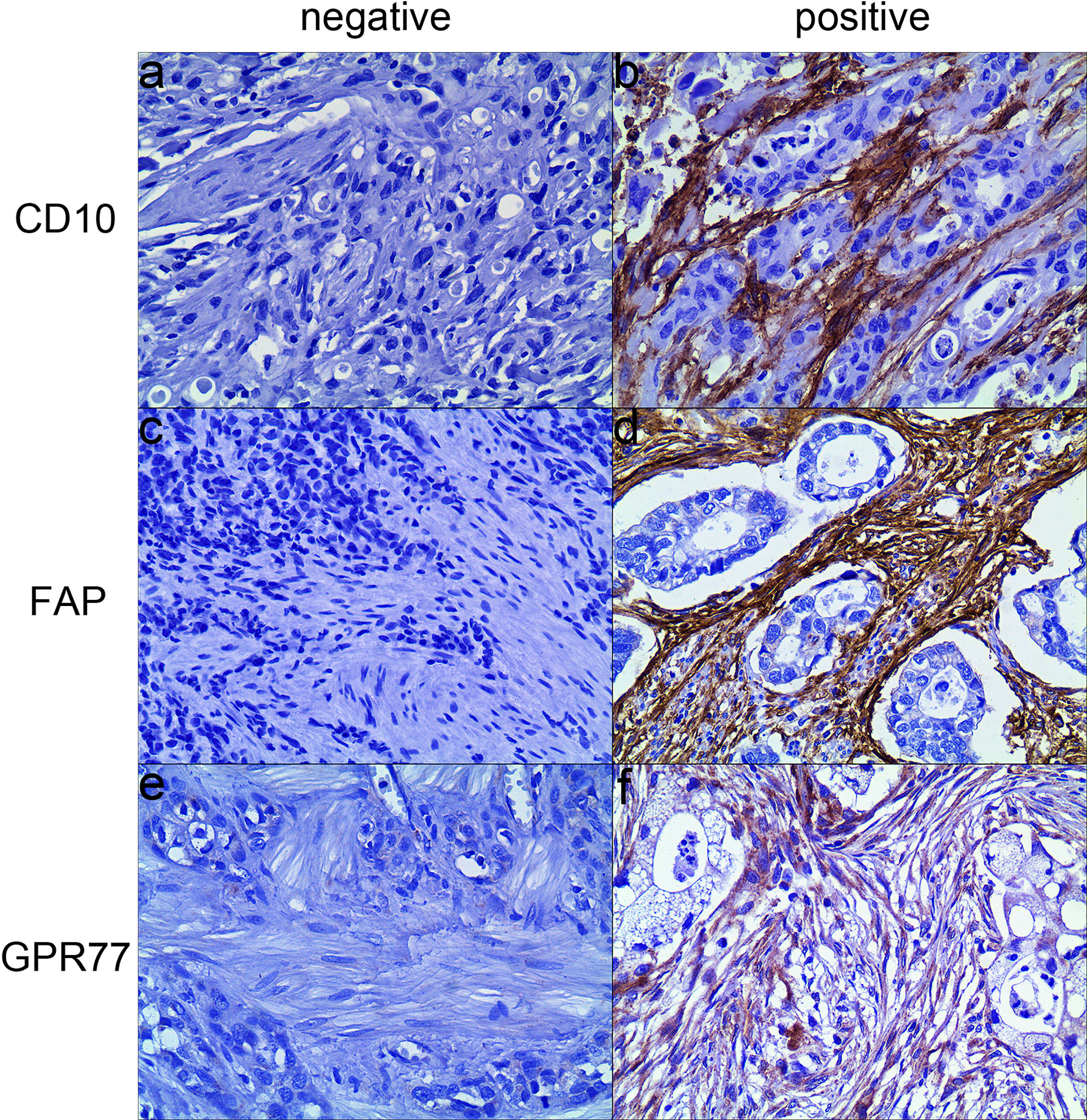
Figure 2 Representative examples of three biomarkers expression by immunohistochemistry (200×). (A) CD10 was negatively expressed in CAFs; (B) CD10 was positively expressed in the cytoplasm and membrane of CAFs; (C) FAP was negatively expressed in CAFs; (D) FAP was positively expressed in the cytoplasm of CAFs; (E) GRP77 was negatively expressed in CAFs; (F) GPR77 was positively expressed in the cytoplasm and membrane of CAFs.
