- 1Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2Department of Ultrasound, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 4Department of Otolaryngology Head and Neck Surgery, Hainan General Hospital, Haikou, China
Background: Active surveillance has been considered a safe alternative to surgery for low-risk papillary thyroid microcarcinoma. This study aimed to assess the oncological outcomes and psychological status of active surveillance of highly suspicious thyroid nodules ≤10 mm in China.
Methods: This prospective single-center cohort study enrolled 336 patients with highly suspicious thyroid nodules for active surveillance to assess oncological outcomes and psychological status. The psychological status of patients was assessed by two different questionnaires and compared among different patient groups.
Results: During a median follow-up period of 28.5 months, eight patients underwent delayed surgery for tumor enlargement and one for lymph node metastases. The cumulative incidence of disease progression at 5 and 10 years was 6.0% and 12.8%, respectively. Patients who underwent delayed surgery had no permanent complications, and no patient had distant metastasis or death. Patients ≤30 years old had a higher baseline anxiety score (4.9 vs. 3.8, p=0.024), a higher proportion of baseline anxiety score, i.e., ≥8 points (24.0% vs. 12.6%, p=0.033), and a worse baseline emotional function (62.7 vs. 70.7, p=0.013) than patients >30. During AS, patients ≤30 years of age had higher overall anxiety levels (p=0.005) and a worse overall emotional function (p=0.001).
Conclusions: Active surveillance in Chinese patients with highly suspicious subcentimetre thyroid nodules has good oncological outcomes and can be used as a safe alternative to surgery. Younger patients (≤30) show a worse psychological status; therefore, more attention should be paid to younger patients.
Introduction
The worldwide incidence of thyroid cancer has significantly increased over the past three decades (1, 2), and more than 50% of this increase is linked to the identification of papillary thyroid microcarcinoma (PTMC) (3, 4). As shown in several autopsy studies, if never diagnosed and treated, most PTMCs remain stable without influencing overall survival (5, 6). Two prospective studies from Japan have shown that AS is feasible for low-risk PTMC patients, given that most of these tumors remain latent without progression or with very slow progression (7, 8). Other studies have also confirmed the feasibility and safety of AS (9–14). In addition, the prognosis of PTMC was excellent, with a local or regional recurrence rate of 2–6%, a distant metastasis rate of 1–2%, and a 20-year disease-specific survival rate of >99% (15, 16). For most low-risk PTMC patients, immediate surgery may lead to more problems, such as surgical complications, neck scarring, and hormone replacement therapy (17, 18). Therefore, AS can be used as an alternative treatment option to reduce the incidence of adverse events without affecting treatment outcomes (19). The incidence of thyroid cancer has also increased rapidly in China, with around 200,000 new cases in 2015, similar to the global trend (20). In our institution, approximately 70% of thyroid surgery patients are diagnosed with PTMC, and most of these tumors are low-risk PTMCs (21). However, there are no prospective studies reporting AS in patients with low-risk PTMC in China.
Psychological status is important for many diseases, including cancer, and it is especially relevant for chronic diseases, which can significantly influence treatment decisions and outcomes (22). Low-risk PTMC resembles a chronic disease; hence, in addition to its oncological treatment, an assessment of psychological status is also important. Few studies have evaluated the psychological status in low-risk PTMC patients, suggesting that surgical patients experience more psychological problems (23, 24). Therefore, it is valuable to determine how the psychological status of low-risk PTMC patients changes in China during AS and how it interacts with treatment decisions and outcomes.
Fine-needle aspiration biopsy (FNAB) is considered the most accurate and cost-effective method available for evaluating thyroid nodules, recommended for nodules ≥10 mm in the greatest dimension with a high-suspicion sonographic pattern. For highly suspicious nodules ≤10 mm, FNAB could be postponed until therapy is considered (11, 25). However, the rates of non-diagnostic and indeterminate cytological results in thyroid nodules ≤10 mm can reach up to 35.6% and 60%, respectively (26–28). In addition, FNAB is not generally well accepted in China; the evaluation and diagnosis of thyroid nodules ≤10 mm is usually conducted by ultrasound. The 2015 ATA ultrasound malignancy risk stratification of thyroid nodules has a good diagnostic performance and plays a key role in the identification and management of thyroid nodules (29). We used these criteria to reach a “thyroid microcarcinoma” diagnosis of highly suspicious nodules, explore AS outcomes for these highly suspicious nodule patients without high-risk factors, and determine its interaction with psychological status in China.
Materials and methods
Study design and patients
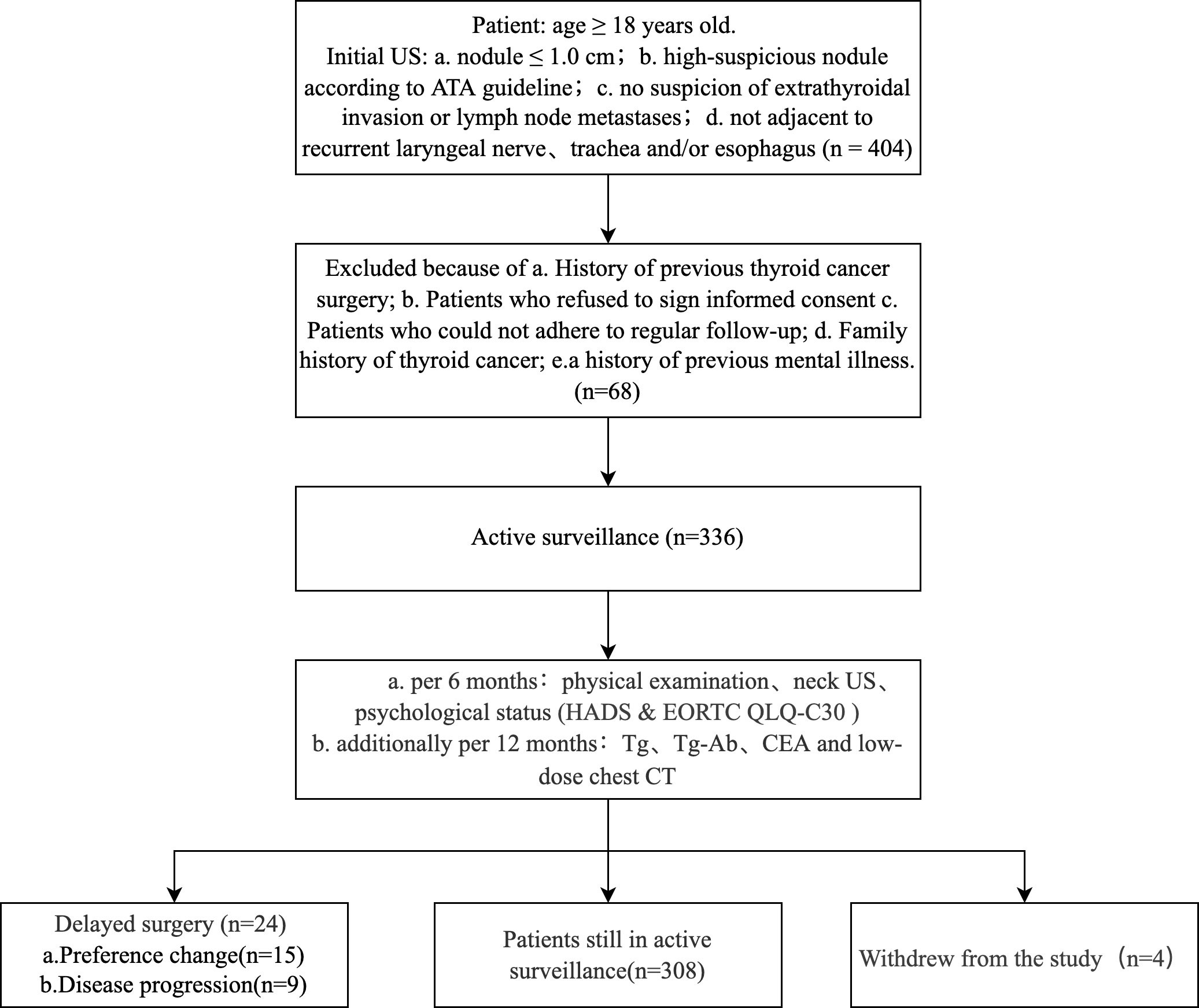
