
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 17 November 2022
Sec. Cancer Immunity and Immunotherapy
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.983892
This article is part of the Research TopicGastrointestinal Cancer Immunotherapy: from Drug Resistance Mechanisms to Overcoming StrategiesView all 16 articles
Objectives: The study aims to summarize publication characteristics of anti-programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) immunotherapy for esophageal cancer and create scientific maps to explore hotspots and emerging trends with bibliometric methods.
Methods: The publications between 2012 and 2021 were retrieved from the Web of Science Core Collection (WoSCC) on June 20, 2022. Bibliometric tools including HistCite, VOSviewer, and CiteSpace were used for statistical analysis. Data on the trend of the annual output, countries/regions, institutions, journals, authors, subject categories, keywords, and co-cited references were presented in this study.
Results: A total of 552 publications written by 3,623 authors of 872 institutions, 44 countries/regions in 250 journals were included in the bibliometric study. China, USA and Japan were the key countries in this field. Kato Ken, Bang Yung-Jue, Frontiers in Oncology, Journal of Clinical Oncology and Natl Canc Ctr were the top 1 productive author, co-cited author, productive journal, co-cited journal and prolific institution, respectively. The top 4 most present keywords were esophageal cancer, immunotherapy, esophageal squamous cell carcinoma and PD-L1. Neoadjuvant chemotherapy, response, PD-1 blockade and CD8+ T cell were four latest research frontiers. The keywords reflected the progress from PD-1/PD-L1 expression to the clinical application of PD-1/PD-L1 inhibitors. The current researches mainly focus on neoadjuvant immunotherapy for esophageal cancer and development of biomarkers. Further research is warranted to determine effective predictive biomarkers or models, illustrate the molecular mechanism of combined treatment, and construct the optimal therapeutic strategy.
Conclusions: This study visually analyzed the global trend and hotspots of anti-PD-1/PD-L1 immunotherapy for esophageal cancer over the past decade. The results could guide scientists to comprehensively understand the global frontiers and determine future directions.
Esophageal cancer is the eighth most common cancer and the sixth major cause of cancer-related death worldwide (1). In 2020, the world witnessed about 604,100 new cases and 544,100 deaths, equaling to the age-standardized morbidity and mortality rates of 6.3/100,000 and 5.6/100,000, respectively (2). Advanced esophageal squamous cell carcinoma (ESCC) is one of devastating tumors with the 5-year survival rate lower than 5% (3, 4). The etiology of esophageal cancer is not completely clear. Recognized risk factors include genetic predisposition, gastroesophageal reflux disease, alcohol consumption, smoking and obesity (5). The alternative clinical treatment for esophageal cancer mainly depends on the stage of the tumor and the specific condition of patients. For esophageal cancer, multidisciplinary approach is an effective strategy for managing this disease, which involves the use of surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy and other treatments (6). In recent years, the emergence of immunotherapy has brought new hope for esophageal cancer. With the recognition of tumor immunotherapy, the application of immune checkpoint inhibitors (ICIs) has gradually shifted from the back-line and second-line treatments to first-line and even perioperative treatments. PD-1/PD-L1 inhibitors have been approved to be used for the first-line treatment of advanced esophageal cancer, which significantly improves patient prognosis (7). The response rate of ICI alone in esophageal cancer varied from 9.9 to 33.3% in the reported studies (8).
A large number of articles regarding anti-PD-1/PD-L1 immunotherapy for esophageal cancer have been published in the past decade. However, no systematic analysis of the data in the available articles has been performed. The increasing number of publications makes it more necessary to illustrate the state of the development by bibliometric methods (9). Bibliometric analysis consists of the quantification and visualization of the data by applying mathematics and statistics (10). In this way, the research trend of the area can be objectively shown. The bibliometric analysis provides researchers valuable information on the development of a specific field from a macro perspective. The most important data source is the Web of Science Core Collection (WoSCC) database (11).
Up to now, there is no published bibliometric study has systematically evaluated the anti-PD-1/PD-L1 immunotherapy for esophageal cancer from 2012 to 2021. In this work, the research tendency and hotspots of anti-PD-1/PD-L1 immunotherapy for esophageal cancer were visually analyzed using HistCite, VOSviewer, and CiteSpace. The aim was to identify the characteristics of publications, build collaboration networks, present hot words, reveal research frontiers and direct the follow-up work (12, 13).
The literature retrieval was performed online using the WoSCC database, which is the most influential citation academic document database worldwide, in order to collect publications on anti-PD-1/PD-L1 immunotherapy for esophageal cancer (14). The search was performed on June 20, 2022 to ensure the same conditions and avoid the bias resulting from daily updates (15). Since all data were downloaded from the public database, no ethical approval was needed for this work. The following formula was used to perform the advanced search: TS = ((esophageal neoplasm OR esophagus neoplasm OR esophageal cancer OR esophagus cancer OR esophageal tumor OR esophagus tumor OR esophageal carcinoma OR esophagus carcinoma OR ESCC) AND (programmed cell death 1 receptor OR programmed cell death ligand 1 OR CD274 OR B7H1 OR PD-1 OR PD-L1 OR Nivolumab OR Pembrolizumab OR Lambrolizumab OR Avelumab OR Atezolizumab OR Nivolizumab OR Durvalumab OR Pidilizumab OR Cemiplimab OR Camrelizumab OR Sintilimab OR Tisleizumab OR Toripalimab)). The detailed retrieval process and analysis procedure are shown in Figure 1.
The inclusion criteria are as follows: (a) literature on anti-PD-1/PD-L1 immunotherapy for esophageal cancer; (b) literature types include articles and reviews; (c) literature published between 2012 and 2021; (d) literature indexed in WoSCC.
The exclusion criteria are as follows: (a) Unpublished documents; (b) Duplicate reports.
WoSCC was used to collect publications for bibliometric analysis and visualization. All the data retrieved from WoSCC were exported in plain text format. HistCite (Clarivate Analytics, Philadelphia, PA, the USA), VOSviewer 1.6.14 (Leiden University, Leiden, the Netherlands) and CiteSpace 5.3.R4 (Drexel University, Philadelphia, the USA) were used for statistical analysis (16). HistCite is a citation analysis software that summarizes and processes data quickly (17). In this study, annual output, language type, document type and total number of citations were analyzed by HistCite. VOSviewer is available for building and viewing bibliometric maps, and displays the results of the cluster analysis, including research characteristics, distribution and hotspots (18). The visual maps of countries/regions, institutions, journals, authors, keywords and references were generated by VOSviewer. CiteSpace, a Java application software, was used to explore the collaboration among countries/institutions/authors, identify co-cited authors/references, detect burst keywords and construct visualization maps (19). The software was effective in revealing the trends and dynamics of publications as well as capturing hotspots in a given research field (20). Due to its rich functions, CiteSpace has been widely used for bibliometric analysis. The CiteSpace parameters were as follows: time slicing (2012–2021), years per slice (1), term source (all selection), term type (burst terms), node type (choose one at a time), links (strength: cosine; scope: within slices), selection criteria (top 50 objects), and pruning (pathfinder and pruning sliced networks).
