- Department of Thoracic Surgery, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
Background: Recently, the new World Health Organization (WHO) tumor classification removed adenocarcinoma in situ (AIS) from the diagnosis of lung cancer. However, it remains unclear whether the “malignancy” item should be assessed when the modified Caprini Risk Assessment Model (RAM) is used to assess venous thromboembolism (VTE) risk in AIS. The purpose of our study is to assess differences between AIS and stage IA adenocarcinoma (AD) from a VTE perspective.
Methods: A retrospective study was performed on AIS and IA adenocarcinoma in our hospital from January 2018 to December 2021, and divided into AIS group and AD group. Propensity score matching (PSM) was used to compare the incidence of VTE and coagulation function, and to analyze whether the RAM is more effective when the “malignancy” item is not evaluated in AIS.
Results: 491 patients were included after screening, including 104 patients in the AIS group and 387 patients in the AD group. After PSM, 83 patients were matched. The incidence of VTE and D-dimer in the AIS group was significantly lower than that in the AD group (P<0.05).When using the RAM to score AIS, compared with retaining the “malignancy” item, the incidence of VTE in the intermediate-high-risk group was significantly higher after removing the item (7.9% vs. 36.4%, P=0.018), which significantly improved the stratification effect of the model.
Conclusions: The incidence of postoperative VTE in AIS was significantly lower than that in stage IA adenocarcinoma. The stratification effect was more favorable when the “malignancy” item was not evaluated in AIS using the RAM.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with malignant tumors, mainly including deep vein thrombosis (DVT) and pulmonary embolism (PE). Once it occurs, it significantly increases the patient’s risk of postoperative complications and death (1, 2). The risk of VTE in cancer patients is significantly higher than that in the general population, especially lung cancer, which is the malignant tumor with the highest incidence of VTE (3, 4). This was confirmed in our previous study, with a 15% incidence of VTE after lung cancer surgery (5). Therefore, lung cancer patients undergoing surgical treatment are a high-risk group for VTE.
To better identify patients at high risk of VTE, a variety of VTE risk assessment models have been developed, among which the modified Caprini RAM is widely used in thoracic surgery (6). High-risk factors for VTE include patient-related risk factors (age, body mass index, abnormal lung function, varicose veins, confined to bed, etc.), tumor-related risk factors (current malignant tumor, history of tumor, etc.), and treatment-related factors (operation time, central venous access, chemotherapy, etc.). In addition, the guidelines recommend perioperative VTE prophylaxis in patients after surgery at intermediate and high risk as assessed by the use of the modified Caprini RAM (7, 8). In the current real world, with the improvement of health awareness and the promotion of low-dose chest CT in lung cancer screening, pulmonary nodules are becoming increasingly common in the clinical work of thoracic surgery, especially ground glass opacities (GGOs) (9–11). Among them, the number of patients with the postoperative pathology of AIS is also increasing.
At present, the tumor classification of the World Health Organization (WHO) is regarded as an internationally recognized tumor diagnostic standard, which is crucial for guiding clinical treatment. Compared with the fourth edition of the WHO classification released in May 2015 (12), the fifth edition of the WHO classification released in April 2021 rearranged lung tumors and removed AIS from the list of adenocarcinomas (13). AIS is no longer classified as a malignant tumor and is classified as a precursor gland lesion. Whether the “malignancy” item should be evaluated in patients with AIS using the modified Caprini RAM is unclear. Therefore, this study aimed to compare the incidence of VTE between patients with AIS and stage IA adenocarcinoma by propensity score matching (PSM) and to evaluate the effectiveness of the modified Caprini RAM in predicting postoperative VTE in patients with AIS.