This prospective cohort study included 336 patients diagnosed with highly suspicious thyroid nodules by ultrasound and followed up by AS without immediate surgery from 2018 to 2021 at Peking Union Medical College Hospital (PUMCH), Beijing, China. We included highly suspicious nodules ≤10 mm assessed by the 2015 ATA ultrasound malignancy risk stratification of thyroid nodules without the following high-risk factors: a) extrathyroidal invasion or clinical lymph node metastasis (LNM) and b) nodules adjacent to recurrent laryngeal nerve, trachea, or esophagus (11). Exclusion criteria were: a) a history of previous thyroid cancer surgery, b) patients refusing to sign informed consent, c) patients who could not adhere to regular follow-ups, d) a family history of thyroid cancer (≥2 family members diagnosed with thyroid cancer) and e) a history of previous mental illness. The following parameters were recorded in this study: gender, age at diagnosis, follow-up time, educational level, marital status, economic level, past medical history, family history, hormone replacement therapy, ultrasound features, the results of the FNAB if performed, surgical approach, postoperative pathology, surgical complications, follow-up outcomes, laboratory tests (TSH, Tg, Tg-Ab, Tpo-Ab, CEA), chest low-dose computed tomography (CT), and two psychological status questionnaires: the Hospital Anxiety and Depression Scale (HADS) Questionnaire and the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (EORTC QLQ-C30) (30). Neck Ultrasound was performed using a Philips iU 22 machines with a 5–12-MHz transducer by two radiologists (X.Y, G.L.Y). This study was approved by the Ethics Review Committee of PUMCH (Ethics number: JS-2454), and each patient signed an informed consent form and was informed that the lesion was clinically diagnosed as a malignancy.
Follow-up plan
Patients were regularly followed up with physical examinations and neck ultrasounds every six months. Psychological status questionnaires were also collected every six months. In addition, serum Tg, Tg-Ab, CEA, and chest low-dose CT examinations were performed every 12 months (Figure 1). Delayed surgery was performed when the patient showed the following conditions: a) a tumor enlargement defined by a size increase of 3 mm or more compared with the size at the initiation of observation and subsequent FNAB confirming malignancy, b) novel LNM or distant metastasis, c) invasion of recurrent laryngeal nerve, trachea or esophagus, and d) changes in the preference of the patient. Patients’ surgical approach and postoperative follow-up plan were implemented in accordance with Chinese guidelines (31). The primary endpoints in this study included the disease progression rate and the patient’s psychological status. The secondary endpoints included the preference change rate and tumor persistence/recurrence rate.
Psychological status questionnaires
The Hospital Anxiety and Depression Scale (HADS) Questionnaire consists of fourteen items. Seven of them indicated anxiety, and the remaining seven indicated depression. The scores of the anxiety and depression subscale were divided into 0–7, no problems of clinical relevance (non-cases); 8–10, cases that warrant further psychiatric investigation (possible cases); and ≥11, clinical level of anxiety/depression (probable cases) (32).
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (EORTC QLQ-C30) consists of five functional scales (physical, role, emotional, social, and cognitive), three symptom scales (fatigue, pain, and nausea/vomiting), a global health status scale, and six single-item scales (dyspnea, loss of appetite, insomnia, constipation, diarrhea, and financial difficulties) (30).
Statistical analyses
Statistical analyses were performed using SPSS (IBM, Version 26.0), GraphPad Prism (Version 9.1.1), and R studio (Version 4.1.0). Categorical variables were expressed as numbers and percentages; continuous variables were expressed as mean ± standard deviation (SD), or median (range). A standard chi-squared test was used to compare categorical variables. The t-test or the Wilcoxon’s test were used to compare continuous variables. A competing risk model was used to analyze the cumulative incidence of disease progression and preference change and associated risk factors. Mixed linear models were used to analyze validated psychological scale results and associated risk factor analysis. All p-values were two-sided, and p<0.05 was considered statistically significant.
Results
Baseline clinical features
Clinical features of the 336 patients under active surveillance (AS) are listed in Table 1. The median age was 43 years old (range: 19–75). In total, 264 patients (78.6%) were female. According to age at diagnosis, 187 patients (55.7%) were ≤45 years old, and 149 (44.3%) were >45 years old. The mean maximal tumor diameter at initial diagnosis was 0.58 ± 0.19 cm, and 184 patients (54.8%) had tumors >0.5 cm in diameter. Multifocality was found in 82 patients (24.7%). The mean serum TSH concentration was 2.05 mIU/L; three patients (0.9%) received levothyroxine replacement therapy, and four (1.2%) had a family history of thyroid cancer (non-medullary thyroid cancer, and only one family member was diagnosed). Thirteen patients (3.9%) had a history of other malignancies. FNAB was performed in 62 patients, and the percentage of malignancy and suspicion for malignancy was 87.1%. CEA was positive in two cases (0.7%), one case had undergone surgery and was confirmed to be PTC, and one case was tested with normal calcitonin levels.
Outcome of patients with delayed surgery
During a median follow-up of 28.5 months (range: 4.3–138), 24 patients (7.1%) underwent delayed surgery. The median age of the patients was 41 years old (range: 25–66), and 87.5% were female. Four patients (1.2%) withdrew from the study. The most common reason for delayed surgery was a change in patient’s preference (15 cases), followed by disease progression (eight cases of tumor enlargement and one case of novel LNM). Of the 24 patients who underwent delayed surgery, 17 underwent lobectomy with central neck dissection, and seven patients underwent total thyroidectomy with central neck dissection. Postoperative pathology showed 15 cases of classic PTC, nine cases of follicular variant PTC, eight cases of LNM, and two cases of high-volume LNM. Only three patients developed transient hypocalcemia after surgery, and no other complications occurred. During a median postoperative follow-up of 7.3 months (range: 0.2–23.3), one patient was found to have lateral neck LNM after one year of follow-up, underwent lateral cervical lymph node dissection (LNM ratio: 1/28), and then reached disease-free survival. No distant metastasis or death occurred. There were no significant differences in gender, capsular invasion, pathological subtype, multifocality, and LNM between the preference change group and disease progression group (Supplementary Table S1).
