
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 24 October 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.983585
This article is part of the Research Topic Case Reports in Hematological Malignancies : 2022 View all 34 articles
Angioimmunoblastic T-cell lymphoma (AITL) is a common type of nodal peripheral T-cell lymphoma, which always presents with extensive follicular dendritic cell (FDC) meshwork. Here, we report a case of AITL combined with extensive spindle cell meshwork. Spindle cells occupied were positive for the FDC markers CD21, CD23, and CD35. Furthermore, some cells were positive for desmin and smooth muscle actin (SMA), suggesting the differentiation of fibroblastic reticular cell (FRC). Interestingly, the proliferation of spindle cells was so extensive that was easily misdiagnosed as FDC sarcoma (FDCS). Next-generation sequencing showed that the common mutations reported in AITL, including RHOA, TET2, and IDH2, were also detected in this case, while the genes that are recurrently mutated in FDCS were not detected. Regrettably, the patient died 19 months later. Overall, we highlight the unusual morphologic features in an AITL patient with extensive FDC and FRC network that may be misdiagnosed as FDCS, and careful morphological observation and immunochemical and molecular examinations are crucial for an accurate diagnosis.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare aggressive malignant tumor derived from mature T-follicular helper (TFH) cells. It is the second most common type of peripheral T-cell lymphomas (PTCLs) and accounts for about 20% (1). Patients with AITL are always diagnosed with advanced stages, and the 5-year survival is only about 30% (2). Generally, the diagnosis of AITL is confirmed by experienced pathologists according to the results of biopsies. The lymph node architecture is partially or totally effaced by small to medium atypical T cells that are typically positive for CD2, CD3, CD4, CD5, CD10, BCL6, C-X-C motif chemokine ligand 13 (CXCL13), ICOS, and programmed cell death 1 (PD-1). The neoplastic cells always congregate near high endothelial venules and are surrounded by expended follicular dendritic cell (FDC) meshwork and inflammatory cell infiltrates (3). However, a small subset of AITL cases still remains difficult to diagnose, and exploitation of the potent supplementary diagnostic methods is of importance.
Nodal FDC sarcoma (FDCS) is a very rare entity usually affecting cervical and abdominal lymph nodes and is generally considered as an indolent tumor with weak aggressiveness (4). Expansion of FDC is common in AITL patients, but discrimination of the unusual expanded FDC meshwork and FDCS is quite difficult. Previously, Benharroch et al. (5) reported a Jewish case who presented with the combination of AITL and FDCS, but the possibility of AITL with an excessively expanded FDC meshwork could not be excluded by the author based on the results of histological examination. Rogges et al. (6) recently reported an Italian patient diagnosed with AITL and FDC expansion, who excluded FDCS based on the results of histopathology and mutation profile. Similarly, we report a Chinese female patient who was diagnosed with AITL with extensive FDC networks mimicking FDCS. She progressed to recurrence and refractory disease and died from AITL after 19 months of the diagnosis.
A 40–45-year-old woman arrived for consultation with facial swelling that affected eating and breathing as well as the palpable “nodules” in both groins in June 2017. Figure 1 summarizes the important events of this patient according to the timeline. Generalized lymphadenopathy and multiple nodules in the bilateral parotid glands [standard uptake value (SUV)max 17.2] were found, as examined by the PET-CT. However, nasopharyngeal biopsy and right cervical lymph node fine-needle aspiration showed no evidence of tumor. In addition, no monoclonal bands were found as detected by serum immunofixation electrophoresis. Thus, she was clinically diagnosed as having plasmacytosis and given prednisone and methotrexate. Meanwhile, she received Chinese herbal medicine from another hospital. Cervical lymph node enlargement regressed, while the bilateral inguinal lymph node size remained unchanged after the treatment.
