- 1Department of Radiation Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat−sen University Cancer Center, Guangzhou, China
- 2Guangdong Association Study of Thoracic Oncology, Guangzhou, China
Background: To evaluate longitudinal changes of concurrent chemoradiotherapy (CCRT) related lymphopenia and its association with survival in locally advanced non-small cell lung cancer (LA-NSCLC) patients.
Methods: Total lymphocyte count (TLC) at baseline, weekly intervals during CCRT and monthly intervals up to 12 months after CCRT were documented. The Common Terminology Criteria for Adverse Events version 5.0 was used to grade the severity of lymphopenia. Cox regression analysis was performed to evaluate the association between overall survival (OS) and CCRT related lymphopenia at different timepoints. Logistic regression model was used to determine the clinical factors associated with TLC level.
Results: 381 LA-NSCLC patients treated with definitive CCRT without consolidation therapy (NCT02573506/NCT02577341) between 2011 to 2020 were analyzed. With a median follow-up of 45.8 months, the median OS was 41.0 months for all patients. Univariable analysis demonstrated that the 3 weeks during CCRT Grade (G) 4 lymphopenia (P=0.018), 2 months after CCRT G1-4 lymphopenia (P=0.004), 6 months after CCRT (6m-post-CCRT) G1-4 lymphopenia (P=0.001), and TLC nadir (P=0.020) were significantly associated with poorer OS. Multivariable analysis suggested that 6m-post-CCRT G1-4 lymphopenia (HR 2.614; P=0.041) were one of the independent predictors of OS. Further analysis inferred that radiation dose (OR: 1.328; P=0.005), GTV volume (OR: 1.004; P=0.036), and baseline TLC (OR: 0.288; P=0.001) were associated with 6m-post-CCRT lymphopenia.
Conclusion: The persistent lymphopenia at 6 months after CCRT was an independent prognostic factor of OS in LA-NSCLC patients. Higher radiation dose, larger gross tumor volume and lower baseline TLC were significantly related to 6m-post-CCRT lymphopenia.
Introduction
Lymphocytes play a crucial role in the host antitumor immune response, which helps suppress cancer progression. Total lymphocyte count (TLC) has been recognized as an important prognostic factor for multiple solid tumors in clinical studies (1, 2). Although concurrent chemoradiotherapy (CCRT) is a standard treatment modality for unresectable locally advanced non-small cell lung cancer (LA-NSCLC), it frequently results in lymphopenia from directly destructing mature circulating lymphocytes, or interfering lymphopoiesis in radiation-sensitive organs, such as bone marrow (3, 4). The reduction of TLC caused by CCRT is associated with poor prognosis in various cancers (5–8). Previous studies have demonstrated that lymphopenia was a predictor of inferior survival as well as acute/chronic inflammatory pneumonitis among patients with NSCLC (7, 9, 10). Nevertheless, the dynamic change of TLC during and after CCRT and its role on prognosis has not been fully elucidated in NSCLC.
CCRT with consolidative immunotherapy offers the best chance for cure in patients with unresectable LA-NSCLC. Moreover, ex vivo studies suggested that radiotherapy can exert immunostimulatory effects through increased expression of cytokines and tumor associated antigens, and through recruitment of effector cells into tumor microenvironment (4, 11). However, CCRT induced lymphopenia might have negative impact on NSCLC patients receiving immunotherapy. Previous study also indicated that lymphopenia could be regarded as an early indicator of immune-related adverse events (12), and severe lymphopenia at the time of immunotherapy initiation was an independent predictor of worse progression-free survival (PFS) (13). Therefore, to explore the longitudinal changes of TLC during and after CCRT is crucial for maximizing the efficacy of combined therapy. Clinical factors associated with persistent lymphopenia and strategies to mitigate lymphopenia effects should be explored.
Given the association between TLC and survival in multiple solid tumors as well as the important role of the immune response in patients with LA-NSCLC, we hypothesized that not only the TLC nadir during CCRT but also the persistent lymphopenia after CCRT could negatively affect survival outcomes in patients with LA-NSCLC treated with CCRT. The objectives of this study were two-fold: (a) to investigate the relationship between longitudinal TLC changes and survival outcome in LA-NSCLC patients undergoing definitive CCRT and (b) to evaluate the clinical factors associated with persistent lymphopenia induced by CCRT in the patient population.
Materials and methods
Patient population
A total of 381 patients diagnosed as unresectable LA-NSCLC and treated with definitive CCRT were identified, including the patient cohorts from two clinical trials (NCT02573506, NCT02577341) as well as patients treated as the protocol of the trials. The inclusive criteria were (1): biopsy-proven NSCLC; (2) stage IIIA-IIIC according to the eighth edition of the International Union against Cancer/American Joint Committee on Cancer staging system. (3) receipt of CCRT with curative intent; (4) without adjuvant or consolidation therapy after CCRT; (5) age of 18 years or older; (6) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 2. (7) complete blood count (CBC) with differential available prior to CCRT, weekly intervals during CCRT and monthly intervals up to 12 months after CCRT. Patients were excluded if they had metastatic disease at the time of diagnosis. Patient and disease characteristics including age, gender, ECOG score, family history of cancer, smoking history, histologic grade, TNM staging, pre-CCRT body mass index (BMI), pre-CCRT albumin were documented. Radiotherapy regimen information including gross tumor volume (GTV), total radiation dose, mean body dose (MBD), mean heart dose (MHD), and mean lung dose (MLD) were collected.
This study was approved by the review board of Sun Yat-sen University Cancer Center. Written informed consent for the use of clinical data was obtained from all patients.
Treatment
All the patients underwent definitive CCRT. A four-dimensional simulation CT was created and the GTV were composite volumes from CT scans of all breathing phases. The GTV was defined as the visible primary tumor and positive lymph nodes on CT scans. The planning target volumes for GTV were created by a uniform expansion of 5-6 mm surrounding the GTV for all patients. Involved nodal irradiation was adopted in our patients. Definitive CCRT was prescribed per clinical trial protocols (NCT02573506, NCT02577341). Radiotherapy was delivered using intensity modulated radiotherapy technique with a total dose of 60-68 Gy to PTV-GTV in 16-23 daily fractions. Concurrent chemotherapy regimens included docetaxel/paclitaxel plus platinum (14, 15). Three to five cycles of weekly concurrent chemotherapy were delivered depending on the fractions.