Materials and methods
Patient selection
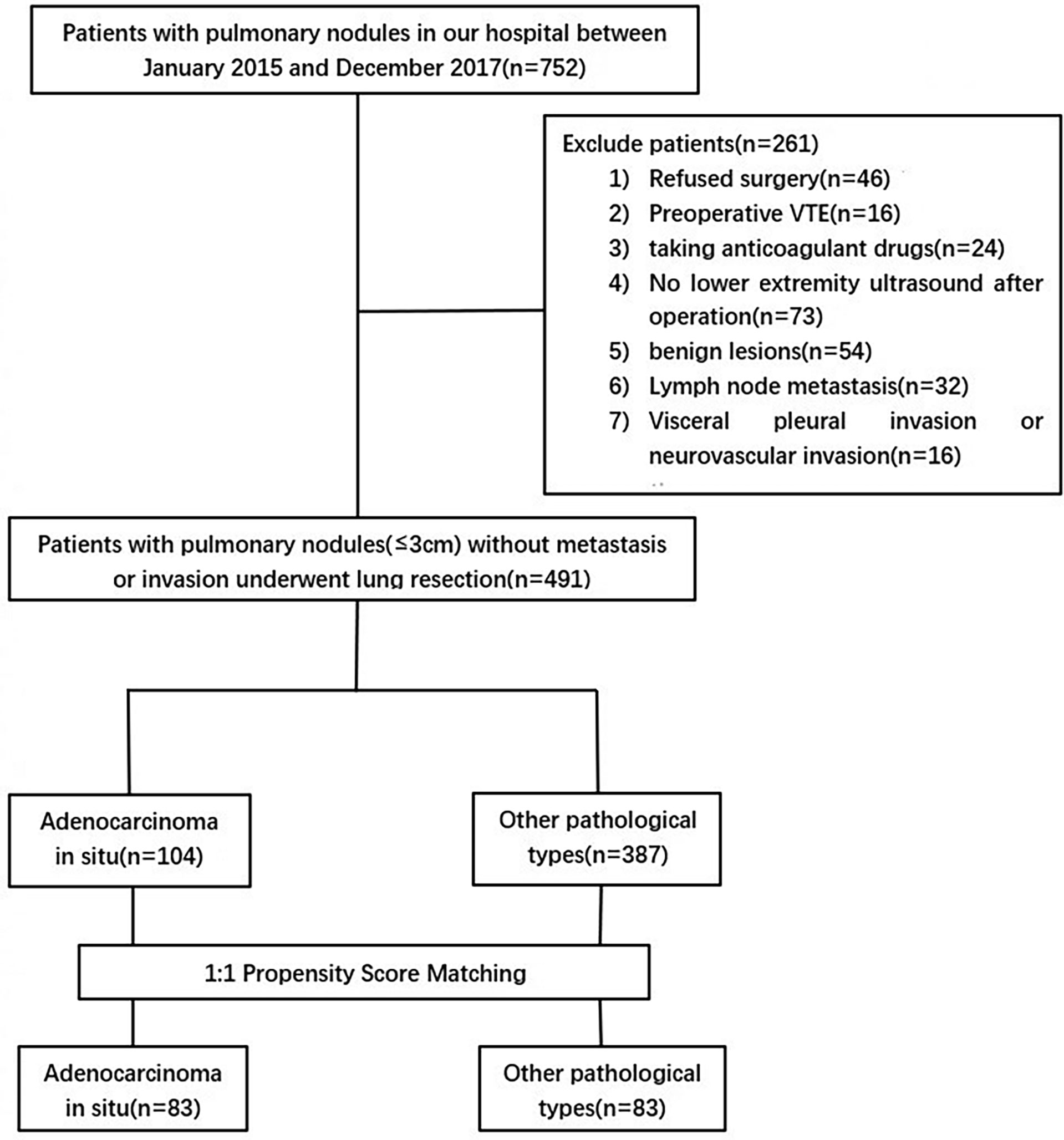
This study is a single-center retrospective study that was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (2017-Ke-1), and patients were exempted from informed consent. The clinical data of 752 patients with clinical stage IA disease from January 2018 to December 2021 were retrospectively collected. Clinical and pathological TNM staging was determined according to the eighth edition of lung cancer staging published by the American Joint Committee on Cancer/International Union Against Cancer (14). The inclusion criteria were as follows: (1) postoperative pathological diagnosis of primary AIS or stage IA adenocarcinoma; (2) lower extremity venous ultrasound before and after surgery; and (3) preoperative lower extremity venous ultrasound showed no DVT. The exclusion criteria were as follows: (1) postoperative pathological diagnosis of benign lesions; (2) pathological diameter greater than 3 cm, lymph node metastasis or invasion of pleura, neurovascular, etc.; (3) preoperative lower extremity ultrasonography diagnosed as DVT; (4) no lower extremity venous ultrasound was performed postoperatively; (5) anticoagulant drugs were required for any reason during the perioperative period; and (6) the patient refused surgical treatment. Finally, a total of 491 patients were included in this study (Figure 1). The follow-up period ended at discharge.
Data collection
The following clinical data were collected through the electronic medical record system: age, sex, body mass index (BMI), hospital stay, acute myocardial infarction (<1 month), congestive heart failure (<1 month), history of inflammatory bowel disease, history of prior major surgery (<1 month), complications of pregnancy, oral contraceptives or hormone replacement therapy (HRT), sepsis (<1 month), severe acute lung disease (<1 month), comorbidities (hypertension, diabetes, coronary heart disease, hyperlipidemia), smoking history, drinking history, family history, history of malignant tumor, previous history of VTE, confined to bed >72 hours, central venous access, history of chemotherapy, abnormal pulmonary function, swollen legs (current), varicose veins, intermuscular vein dilation, positive anticardiolipin antibody, positive lupus anticoagulant, acute spinal cord injury (<1 month), operation-related information (surgical approach, resection range, operation time, blood loss, and number of lymph nodes removed), pathological diameter, tumor location, nodule morphology, forced expiratory volume in one second (FEV1), forced vital capacity (FVC), maximal voluntary ventilation (MVV), platelets (PLT), prothrombin time (PT), partial thromboplastin time (APTT), D-dimer, and VTE events.