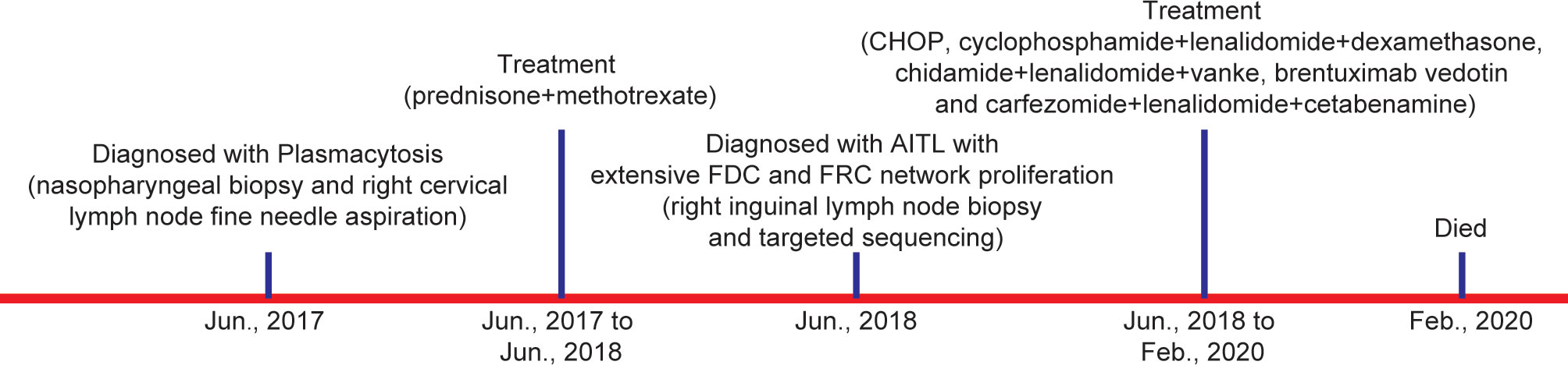
Figure 1 Diagnosis and treatment process of the patient. (AITL, angioimmunoblastic T-cell lymphoma; FDC, follicular dendritic cell; FRC, fibroblastic reticular cell).
One year later, the patient presented with significantly enlarged inguinal lymph nodes on both sides, increased local skin temperature, local tenderness, surface redness, and left lower extremity edema. A right inguinal lymph node biopsy was carried out. The normal structure of the lymph node was effaced (Figure 2A), with hyperplasia of several kinds of cells and perinodal infiltration. In most of the areas, the proliferation of spindle cells was dominant, with vortex distribution (Figure 2B). Some areas were dominated by medium-sized lymphoid tumor cells with mild atypia, unclear cell boundary, and abundant pale cytoplasm (Figure 2C). Affluent branching of high endothelial small vessels, mixed with small lymphocytes, plasma cells (Figure 2D), eosinophils, and neutrophils was seen in the background (Figure 2E), together with few scattered large immunoblastic cells (Figure 2F). The immunohistochemistry (IHC) staining demonstrated that the spindle cells were positive for complement C3d receptor 2 (CD21) (Figure 3A), CD23 (Figure 3B), CD35 (Figure 3C), and desmin (Figure 3D) and partially positive for SMA (Figure 3E). The lymphoid tumor cells were positive for CD3 (Figure 4A), CD4, CD10 (Figure 4B), PD-1 (Figure 4C), CXCL13 (Figure 4D), and T cell receptor, bF1 (TCRbF1) and negative for CD20, CD8, and T cell receptor, g (TCRg). The Ki-67 index of the tumor cells was about 60%, while majority of the spindle cells were negative for Ki-67 staining (Figures 4E, F). In situ hybridization for Epstein-Barr virus (EBV)-encoded small RNA (EBER) showed that a few scattered immunoblastic large cells were positive for EBER (Figure 4G). Clonal rearrangement of TCRβ gene was detected (Figure 4H).