Longitudinal complete blood count
White blood cell, neutrophil, hemoglobin, and TLC prior to CCRT, weekly intervals during CCRT and monthly intervals up to 12 months after CCRT were documented. Longitudinal TLCs, including the baseline (pre-CCRT) TLC (within 2 weeks prior to RT), the 3 weeks during CCRT (mid-CCRT) TLC, the end of CCRT (end-CCRT) TLC, 2 months after CCRT (2m-post-CCRT) TLC, 6 months after CCRT (6m-post-CCRT) TLC and TLC nadir, were collected for analysis. TLC nadir was recorded as the minimum value from the onset till 12 months after CCRT. As treatment beyond progression was supposed to affect TLC, 2m-post-CCRT TLC and 6m-post-CCRT TLC were recorded for those patients who were progression-free for at least 6 months after CCRT. The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to grade the severity of lymphopenia (G0: ≥1.00 k/µL, G1: <1.00-0.80 k/µL, G2: <0.80–0.50 k/µL, G3: <0.50–0.20 k/µL, and G4: <0.20 k/µL).
Follow-up
Patients were firstly followed up every 3 months during the first 2 years, every 6 months in 3-5 years, and every year thereafter until progression or death. Medical history, physical examination, CT scans of the chest and upper abdomen were performed at each follow-up. Brain magnetic resonance imaging, bone scan or positron emission tomography/CT were performed when tumor recurrence or metastasis was suspected.
Statistical analysis
The characteristics of patients, disease and treatments were summarized by descriptive analysis. Continuous variables were presented with median (range) and compared by Mann-Whitney U test. The primary endpoint of the study was overall survival (OS) which calculated from the date of initial treatment to death of any cause or time of last follow-up. Progression free survival was calculated as the time interval between the initial treatment to the date of disease progression or death. OS, PFS and local control were analyzed using the Kaplan-Meier method and log-rank test. Univariable and multivariable Cox regression model were performed to select the independent predictors of OS. The hazard ratio (HR) with their 95% confidence interval (CI) were calculated. Clinical and dosimetric factors were analyzed by univariable and multivariable binary logistic regression model to identify independent baseline variables associated with 6m-post-CCRT lymphopenia. The odds ratio (OR) with their 95% CI were calculated. All data were analyzed using the SPSS statistics, version 26.0 (IBM Corp., Armonk, NY). Two-tailed P value <0.05 was considered statistically significant.
Results
Patient characteristics
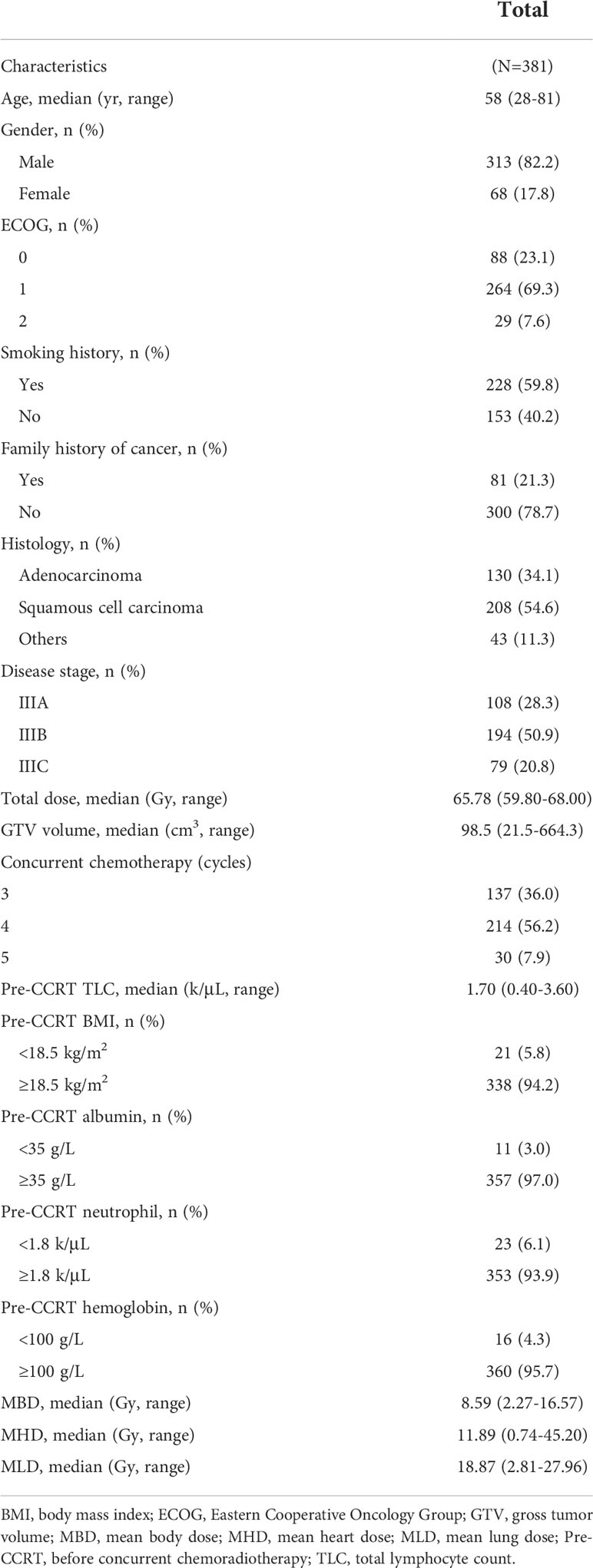
The baseline characteristics of 381 patients are summarized in Table 1. For the entire patient cohort, the median age was 58 (range, 28-81). The majority were male (82.2%) and had an ECOG performance status of 0-1 (92.4%). At diagnosis, 59.8% of patients had a smoking history. There were 208 (54.6%) patients had squamous cell carcinoma, and 194 (50.9%) patients had stage IIIB disease. The median radiation dose was 65.78 Gy (range, 59.80-68.00). Patients received weekly concurrent chemotherapy (25 mg/m2 docetaxel and 25 mg/m2 platinum) at 3~5 cycles. The median GTV volume was 98.5 cm3 (range, 21.5-664.3). The median values of MBD, MHD and MLD were 8.59 Gy (range, 2.27-16.57), 11.89 Gy (range, 0.74-45.20), and 18.87 Gy (range, 2.81-27.96), respectively.