The expressions of biomarkers after treatment (post-) and before treatment (pre-) of all 171 patients are shown in Table 1 and Supplementary Table 1, respectively. For biomarkers after treatment, high expressions of all biomarkers were related to worse pathological response (all p<0.005) (Table 1). However, before treatment, only high expression of pre-CD10 was related to worse pathological response (p=0.030), while pre-FAP (p=0.067) and pre-GPR77 (p=0.233) were not (Supplementary Table 1). The details of relationships between biomarkers and the TRG were shown in Supplementary Table 2.
The relationships between biomarkers after treatment and other clinicopathological characteristics are shown in Table 1. Post-CD10 and post-FAP were related to tumor size (p=0.006, p=0.002, respectively), ypT (p=0.047, p<0.001, respectively), and ypN (p=0.034, p=0.034, respectively). Post-CD10, post-FAP and post-GPR77 were related to TNM stage (p=0.003, p<0.001, p=0.014, respectively).
The relationships between biomarkers before treatment and other clinicopathological characteristics are shown in Supplementary Table 1. Pre-CD10 was related to tumor size (p=0.032), while pre-FAP was not (p=0.413). None of biomarkers before treatment were related to ypT and ypN. Only pre-GPR77 was related to ypTNM (p=0.014).
In univariable analysis for pathological response, for biomarkers after treatment, post-CD10 (odds ratio [OR], 20.602; p=0.003), post-FAP (OR, 24.826; p<0.001), and post-GPR77 (OR, 12.258; p=0.016) were predictors to pathological response. However, for biomarkers before treatment, only pre-CD10 (OR, 3.264; p=0.038) were related to pathological reaction (Table 2).
For other clinicopathological factors, tumor size (OR, 6.502; p<0.001), ypN (OR, 4.473; p<0.001), ypTNM (OR, 6.364; p<0.001), histological type (OR, 2.275; p=0.020), grade of differentiation (OR, 4.436; p<0.001), vascular or lymphatic invasion (OR, 3.575; p=0.008), nervous invasion (OR, 4.007; p=0.007) were related to pathological response (Table 2).
Four groups of biomarkers were included in multivariable analyses for pathological response respectively, with other statistically significant factors. Post-FAP (OR, 12.805; p<0.001) and pre-FAP (OR, 5.672; p=0.009) were independent predictors for pathological response, respectively, while CD10 and GPR77 were not (Table 3).