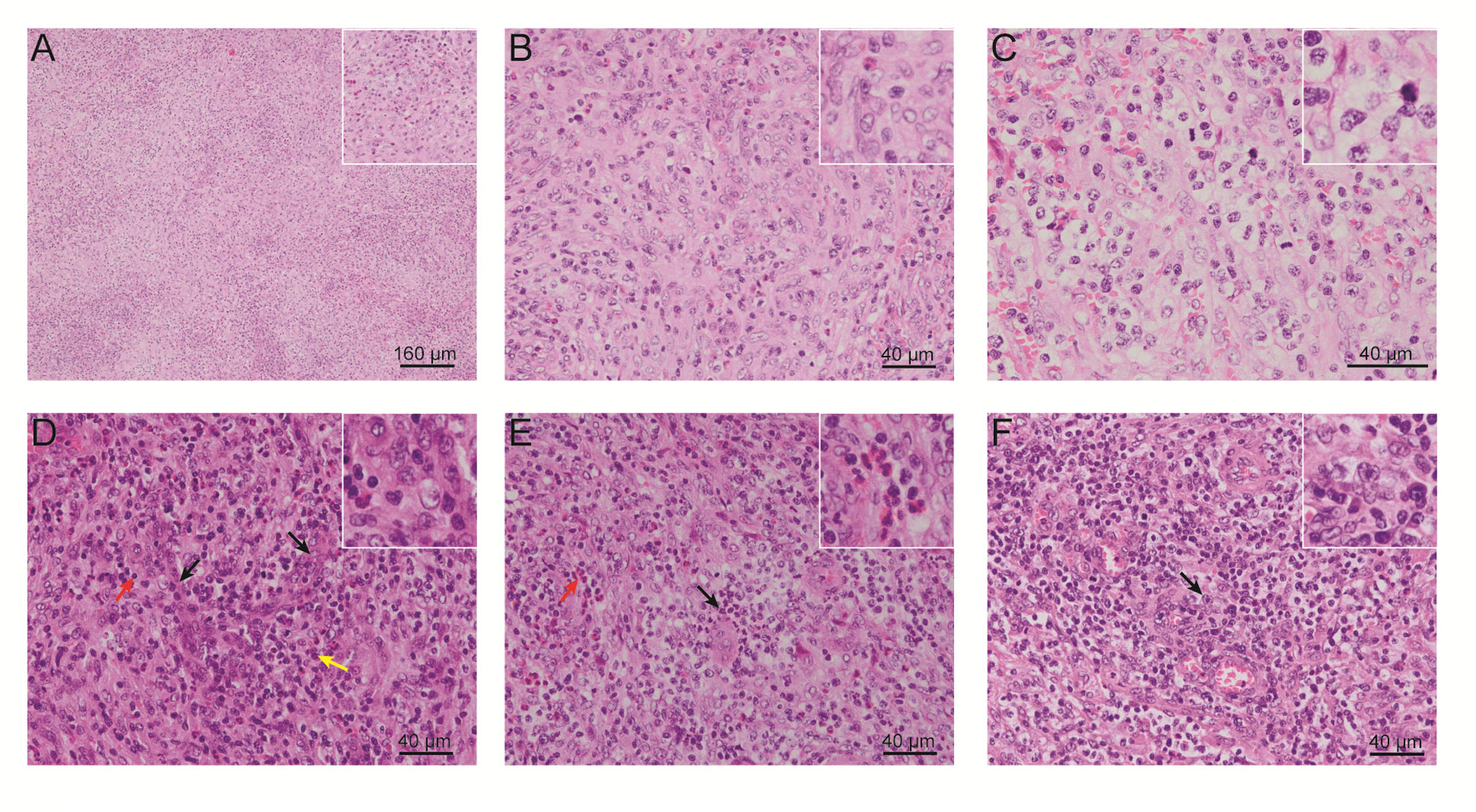
Figure 2 Representative H&E-stained images of the patient’s right inguinal lymph node. (A) Cell hyperplasia and perinodal infiltration. (B) Vortex distribution of spindle cells. (C) Medium-sized lymphoid tumor cells with mild atypia, unclear cell boundary, and abundant pale cytoplasm. Abundant branching of high endothelial small vessels, mixed with (D) small lymphocytes and plasma cells, (E) eosinophils and neutrophils, and (F) few scattered large immunoblastic cells. (H&E, Eosin & Hematoxylin).