Longitudinal total lymphocyte counts
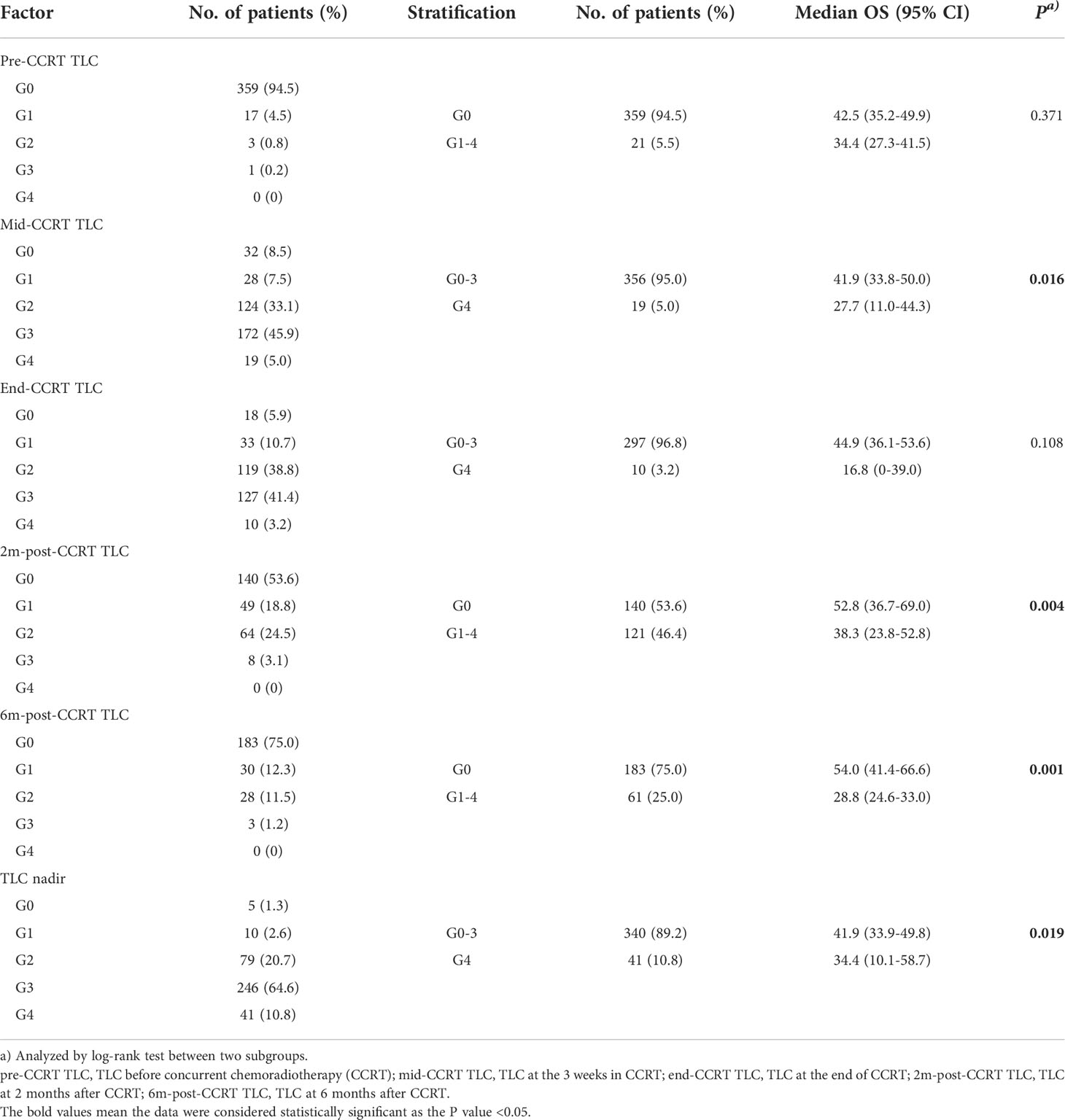
The longitudinal dynamic change of TLC was shown in Figure 1. The median pre-CCRT TLC was 1.70 k/μL (range, 0.40-3.60). At 3 weeks during CCRT (mid-CCRT), the median TLC was 0.49 k/μL (range, 0.10-1.55), and the rate of G4 lymphopenia was 5.0%. The median end-CCRT TLC was 0.50 k/μL (range, 0-1.49), and the rate of G4 lymphopenia was 3.2%. The median 2m-post-CCRT TLC was 1.04 k/μL (range, 0.24-3.43), however, 46.4% of patients had G1-4 lymphopenia. The median 6m-post-CCRT TLC was 1.22 k/μL (range, 0.36-3.60). 75.0% of patients had TLC returned to >1.0 k/μL (G0) by 6 months, whereas 25.0% had persistent lymphopenia beyond 6 months. The median TLC nadir was 0.40 k/μL (range, 0-1.21), and 10.8% (41/381) of patients experienced G4 lymphopenia during the whole course of CCRT (Table 2).