In this study, all enrolled patients underwent preoperative and postoperative lower extremity venous ultrasonography to evaluate the presence or absence of DVT. If the patient had typical symptoms of PE (chest pain, hemoptysis, dyspnea or persistent unexplained hypoxemia), a modified Caprini score ≥9, or newly diagnosed DVT after surgery, computed tomography pulmonary angiography (CTPA) was performed.
Evaluation of VTE and coagulation
A VTE event was defined as postoperative VTE in patients with no preoperative diagnosis of VTE. The evaluation of coagulation indicators included preoperative and postoperative PLT, PT, APTT, and D-dimer. The preoperative coagulation results of all patients were blood drawn and submitted on the first day of hospitalization; the postoperative coagulation results were blood drawn and submitted on the first postoperative day. The PSM method was used to evaluate the differences in the incidence of VTE and coagulation between the two groups of patients with AIS and stage IA adenocarcinoma.
Evaluation of the modified Caprini RAM
The modified Caprini RAM was used to assess the risk of VTE according to clinical parameters (Supplementary Table). In this study, all clinical parameters in the model were collected for PSM, and the incidence of VTE in patients with AIS and stage IA adenocarcinoma was compared.
All patients underwent modified Caprini RAM risk stratification, with ≤4 scores indicating low risk, 5-8 scores indicating risk, and ≥9 scores indicating high risk. In this study, patients with AIS were divided into two groups according to the score with keeping and removing the “malignant tumor” item, and the proportion of the intermediate-high-risk population in the two groups was compared to evaluate the effectiveness of the modified Caprini RAM for AIS patients.
Statistical analysis
Continuous variables with a normal distribution are expressed as the mean ± standard deviation (mean ± SD), and a t-test was used for comparisons between groups. Continuous variables that did not conform to a normal distribution are represented by M (Q1, Q3), and comparisons between groups were performed using the Mann–Whitney U test. Categorical variables are represented by percentages using the χ2 test or Fisher’s exact test. To eliminate the effects of confounding factors and selection bias, 1:1 PSM was used to match the two groups of patients (PS = 0.02). Matched variables included clinical parameters in the modified Caprini RAM scale, comorbidities (hypertension, diabetes, coronary heart disease, and hyperlipidemia), smoking history, drinking history, family history, intermuscular vein dilatation, surgery-related information (surgical approach, resection range, operation time, blood loss, and number of lymph nodes removed), pathological diameter, tumor location, nodule morphology, FEV1, FVC, and MVV. Outcome variables were the above coagulation indicators and VTE events. All analyses in this study were performed using SPSS version 26.0 software (IBM, Armonk, NY, USA), and a two-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