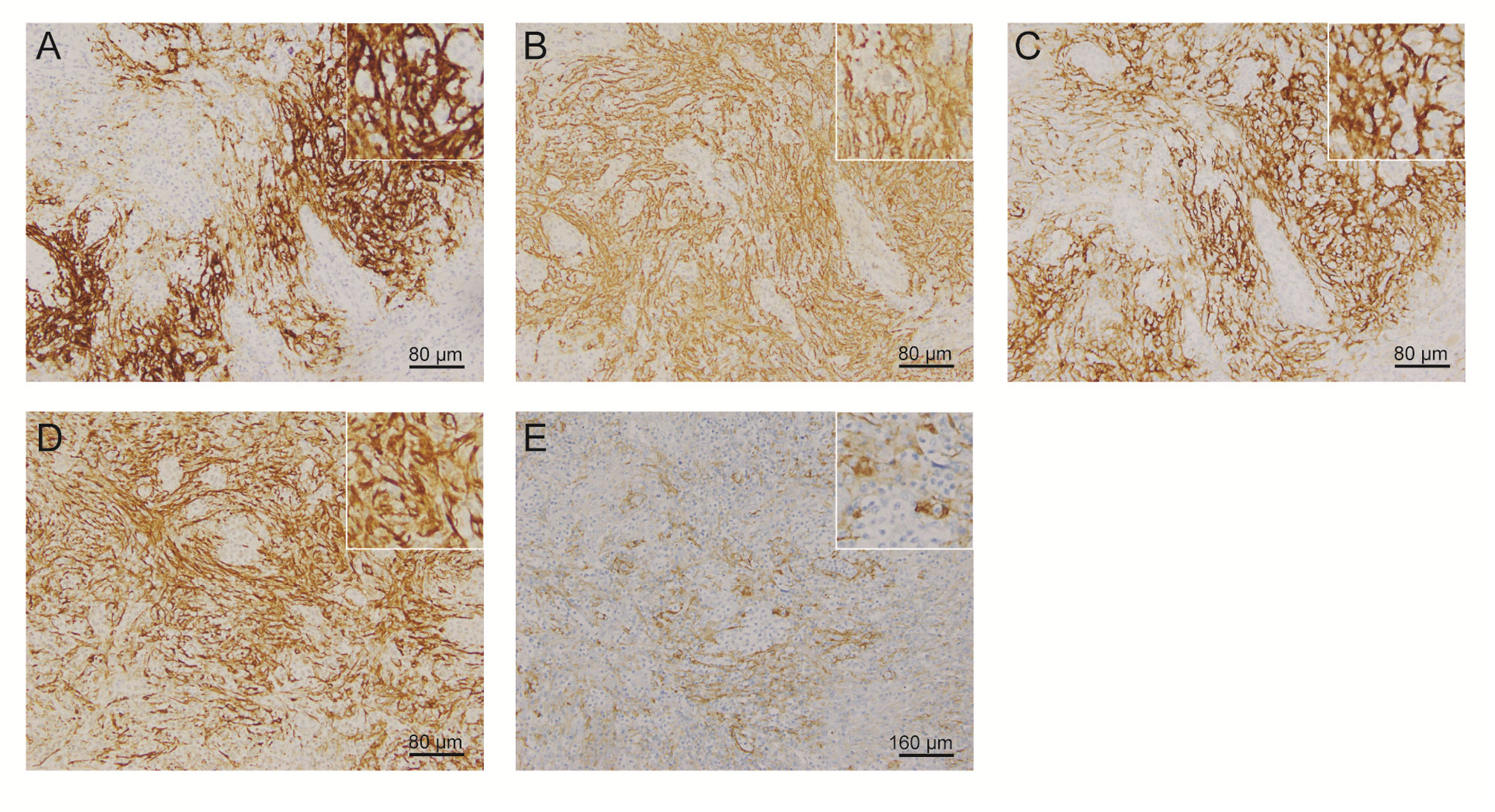
Figure 3 Representative IHC staining images of spindle cells. Spindle cells were positive for (A) CD21, (B) CD23, (C) CD35, (D) desmin, and (E) SMA. (IHC, immunohistochemistry; SMA, smooth muscle actin).