A competing risk model analysis showed that the 5- and 10-year cumulative incidence of patients with disease progression were 6.0% and 12.8%, respectively, and the 5- and 10-year cumulative incidence of patients with preference change were 8.2% and 8.2%, respectively (Figure 2A). Regarding gender, age (45 years old as the boundary) and tumor size (0.5 cm as the boundary), the cumulative incidences were not significantly different in either the disease progression group or the preference change group (Figures 2B–D).
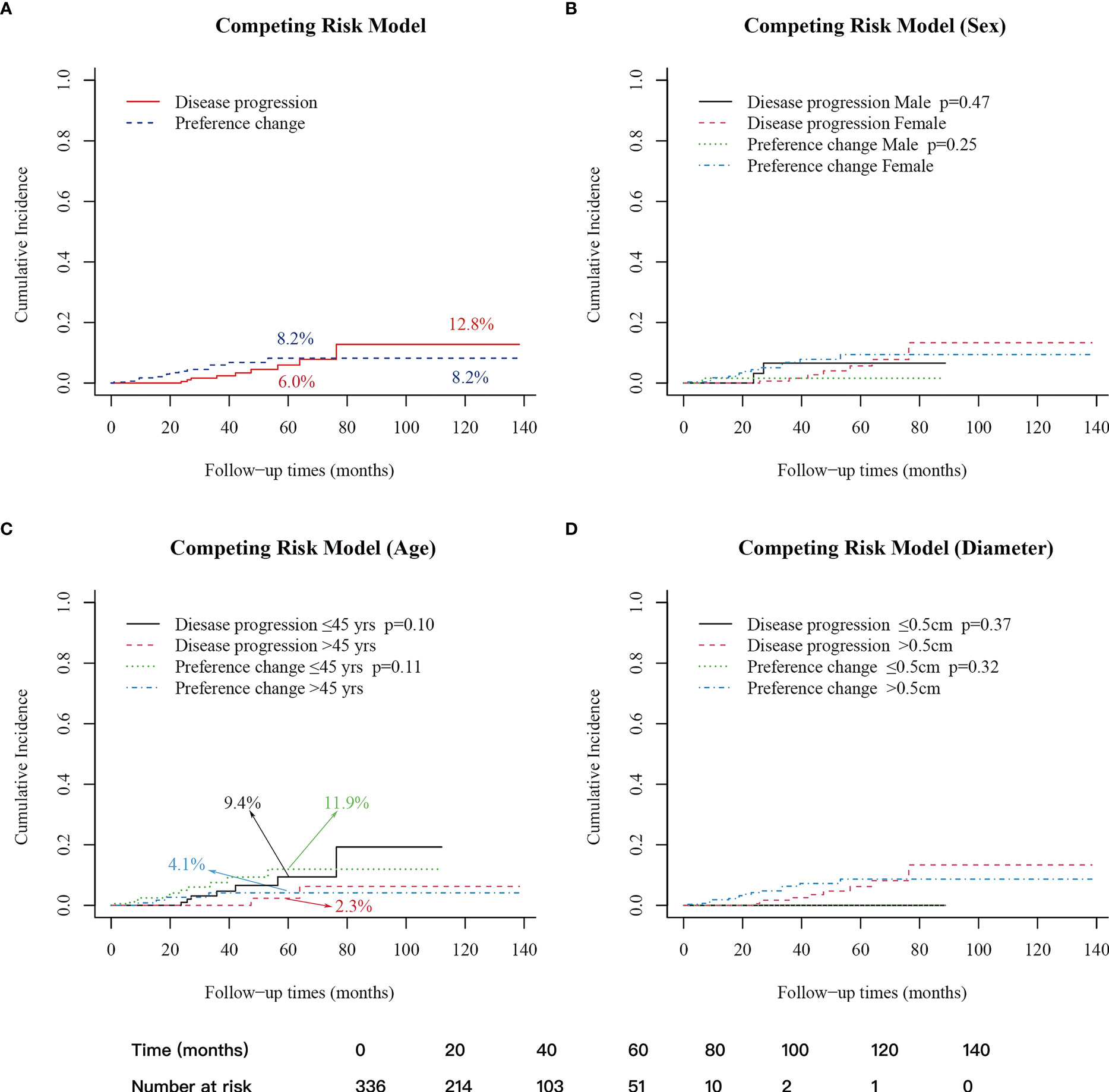
Figure 2 Cumulative incidence of patients with disease progression and preference change. (A) 5-year cumulative incidence: disease progression (6.0%); preference change (8.2%); 10-year cumulative incidence: disease progression (12.8%); preference change (8.2%). (B) Between male and female groups. (C) Between age>45 years old and age ≤45 years old groups, 5-year cumulative incidence: disease progression ≤ 45 years old (9.4%); disease progression>45 years old (2.3%); preference change ≤ 45 years old (11.9%); preference change>45 years old (4.1%). (D) Between diameter ≤ 0.5cm and>0.5cm. A p-value < 0.05 was considered significant.