A total of 552 publications were included in the bibliometric study from 2012 to 2021. These publications were written by 3,623 authors of 872 institutions, 44 countries/regions in 250 journals, with 16,778 citations totally. All publications involved were made up of original articles (n = 410, 74.28%) and reviews (n = 142, 25.72%). The annual output generally maintained an increase trend in the past decade (Figure 2). The most prolific year was 2021 with 216 publications, while the minimum annual output occurred in 2012 with one article. 2014 is the fastest growing year in the past decade. As regard the total citations, the figure peaked at 1,886 in 2018 and bottomed out in 2012.
A total of 44 countries/regions participated in the publication of anti-PD-1/PD-L1 immunotherapy for esophageal cancer in the last 10 years. Among them, China (n = 227, 32.76%) was the most prolific country/region, followed by the USA (n = 153, 22.08%) and Japan (n = 101, 14.57%). In terms of citations, the USA had the most total citations and France had the highest ratio of Citations/Paper. Table 1 lists the top 8 most prolific countries/regions. A network map was constructed for countries with more than 5 publications. Figure 3 shows that the map had 18 nodes. The 3 largest nodes respectively represented China, the USA and Japan for their huge number of publications. The USA had the most active cooperation with others, with the strongest total link strength (TLS, TLS = 125). The closest cooperation was between China and the USA (TLS = 27).
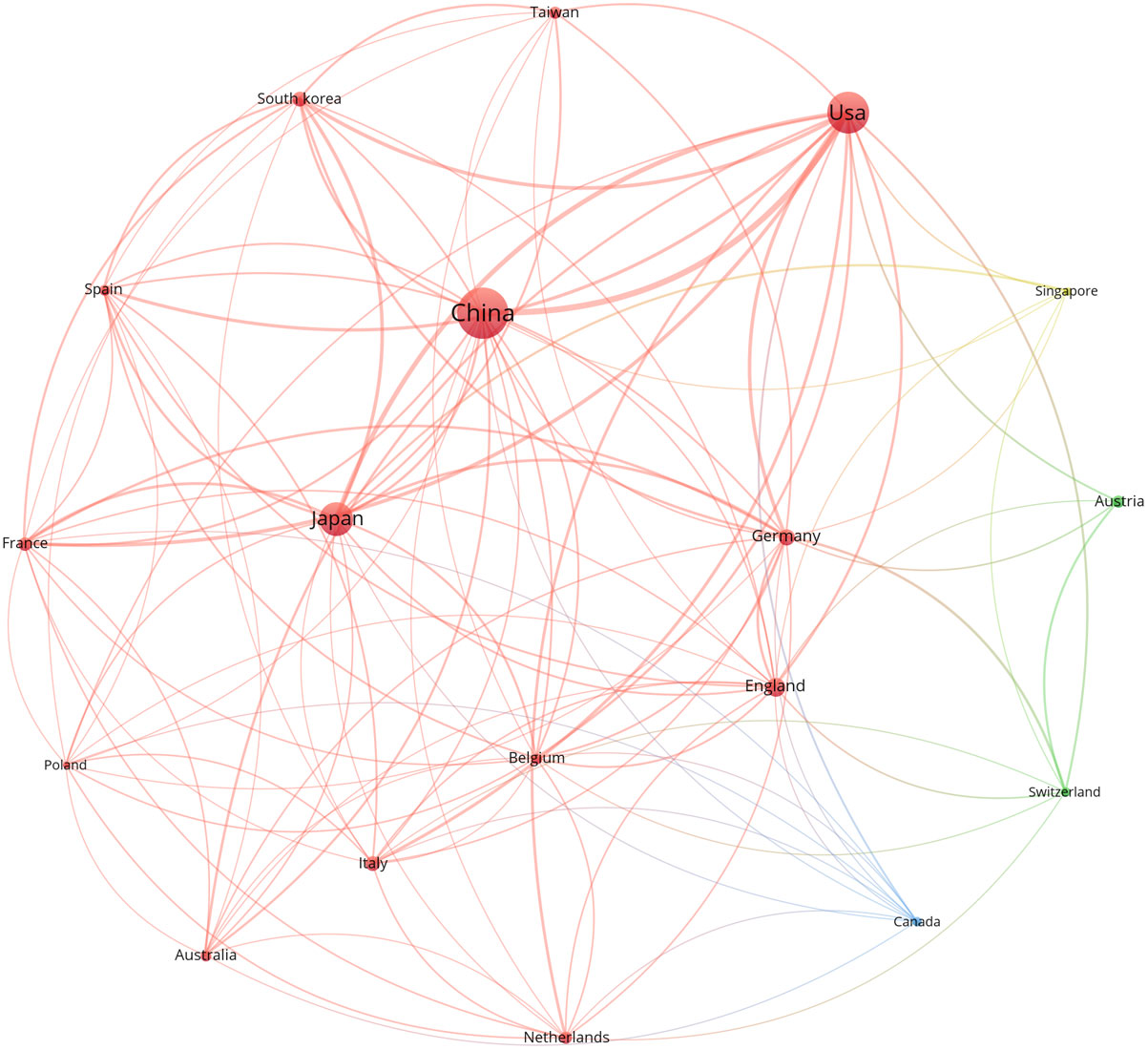
Figure 3 The co-authorship network visualization map of countries/regions. Larger nodes represent more publications of the term. Lines between nodes represent the connection between them.
A total of 872 institutions contributed to the research of anti-PD-1/PD-L1 immunotherapy for esophageal cancer. The top 10 most productive institutions from 2012 to 2021 are listed in Table 2. Natl Canc Ctr (Japan, 23 publications) and Zhengzhou Univ (China, 23 publications) tied first place, followed by Sun Yat Sen Univ (China, 21 publications), Natl Canc Ctr Hosp East (Japan, 19 publications) and Fudan Univ (China, 16 publications). The publications of the top 10 institutions accounted for more than 29% of the total publications. As regard the citations, Dana Farber Canc Inst (the USA, 1,108 citations) ranked first. Natl Canc Ctr Hosp East (Japan, 733 citations) and Natl Canc Ctr (Japan, 725 citations) came second and third, respectively. Figure 4 shows the co-authorship network among institutions with 10 or more publications. It is evident that institutions in the same district always closely cooperate with each other. Natl Canc Ctr Hosp East (TLS = 78) had the most active cooperation with others. The closest cooperation was between Natl Canc Ctr Hosp East and Natl Canc Ctr (TLS = 14).
All the 552 papers were published in 250 journals. The top 10 prolific journals and co-cited journals are listed in Table 3. The 10 most prolific journals published 164 papers, accounting for 29.71% papers involved in this study. The IF of these journals ranged from 3.111 to 13.801, half of which were higher than 6. Among these journals, Frontiers in Oncology (IF = 5.738; 22 publications) had most publications, followed by Cancer Science (IF = 6.518; 14 publications) and Journal for Immunotherapy of Cancer (IF = 12.469; 13 publications). The top 3 co-cited journals were as follows: Journal of Clinical Oncology (IF = 50.717; 1,788 co-citations), New England Journal of Medicine (IF = 176.079; 1,232 co-citations) and Lancet Oncology (IF = 54.433; 996 co-citations). The co-citations of the 50% of the listed journals were greater than 700 and 7 of the top 10 co-cited journals had IF higher than 50.