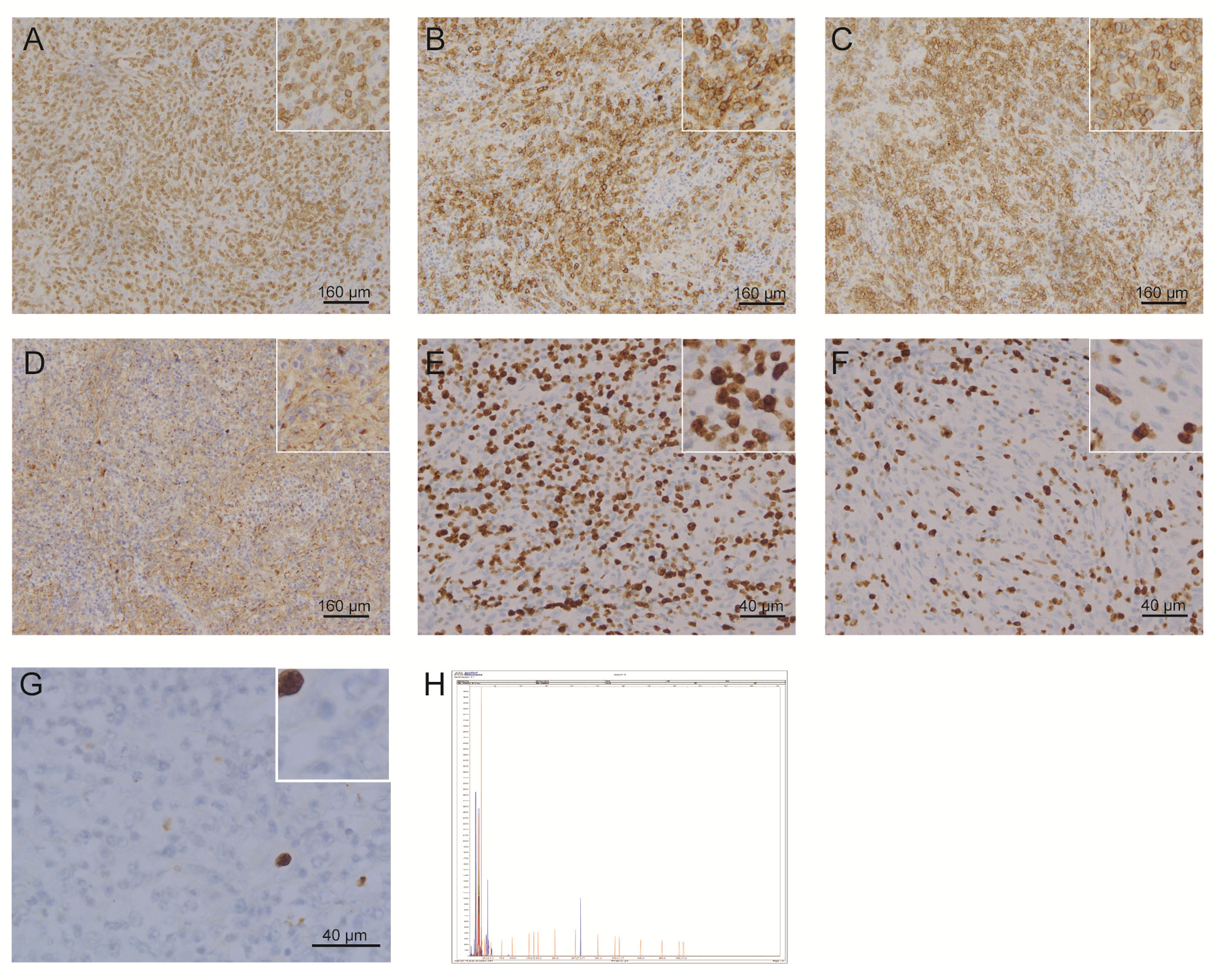
Figure 4 Representative IHC staining images and detection of clonal rearrangement of the TCR gene in lymphoid tumor cells. Lymphoid tumor cells were positive for (A) CD3, (B) CD10, (C) PD-1, and (D) CXCL13. (E, F) Lymphoid tumor cells were positive for Ki-67, while most of the spindle cells were negative for Ki-67 staining. (G) A few scattered immunoblastic large cells were positive for EBER. (H) Clonal rearrangement of TCRβ was observed. (IHC, immunohistochemistry; TCR, T cell receptor; PD-1, programmed cell death 1; CXCL13, C-X-C motif chemokine ligand 13; EBER, Epstein-Barr virus encoded small RNA).
To further describe the characteristics of this case, we also carried out next-generation sequencing (DNA sequencing) to detect the mutation profile of the lymph node sample. Mutations of 571 lymphoma-related genes (Supplementary Table S1) were detected on Novaseq (Illumina, San Diego, CA, USA). Variants, including single-nucleotide variations (SNVs) and Indels (insertion and deletion), were screened by Shanghai Rightongene Biotechnology Co., Ltd. (Shanghai, China), based on the following filter conditions: 1) SNVs or Indels with a variant allele frequency (VAF) ≥1% were retained; 2) SNVs or Indels with a mutation allele frequency (MAF) ≥0.001 in databases of the 1000 Genomes (1KG) Project (7), ExAC_ALL, and ExAC_EAS (8) were removed; 3) SNVs or Indels including stopgain, stoploss, frameshift, non-frameshift, and splicing sites were retained; 4) missense variants that are predicted to be deleterious by SIFT and PolyPhen-2 were retained. According to this, mutations in RHOA, TET2, IDH2, ERG, JAK1, EGFR, MUC4, MYH11, PKP2, ARHGAP29, CCND1, and SAMD9 genes were detected (Table 1).