Baseline questionnaire score and risk factor analysis
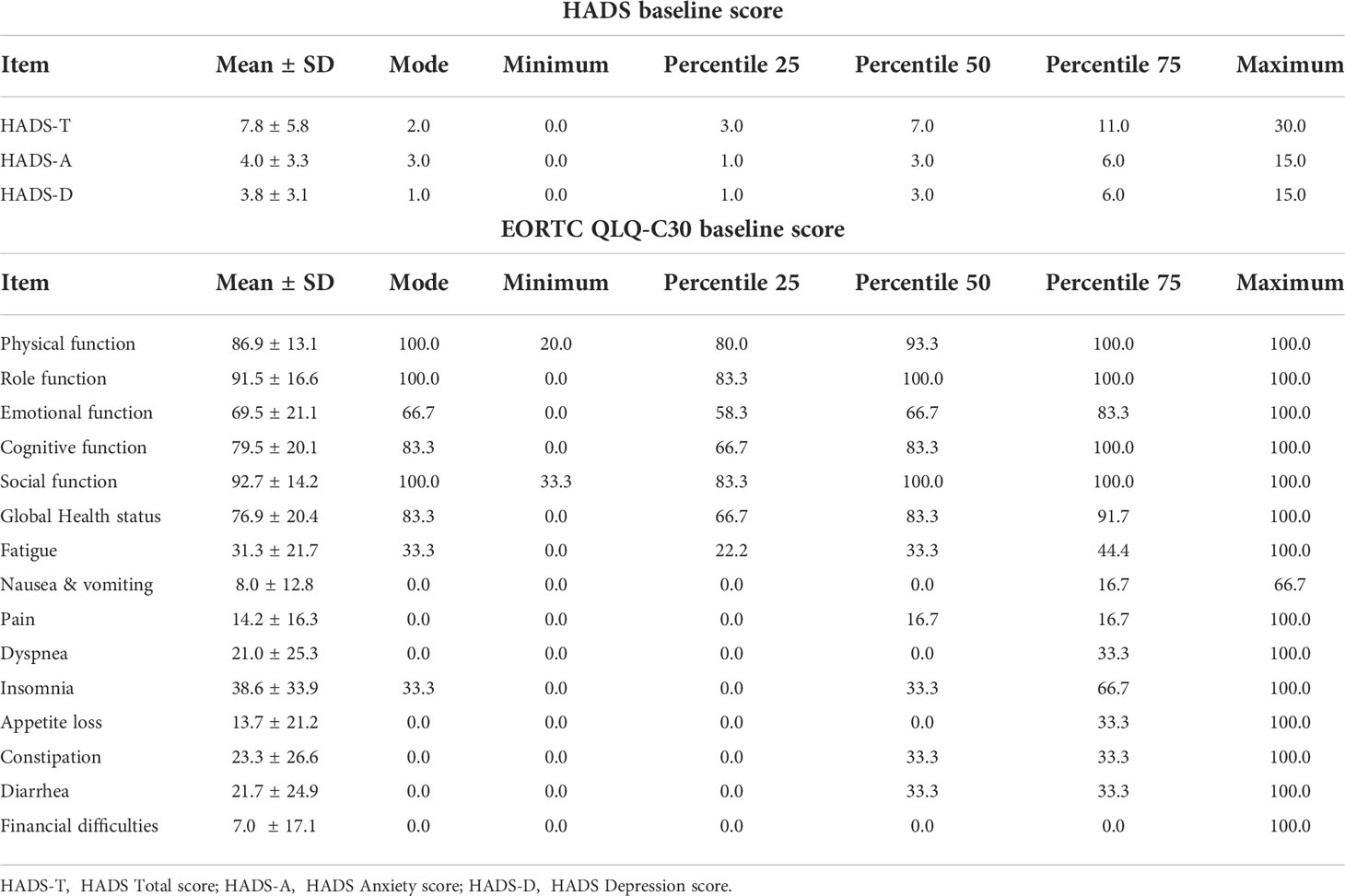
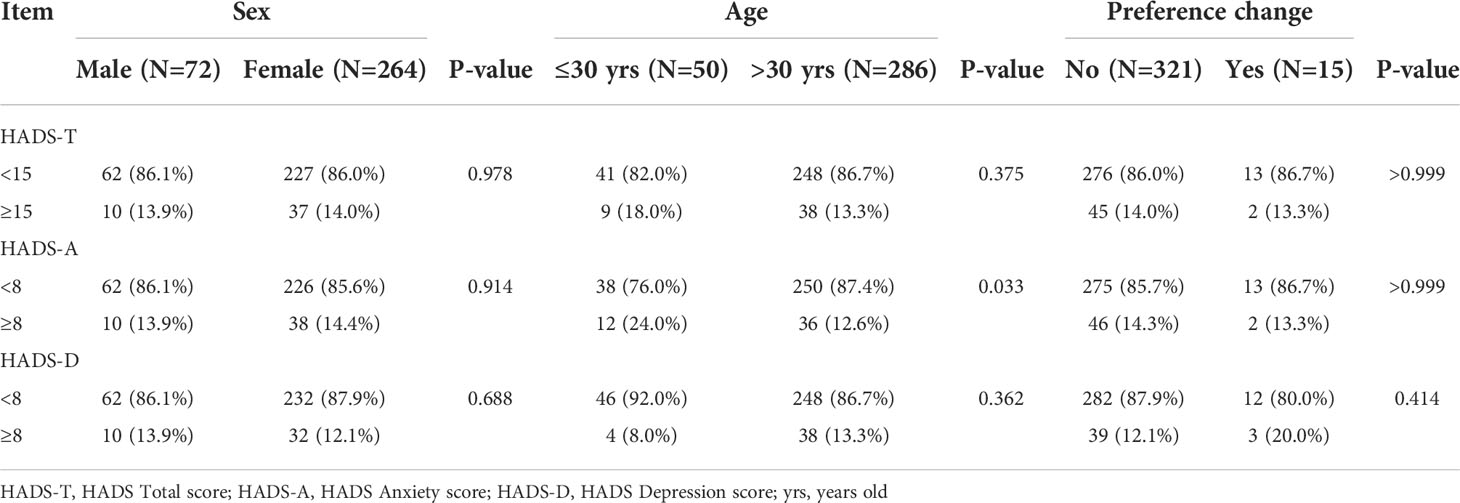
The mean Hospital Anxiety and Depression Scale A (HADS-A) score was 4.0 ± 3.3 (range: 0–15), and there were 47 (14.3%) cases with a HADS-A score of ≥8. The mean Hospital Anxiety and Depression Scale D (HADS-D) score was 3.8 ± 3.1 (range: 0–15), and there were 42 (12.5%) cases with a HADS-D score of ≥8 (Tables 2, 3). Compared with patients still under AS, the HADS-A score (4.0 ± 3.2 vs. 4.2 ± 3.2, p=0.794) and HADS-D score (3.9 ± 3.1 vs. 3.8 ± 3.1, p=0.924) of patients who changed their preference to surgery were not statistically different (Table 4). The proportion of HADS-A score ≥8 (13.3% vs. 14.4%, p>0.999) and HADS-D score ≥8 (20.0% vs. 12.1%, p=0.415) were also not statistically different according to preference change or not (Table 3). Patients ≤30 years of age had a higher HADS-A score than patients >30 (4.9 ± 3.3 vs. 3.8 ± 3.2, p=0.024) (Table 4) and represented a higher proportion of HADS-A score of ≥8 (24.0% vs. 12.6%, p=0.033) (Table 3). The baseline score of each item from the EORTC QLQ-C30 was not significantly different in terms of preference change. Patients ≤30 years of age had a worse emotional function than those >30 (62.7 ± 20.4 vs. 70.7 ± 21.1, p=0.013) (Table 5).

Table 4 HADS mean baseline scores of patients under AS, depending on Sex, Age and Treatment options.