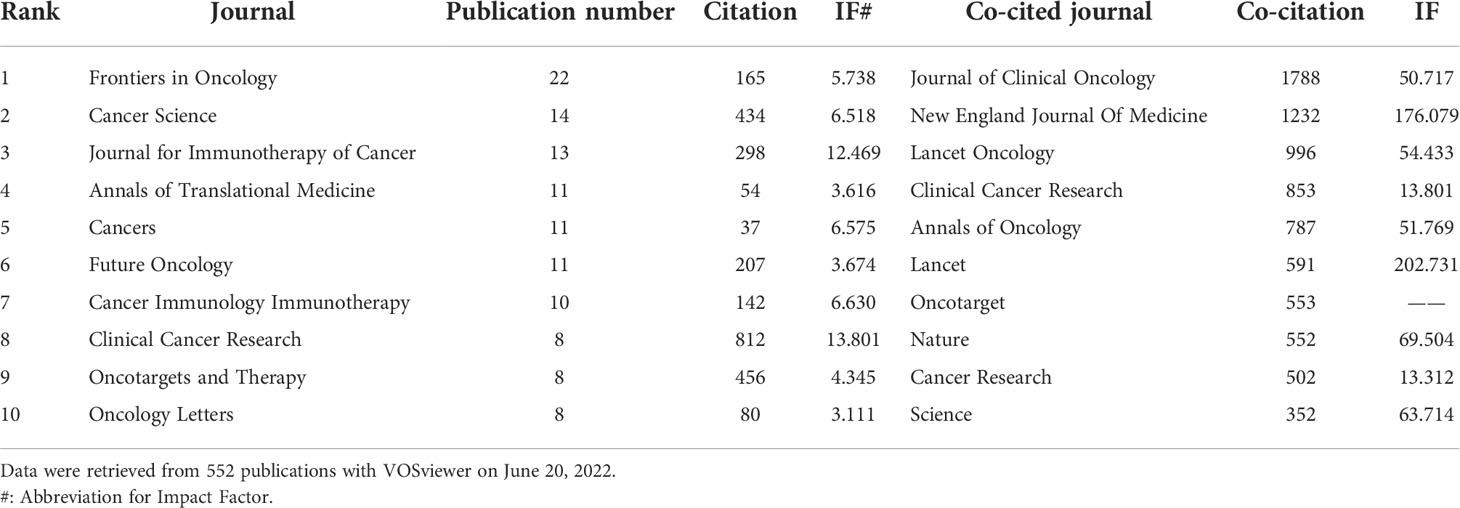
Table 3 The top 10 journals and co-cited journals on anti-PD-1/PD-L1 immunotherapy for esophageal cancer from 2012 to 2021.
A total of 3,623 authors contributed to the involved publications. Table 4 shows that the top 10 authors were all from Japan. Among them, Kato Ken with 16 publications and 410 citations was the most productive author, followed by Kojima Takashi (12 publications, 440 citations) and Doi Toshihiko (10 publications, 394 citations). As regard the co-cited authors, Bang Yung-Jue from South Korea ranked first with 197 co-citations, followed by Kato Ken (193 co-citations) and Fuchs Charles S. (184 co-citations).
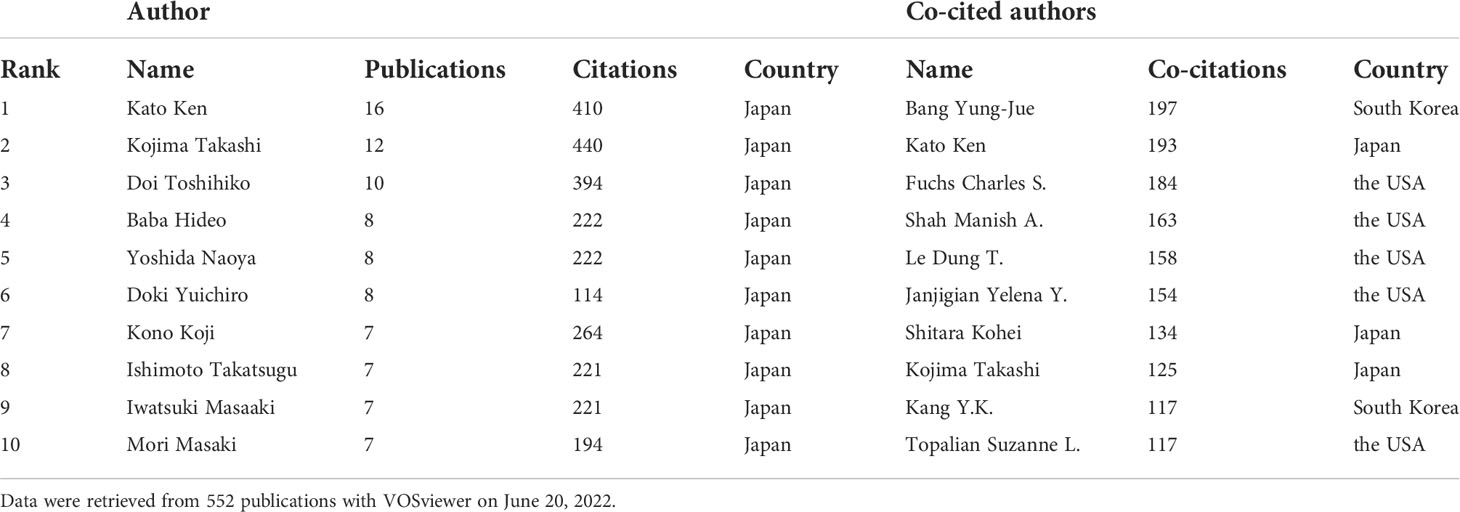
Table 4 The top 10 prolific authors and co-cited authors on anti-PD-1/PD-L1 immunotherapy for esophageal cancer research from 2012 to 2021.
VOSviewer analyzed the information of authors and co-cited authors, then visualized it in a network map to explore influential researchers and potential collaborators (Figure 5) (21). The 42 authors with more than 5 publications formed several clusters and almost no collaboration was present among the clusters. The linkages among the authors were clearly less robust. However, the linkages among authors from same cluster were relatively close. When it comes to the co-cited authors, the minimum number of co-citations was set as 60. Unlike authors, the collaborations among 31 co-cited authors were quite active.
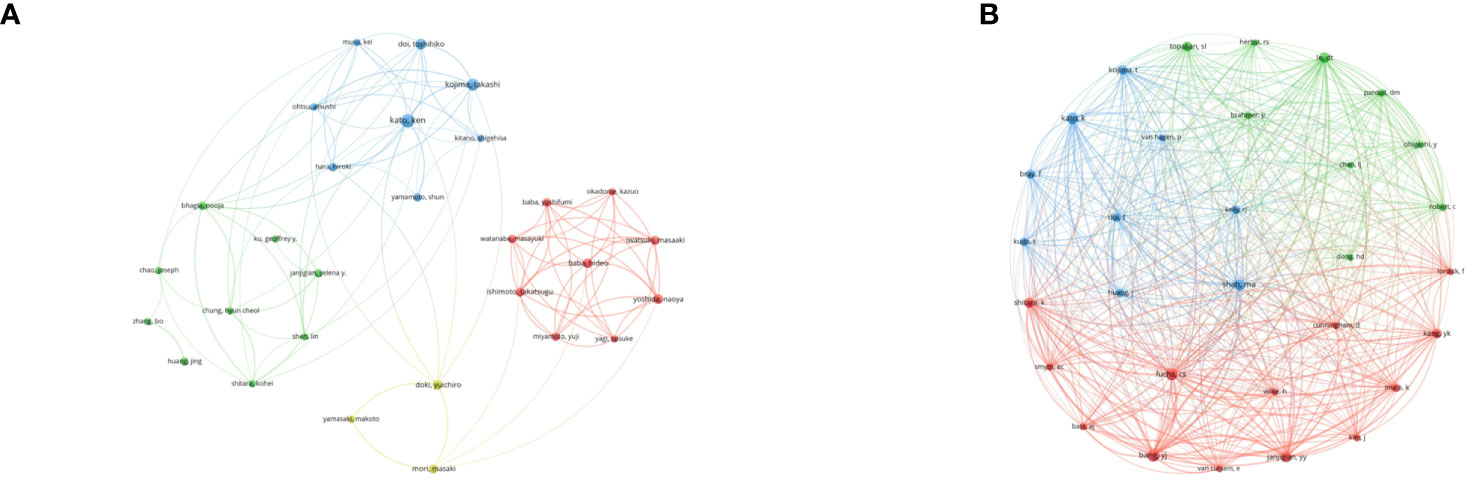
Figure 5 (A) Co-authorship network visualization map of authors. (B) Co-citation network visualization map of authors.