The survival curves of all biomarkers after treatment were shown in Figure 3. When divided into two groups, post-CD10 (p=0.030), post-FAP (p=0.011) were related to OS (Figure 3). However, for biomarkers before treatment, only pre-FAP was related to OS (p=0.024) (Supplementary Figure 2).
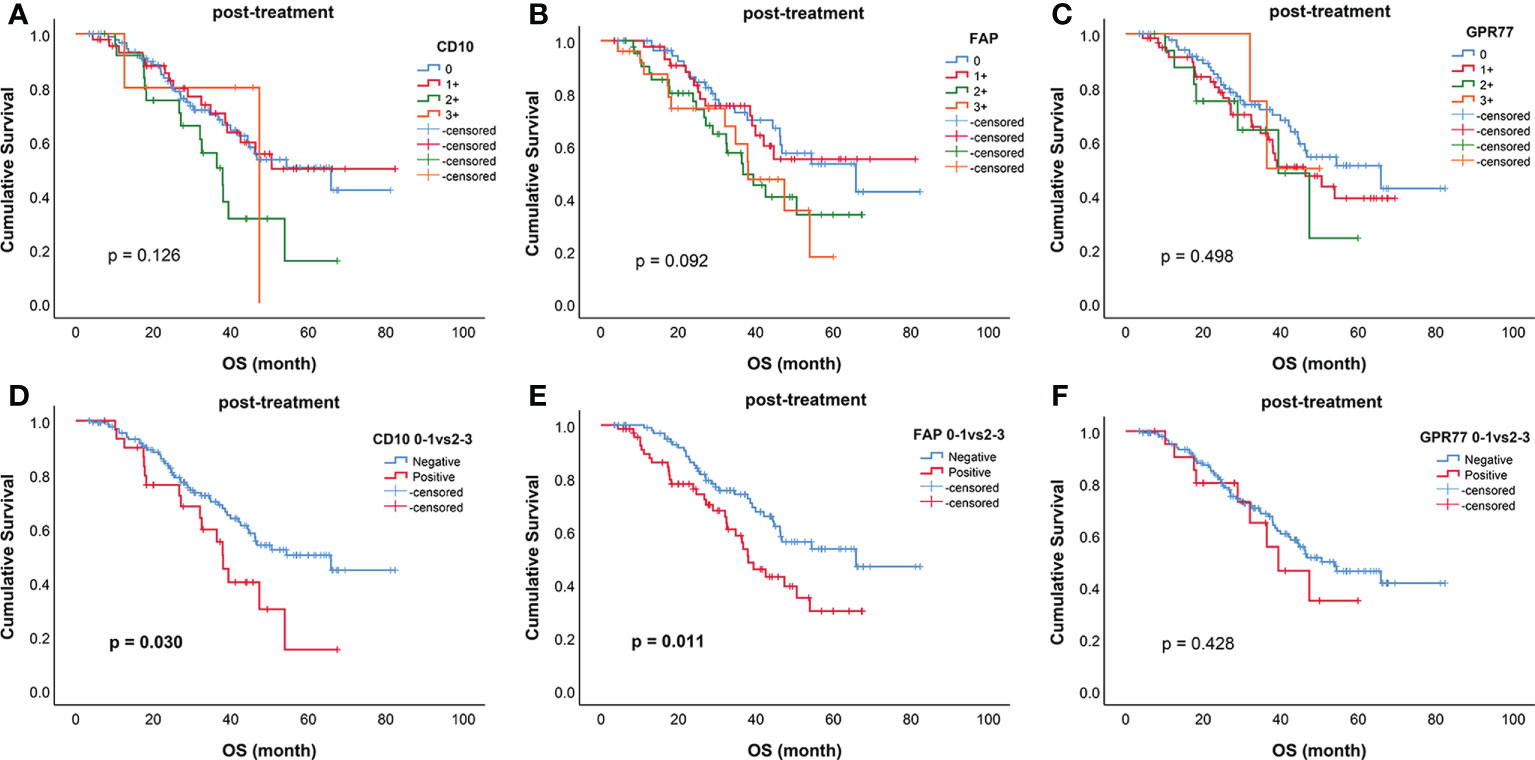
Figure 3 Kaplan–Meier curves for overall survival (OS) of biomarkers after treatment. Survival curves for (A) CD10; (B) FAP; (C) GPR77; (D) CD10 (0-1 vs 2-3); (E) FAP (0-1 vs 2-3); (F) GPR77 (0-1 vs 2-3). CD10 (0-1 vs 2-3) (p=0.030) and FAP (0-1 vs 2-3) (p=0.011) were significantly related to OS.
In univariable analysis for OS, post-CD10 (hazard ratio [HR], 1.832; p=0.032), post-FAP (HR, 1.843; p=0.013) were related to the prognosis. None of biomarkers before treatment were related to the prognosis (all p > 0.05) (Table 4).
In multivariable analysis, four groups of biomarkers were included respectively. None of biomarkers were independent factors for OS (all p > 0.05) (Table 5).
In this study, we investigated the relationships between expressions of CD10, FAP, GPR77 in CAFs and clinicopathological characteristics; and explored the predictive values of these biomarkers before and after treatment for pathological response and OS. We found that high expressions of post-CD10, post-FAP, and post-GPR77 predicted a worse pathological response, and post-FAP was independent predictive factor to pathological response. These results are consistent with other studies, in which FAP was proved to be related to drug chemoresistance (15, 24, 25). The mechanisms of FAP increasing drug resistance are various, such as promoting immunosuppression (13), interacting with membrane proteins (14) and producing chemokine (15). Drugs targeting FAP have shown great effect in vitro experiments (26, 27), but in vivo, these drugs did not show satisfactory effectiveness (28). CD10 has also been proved to promote cancer formation and chemoresistance in breast cancer (8), colorectal cancer (29) and malignant melanoma (20), involving mechanisms such as providing a survival niche for cancer stem cells (8) and promoting epithelial–mesenchymal transition (29). For GPR77, one study suggested it was related to cancer formation and chemoresistance (8).