Based on the results of histological examination and mutational characteristics, the diagnosis of AITL (at a stage of IV) with extensive FDC and fibroblastic reticular cell (FRC) network proliferation was confirmed in July 2018.
The anti-AITL therapeutic schedules for this patient were complex (Figure 1). First, she received CHOP (cyclophosphamide, Adriamycin, vincristine, and prednisone) combined with 20 mg chidamide for six cycles. After the first cycle of chemotherapy, skin pruritus was relieved, as well as the swelling of the left lower extremity. However, the disease progressed. Then, she was injected with gemcitabine hydrochloride. Two cycles later, the CT examination indicated that she achieved stable disease (SD). Unluckily, the patient developed fever and infection and was treated with moxifloxacin. Also, she was given cyclophosphamide, lenalidomide, and dexamethasone owing to the increased pleural effusion and poor mental state. Following one cycle of the treatment, she achieved SD but had a cough that was so frequent to lie at night. Then, the treatment regimen was changed into chidamide, lenalidomide, and Velcade. Disappointingly, the disease progressed again, and brentuximab vedotin (a monoclonal antibody of CD30) was given. Three cycles later, the symptoms were not improved, and multiple lung lesions in both lungs were detected. Based on this, she was diagnosed as having recurrent refractory AITL. Subsequently, salvage therapy (carfezomide, lenalidomide, and cetabenamine) was recommended. Cough and sputum were improved after the treatment. Regrettably, she died from AITL in February 2020.
In the present study, we reported a Chinese female patient who was diagnosed as having AITL combined with extensive spindle cell network. This patient was diagnosed as having plasmacytosis without detectable tumor cells 1 year before the diagnosis. Several research groups assessed the behavior of plasma cells in AITL, which ranged from reactive plasmacytosis to striking clonal proliferation (9–11). The performance status of AITL patients with exuberant polyclonal plasmacytosis was significantly worse compared to those in AITL patients without exuberant polyclonal plasmacytosis, but patients with plasmacytosis responded well to chemotherapy and immunosuppressants and had a favorable outcome (10). This case was biopsied twice at the initial onset of disease. However, the nasopharyngeal site was often infected and inguinal lymph nodes presented with more necrotic area, which affected the diagnosis. Thus, how to take biopsy to improve the diagnostic success rate still remains a big challenge for clinicians.
It was uncertain whether the extensive proliferation of spindle cells was an accompanying tumor or not at first. The proliferation of spindle cells was more extensive than other AITL cases, which was easily misdiagnosed as FDCS. In this case, the morphology of spindle cells was gentle without atypia and mitosis, and most of the spindle cells were negative for Ki-67 staining while the Ki-67 index of tumor cells was about 60%. Thus, FDCS was excluded for which the Ki-67 index was always less than 30%. Moreover, parts of the spindle cells expressed desmin and SMA, suggesting that FRCs existed. Similarly, the proliferation of both FDC and FRC in AITL has been reported by Jones et al. (12). Recently, Benharroch et al. (5) reported a Jewish case who was diagnosed with the combination of AITL and FDCS only based on the histopathology. However, the authors acknowledged that they cannot provide sufficient evidence to exclude excessive FDC meshwork proliferation from FDCS. Also, Starkey et al. (13) described an elderly male patient with peripheral T-cell lymphoma (PTCL) accompanied by an excessive FDC network mimicking FDCS. Such cases of extensive FDC hyperplasia challenge pathological examination alone.