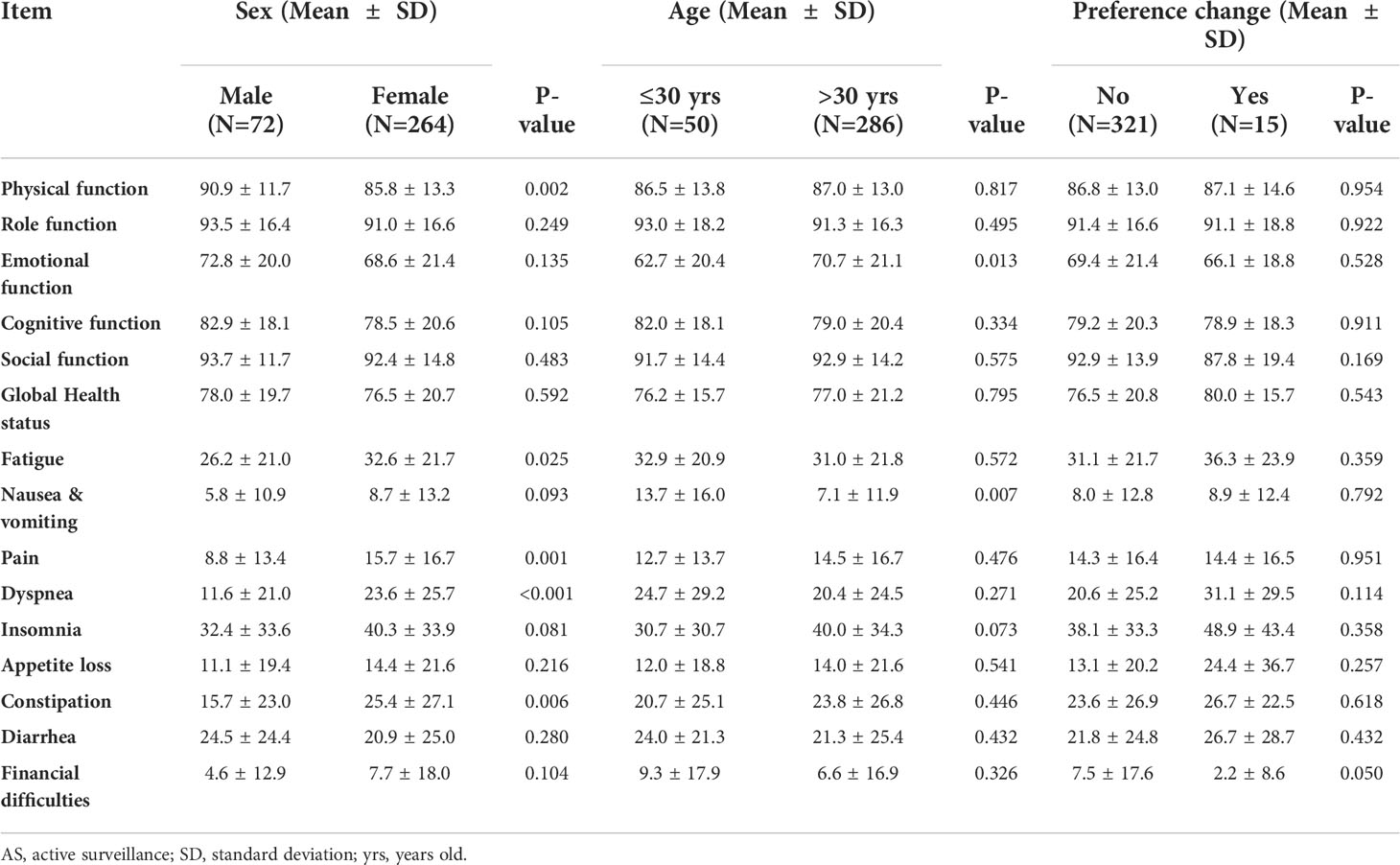
Table 5 EORTC QLQ-C30 baseline mean scores of patients under AS, depending on Sex, Age and Treatment options.
Mixed linear model analysis of HADS and EORTC QLQ-C30
The mixed linear model analysis of HADS showed no significant differences in the HADS-T, HADS-A, and HADS-D at different follow-up times (all p>0.05; Supplementary Table S2, Figures 3A-C). Risk factor analysis showed that during the follow-up, the overall mean anxiety score in patients aged ≤30 was higher than that of patients >30 (Estimate=0.89, 95% CI: 0.27~1.52, p=0.005) (Supplementary Table S2, Figure 4A). After adjusting the baseline HADS-A score as a covariate, there were no significant differences between the overall mean anxiety score of patients ≤30 years old and that of patients >30 (Estimate=0.064, 95% CI: -0.54~0.67, p=0.834) (Supplementary Table S4, Figure 4B). There were no significant differences in the overall mean scores of HADS-T, HADS-A, and HADS-D between male and female groups (Supplementary Table S2).
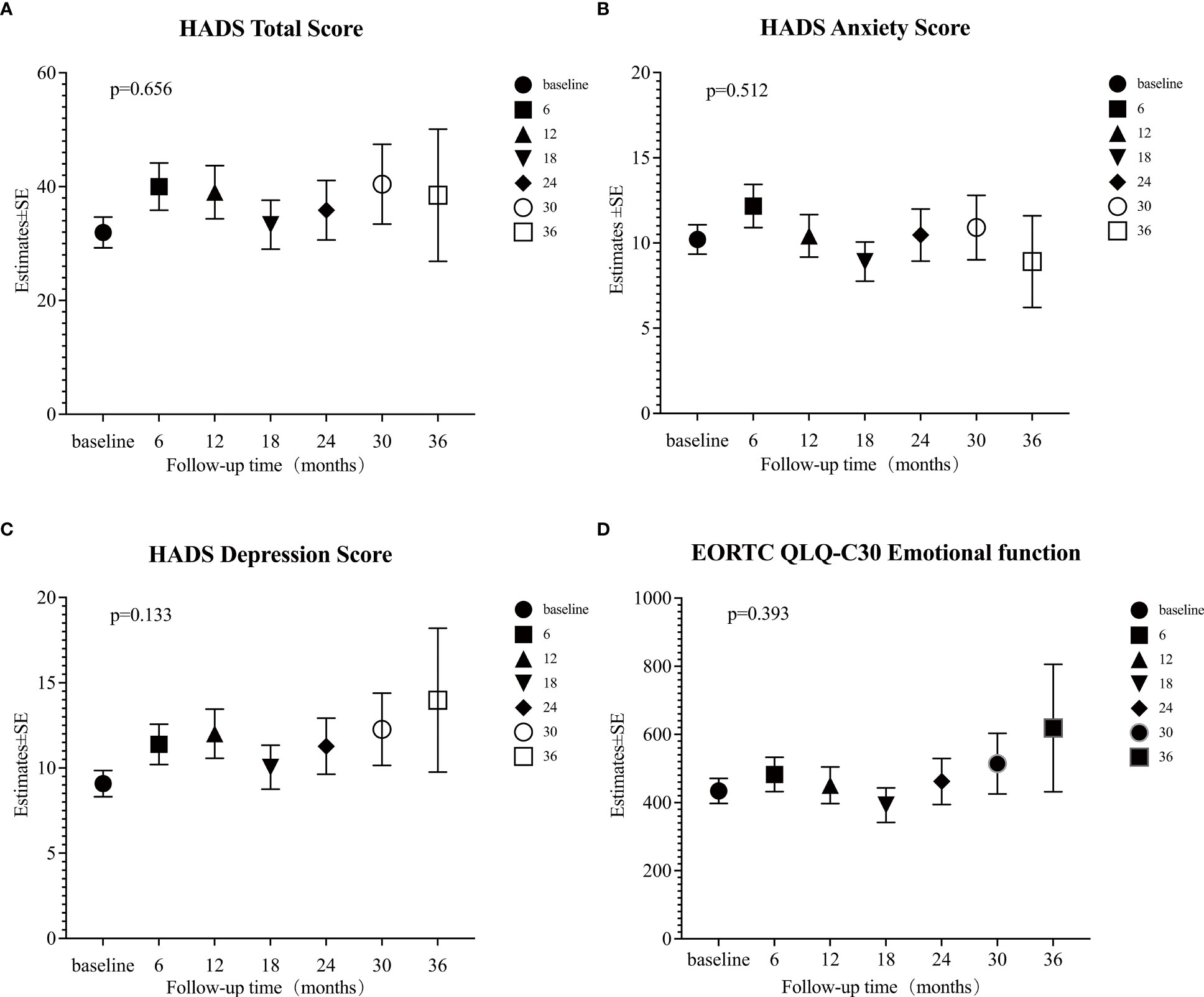
Figure 3 Estimates of four psychological scales. (A) Hospital Anxiety and Depression Scale (HADS) total score; (B) HADS anxiety score; (C) HADS depression score; (D) European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire (EORTC QLQ-C30) Emotional function was calculated at each time point (baseline, and 6, 12, 18, 24, 30, and 36 months) by mixed linear model. A p-value < 0.05 was considered significant.