In the present study, CiteSpace was used to analyze the information regarding publication categories and construct a knowledge map (Figure 6). The larger node represented more publications of the term. Nodes with high centrality were usually considered as pivotal points in the field (22). In this work, the top 5 subject categories were selected according to the publication number and centrality. Table 5 shows that ONCOLOGY and IMMUNOLOGY ranked first and second, respectively.

Figure 6 The visualization map of subject categories. The tree ring-shaped nodes represented different subject categories. The lines between two nodes meant co-occurrence. The area of the nodes referred to the number of publications. Nodes with high centrality were deemed as the hot field.

Table 5 Top 5 subject categories in terms of publication number and centrality related to anti-PD-1/PD-L1 immunotherapy for esophageal cancer.
High-frequency keywords represent the hot topics in a particular field. Fifty-nine keywords with more than 5 occurrences were extracted from 552 publications. The top 4 keywords with most occurrences were listed as follows: esophageal cancer (n = 123), immunotherapy (n = 119), esophageal squamous cell carcinoma (n = 89), and PD-L1 (n = 79). VOSviewer was used to construct the network map of keywords, including esophageal cancer, immunotherapy, esophageal squamous cell carcinoma, PD-L1, PD-1, prognosis and so on (Figure 7A).
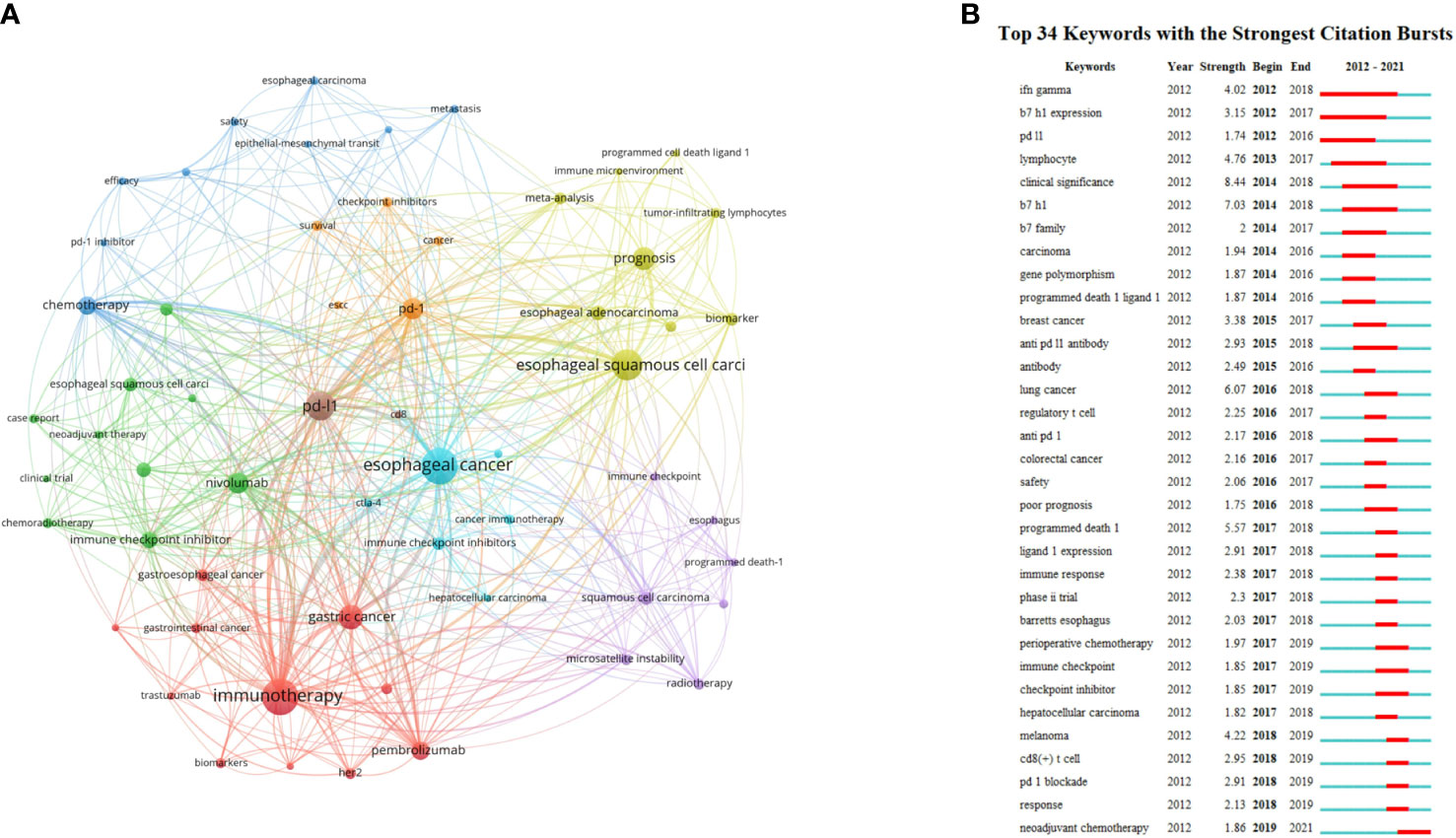
Figure 7 (A) The co-occurrence network visualization map of keywords. Keywords in the same color represent were sorted into the same cluster. (B) The top 34 keywords with the strongest citation bursts from 2012 to 2021. The red segment of the blue line denoted the burst duration of a keyword.
CiteSpace was used to identify and analyze keywords with citation bursts, thereby indicating the research hotspots and emerging trends over a period time. The minimum burst duration was set as 1 year. The top 34 keywords with the strongest citation burst are listed in Figure 7B. Among them, clinical significance had the highest burst strength (8.44). Response, PD-1 blockade, CD8+ T cell, and melanoma were the four keywords with the highest burst strength from 2018 to 2019. Neoadjuvant chemotherapy (NCT) was the top 1 keyword with the strongest citation bursts recently.