Next-generation sequencing emerges as a reliable method for the adjuvant diagnosis of lymphoma. It has been demonstrated that TET2, RHOAG17V, DNMT3A, and IDH2 are the most common mutated genes in AITL, with a mutation frequency of 80% (14–18), 50%–70% (15–19), 20%–40% (15–18, 20), and 20%–30% (15–18, 21), respectively. Among them, TET2, DNMT3A, and IDH2 are three genes encoding epigenetic enzymes (18). RHOA encodes a small GTPase in either active GTP-bound or inactive GDP-bound forms. The mutation of RHOAG17V induces the phosphorylation of VAV1 (a guanine exchange factor) and then promotes the enhancement of T-cell receptor (TCR) signaling in vitro (22). In this case, mutations in TET2, RHOAG17V, and IDH2 genes were detected, further confirming the diagnosis of AITL.
Recently, Rogges et al. (6) reported an expansion of FDC in a patient with AITL based on the results of both histopathology and mutational profile. Mutations in genes (i.e., RHOA, DNMT3A, TET2, and IDH2) associated with AITL were detected, while the mutations in genes recurrent in FDCS, such as the nuclear factor (NF)κB pathway-involving genes (BIRC3, NFKBIA, TRAF3, SOCS3, CYLD, and TNFAIP3) and tumor suppressor genes (CDKN2A, RB1, and TP53) (23), were detected. Consistently, mutations in RHOA, TET2, IDH2, ERG, JAK1, EGFR, MUC4, MYH11, PKP2, ARHGAP29, CCND1, and SAMD9 genes were detected in our patient, none of which was involved in FDCS. These results emphasize the importance of next-generation sequencing in the diagnosis of lymphoma, as well as the differential diagnosis of the expanded FDC meshwork and FDCS.
This patient received various anticancer regimens, including CHOP, gemcitabine hydrochloride, chidamide, lenalidomide, Velcade, and monoclonal antibodies against CD30. However, the curative effect was unsatisfactory, the disease progressed, and the patient died, suggesting a poor prognosis of this case with AITL and extensive FDC and FRC networks.
Taken together, this study described a rare case of AITL combined with extensive FDC and FRC networks mimicking FDCS in a female patient. This case highlights the heterogeneity of the clinical presentation of AITL, and a combination of histological examination and next-generation sequencing may help the clinical diagnosis and initiate treatment of this kind of disease. It is crucial to include more of the same cases to summarize the features of AITL combined with extensive FDC and FRC networks and thereafter to develop efficient treatment methods.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Guangdong Provincial People’s Hospital/Guangdong Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL, FZ, and WL contributed to the study conception and design. Material preparation, data collection and analysis were performed by YL, FZ, WL, QC, YC, and YL. The first draft of the manuscript was written by FZ and WL. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Natural Science Foundation of Guangdong Province (no. 2019A1515011643).
We thank Shanghai Rightongene Biotechnology Co. Ltd. (Shanghai, China) for assisting in molecular detection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.983585/full#supplementary-material
1. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural Killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol: Off J Am Soc Clin Oncol (2008) 26(25):4124–30. doi: 10.1200/JCO.2008.16.4558
2. Bellei M, Federico M. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: A report from the prospective international T-cell project. Haematologica (2019) 104(4):e178. doi: 10.3324/haematol.2019.218305
3. Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract (2019) 15(3):137–43. doi: 10.1200/JOP.18.00511
4. Dalia S, Jaglal M, Chervenick P, Cualing H, Sokol L. Clinicopathologic characteristics and outcomes of histiocytic and dendritic cell neoplasms: The Moffitt cancer center experience over the last twenty five years. Cancers (2014) 6(4):2275–95. doi: 10.3390/cancers6042275
5. Benharroch D, Zekzer M, Nalbandyan K. Angioimmunoblastic T-cell lymphoma: A questionable association with follicular dendritic cell sarcoma. Case Rep Hematol (2017) 2017:9601094. doi: 10.1155/2017/9601094