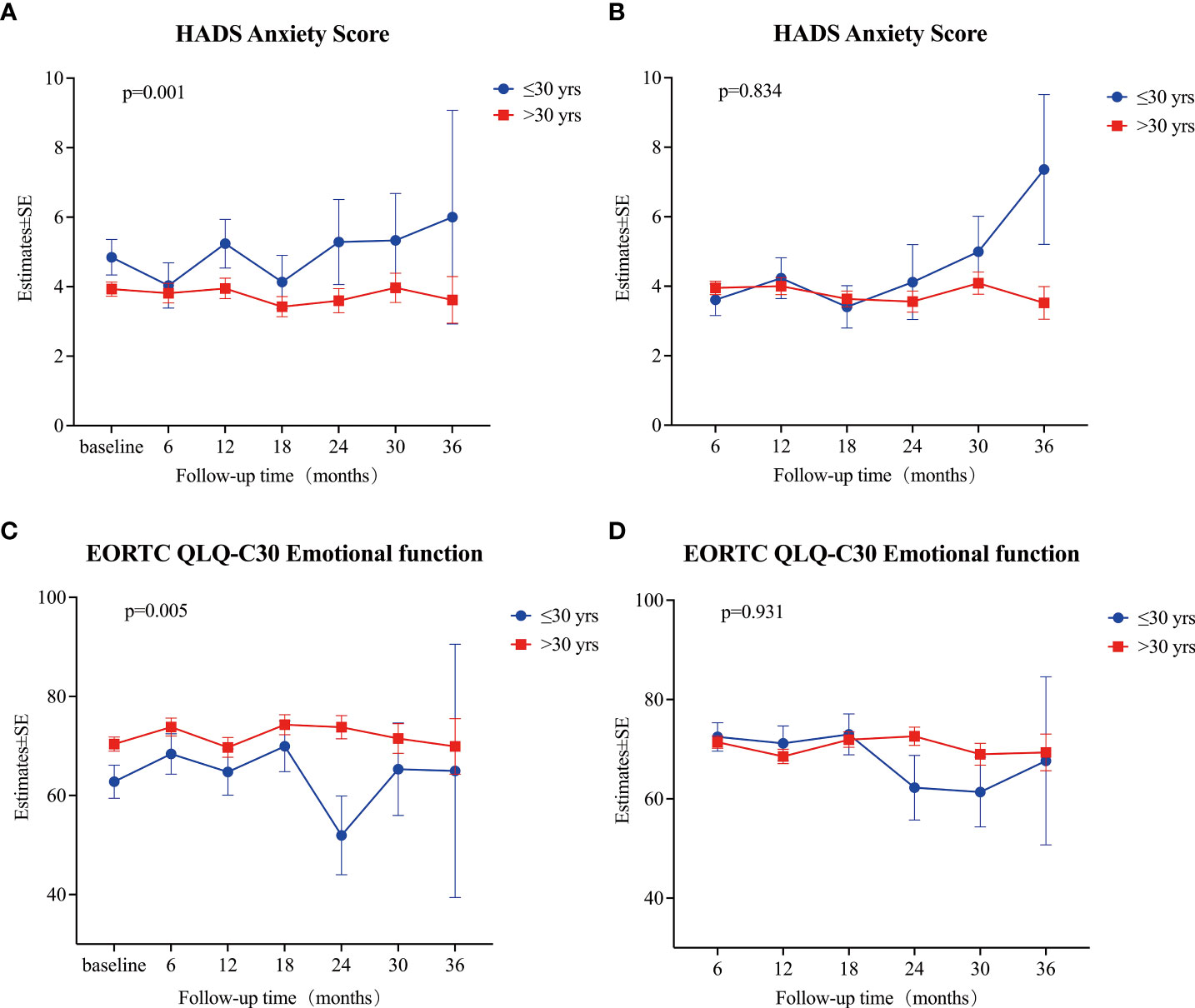
Figure 4 (A) Estimates of HADS anxiety score at each time point (baseline, and 6, 12, 18, 24, 30, and 36 months) between patients ≤30 years old and patients > 30 years old. (B) Estimates of HADS anxiety score at each time point (6, 12, 18, 24, 30, and 36 months) between patients ≤30 years old and patients > 30 years old after adjusting baseline score. (C) Estimates of EORTC QLQ-C30 Emotional function at each time point (baseline, and 6, 12, 18, 24, 30, and 36 months) between patients ≤30 years old and patients > 30 years old. (D) Estimates of EORTC QLQ-C30 Emotional function at each time point (6, 12, 18, 24, 30, and 36 months) between patients ≤30 years old and patients > 30 years old after adjusting baseline score. All estimates were calculated using a mixed linear model. A p-value < 0.05 was considered significant.
The mixed linear model analysis of EORTC QLQ-C30 showed no significant differences at different follow-up times, except for nausea/vomiting and financial difficulties (Supplementary Table S3, Figure 3D). Risk factor analysis showed that men had better scores than women in physical, role, cognitive, and social functions, fatigue, pain, dyspnea, insomnia, constipation, and financial difficulties (Supplementary Table S3). The overall mean score of emotional function was lower in patients ≤30 years old than in those >30 (Estimate = 6.90, 95% CI: 2.82–10.98, p = 0.001) (Supplementary Table S3, Figure 4C). After adjusting baseline emotional function as a covariate, the overall mean of the emotional function of patients ≤30 years old was not significantly different from that of patients >30 (Estimate= -0.168, 95% CI: -3.99~3.66, p =0.931) (Supplementary Table S4, Figure 4D).