Co-cited reference is regarded as one of the most valuable indicators in bibliometrics that displays the key landmark articles of this field (23–25). Table 6 lists the top 10 co-cited references. Among them, 8 articles were clinical trials, 2 were original articles. The article written by Freddie Bray et al. published in CA Cancer J Clin ranked first (n = 111) (26), followed by a clinical trial written by Yoon-Koo Kang et al. in Lancet (n = 99) (27) and another clinical trial written by Ken Kato et al. in Lancet Oncol (n = 97) (28). A co-citation network map was created using articles with more than 40 co-citations and explored the connection among these articles. The map contained 27 nodes, which clearly indicated the scientific relevance among these references. Figure 8A shows that the largest node represented the most co-cited reference. “Yoon-Koo Kang, 2017, Lancet, V390, P2461” (TLS = 606) (27) had the most active association with other references, followed by “Charles S Fuchs, 2018, JAMA Oncol, V4” (TLS = 529) (32) and “Manish A Shah, 2019, JAMA Oncol, V5, P546” (TLS = 480) (30).
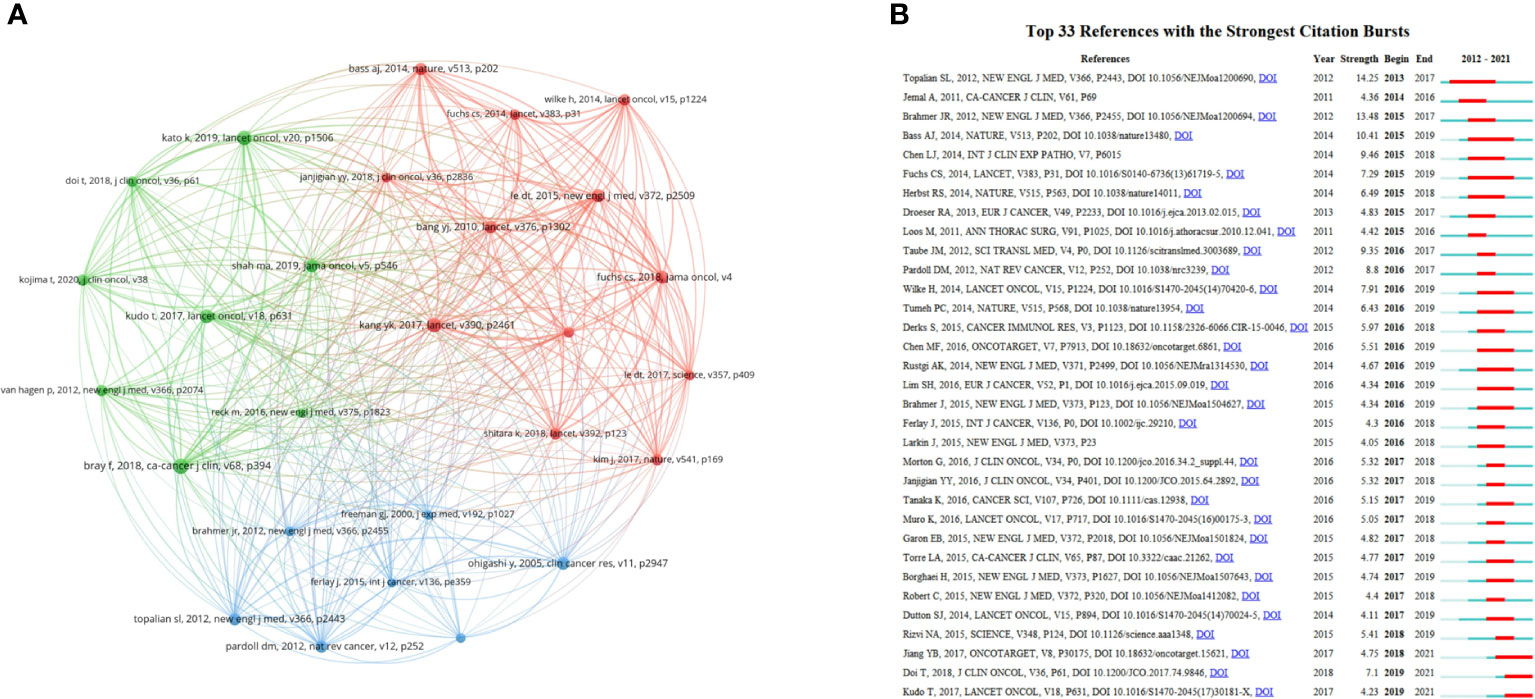
Figure 8 (A) The co-citation network visualization map of references from 2012 to 2021. (B) The top 33 references with the strongest citation bursts from 2012 to 2021. The red segment of the blue line denoted the burst duration of a keyword.
References with citation bursts refer to those that are frequently cited during certain a period of time (36). CiteSpace was used to perform references with citation bursts, and the minimum burst duration was set as 1 year. The blue line in Figure 8B represents the timeline in years, while the red line represents the time range in which a reference had citation burst (37). The burst strength of the top 33 references ranged from 4.05 to 14.25. Among them, “Topalian SL, 2012, New Engl J Med, V366, P2443 (35)“ had the highest burst strength (14.25), which ranked tenth in the list of co-citations, indicating the great influence of this study. The article assessed the antitumor activity and safety of anti–PD-1 antibody in cancer, showing that the adverse-event profile does not appear to preclude its use. “Kudo T, 2017, Lancet Oncol, V18, P631” (29), “Doi T, 2018, J Clin Oncol, V36, P61” (38) and “Jiang YB, 2017, Oncotarget, V8, P30175” (39) were 3 co-cited references with recent bursts. Toshihiro Kudo et al. conducted a phase II clinical trial and suggested that nivolumab exhibited favorable activity and controllable safety in ESCC (29). Toshihiko Doi et al. reported the results of KEYNOTE-028, a phase Ib study on PD-L1(+) patients with advanced solid tumors (38). Pembrolizumab displayed controllable toxicity and persistent antitumor activity in these patients. Yubo Jiang et al. revealed the prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in ESCC (39).
Esophageal cancer has a high degree of malignancy and poor prognosis. As a new therapeutic method, immunotherapy can significantly improve the prognosis of patients (40, 41). The anti-PD-1/PD-L1 antibody is the most commonly used ICI. Therefore, it is important to build an in-depth understanding of publications in this field. In this study, a bibliometric analysis of anti-PD-1/PD-L1 immunotherapy for esophageal cancer from 2012 to 2021 was performed, presenting the research hotspots and trends.
Figure 2 shows that the annual output maintained a rapid growth over the last decade. Literature published between 2012 and 2016 mostly focused on the expression and prognostic value of PD-1/PD-L1. In 2017, results of clinical trials of ICIs for esophageal cancer began to be published. From then on, the annual output increased rapidly, from 40 in 2017 to 216 in 2021. The annual growth rate also increased year by year.