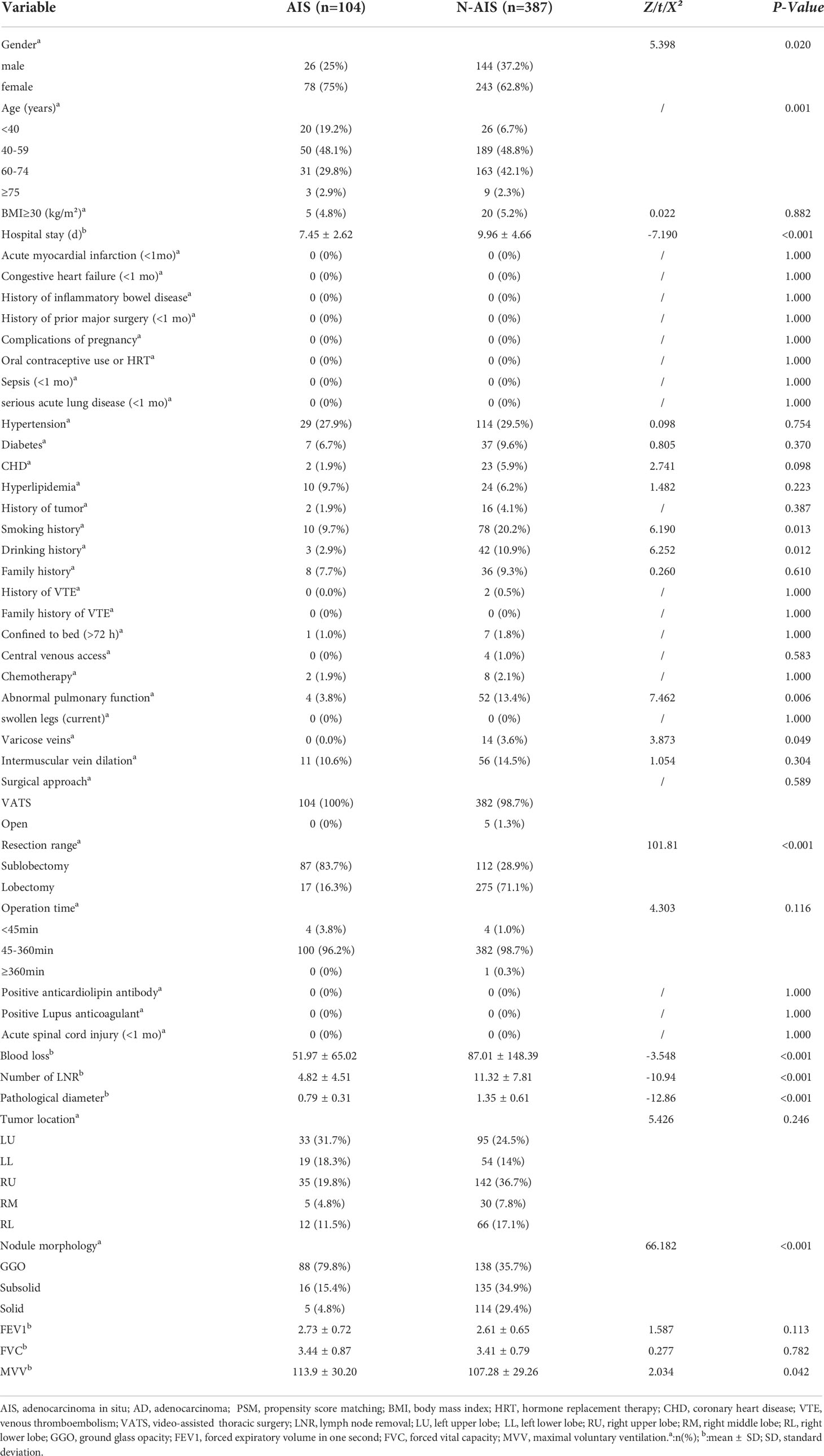
Among the 491 patients enrolled, 104 patients (21%) were diagnosed with AIS postoperatively, and 387 patients (79%) were diagnosed with stage IA adenocarcinoma. The baseline data of all patients are shown in Table 1. There were significant differences in age, sex and length of hospital stay between the two groups (P<0.05). Compared with the AD group, the AIS group had a higher proportion of females, younger age, and shorter hospital stay. However, there was no significant difference in BMI or comorbidities (hypertension, diabetes, CHD, and hyperlipidemia) between the two groups (P>0.05). Comparing the past histories of the two groups, the results showed that the proportions of smoking and drinking history in the AD group were significantly higher than those in the AIS group (20.2% vs. 9.7%, 10.9% vs. 2.9%, P<0.05), but in acute myocardial infarction (<1 month), congestive heart failure (<1 month), inflammatory bowel disease, previous major surgery (<1 month), complications of pregnancy, oral contraceptives or HRT, sepsis (<1 month), severe acute lung disease (<1 month), tumor history, family history, history of VTE, and acute spinal cord injury (<1 month) the results were not significantly different (P>0.05). There were significant differences in abnormal pulmonary function, MVV, and varicose veins between the two groups (P<0.05). The AD group had significantly more patients with abnormal lung function (13.4% vs. 3.8%, P=0.006) and varicose veins (3.6% vs. 0%, P=0.049) than the AIS group, and the MVV in the AD group was significantly lower than that in the AIS group (107.28 ± 29.26 vs. 113.9 ± 30.2, P=0.042); however, there were no significant differences in FEV1, FVC, confined to bed >72 hours, central venous access, history of chemotherapy, intermuscular vein dilation, positive anticardiolipin antibody, and positive lupus anticoagulant in both groups. The surgical and pathological information of the two groups was compared. The results showed that the pathological diameter (0.79 ± 0.31 vs. 1.35 ± 0.61, P<0.001), the number of lymph nodes removed (4.82 ± 4.51 vs. 11.32 ± 7.81, P<0.001) and blood loss in the AIS group (51.97 ± 65.02 vs. 87.01 ± 148.39, P<0.001) were significantly lower than those in the AD group, and there were also significant differences in nodule morphology and resection range between the two groups. The nodules in the AIS group were mainly ground glass opacities (GGOs) (79.8%) upon imaging, and the resection range was mostly sublobar resection (83.7%). However, the operation approach, operation time and tumor location were all comparable (P>0.05).