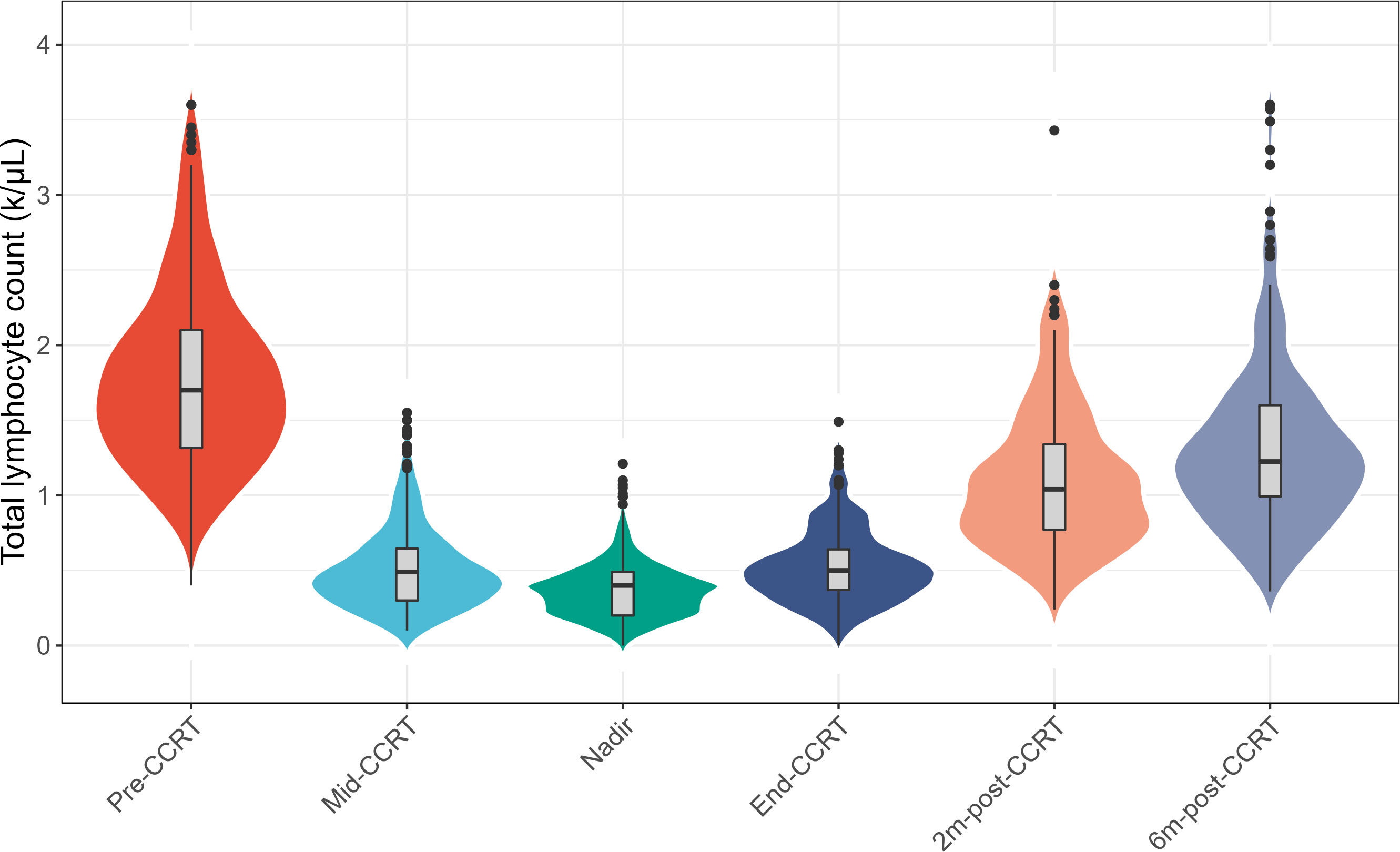
Figure 1 Changes of the total lymphocyte count (TLC) at longitudinal timepoints during concurrent chemoradiotherapy (CCRT) and follow-up. Abbreviations: pre-CCRT, the baseline; mid-CCRT, the 3 weeks during CCRT; end-CCRT, the end of CCRT; 2m-post-CCRT, 2 months after CCRT; 6m-post-CCRT, 6 months after CCRT.
Clinical and dosimetric factors associated with overall survival
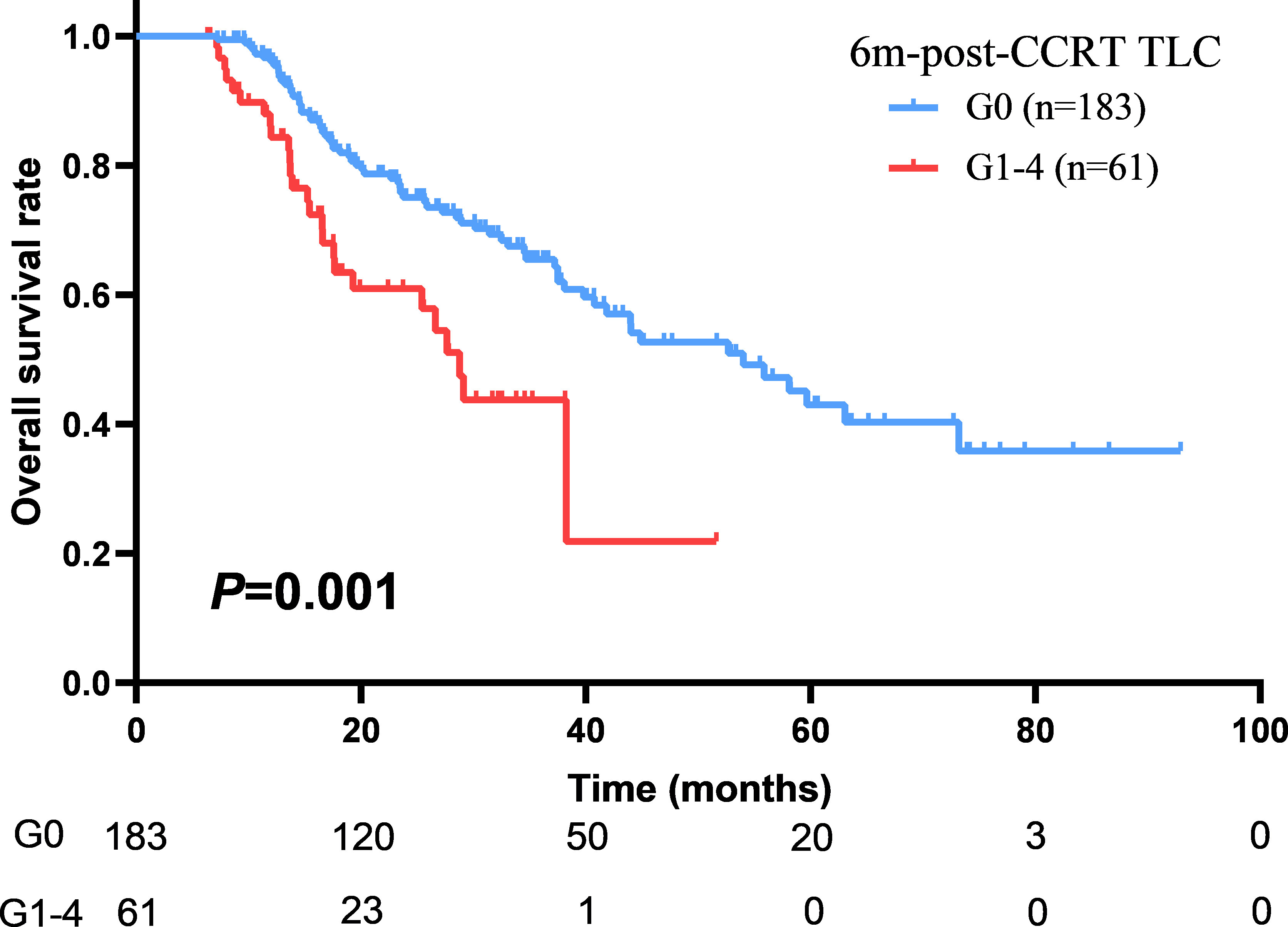
With a median follow up of 45.8 months (range: 1.5-92.9 months), the median OS and PFS were 41.0 months (95%CI: 36.2–45.9 months) and 12.7 months (95%CI: 11.3–14.2 months) for all patients. The 1-year and 2-year of local control rate were 70.2% and 50.9%, respectively. Age (HR: 1.542; P=0.005), gender (HR: 1.548; P=0.046), ECOG (HR: 1.496; P=0.008), disease stage (HR: 1.325; P=0.010), radiation dose (HR: 1.083; P=0.037), GTV volume (HR: 1.004; P<0.001), mid-CCRT TLC (HR: 2.040; P=0.018), 2m-post-CCRT TLC (HR: 1.754; P=0.004), 6m-post-CCRT TLC (HR: 2.189; P=0.001), TLC nadir (HR: 1.655; P=0.020), MBD (HR: 1.110; P=0.002), and MLD (HR: 1.085; P=0.001) were significantly associated with OS on univariable Cox regression analysis. Subsequently multivariable analysis demonstrated that gender (HR: 3.797; P=0.009), disease stage (HR: 1.620; P=0.045), GTV volume (HR: 1.004; P=0.035), 6m-post-CCRT TLC (HR: 2.614; P=0.041) were independent predictors of OS (Table 3, Figure 2). The relationship between progression free survival and local control rates with 6m-post-CCRT TLC were shown in Figure 3.