Discussion
The global incidence of papillary thyroid cancer has substantially increased over the past few decades, with more than half of this increase linked to PTMC (33, 34). Researchers from Japan firstly proposed AS as an alternative strategy to immediate surgery for low-risk PTMC patients (35). Subsequently, several other research teams also successively reported studies on AS of low-risk PTMC (36–38). Our study is the first prospective active surveillance cohort study in China reporting oncological outcomes and psychological burden of low-risk thyroid microcarcinomas diagnosed clinically according to the 2015 ATA ultrasound malignancy risk stratification of thyroid nodules (11). As the acceptance of FNAB in China is less favorable, in this study, only 62 patients (18.5%) underwent FNAB. A low rate of FNAB may lead to misdiagnosis, but ultrasound still has relatively high diagnostic sensitivity (about 95%) and accuracy (about 85%) values for malignant nodules (29, 39). A total of 336 patients were included in this study. The median follow-up time was 28.5 months. Nine patients (2.7%) showed disease progression: eight patients (2.4%) showed tumor enlargement, and one patient (0.3%) showed newly identified LNM. The cumulative incidence of disease progression at five years was 6.0%, similar to the incidence reported (4.4–6.4%) in a systematic review (40). Delayed surgery was performed in 24 patients. The primary reason for delayed surgery was a change in patient’s preference (15/24), followed by tumor enlargement (8/24) and LNM (1/24), which are similar to previous studies (12, 36). The overall LNM rate of patients with delayed surgery of 33.3% was comparable to the LNM rate from previous studies (41). Patients with disease progression had no worse postoperative pathological outcomes compared to patients who changed their preference, and none of the patients had permanent surgery-related complications. During a median postoperative follow-up of 7.3 months (0.2-23.3), only one patient was found to have lateral neck LNM, and an excellent outcome was achieved after the second surgery. The preliminary outcome of this study showed that AS is safe and feasible for patients with highly suspicious thyroid nodules in China
Several studies have suggested that PTMC patients who undergo immediate surgery experience more health-related problems, including mental health issues, than patients who are managed by AS (23, 24), which may influence patients’ treatment decisions and outcomes. The main reason for delayed surgery was a change in preference rather than disease progression in previous studies and this study (12, 36). Although we did not find significant differences in baseline anxiety score (HADS) and emotional function (EORTC QLQ-C30) between patients who changed their preference for surgery and those who did not, we showed that patients ≤30 years of age had a significantly higher baseline anxiety score (4.9 ± 3.3 vs. 3.8 ± 3.2, p=0.024), a significantly worse baseline emotional function (62.7 ± 20.4 vs. 70.7 ± 21.1, p=0.013), and a significantly higher baseline proportion of patients with a HADS-A score of ≥8 (24.0% vs. 12.6%, p=0.033) than patients >30 (Tables 3, 4, and Supplementary Table S2). Among patients with a preference change, 13.3% of patients were younger than or equal to 30 years old, and 15.0% for patients still under AS, the similar percentage might explain no differences in scores. On the other hand, this study also showed that during follow-ups, patients ≤30 years of age had a higher anxiety score (Supplementary Table S2, Figure 4A) and a worse emotional function (Supplementary Table S3, Figure 4C). After adjusting the effect of the baseline score, we observed that for patients ≤30 years of age, a lower overall psychological status was mainly related to a worse baseline psychological status. For the first time, we found that younger patients had a worse baseline psychological status, which led to a worse psychological status during the follow-up period. Therefore, psychological counseling might be necessary for patients with highly suspicious thyroid nodules managed by AS, and it might improve the psychological status during the follow-up period, especially in younger patients.
Age is an important prognostic factor of differentiated thyroid cancer (11, 41, 42). Previous studies have found that PTC in young patients may be more progressive than in older patients during AS (7, 36, 43). This study compared the cumulative incidence of disease progression across different age stratifications, which showed no significant difference. However, the 5-year cumulative incidence of disease progression between patients aged ≤45 and patients >45 was significantly different (9.4% vs. 2.3%, p=0.1) (Figure 2C). Also, among patients who changed their preference, the 5-year cumulative incidence was higher in patients aged ≤45 compared with patients >45 (11.9% vs. 4.1%, p=0.11) (Figure 2C). These findings show no significant differences, which may be related to the small number of patients and the shorter follow-up time. According to previous studies and the data from this study, the disease progression still requires special attention for younger patients, especially for those with a longer life expectancy and a longer follow-up time. Moreover, these patients may be more likely to change their preference, requiring attention not only to oncological outcomes but also to psychological status assessment.
This study had certain limitations, such as the malignancy criteria of highly suspicious thyroid nodules (they have mainly been diagnosed by ultrasound, which may have low misdiagnosis rates), the relatively short follow-up period, and a small number of cases (it may be necessary to continue to expand the sample size and increase the follow-up time to obtain more robust data). In addition, most patients do not have their initial visit to our institution. Therefore, there may be some bias when conducting the psychological questionnaire assessment.
In conclusion, active surveillance of highly suspicious subcentimetre thyroid nodules in patients without high-risk factors has good oncological outcomes and could be a safe alternative to surgery in China. However, younger patients may not only be more prone to disease progression but also have a worse psychological status, which may affect their treatment process; therefore, more attention should be paid to younger patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CL: Conceptualization, methodology, validation, formal analysis, data curation, visualization, writing - original draft. HZ: Investigation, formal analysis, data curation. YX: Conceptualization, and, methodology, writing - review and editing. YC: Investigation, Data Curation. LZ: Investigation, Data Curation. YZ: Investigation, Data Curation. LG: Investigation, Data Curation. RL: Investigation, Data Curation. YL: Conceptualization, methodology, writing - review and editing. HL: investigation, data curation. ZM: Methodology. SL: Investigation, Data Curation. XL: Conceptualization, investigation, methodology, Project administration, supervision, Funding acquisition, writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant numbers: 2019XK320011).
Acknowledgments
The authors thank Mr. Yanlong Li for statistical guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.981495/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol (2020) 8(6):468–70. doi: 10.1016/S2213-8587(20)30115-7
3. Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol (2021) 9(4):193–4. doi: 10.1016/S2213-8587(21)00049-8
4. Davies L, Hoang JK. Thyroid cancer in the USA: current trends and outstanding questions. Lancet Diabetes Endocrinol (2021) 9(1):11–2. doi: 10.1016/S2213-8587(20)30372-7
5. Robenshtok E, Neeman B, Reches L, Ritter A, Bachar G, Kaminer K, et al. Adverse histological features of differentiated thyroid cancer are commonly found in autopsy studies: Implications for treatment guidelines. Thyroid (2022) 32(1):37–45. doi: 10.1089/thy.2021.0268