Propensity score matching
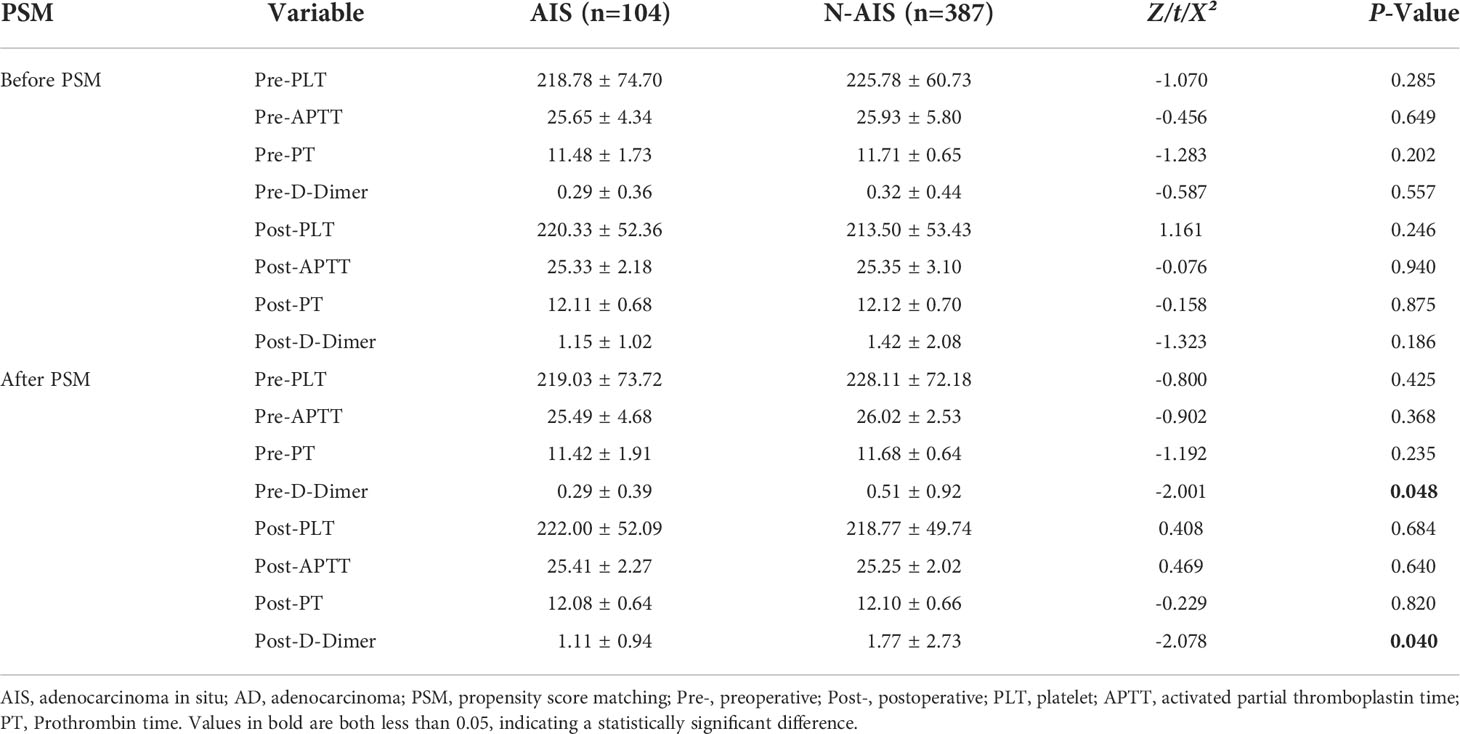
Before PSM, there were significant differences in age, sex, length of hospital stay, smoking history, drinking history, abnormal lung function, MVV, varicose veins, pathological diameter, number of lymph nodes removed, blood loss, nodule morphology, and resection range between the two groups of patients (Table 1). The incidence of VTE and coagulation indicators were compared between the two groups. The results showed that (Figure 2A) there were 7 and 35 patients with VTE events in the AIS group and AD group, respectively, and there was no significant difference in the incidence of VTE between the two groups (6.7% vs. 9.0%, P>0.05). There was also no significant difference in coagulation indices preoperatively and postoperatively between the two groups (Table 2).