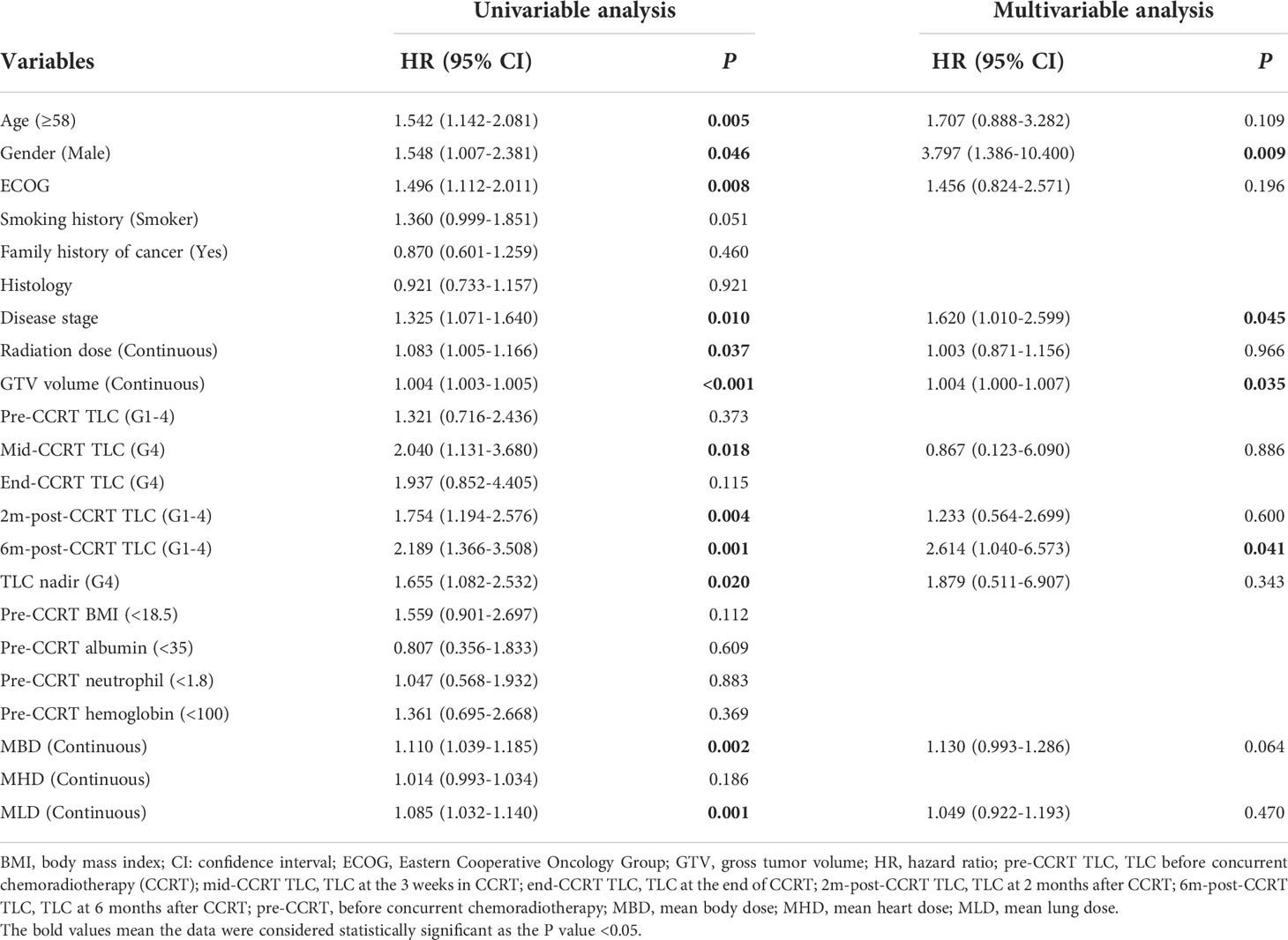
Table 3 Univariable and multivariable Cox regression analysis of potential variables associated with OS in LANSCLC patients.