In 2017, Toshihiko Doi (38) and Toshihiro Kudo (29) released their respective clinical trial results, which respectively demonstrated that Pembrolizumab and Nivolumab had certain anti-tumor effect in PD-L1(+) patients who failed second-line or back-like treatment. In 2018, Huang Jing et al. conducted a study on 30 patients with relapsed or metastatic advanced ESCC that showed chemoresistance previously (42). According to their results, the anti-PD-1drug SHR-1210 exhibited definite antitumor activity, with tolerable toxic and side effects. In 2019, the research data from multiple clinical trials were released, including KEYNOTE-180 (30), KEYNOTE-181 (43) and ATTRACTION-03 (28). As revealed by KEYNOTE-180 and KEYNOTE-181, Pembrolizumab had remarkable therapeutic effect and favorable safety on patients with PD-L1(+) advanced esophageal carcinoma, supporting the application of Pembrolizumab as the new second-line standard treatment for PD-L1(+) metastatic esophageal carcinoma. In 2019, Pembrolizumab was approved by the USA FDA to be used to treat relapsed, locally advanced or metastatic ESCC patients who had received first-line or later-line systemic treatment, with positive PD-L1 expression in tumor tissues (CPS≥10). In 2020, a breakthrough was made in the immunotherapy for esophageal carcinoma. The preliminary research results from KEYNOTE-590 demonstrated the satisfactory therapeutic effect and safety of Pembrolizumab combined with chemotherapy in the first-line treatment for advanced esophageal carcinoma (44). In the 2020 V5 version of NCCN guidelines, Pembrolizumab combined with platinum-based chemotherapeutic regimens was recommended in the first-line treatment of unresectable, locally advanced, locally relapsed or metastatic esophageal carcinoma with PD-L1 CPS≥10 and negative HER-2 expression. Additionally, CheckMate-577 ushered in the new chapter of adjuvant immunotherapy for esophageal carcinoma, which comprehensively evaluated the therapeutic effect of adjuvant nivolumab on patients with esophageal carcinoma and gastroesophageal junction carcinoma who did not achieve complete pathological remission after neoadjuvant radiochemotherapy (NRCT) (45). Clinical trials of neoadjuvant immunotherapy, such as NICE, KEEP-G 03, and PALACE-1, also reported the preliminary results. In March 2021, based on the KEYNOTE-590 research results, the USA FDA approved the use of Pembrolizumab combined with platinum-based chemotherapy as the first-line treatment for unresectable locally advanced or metastatic esophageal carcinoma or gastrointestinal junction carcinoma or those not suitable for radical radiochemotherapy, regardless of the PD-L1 expression status. The results of neoadjuvant immunotherapy combined with chemotherapy or radiochemotherapy were also released in 2021. The rapid development of immunotherapy for esophageal cancer suggests the great potential of the field in the future. Given that the feasibility and safety of anti-PD-1/PD-L1 immunotherapy have been confirmed, there might be more publications in the following years. The development prospects of immunotherapy for esophageal cancer could be expected.
China was the top 1 country ranked by total publications, which was consistent with epidemiological status that the incidence rate and fatality rate were high in this country. Although China had a huge number of publications, its citation was not impressive. Germany and France with less publications had high ratio of Citations/Paper, reflecting the high quality of their publications. The USA was the most active collaborator in Figure 3 and played an important role in international cooperation.
As China is one of the high-risk areas of esophageal cancer, 6 of the top 10 institutions are from China. However, the articles published in China were scarcely cited, reflecting the weak influence of these publications. Therefore, Chinese institutions need to find methods to improve the quality of publications.
Natl Canc Ctr and Natl Canc Ctr Hosp East located in Japan were institutions that not only productive but also influential. They were both participating institutions of several important clinical trials, including KEYNOTE-180, KEYNOTE-181 and KEYNOTE-590. Ken Kato from National Cancer Center Hospital together with Toshihiko Doi and Takashi Kojima from National Cancer Center Hospital East were all contributed to the three clinical trials. They were also the top 3 prolific authors. Kato Ken who was the most prolific author ranked second in terms of co-cited authors, and he was a key figure of ATTRACTION-3. The top 10 prolific authors were all from Japan, indicating that Japanese scientists made a tremendous contribution in this field.
The analysis of prolific journals guides scientists in identifying core journals for information access and manuscript submissions. Three journals were highly recommended to scientists in the field: Frontiers in Oncology, Cancer Science and Journal for Immunotherapy of Cancer. Moreover, Journal of Clinical Oncology, Lancet Oncology, and New England Journal of Medicine, were the most authoritative journals in this field according to the co-citation amount shown in Table 3. As regard the subject categories shown in Figure 6 and Table 5, ONCOLOGY and IMMUNOLOGY occupied central positions in this field, which were consistent with the analyzing results of the journals.
The current research hotspots were obtained from the high frequency keywords and cited references, which helped researchers to rapidly understand the direction of the research. This work lists the remarkable highlights of the research field as follows.
Clinical significance had the highest burst strength among the 34 keywords. It has always been significant in the field from 2014 to 2018. During this period of time, many studies focused on the prognostic value of PD-1/PD-L1 in patients with esophageal cancer. Numerous studies have suggested that, PD-L1 expression is related to the adverse clinical outcomes of esophageal cancer, supporting its role as a prognostic biomarker (39, 46–49). Further study found that PD-L1 expression in ESCC tumor cells was significantly associated with worse survival while no statistical significance was found between PD-L1 expression in ESCC tumor-infiltrating immune cells and survival (50). Recently, Peipei Wang et al. claimed that increased co-expression of PD-L1 and TIM3/TIGIT was associated with poor overall survival of ESCC (51).
Neoadjuvant chemotherapy was the hottest keywords in the last two years. With the moving forward of immunotherapy, more and more publications about NCT or NRCT plus immunotherapy are available at present. Multiple studies have evaluated the safety, feasibility and efficacy of neoadjuvant PD-1/PD-L1 inhibitors combined with chemotherapy in treating esophageal cancer patients (52–62). The neoadjuvant treatment of PD-1/PD-L1 inhibitor with chemotherapy produced satisfactory outcomes, indicating its potential as a promising neoadjuvant treatment for esophageal cancer. Besides, Wenqun Xing et al. designed a study to explore the impact of chemotherapy and toripalimab sequence on the pathological complete response (pCR) rate and safety of locally advanced ESCC patients (63). The initial results showed that delaying toripalimab to day 3 in chemoimmunotherapy might achieve a higher pCR rate than that on the same day. In the PERFECT trial, we investigated the feasibility and efficacy of NCRT combined with PD-L1 inhibitor (64). However, most of these studies were single-arm, phase I or II clinical trials. The long-term efficiency of this novel treatment and the validity of the present findings should be confirmed with more large-scale, longer follow-up and prospective comparative trials. In the existing clinical trials on neoadjuvant immunotherapy for esophageal carcinoma, no definite molecular biomarkers are available for selecting the possibly beneficial population. The previous research mainly focused on PD-L1, TMB, EGFR and CD8+ T cells. These studies not only help to identify the molecular biomarkers, but also provide ideas for the design of phase III clinical trials. Further studies should confirm more predictive biomarkers as well as indicators for the selection of a specific treatment.
The application of PD-1/PD-L1 inhibitors in esophageal cancer has achieved unprecedented successes. However, some treated patients exhibit non-response and severe immune-related adverse events. Therefore, immunotherapeutic markers are needed to assist in screening populations who can gain benefits from immunotherapy. At present, PD-L1 expression is used as the major biomarker for efficacy prediction in the application of PD-L1 inhibitors (65). With the deepening of research, DNA mismatch repair-deficient/microsatellite instability (dMMR/MSI) (66), tumor mutational burden (TMB) (67), copy number variation (CNV), polymerase epsilon (POLE) (68, 69), circulating tumor DNA (ctDNA) (70), inflamed gene expression profile (71, 72), tumor-infiltrating lymphocytes (TILs) (73), and immune gene signatures (74) have been suggested to show certain potential in predicting efficacy, which deserve further verification. Park R et al. Mentioned that, for esophageal cancer, there is no highly sensitive or specific marker apart from dMMR/MSI-H (75). Consequently, it is necessary to develop biomarkers for immunotherapy.