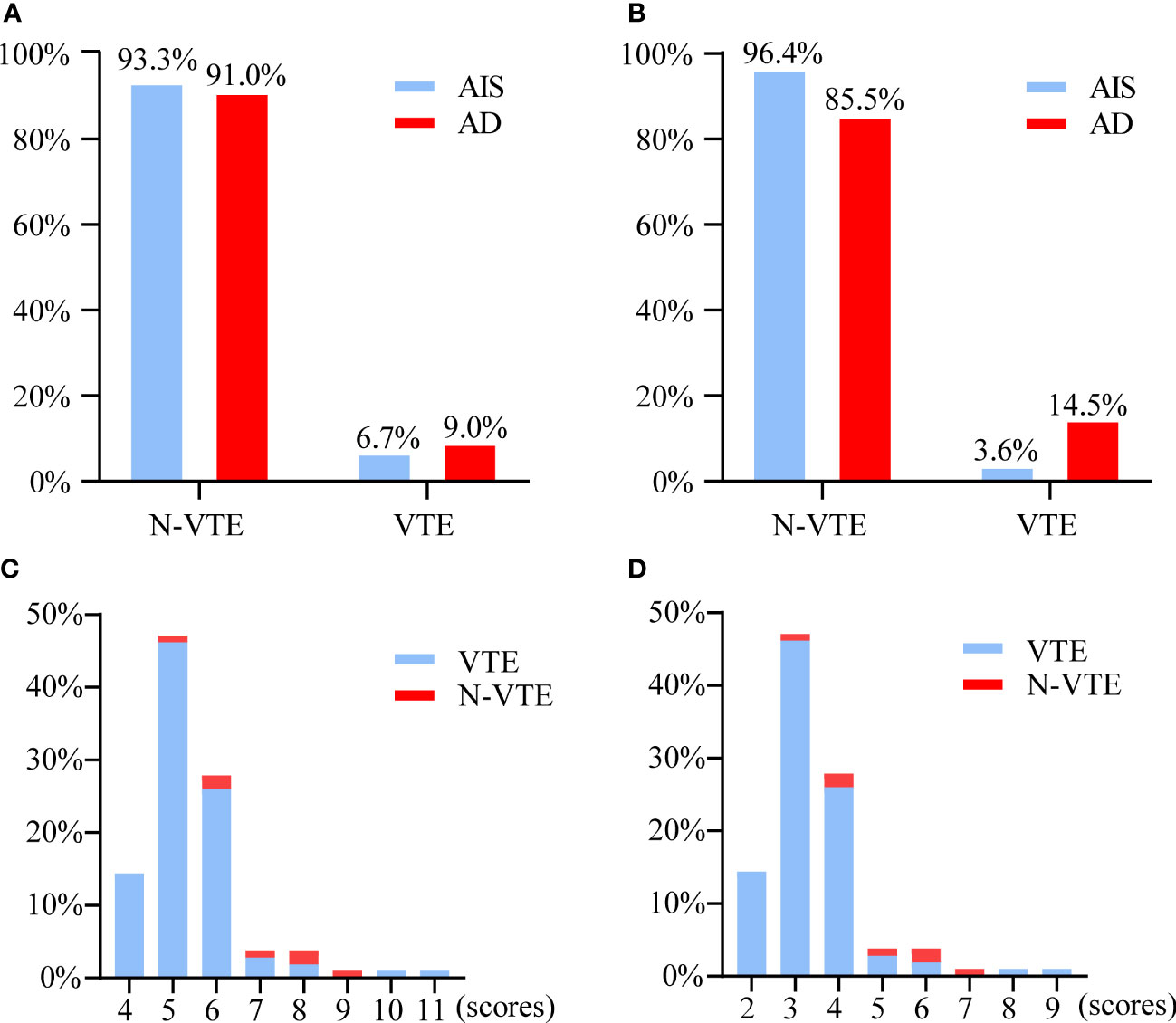
Figure 2 (A) Proportion of VTE and N-VTE patients between AIS and AD groups before PSM. (B) Proportion of VTE and N-VTE patients between AIS and AD groups after PSM. (C) Distribution of Caprini scores in patients with AIS while remaining the “malignant tumor” item. (D) Distribution of Caprini scores in patients with AIS while removing the “malignant tumor” item. VTE, venous thromboembolism. AIS, adenocarcinoma in situ.PSM, propensity score matching. AD, adenocarcinoma.