6. Rogges E, Pelliccia S, Lopez G, Barresi S, Tafuri A, Alaggio R, et al. Angioimmunoblastic T-cell lymphoma with exuberant Cd30-positive follicular dendritic cell proliferation in a sars-Cov-2 patient: The role of mutational analysis to exclude an associated follicular dendritic cell sarcoma. Int J Mol Sci (2022) 23(16):9346. doi: 10.3390/ijms23169349
7. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature (2010) 467(7319):1061–73. doi: 10.1038/nature09534
8. Zou J, Valiant G, Valiant P, Karczewski K, Chan SO, Samocha K, et al. Quantifying unobserved protein-coding variants in human populations provides a roadmap for Large-scale sequencing projects. Nat Commun (2016) 7:13293. doi: 10.1038/ncomms13293
9. Singh N, Sharma A, Pasricha S, Agrawal N, Bhurani D, Gupta G, et al. Florid plasmacytosis in angioimmunoblastic T cell lymphoma: A diagnostic conundrum. Indian J Hematol Blood Transfusion: Off J Indian Soc Hematol Blood Transfusion (2018) 34(1):188–90. doi: 10.1007/s12288-017-0824-x
10. Nagoshi H, Kuroda J, Kobayashi T, Maegawa S, Chinen Y, Kiyota M, et al. Clinical manifestation of angioimmunoblastic T-cell lymphoma with exuberant plasmacytosis. Int J Hematol (2013) 98(3):366–74. doi: 10.1007/s12185-013-1411-z
11. Sokol K, Kartan S, Johnson WT, Alpdogan O, Nikbakht N, Haverkos BM, et al. Extreme peripheral blood plasmacytosis mimicking plasma cell leukemia as a presenting feature of angioimmunoblastic T-cell lymphoma (Aitl). Front Oncol (2019) 9:509. doi: 10.3389/fonc.2019.00509
12. Jones D, Jorgensen JL, Shahsafaei A, Dorfman DM. Characteristic proliferations of reticular and dendritic cells in angioimmunoblastic lymphoma. Am J Surg Pathol (1998) 22(8):956–64. doi: 10.1097/00000478-199808000-00005
13. Starkey CR, Corn AI, Porensky RS, Viswanatha D, Wilson CS. Peripheral T-cell lymphoma with extensive dendritic cell network mimicking follicular dendritic cell tumor: A case report with pathologic, immunophenotypic, and molecular findings. Am J Clin Pathol (2006) 126(2):230–4. doi: 10.1309/Q1YK-AU1X-XEN3-NVKQ
14. Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, et al. Tet2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell (2011) 20(1):25–38. doi: 10.1016/j.ccr.2011.06.003
15. Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, et al. Somatic rhoa mutation in angioimmunoblastic T cell lymphoma. Nat Genet (2014) 46(2):171–5. doi: 10.1038/ng.2872
16. Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood (2014) 123(9):1293–6. doi: 10.1182/blood-2013-10-531509
17. Wang C, McKeithan TW, Gong Q, Zhang W, Bouska A, Rosenwald A, et al. Idh2r172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood (2015) 126(15):1741–52. doi: 10.1182/blood-2015-05-644591
18. Chiba S, Sakata-Yanagimoto M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia (2020) 34(10):2592–606. doi: 10.1038/s41375-020-0990-y
19. Yoo HY, Sung MK, Lee SH, Kim S, Lee H, Park S, et al. A recurrent inactivating mutation in rhoa gtpase in angioimmunoblastic T cell lymphoma. Nat Genet (2014) 46(4):371–5. doi: 10.1038/ng.2916
20. Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, rhoa and fyn kinase in peripheral T cell lymphomas. Nat Genet (2014) 46(2):166–70. doi: 10.1038/ng.2873
21. Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, et al. Idh2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood (2012) 119(8):1901–3. doi: 10.1182/blood-2011-11-391748
22. Fujisawa M, Sakata-Yanagimoto M, Nishizawa S, Komori D, Gershon P, Kiryu M, et al. Activation of rhoa-Vav1 signaling in angioimmunoblastic T-cell lymphoma. Leukemia (2018) 32(3):694–702. doi: 10.1038/leu.2017.273
Keywords: angioimmunoblastic T-cell lymphoma, follicular dendritic cell, mutation, diagnosis, morphology
Citation: Zhang F, Li W, Cui Q, Chen Y and Liu Y (2022) Angioimmunoblastic T-cell lymphoma with extensive follicular dendritic cell and fibroblastic reticular cell network proliferation mimicking follicular dendritic cell sarcoma: A case report with pathologic, immunophenotypic, and molecular findings. Front. Oncol. 12:983585. doi: 10.3389/fonc.2022.983585
Received: 01 July 2022; Accepted: 03 October 2022;
Published: 24 October 2022.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Julie Bruneau, Assistance Publique Hopitaux De Paris, FranceCopyright © 2022 Zhang, Li, Cui, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui Liu, WWFuaHVpbGl1MTEyNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.