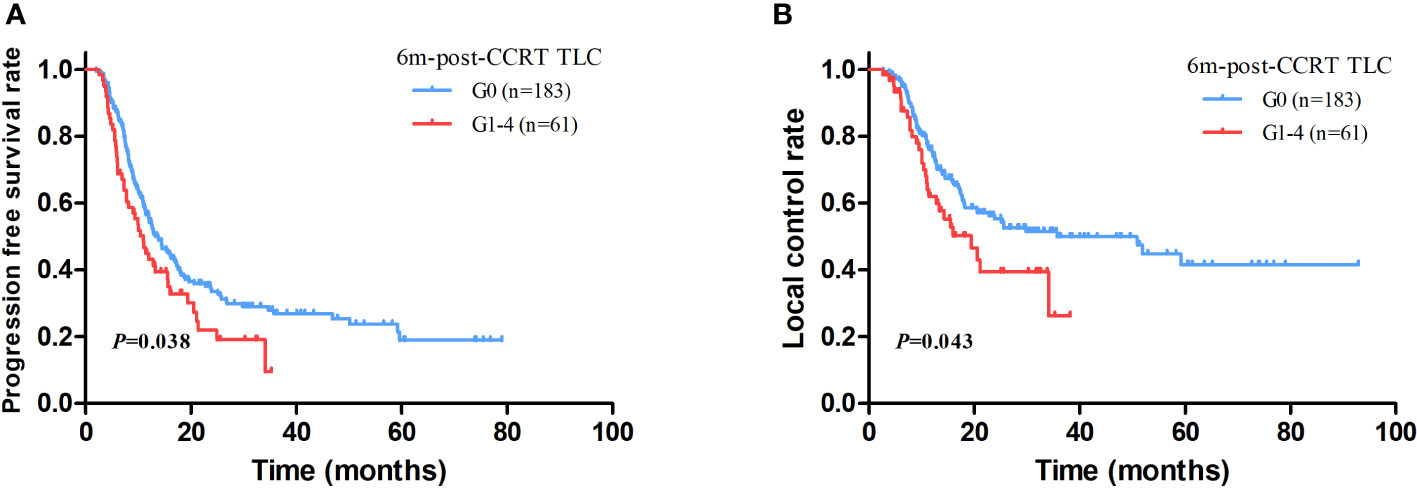
Figure 3 The association between progression free survival (PFS) (A) and local control (B) with 6m-post-CCRT TLC.
Clinical and dosimetric factors related to 6m-post-CCRT lymphopenia
Clinical and dosimetric factors affecting 6m-post-CCRT lymphopenia were shown in Table 4. Total radiation dose (OR: 1.307; P=0.002), GTV volume (OR: 1.005; P=0.002), pre-CCRT TLC (OR: 0.339; P<0.001), pre-CCRT BMI (OR: 3.044, P=0.040), and MLD (OR: 1.118, P=0.027) were found to be significantly associated with 6m-post-CCRT lymphopenia on the univariate analysis. Subsequently multivariable analysis revealed that radiation dose (OR: 1.328; P=0.005), GTV volume (OR: 1.004; P=0.036), and pre-CCRT TLC (OR: 0.288; P=0.001) were independent predictors of persistent lymphopenia at 6 months after CCRT.
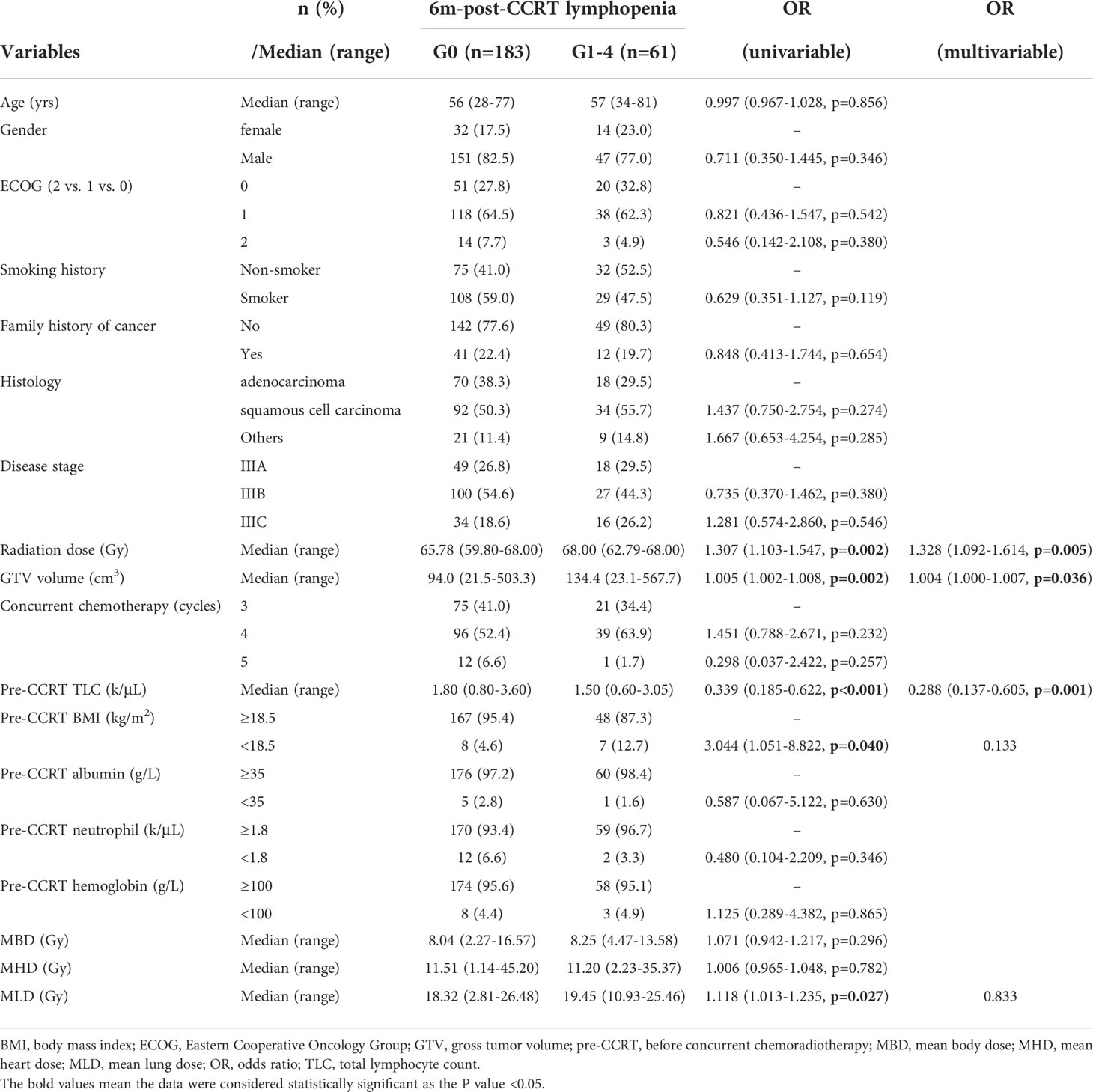
Table 4 Binary logistic regression results of variables related to persistent lymphopenia at 6 months after CCRT.