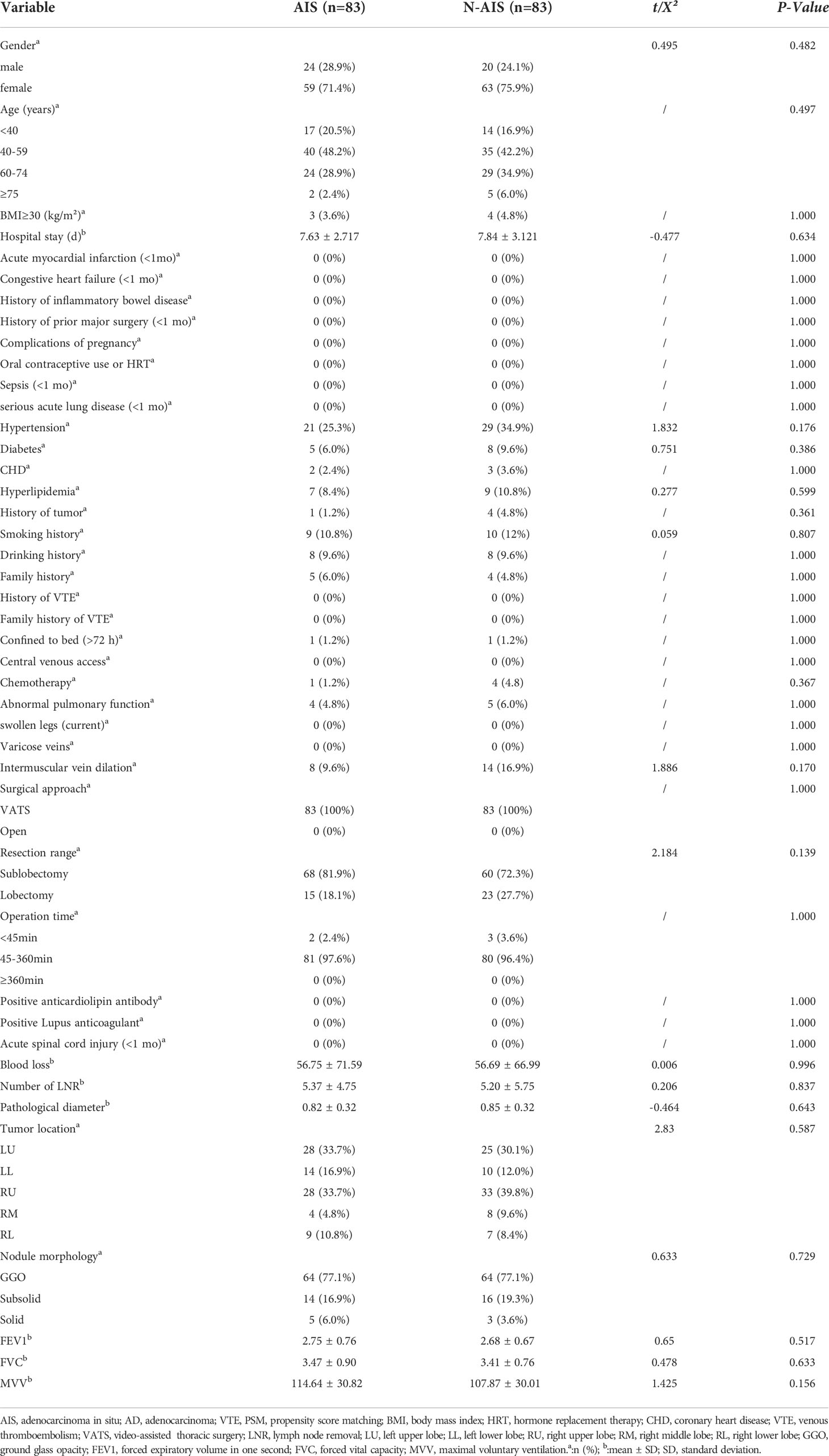
After PSM, the baseline data of 83 patients were re-evaluated. The results are shown in Table 3. There was no significant difference in any baseline variable between the two groups (P>0.05). Re-evaluation of the incidence of VTE and coagulation indicators showed that (Figure 2B) there were 3 and 12 patients with VTE events in the AIS group and AD group, respectively, and there was a significant difference in the incidence of VTE between the two groups (3.6% vs. 14.5%, P =0.015). In terms of coagulation indicators (Table 2), the preoperative and postoperative D-dimer levels in the AIS group were significantly lower than those in the AD group (0.29 ± 0.39 vs. 0.51 ± 0.92; 1.11 ± 0.94 vs. 1.77 ± 2.73, P<0.05).
Effect of the modified Caprini RAM
All patients with AIS (n=104) were assigned a risk score according to the modified Caprini RAM, in which “malignant tumor” accounted for 2 scores. When the “malignant tumor” item score was retained, the distributions of the Caprini score and VTE are shown in Figure 2C. There were 15, 86, and 3 patients in the low-, intermediate-, and high-risk groups, respectively. In addition, 0, 6, and 1 patients in the three groups had VTE events, mainly in the intermediate-risk group (5-8 scores). When the “malignant tumor” item was not evaluated, the distributions of the Caprini score and VTE are shown in Figure 2D. There were 93, 10, and 1 patients in the low-, intermediate-, and high-risk groups, respectively. Among the three groups, VTE events occurred in 3, 4, and 0 patients, respectively. The incidence of VTE in the intermediate-high-risk patients was compared between the above two scores. The results are shown in Figure 3. The incidence of VTE was 7.9% and 36.4%, respectively, with a significant difference (P=0.018). For AIS, the modified Caprini RAM was more effective in predicting postoperative VTE in intermediate-high-risk patients when the “malignancy” item was not evaluated.