For the time being, the sensitivity and specificity of single biomarkers are not high enough. As a result, they may not be used as biomarkers alone. The combination of multiple biomarkers contributes more to predicting the immunotherapeutic efficacy in esophageal cancer. Moreover, when immunotherapy is used in combination with other treatments, whether a specific biomarker can maintain its prediction ability should be further analyzed. Whether predictors verified in advanced tumors can be applied in perioperative treatment is another important problem to be answered by on-going and future trials. In the future, it is promising to develop more precise tools to predict the anti-PD-1/PD-L1 therapeutic efficacy in esophageal cancer patients by standardizing and normalizing diverse biomarkers, intensively investigating the relations of different biomarkers, and applying computer technologies and medical databases, which is of great significance for individualized immunotherapy.
As far as we know, this is the first bibliometric study regarding anti-PD-1/PD-L1 immunotherapy for esophageal cancer in the past decade. The article hopes to guide scholars select research direction, references, cooperative institutions and authoritative journals. The data analysis was relatively objective and comprehensive, clearly displaying the research status visually. However, here are some limitations as follows.
1. The study included articles and reviews retrieved from WoSCC. Articles of other types or from other databases could not be involved in our study, thus limit the comprehensiveness of the study.
2. Papers published from 2012 to 2021 were retrieved on June 20, 2022. However, the database is still updating the data. Therefore, some recent publications could not be included. Besides, the number of citations of recent literature might be affected.
3. All of the publications included were in English, which might lead to a linguistic bias. Languages like Chinese, Japanese, French, German, Polish, Hungarian, Portuguese, Rumanian and Korean were not involved in the database. Therefore, it is likely that our results may not be applicable to publications in other languages.
4. Although analysis process was performed by software objectively, the method to explain these results had inherent subjective bias by individuals.
Still, it is believed that this article provides the overall situation and research trend of anti-PD-1/PD-L1 immunotherapy for esophageal cancer. The results could provide readers a general overview of the landscape, especially to those without in-depth knowledge. The information could also be used to explore possible collaboration partners, potentially relevant publications, and promising research directions. Our study not only exhibits important milestones of esophageal cancer immunotherapy but also offers a better guide to the future. We sincerely hope that bibliometric and visual analyses will give us more ideas in this field.
To conclude, this article provides a comprehensive understanding of publications on anti-PD-1/PD-L1 immunotherapy for esophageal cancer from 2012 to 2021, providing valuable information to researchers in this field. This article presents data on the trend of annual output, countries/regions, institutions, journals, authors, subject categories, keywords, and co-cited references obtained using bibliometric analysis. Neoadjuvant chemotherapy, response, PD-1 blockade and CD8+ T cell were four latest research frontiers. Further studies and more cooperation are needed worldwide. Overall, our results could help the discovery of new perspectives and determine future directions.
Data were retrieved from 552 publications with VOSviewer on June 20, 2022. The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ supplementary material.
Study conception, design and data analysis: YY. Paper writing: YY. Language polishing, paper review and editing: FW. All authors read and approved the submitted version. All authors contributed to the article and approved the submitted version.
This study was supported by Health Training Program Foundation for Young and Middle-Aged Innovative Talents of Science and Technology (YXKC2020017) and Programs for Medical Science and Technology Development of Henan Province of China (SBGJ202002080).
We thank Danyang Chen for assistance with manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology (2022) 163(3):649–58.e2. doi: 10.1053/j.gastro.2022.05.054
3. Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992-2013. Cancer Epidemiol Biomarkers Prev (2017) 26(4):632–41. doi: 10.1158/1055-9965.EPI-16-0520
4. Enomoto N, Yamada K, Terayama M, Kato D, Yagi S, Wake H, et al. Current status of immune checkpoint inhibitor therapy for advanced esophageal squamous cell carcinoma. Glob Health Med (2021) 3(6):378–85. doi: 10.35772/ghm.2020.01112
5. Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg (2018) 41(3):210–5. doi: 10.1016/j.asjsur.2016.10.005
6. Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today (2020) 50(1):12–20. doi: 10.1007/s00595-019-01878-7
7. Huang R, Qiu Z, Zheng C, Zeng R, Chen W, Wang S, et al. Neoadjuvant therapy for locally advanced esophageal cancers. Front Oncol (2022) 12:734581. doi: 10.3389/fonc.2022.734581
8. Alsina M, Moehler M, Lorenzen S. Immunotherapy of esophageal cancer: Current status, many trials and innovative strategies. Oncol Res Treat (2018) 41(5):266–71. doi: 10.1159/000488120
9. Ozen Cinar I. Bibliometric analysis of breast cancer research in the period 2009-2018. Int J Nurs Pract (2020) 26(3):e12845. doi: 10.1111/ijn.12845
10. Miao Y, Zhang Y, Yin L. Trends in hepatocellular carcinoma research from 2008 to 2017: a bibliometric analysis. PeerJ (2018) 6:e5477. doi: 10.7717/peerj.5477
11. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
12. Wrigley J, Carden V, von Isenburg M. Bibliometric mapping for current and potential collaboration detection. J Med Libr Assoc (2019) 107(4):597–600. doi: 10.5195/jmla.2019.764
13. Zhai F, Zhai Y, Cong C, Song T, Xiang R, Feng T, et al. Research progress of coronavirus based on bibliometric analysis. Int J Environ Res Public Health (2020) 17(11):3766. doi: 10.3390/ijerph17113766
14. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, scopus, web of science, and Google scholar: strengths and weaknesses. FASEB J (2008) 22(2):338–42. doi: 10.1096/fj.07-9492LSF
15. Balel Y, Tumer MK. A bibliometric analysis of international publication trends in total temporomandibular joint replacement research (1986-2020). J Oral Maxillofac Surg (2021) 79(7):1458.e1–12. doi: 10.1016/j.joms.2021.02.038
16. Chen D, Zhang G, Wang J, Chen S, Wang J, Nie H, et al. Mapping trends in moyamoya angiopathy research: A 10-year bibliometric and visualization-based analyses of the web of science core collection (WoSCC). Front Neurol (2021) 12:637310. doi: 10.3389/fneur.2021.637310
17. Garfield E, Paris SW, Stock WG. HistCiteTM: a software tool for informetric analysis of citation linkage. Information-Wissenschaft und Praxis (2006) 57:391–400.
18. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
19. Chen. C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol (2006) 57(3):359–77. doi: 10.1002/asi.20317
20. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA (2004) 101 Suppl 1:5303–10. doi: 10.1073/pnas.0307513100
21. Liang YD, Li Y, Zhao J, Wang XY, Zhu HZ, Chen XH. Study of acupuncture for low back pain in recent 20 years: a bibliometric analysis via CiteSpace. J Pain Res (2017) 10:951–64. doi: 10.2147/JPR.S132808
22. Zheng K, Wang X. Publications on the association between cognitive function and pain from 2000 to 2018: A bibliometric analysis using CiteSpace. Med Sci Monit (2019) 25:8940–51. doi: 10.12659/MSM.917742
23. Lu C, Li X, Yang K. Trends in shared decision-making studies from 2009 to 2018: A bibliometric analysis. Front Public Health (2019) 7:384. doi: 10.3389/fpubh.2019.00384
24. Miao Y, Liu R, Pu Y, Yin L. Trends in esophageal and esophagogastric junction cancer research from 2007 to 2016: A bibliometric analysis. Med (Baltimore) (2017) 96(20):e6924. doi: 10.1097/MD.0000000000006924
25. Huang X, Fan X, Ying J, Chen S. Emerging trends and research foci in gastrointestinal microbiome. J Transl Med (2019) 17(1):67. doi: 10.1186/s12967-019-1810-x
26. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
27. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
28. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
29. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/S1470-2045(17)30181-X
30. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441
31. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
32. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
33. Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res (2005) 11(8):2947–53. doi: 10.1158/1078-0432.CCR-04-1469
34. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X
35. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
36. Gao Y, Shi S, Ma W, Chen J, Cai Y, Ge L, et al. Bibliometric analysis of global research on PD-1 and PD-L1 in the field of cancer. Int Immunopharmacol (2019) 72:374–84. doi: 10.1016/j.intimp.2019.03.045
37. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther (2014) 14(9):1295–317. doi: 10.1517/14712598.2014.920813
38. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol (2018) 36(1):61–7. doi: 10.1200/JCO.2017.74.9846
39. Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget (2017) 8(18):30175–89. doi: 10.18632/oncotarget.15621
40. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg (2021) 161(3):836–43.e1. doi: 10.1016/j.jtcvs.2020.11.106
41. Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ Book (2017) 37:292–300. doi: 10.1200/EDBK_175231
42. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res (2018) 24(6):1296–304. doi: 10.1158/1078-0432.CCR-17-2439
43. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
44. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
45. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
46. Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Paireder M, et al. Expression of programmed cell death protein 1 by tumor-infiltrating lymphocytes and tumor cells is associated with advanced tumor stage in patients with esophageal adenocarcinoma. Ann Surg Oncol (2017) 24(9):2698–706. doi: 10.1245/s10434-017-5858-7
47. Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Baierl A, et al. PD-L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology (2018) 7(6):e1435226. doi: 10.1080/2162402X.2018.1435226
48. Akutsu Y, Murakami K, Kano M, Toyozumi T, Matsumoto Y, Takahashi M, et al. The concentration of programmed cell death-ligand 1 in the peripheral blood is a useful biomarker for esophageal squamous cell carcinoma. Esophagus (2018) 15(2):103–8. doi: 10.1007/s10388-018-0604-1
49. Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg (2019) 269(3):471–8. doi: 10.1097/SLA.0000000000002616
50. Rong L, Liu Y, Hui Z, Zhao Z, Zhang Y, Wang B, et al. PD-L1 expression and its clinicopathological correlation in advanced esophageal squamous cell carcinoma in a Chinese population. Diagn Pathol (2019) 14(1):6. doi: 10.1186/s13000-019-0778-4
51. Wang P, Chen Y, Long Q, Li Q, Tian J, Liu T, et al. Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J Immunother Cancer (2021) 9(10):e002836. doi: 10.1136/jitc-2021-002836
52. Huang B, Shi H, Gong X, Yu J, Xiao C, Zhou B, et al. Comparison of efficacy and safety between pembrolizumab combined with chemotherapy and simple chemotherapy in neoadjuvant therapy for esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(5):2013–21. doi: 10.21037/jgo-21-610
53. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(3):e004291. doi: 10.1136/jitc-2021-004291
54. Duan H, Shao C, Pan M, Liu H, Dong X, Zhang Y, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: An open-label, single-arm study (PEN-ICE). Front Immunol (2022) 13:849984. doi: 10.3389/fimmu.2022.849984
55. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer (2022) 10(1):e003497. doi: 10.1136/jitc-2021-003497
56. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death-1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med (2021) 9(15):1254. doi: 10.21037/atm-21-3352
57. Cheng J, Guo M, Yang Y, Liu Y, Hu W, Shang Q, et al. Perioperative outcomes of minimally invasive esophagectomy after neoadjuvant immunotherapy for patients with locally advanced esophageal squamous cell carcinoma. Front Immunol (2022) 13:848881. doi: 10.3389/fimmu.2022.848881
58. Hong ZN, Gao L, Weng K, Huang Z, Han W, Kang M. Safety and feasibility of esophagectomy following combined immunotherapy and chemotherapy for locally advanced esophageal squamous cell carcinoma: A propensity score matching analysis. Front Immunol (2022) 13:836338. doi: 10.3389/fimmu.2022.836338
59. Gu YM, Shang QX, Zhang HL, Yang YS, Wang WP, Yuan Y, et al. Safety and feasibility of esophagectomy following neoadjuvant immunotherapy combined with chemotherapy for esophageal squamous cell carcinoma. Front Surg (2022) 9:851745. doi: 10.3389/fsurg.2022.851745
60. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol (2021) 12(1):1–10. doi: 10.21037/jgo-20-599
61. Gao L, Lu J, Zhang P, Hong ZN, Kang M. Toripalimab combined with docetaxel and cisplatin neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma: a single-center, single-arm clinical trial (ESONICT-2). J Gastrointest Oncol (2022) 13(2):478–87. doi: 10.21037/jgo-22-131
62. He W, Leng X, Mao T, Luo X, Zhou L, Yan J, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. Oncologist (2022) 27(1):e18–28. doi: 10.1093/oncolo/oyab011
63. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-a phase II study. Front Immunol (2021) 12:772450. doi: 10.3389/fimmu.2021.772450
64. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443
65. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther (2015) 14(4):847–56. doi: 10.1158/1535-7163.MCT-14-0983
66. Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: A predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol (2018) 26(2):e15–21. doi: 10.1097/PAI.0000000000000575
67. Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell (2021) 39(2):154–73. doi: 10.1016/j.ccell.2020.10.001
68. Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol (2019) 5(10):1504–6. doi: 10.1001/jamaoncol.2019.2963
69. He J, Ouyang W, Zhao W, Shao L, Li B, Liu B, et al. Distinctive genomic characteristics in POLE/POLD1-mutant cancers can potentially predict beneficial clinical outcomes in patients who receive immune checkpoint inhibitor. Ann Transl Med (2021) 9(2):129. doi: 10.21037/atm-20-7553
70. Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: A broad overview. Crit Rev Oncol Hematol (2020) 155:103109. doi: 10.1016/j.critrevonc.2020.103109
71. Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-Cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol (2019) 37(4):318–27. doi: 10.1200/JCO.2018.78.2276
72. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science (2018) 362(6411):eaar3593. doi: 10.1126/science.aar3593
73. Li F, Li C, Cai X, Xie Z, Zhou L, Cheng B, et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine (2021) 41:101134. doi: 10.1016/j.eclinm.2021.101134
74. Sundar R, Smyth EC, Peng S, Yeong JPS, Tan P. Predictive biomarkers of immune checkpoint inhibition in gastroesophageal cancers. Front Oncol (2020) 10:763. doi: 10.3389/fonc.2020.00763
Keywords: bibliometrics, anti-PD-1/PD-L1, immunotherapy, esophageal cancer, CiteSpace, HistCite, VOSviewer, Web of Science (WOS)
Citation: Yang Y and Wang F (2022) Research trends on anti-PD-1/PD-L1 immunotherapy for esophageal cancer: A bibliometric analysis. Front. Oncol. 12:983892. doi: 10.3389/fonc.2022.983892
Received: 01 July 2022; Accepted: 31 October 2022;
Published: 17 November 2022.
Edited by:
Yibo Fan, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jinbo Zhao, Tangdu Hospital, ChinaCopyright © 2022 Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, ZmVuZ3cwMTBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.