6. Lee YS, Lim H, Chang HS, Park CS. Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J Korean Med Sci (2014) 29(5):676–9. doi: 10.3346/jkms.2014.29.5.676
7. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid (2014) 24(1):27–34. doi: 10.1089/thy.2013.0367
8. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg (2010) 34(1):28–35. doi: 10.1007/s00268-009-0303-0
9. Ito Y, Miyauchi A. Active surveillance as first-line management of papillary microcarcinoma. Annu Rev Med (2019) 70:369–79. doi: 10.1146/annurev-med-051517-125510
10. Ito Y, Miyauchi A, Kudo T, Oda H, Yamamoto M, Sasai H, et al. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at kuma hospital: Gradual increase and heterogeneity in the acceptance of this new management option. Thyroid (2018) 28(4):488–95. doi: 10.1089/thy.2017.0448
11. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
12. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, et al. Active surveillance for patients with papillary thyroid microcarcinoma: A single center's experience in Korea. J Clin Endocrinol Metab (2017) 102(6):1917–25. doi: 10.1210/jc.2016-4026
13. Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: Experience at a single Italian center. J Clin Endocrinol Metab (2020) 105(3):e162–70. doi: 10.1210/clinem/dgz113
14. Saravana-Bawan B, Bajwa A, Paterson J, McMullen T. Active surveillance of low-risk papillary thyroid cancer: A meta-analysis. Surgery (2020) 167(1):46–55. doi: 10.1016/j.surg.2019.03.040
15. Londero SC, Krogdahl A, Bastholt L, Overgaard J, Pedersen HB, Hahn CH, et al. Papillary thyroid carcinoma in Denmark, 1996-2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort. Thyroid (2015) 25(1):78–84. doi: 10.1089/thy.2014.0294
16. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol (2016) 4(11):933–42. doi: 10.1016/S2213-8587(16)30180-2
17. Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR. Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab (2017) 102(7):2543–51. doi: 10.1210/jc.2017-00255
18. Papaleontiou M, Haymart MR. Too much of a good thing? a cautionary tale of thyroid cancer overdiagnosis and overtreatment. Thyroid (2020) 30(5):651–2. doi: 10.1089/thy.2020.0185
19. Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid (2016) 26(1):150–5. doi: 10.1089/thy.2015.0313
20. Du L, Zhao Z, Zheng R, Li H, Zhang S, Li R, et al. Epidemiology of thyroid cancer: Incidence and mortality in China, 2015. Front Oncol (2020) 10:1702. doi: 10.3389/fonc.2020.01702
21. Liu C, Zhang L, Liu Y, Xia Y, Cao Y, Liu Z, et al. Ultrasonography for the prediction of high-volume lymph node metastases in papillary thyroid carcinoma: Should surgeons believe ultrasound results? World J Surg (2020) 44(12):4142–8. doi: 10.1007/s00268-020-05755-0
22. Lardas M, Liew M, van den Bergh RC, De Santis M, Bellmunt J, Van den Broeck T, et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: A systematic review. Eur Urol (2017) 72(6):869–85. doi: 10.1016/j.eururo.2017.06.035
23. Jeon MJ, Lee YM, Sung TY, Han M, Shin YW, Kim WG, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: A cross-sectional study. Thyroid (2019) 29(7):956–62. doi: 10.1089/thy.2018.0711
24. Nakamura T, Miyauchi A, Ito Y, Ito M, Kudo T, Tanaka M, et al. Quality of life in patients with low-risk papillary thyroid microcarcinoma: Active surveillance versus immediate surgery. Endocr Pract (2020) 26(12):1451–7. doi: 10.4158/EP-2020-0201
25. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur Thyroid J (2017) 6(5):225–37. doi: 10.1159/000478927
26. Partyka KL, Wu HH. Fine-needle aspirates of thyroid microcarcinoma. J Am Soc Cytopathol (2017) 6(6):236–41. doi: 10.1016/j.jasc.2017.06.006
27. Moon HJ, Son E, Kim EK, Yoon JH, Kwak JY. The diagnostic values of ultrasound and ultrasound-guided fine needle aspiration in subcentimeter-sized thyroid nodules. Ann Surg Oncol (2012) 19(1):52–9. doi: 10.1245/s10434-011-1813-1
28. Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab (1999) 84(1):24–8. doi: 10.1210/jcem.84.1.5418
29. Zhang WB, Xu HX, Zhang YF, Guo LH, Xu SH, Zhao CK, et al. Comparisons of ACR TI-RADS, ATA guidelines, kwak TI-RADS, and KTA/KSThR guidelines in malignancy risk stratification of thyroid nodules. Clin Hemorheol Microcirc (2020) 75(2):219–32. doi: 10.3233/CH-190778
30. Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European organization for research and treatment of cancer core quality-of-Life questionnaire: the QLQ=C30 (+ 3). J Clin Oncol (1998) 16(3):1188–96. doi: 10.1200/JCO.1998.16.3.1188
31. Teng W, Liu Y, Gao M, Huang G, Wu Y, Zhao J, et al. Guidelines for the diagnosis and management of thyroid nodules and differentiated thyroid cancer. Chin J Endocrinol Metab (2012) 28(10):779–97. doi: 10.3760/cma.j.issn.1000-6699.2012.10.002
32. Arving C, Glimelius B, Brandberg Y. Four weeks of daily assessments of anxiety, depression and activity compared to a point assessment with the hospital anxiety and depression scale. Qual Life Res (2008) 17(1):95–104. doi: 10.1007/s11136-007-9275-4
33. Davies L, Welch HG. Current thyroid cancer trends in the united states. JAMA Otolaryngol Head Neck Surg (2014) 140(4):317–22. doi: 10.1001/jamaoto.2014.1
34. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"–screening and overdiagnosis. N Engl J Med (2014) 371(19):1765–7. doi: 10.1056/NEJMp1409841
35. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid (2003) 13(4):381–7. doi: 10.1089/105072503321669875
36. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: A multi-center cohort study in Korea. Thyroid (2018) 28(12):1587–94. doi: 10.1089/thy.2018.0263
37. Sanabria A. Active surveillance in thyroid microcarcinoma in a Latin-American cohort. JAMA Otolaryngol Head Neck Surg (2018) 144(10):947–8. doi: 10.1001/jamaoto.2018.1663
38. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg (2017) 143(10):1015–20. doi: 10.1001/jamaoto.2017.1442
39. Ha EJ, Na DG, Moon WJ, Lee YH, Choi N. Diagnostic performance of ultrasound-based risk-stratification systems for thyroid nodules: Comparison of the 2015 American thyroid association guidelines with the 2016 Korean thyroid Association/Korean society of thyroid radiology and 2017 American college of radiology guidelines. Thyroid (2018) 28(11):1532–7. doi: 10.1089/thy.2018.0094
40. Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Chung KW, et al. Active surveillance for small papillary thyroid cancer: A systematic review and meta-analysis. Thyroid (2019) 29(10):1399–408. doi: 10.1089/thy.2019.0159
41. Liu C, Liu Y, Zhang L, Dong Y, Hu S, Xia Y, et al. Risk factors for high-volume lymph node metastases in cN0 papillary thyroid microcarcinoma. Gland Surg (2019) 8(5):550–6. doi: 10.21037/gs.2019.10.04
42. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and Male sex are predictors of Large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid (2017) 27(10):1285–90. doi: 10.1089/thy.2017.0250
43. Koshkina A, Fazelzad R, Sugitani I, Miyauchi A, Thabane L, Goldstein DP, et al. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg (2020) 146(6):552–60. doi: 10.1001/jamaoto.2020.0368
Keywords: highly suspicious thyroid nodules, active surveillance, management, outcome, psychological status
Citation: Liu C, Zhao H, Xia Y, Cao Y, Zhang L, Zhao Y, Gao L, Liu R, Liu Y, Liu H, Meng Z, Liu S and Li X (2022) Active surveillance of highly suspicious thyroid nodules cohort in China shows a worse psychological status in younger patients. Front. Oncol. 12:981495. doi: 10.3389/fonc.2022.981495
Received: 29 June 2022; Accepted: 27 July 2022;
Published: 26 August 2022.
Edited by:
Jonathan Robert Clark, Chris O’Brien Lifehouse, AustraliaReviewed by:
Jonathan Mark Fussey, Queen Elizabeth Hospital Birmingham, United KingdomYin Detao, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2022 Liu, Zhao, Xia, Cao, Zhang, Zhao, Gao, Liu, Liu, Liu, Meng, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyi Li, bGkueGlhb3lpQDI2My5uZXQ=
 Chunhao Liu1
Chunhao Liu1 Hao Zhao
Hao Zhao Yu Xia
Yu Xia Xiaoyi Li
Xiaoyi Li