In our study, post-CD10 and post-FAP were related to prognosis. This result is in line with other studies. High expression of CD10 has been proved to be related to a poor prognosis in breast cancer (22), malignant melanoma (20) and esophageal carcinoma (30). Nevertheless, CD10 did not show similar predictive value in papillary thyroid carcinoma (31). In gastric cancer, few articles verified this conclusion, especially based on patients who underwent NCT. High expression of FAP has been proved to be related to a poor prognosis in pancreatic ductal adenocarcinoma (32), colorectal cancer (17) and gastric cancer (16, 18, 33), but none of their patients underwent NCT. In Wen et al. (16) study, high FAP expression was an independent prognostic factor of poor survival in GC patients, but in our study, none of biomarkers were independent prognostic factors. This might be attributed to the influence of NCT, or the collinearity among biomarkers. In our study, GPR77 did not show prognostic predictive value, but Su et al. (8) suggested GPR77 was related to chemoresistance in breast cancer. Therefore, more evidences are needed to verify these results.
It is worthy to mention that in our study, predictive values of biomarkers after treatment were more significant than those before treatment. This might because the roles of biomarkers changed due to NCT, or because the preoperative biopsy specimens were not enough to show the roles of biomarkers completely. Nevertheless, these results suggested that biomarkers after treatment had better predictive values for efficacy of NCT and prognosis.
In addition, we found that after treatment, high expressions of CD10, FAP and GPR77 were related to T stage and TNM stage, which is in accordance with other articles (18, 33, 34). Hu et al. (18) suggested FAP was related to histological type, while FAP did not show similar result in our study. This difference might due to the influence of NCT.
We explored the relationships between these biomarkers of CAFs and chemoresistance clinically, but more researches on mechanism are needed. In addition, in our study, CD10, and FAP before treatment are related to pathological reaction, which means these biomarkers have the potential to predict the efficacy of NCT and could be helpful to further clinical decision-making.
This study has several limitations. It was a retrospective study from a single institution and the sample size was not large, which might cause bias. The relationships between biomarkers and pathological response and prognosis were investigated clinically, but we have not yet revealed the mechanism that lead to these results. When taking pre-treatment specimens, due to the limitation of the amount of pre-treatment specimens, false-negative results might occur, which might be the reason that some pre-treatment markers did not show prognostic predictive value. Because of correlations of expression locations of biomarkers, collinearity might be the reason why some biomarkers lost predictive values in multivariable analysis. Nevertheless, we concentrated on a specific group of patients and verified the clinical values of CAFs biomarkers, including CD10, FAP and GPR77 in locally advanced gastric cancer patients who underwent NCT, and explored the association of these biomarkers with drug resistance and prognosis. These results could be helpful to clinical decision making and could provide evidence for future researches.
In conclusion, the expressions of CD10, FAP and GPR77 in CAFs were related to drug resistance and overall survival, and they could be used as predictors for pathological reaction and prognosis in locally advanced gastric cancer patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All experimental protocols were approved by the Institutional Review Board (IRB) at Liaoning Cancer Hospital and Institute (Cancer Hospital of China Medical University). All methods were carried out in accordance with relevant guidelines and regulations. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YT and ZZ performed the majority of experiments and analyzed the data and drafted the manuscript. JZ reviewed and revised the manuscript. WW assisted in collecting and analyzing the data. YZ supervised the study and provided critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Natural Science Foundation of Liaoning Province (2020-ZLLH-45), Shenyang High-level Innovative Talents Program (RC190447) and Liaoning Cancer Hospital & Institute- Dalian University of Technology “Medical-industrial interdisciplinary research fund” (LD202021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.984817/full#supplementary-material
NCT, neoadjuvant therapy; GC, gastric cancer; TRG, tumor regression grade; CAFs, cancer-associated fibroblasts; ECM, extracellular matrix; FAP, Fibroblast-activation protein; GPR77, G-protein-coupled receptor 77; AJCC, American Joint Committee on Cancer; OS, overall survival; ypTNM, post-neoadjuvant therapy stage.
1. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2016) 14(10):1286–312. doi: 10.6004/jnccn.2016.0137
2. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK.Ajcc cancer staging manual. 8th Ed. Chicago: Springer (2017). 260.
3. Tong Y, Liu D, Zhang J. Connection and distinction of tumor regression grading systems of gastrointestinal cancer. Pathol Res Pract (2020) 216(9):153073. doi: 10.1016/j.prp.2020.153073
4. Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene (2019) 38(25):4887–901. doi: 10.1038/s41388-019-0765-y
5. Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. Il-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res (2018) 78(17):4957–70. doi: 10.1158/0008-5472.CAN-17-2268
6. Maeda M, Takeshima H, Iida N, Hattori N, Yamashita S, Moro H, et al. Cancer cell niche factors secreted from cancer-associated fibroblast by loss of H3k27me3. Gut (2020) 69(2):243–51. doi: 10.1136/gutjnl-2018-317645
7. Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, et al. Cancer-associated fibroblasts lead tumor invasion through integrin-Beta3-Dependent fibronectin assembly. J Cell Biol (2017) 216(11):3509–20. doi: 10.1083/jcb.201702033
8. Su S, Chen J, Yao H, Liu J, Yu S, Lao L, et al. Cd10(+)Gpr77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell (2018) 172(4):841–56.e16. doi: 10.1016/j.cell.2018.01.009
9. Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet (2017) 49(5):708–18. doi: 10.1038/ng.3818
10. Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. Fap-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer (2011) 11:245. doi: 10.1186/1471-2407-11-245
11. Sanchez-Garrido MA, Habegger KM, Clemmensen C, Holleman C, Muller TD, Perez-Tilve D, et al. Fibroblast activation protein (Fap) as a novel metabolic target. Mol Metab (2016) 5(10):1015–24. doi: 10.1016/j.molmet.2016.07.003
12. Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest (2009) 119(12):3613–25. doi: 10.1172/JCI38988
13. Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. Fap promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment Via Stat3-Ccl2 signaling. Cancer Res (2016) 76(14):4124–35. doi: 10.1158/0008-5472.CAN-15-2973
14. Artym VV, Kindzelskii AL, Chen WT, Petty HR. Molecular proximity of seprase and the urokinase-type plasminogen activator receptor on malignant melanoma cell membranes: Dependence on Beta1 integrins and the cytoskeleton. Carcinogenesis (2002) 23(10):1593–601. doi: 10.1093/carcin/23.10.1593
15. Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting Cxcl12 from fap-expressing carcinoma-associated fibroblasts synergizes with anti-Pd-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U.S.A. (2013) 110(50):20212–7. doi: 10.1073/pnas.1320318110
16. Wen X, He X, Jiao F, Wang C, Sun Y, Ren X, et al. Fibroblast activation protein-Alpha-Positive fibroblasts promote gastric cancer progression and resistance to immune checkpoint blockade. Oncol Res (2017) 25(4):629–40. doi: 10.3727/096504016X14768383625385
17. Coto-Llerena M, Ercan C, Kancherla V, Taha-Mehlitz S, Eppenberger-Castori S, Soysal SD, et al. High expression of fap in colorectal cancer is associated with angiogenesis and immunoregulation processes. Front Oncol (2020) 10:979. doi: 10.3389/fonc.2020.00979
18. Hu M, Qian C, Hu Z, Fei B, Zhou H. Biomarkers in tumor microenvironment? upregulation of fibroblast activation protein-alpha correlates with gastric cancer progression and poor prognosis. OMICS (2017) 21(1):38–44. doi: 10.1089/omi.2016.0159
19. Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: Cutting is the key! Blood (1993) 82(4):1052–70. doi: 10.1182/blood.V82.4.1052.1052