Discussion
The study investigated the relationship between the longitudinal TLC changes and survival outcome in LA-NSCLC patients undergoing definitive CCRT and evaluated the clinical factors associated with persistent lymphopenia induced by CCRT in the patient population. The results of current study suggested that the 6m-post-CCRT persistent lymphopenia was the independent predictor of OS among different time points.
94.5% of LA-NSCLC patients had a G0 TLC before CCRT, which dropped to 53.6% and 75.0% at 2 months and 6 months after the completion of CCRT. At 6 months after CCRT, 25% of patients had G1-4 CCRT related lymphopenia, with 1.2% of patients had G3 lymphopenia and no patients had G4 lymphopenia. The degree and timing of CCRT related lymphopenia in our study were consistent with previous studies that 51%-84% of patients had TLC reduction, the incidence of G3-4 lymphopenia was 35%-61% at 2 months after CCRT and persisted throughout one year of observation (5, 16–19).
As lymphocyte plays a vital role in infiltrating and destroying tumor tissues by activation of cellular immune response through T cells, TLC may be recognized as one of biomarkers to represent the immune status and anti-tumor response. Radiation to those circulating blood pool such as heart and lung might reduce tumor infiltrating lymphocytes by depleting circulating lymphocyte subpopulations, including CD8+ cytotoxic T cells, CD4+ helper T cells, and Foxp3+ regulatory T cells which have been proved to strongly correlate with survival outcome in NSCLC. Previous studies found that the TLC nadir, as well as lymphopenia occurred in 1 and 3 months after radiotherapy were significantly associated with OS in limited stage small cell lung cancer (20, 21). A large retrospective analysis of 901 patients with NSCLC demonstrated that TLC nadir with G3-4 lymphopenia was an independent factor of OS (11). Our results also found that TLC nadir was significantly associated with inferior OS in univariable analysis. Moreover, the multivariable analysis of current study suggested that the 6m-post-CCRT lymphopenia was the independent predictor of OS, indicating that the persistent lymphopenia after CCRT was crucial for long-term treatment outcome and regular examination was important for LA-NSCLC patients. The exact mechanism by which CCRT related lymphopenia affects OS is not clear. The impaired anti-tumor immunity associated with lymphopenia is one possible explanation. Recent studies suggested higher tumor infiltrated lymphocytes were associated with better disease control in lung cancer patients treated with immunotherapy (22, 23). In addition, CCRT related lymphopenia may lead to poorer OS due to increased lymphopenia-related complications such as infection (18). In our study, 103 patients (27.0%) used antibiotics for infection or ≥G2 radiation pneumonitis. In those patients, 3 patients (0.8%) experienced a sepsis, and 46 patients (12.1%) experienced infection confirmed by the procalcitonin testing and sputum culture and treated with prolonged antibiotics. For patients who had recurrent infections were more likely to have prolonged antibiotic use, which may induce antibiotic-associated bone marrow suppression through depletion of gut microbiota (24). In this case, lymphopenia as the reflector of myelosuppression was thought to be another prognostic factor for those patients.
PACIFIC study established the role of consolidative immunotherapy after CCRT for unresectable LA-NSCLC. However, not all patients could benefit from this strategy, raising an urgent need to discover important predictive factors. Among them, radiation induced lymphopenia is worthy of attention. Lymphopenia has been shown to negatively impact the treatment outcome of immunotherapy. Yeona et al. (13) demonstrated that patients with peri-immunotherapy lymphopenia showed worse OS and PFS. Friedes et al. (25) found that G3-4 lymphopenia before immunotherapy was associated with disease progression in patients with LA-NSCLC receiving consolidative immunotherapy after CCRT. Therefore, monitoring the recovery of TLC after CCRT was clinically important. Patients with favorable TLC recovery after CCRT and persistent normal TLC during long-term follow up could benefit more from the Pacific paradigm. In the current study, 46.4% and 25.0% of patients had persistent G1-4 lymphopenia at 2 months and 6 months after CCRT, respectively. Considering the impact of persistent lymphopenia on treatment outcome, utilizing TLC as a stratification tool for the initiation time of immunotherapy might help to improve treatment efficacy.
As the 6m-post-CCRT lymphopenia was independently associated with OS, pretreatment clinical factors associated with lymphopenia after CCRT and strategies to reduce the incidence of persistent lymphopenia should be considered. A growing number of studies have demonstrated that the incidence and severity of lymphopenia is associated with GTV volume, duration of radiotherapy, radiation fraction, multiple irradiated sites and receipt of concurrent chemotherapy (13, 26, 27). Abravan et al. has reported that vertebrae V20, MLD and MHD as predictors of lymphopenia by data mining approach (11). Higher doses of radiation to the immune system were associated with the development of G3 lymphopenia, tumor progression and death after the definitive treatment of stage III NSCLC (28). In our study, radiation dose, GTV volume, and pre-CCRT TLC were identified as significantly predictors of persistent lymphopenia. These results indicated that the appropriate radiation dose to gross tumor, effective induction therapy to reduce tumor volume before CCRT and nutrition support to maintain TLC level might be essential for clinical practice.
This study has several limitations aside from its retrospective nature. Firstly, some dosimetric factors were not included in the analysis, such as lung V5, and heart V5 (10, 29). There might be other dosimetric factors which contribute to the recovery of TLCs. Secondly, we included patients irradiated at different doses and fractionations, that might have different impact on the results. Third, all the patients in current study had no consolidative immunotherapy. For patients treated with CCRT followed by consolidation immunotherapy, the TLC after CCRT was supposed to be different and warrants further investigation.
In conclusion, the persistent G1-4 lymphopenia at 6 months after CCRT was found to be a prognostic factor of OS in LA-NSCLC patients. Higher radiation dose, larger gross tumor volume and lower baseline TLC were significantly related to 6m-post-CCRT lymphopenia. The association between the CCRT relate lymphopenia and OS suggests an important role of the host immunity in LA-NSCLC outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the review board of Sun Yat-sen University Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study conception and design: FL, YW, JS, and HL. Literature review: BQ. Data acquisition: YW, QL, FL, SG, JS, JG, DW, CC, and RZ. Statistical analysis: NC, XA, HL, and BQ. Data interpretation: FL, YW and JS. Manuscript preparation: FL, YW, JS, and HL. Manuscript review: All authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number 82073328).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res (2009) 69(13):5383–91. doi: 10.1158/0008-5472.Can-08-3845
2. Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) (2011) 236(5):567–79. doi: 10.1258/ebm.2011.011007
3. Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol (1987) 139(10):3199–206.
4. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol (2018) 123:42–51. doi: 10.1016/j.critrevonc.2018.01.003
5. Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol (2016) 140(1):76–82. doi: 10.1016/j.ygyno.2015.11.013
6. Liu LT, Chen QY, Tang LQ, Guo SS, Guo L, Mo HY, et al. The prognostic value of treatment-related lymphopenia in nasopharyngeal carcinoma patients. Cancer Res Treat (2018) 50(1):19–29. doi: 10.4143/crt.2016.595
7. Tang C, Lee MS, Gomez D, Levy LB, Zhuang Y, Lu C, et al. Effects of chemotherapy regimen and radiation modality on hematologic toxicities in patients receiving definitive platinum-based doublet chemoradiation for non-small cell lung cancer. Am J Clin Oncol (2017) 40(6):625–30. doi: 10.1097/coc.0000000000000206
8. Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol (2015) 38(3):259–65. doi: 10.1097/COC.0b013e3182940ff9
9. Upadhyay R, Venkatesulu BP, Giridhar P, Kim BK, Sharma A, Elghazawy H, et al. Risk and impact of radiation related lymphopenia in lung cancer: A systematic review and meta-analysis. Radiother Oncol (2021) 157:225–33. doi: 10.1016/j.radonc.2021.01.034
10. Zhao Q, Li T, Chen G, Zeng Z, He J. Prognosis and risk factors of radiation-induced lymphopenia in early-stage lung cancer treated with stereotactic body radiation therapy. Front Oncol (2019) 9:1488. doi: 10.3389/fonc.2019.01488
11. Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol (2020) 15(10):1624–35. doi: 10.1016/j.jtho.2020.06.008
12. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget (2017) 8(69):114268–80. doi: 10.18632/oncotarget.23217
13. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2019) 105(5):1065–73. doi: 10.1016/j.ijrobp.2019.08.047
14. Qiu B, Wang D, Li Q, Wu Y, Guo S, Jiang X, et al. Concurrent chemoradiation therapy with or without nimotuzumab in locally advanced squamous cell lung cancer: A phase 2 randomized trial. Int J Radiat Oncol Biol Phys (2021) 111(4):917–25. doi: 10.1016/j.ijrobp.2021.06.032
15. Qiu B, Xiong M, Luo Y, Li Q, Chen N, Chen L, et al. Hypofractionated intensity modulated radiation therapy with concurrent chemotherapy in locally advanced non-small cell lung cancer: A phase II prospective clinical trial (GASTO1011). Pract Radiat Oncol (2021) 11(5):374–83. doi: 10.1016/j.prro.2021.06.004
16. Campian JL, Ye X, Sarai G, Herman J, Grossman SA. Severe treatment-related lymphopenia in patients with newly diagnosed rectal cancer. Cancer Invest (2018) 36(6):356–61. doi: 10.1080/07357907.2018.1499028
17. Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest (2013) 31(3):183–8. doi: 10.3109/07357907.2013.767342
18. Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest (2012) 30(8):571–6. doi: 10.3109/07357907.2012.700987
19. Lee G, Kim DW, Muralidhar V, Mitra D, Horick NK, Eyler CE, et al. Chemoradiation-related lymphopenia and its association with survival in patients with squamous cell carcinoma of the anal canal. Oncologist (2020) 25(12):1015–22. doi: 10.1634/theoncologist.2019-0759
20. Wang X, Lu J, Teng F, Yu J. Lymphopenia association with accelerated hyperfractionation and its effects on limited-stage small cell lung cancer patients' clinical outcomes. Ann Transl Med (2019) 7(16):385. doi: 10.21037/atm.2019.07.58
21. Cho O, Oh YT, Chun M, Noh OK, Lee HW. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol (2016) 37(1):971–8. doi: 10.1007/s13277-015-3888-y
22. Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating b cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol (2019) 16(1):6–18. doi: 10.1038/s41423-018-0027-x
23. Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: A meta-analysis and individual patient-level analysis. JAMA network Open (2019) 2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879
24. Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood (2017) 129(6):729–39. doi: 10.1182/blood-2016-03-708594
25. Friedes C, Chakrabarti T, Olson S, Prichett L, Brahmer JR, Forde PM, et al. Association of severe lymphopenia and disease progression in unresectable locally advanced non-small cell lung cancer treated with definitive chemoradiation and immunotherapy. Lung Cancer (2021) 154:36–43. doi: 10.1016/j.lungcan.2021.01.022
26. Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys (2014) 89(5):1084–91. doi: 10.1016/j.ijrobp.2014.04.025
27. Joseph N, McWilliam A, Kennedy J, Haslett K, Mahil J, Gavarraju A, et al. Post-treatment lymphocytopaenia, integral body dose and overall survival in lung cancer patients treated with radical radiotherapy. Radiother Oncol (2019) 135:115–9. doi: 10.1016/j.radonc.2019.03.008
28. Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys (2019) 105(2):346–55. doi: 10.1016/j.ijrobp.2019.05.064
Keywords: total lymphocyte count, concurrent chemoradiotherapy, overall survival, locally advanced non-small cell lung cancer, lymphopenia
Citation: Liu F, Wu Y, Shao J, Qiu B, Guo S, Luo Q, Guo J, Wang D, Chu C, Zhou R, Chen N, Ai X and Liu H (2022) Hypofractionated concurrent chemoradiotherapy related lymphopenia and its association with survival in locally advanced non-small cell lung cancer patients. Front. Oncol. 12:979384. doi: 10.3389/fonc.2022.979384
Received: 28 June 2022; Accepted: 31 October 2022;
Published: 18 November 2022.
Edited by:
Dawei Chen, Shandong Cancer Hospital, ChinaReviewed by:
Bhanu Prasad Venkatesulu, Loyola University Chicago, United StatesHong In Yoon, Yonsei University, South Korea
Copyright © 2022 Liu, Wu, Shao, Qiu, Guo, Luo, Guo, Wang, Chu, Zhou, Chen, Ai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liu, bGl1aHVpc3lzdWNjQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 FangJie Liu
FangJie Liu YingJia Wu1†
YingJia Wu1† SuPing Guo
SuPing Guo DaQuan Wang
DaQuan Wang Hui Liu
Hui Liu