20. Oba J, Nakahara T, Hayashida S, Kido M, Xie L, Takahara M, et al. Expression of Cd10 predicts tumor progression and unfavorable prognosis in malignant melanoma. J Am Acad Dermatol (2011) 65(6):1152–60. doi: 10.1016/j.jaad.2010.10.019
21. Ogawa H, Iwaya K, Izumi M, Kuroda M, Serizawa H, Koyanagi Y, et al. Expression of Cd10 by stromal cells during colorectal tumor development. Hum Pathol (2002) 33(8):806–11. doi: 10.1053/hupa.2002.125773
22. Desmedt C, Majjaj S, Kheddoumi N, Singhal SK, Haibe-Kains B, El Ouriaghli F, et al. Characterization and clinical evaluation of Cd10+ stroma cells in the breast cancer microenvironment. Clin Cancer Res (2012) 18(4):1004–14. doi: 10.1158/1078-0432.CCR-11-0383
23. Li XX, Clark RJ, Woodruff TM. C5ar2 activation broadly modulates the signaling and function of primary human macrophages. J Immunol (2020) 205(4):1102–12. doi: 10.4049/jimmunol.2000407
24. Kramer N, Schmollerl J, Unger C, Nivarthi H, Rudisch A, Unterleuthner D, et al. Autocrine Wnt2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene (2017) 36(39):5460–72. doi: 10.1038/onc.2017.144
25. Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, et al. Tumor-promoting desmoplasia is disrupted by depleting fap-expressing stromal cells. Cancer Res (2015) 75(14):2800–10. doi: 10.1158/0008-5472.CAN-14-3041
26. Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res (2008) 14(14):4584–92. doi: 10.1158/1078-0432.CCR-07-5211
27. Wen Y, Wang CT, Ma TT, Li ZY, Zhou LN, Mu B, et al. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci (2010) 101(11):2325–32. doi: 10.1111/j.1349-7006.2010.01695.x
28. Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, et al. Phase ii trial of single agent Val-boropro (Talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol Ther (2007) 6(11):1691–9. doi: 10.4161/cbt.6.11.4874
29. Khanh do T, Mekata E, Mukaisho K, Sugihara H, Shimizu T, Shiomi H, et al. Transmembrane mucin Muc1 overexpression and its association with Cd10(+) myeloid cells, transforming growth factor-Beta1 expression, and tumor budding grade in colorectal cancer. Cancer Sci (2013) 104(7):958–64. doi: 10.1111/cas.12170
30. Dzobo K, Dandara C. Broadening drug design and targets to tumor microenvironment? cancer-associated fibroblast marker expression in cancers and relevance for survival outcomes. OMICS (2020) 24(6):340–51. doi: 10.1089/omi.2020.0042
31. Oh EJ, Bychkov A, Cho H, Kim TM, Bae JS, Lim DJ, et al. Prognostic implications of Cd10 and Cd15 expression in papillary thyroid carcinoma. Cancers (Basel) (2020) 12(6):1413. doi: 10.3390/cancers12061413
32. Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res (2014) 2(3):187–93. doi: 10.1158/2326-6066.CIR-14-0002
33. Gao LM, Wang F, Zheng Y, Fu ZZ, Zheng L, Chen LL. Roles of fibroblast activation protein and hepatocyte growth factor expressions in angiogenesis and metastasis of gastric cancer. Pathol Oncol Res (2019) 25(1):369–76. doi: 10.1007/s12253-017-0359-3
Keywords: gastric cancer, tumor regression grade (TRG), neoadjuvant therapy, cancer-associated fibroblasts (CAFs), CD10, FAP, GPR77
Citation: Tong Y, Zhao Z, Zhang J, Wang W and Zhu Y (2022) High expressions of CD10, FAP and GPR77 in CAFs are associated with chemoresistance and worse prognosis in gastric cancer. Front. Oncol. 12:984817. doi: 10.3389/fonc.2022.984817
Received: 02 July 2022; Accepted: 26 September 2022;
Published: 28 October 2022.
Edited by:
Fabiana Conciatori, Hospital Physiotherapy Institutes (IRCCS), ItalyReviewed by:
Hiromu Suzuki, Sapporo Medical University, JapanCopyright © 2022 Tong, Zhao, Zhang, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanmei Zhu, emh1eWFubWVpQGNhbmNlcmhvc3AtbG4tY211LmNvbQ==; Wentao Wang, d2FuZ3dlbnRhb0BjYW5jZXJob3NwLWxuLWNtdS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.