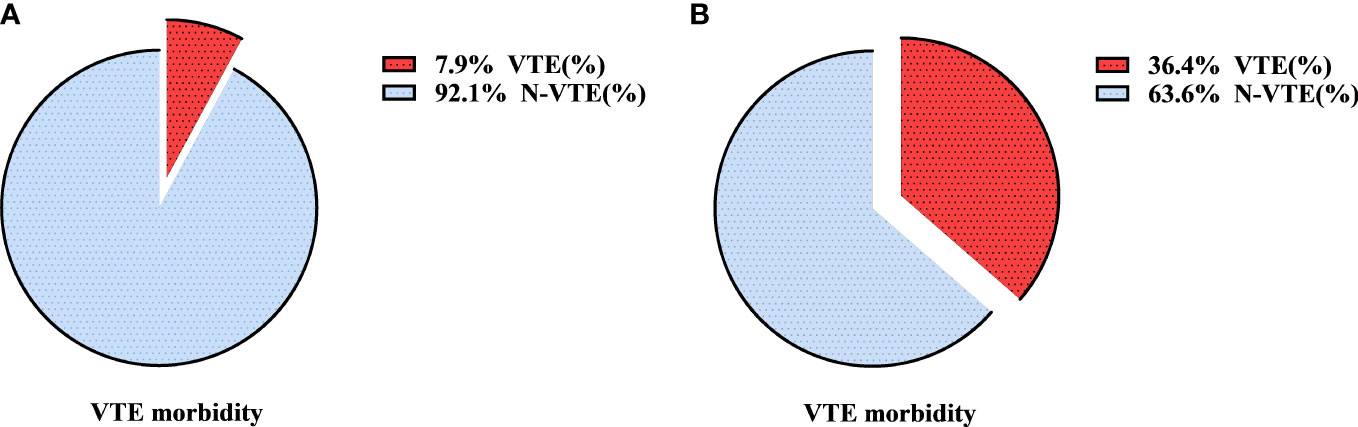
Figure 3 The incidence of VTE in intermediate-high risk with AIS after modified Caprini RAM when remaining the “malignant tumor” item (A) or removing the “malignant tumor” item (B). VTE, venous thromboembolism; RAM, risk assessment model; AIS, adenocarcinoma in situ.
Discussion
At present, the incidence of postoperative VTE in patients with AIS is unclear. This study confirmed that the overall incidence of postoperative VTE was 6.7%, which is similar to the incidence of VTE after lung resection for benign lesions (7%) in our previous study (5). Many previous studies have confirmed that AIS mostly appears as pure ground-glass opacities (p-GGOs) on imaging (15–17). In recent years, the widespread promotion and application of low-dose chest computed tomography (CT) has facilitated the detection of pulmonary nodules in many asymptomatic individuals. Among them, isolated ground-glass opacities (GGOs) have become an emerging group in thoracic surgery (18). This study showed that 79.8% of AIS patients showed p-GGOs on CT, which was consistent with the results of previous studies (15, 16). Ishida et al. (15) reported that 74% of AIS patients showed p-GGOs on CT, and Jia et al. (16) also reported that 78% of AIS patients showed p-GGOs on CT. It can be seen that with the increase in the detection rate of GGOs, an increasing number of patients are diagnosed with AIS. However, there is no relevant research report on the incidence of postoperative VTE in these patients.
The incidence of postoperative VTE in patients with AIS may be significantly lower than in other adenocarcinoma types. Many factors can influence the occurrence of VTE, including patient-related risk factors (age, complications, previous VTE history, etc.), tumor-related risk factors (tumor diameter, nodule morphology, etc.) and treatment-related risk factors (chemotherapy, surgery, center Intravenous access, etc.) (19). In addition, previous studies have confirmed that different TNM stages significantly affect the occurrence of VTE events (20–22). Among them, Lee et al. (21) showed that the risk of VTE in patients with non-small-cell lung cancer increased 2.45 times with the progression of each stage. Cui et al. (22) also showed that patients with advanced stage disease were at increased risk of VTE. Previous studies have suggested that lung adenocarcinoma exhibits a natural progression pattern of AAH-AIS-MIA-IA with different degrees of malignancy (23–25). However, the influence of different degrees of malignancy of adenocarcinoma on the occurrence of VTE events is still unclear. In this study, the propensity score matching method (PSM) was used to eliminate the factors that interfered with the occurrence of VTE events between the two groups of patients with AIS and stage IA adenocarcinoma and confirmed for the first time that the incidence of postoperative VTE in patients with AIS was significantly lower than that in adenocarcinoma patients with other degrees of malignancy. Moreover, the author Ming S. Tsao of the recently released fifth edition of the World Health Organization (WHO) classification of thoracic tumors (13) considered AIS as a precursor gland lesion and removed the diagnosis of lung cancer to obtain a clearer tumor classification. On the other hand, as we all know, thymic tumors have a low grade of malignancy and a good prognosis. A previous study reported the incidence of VTE after thymectomy and evaluated the effectiveness of Caprini RAM (26), in which the incidence of VTE was 4.6% in patients with benign thymic disease and 14.5% in patients with malignant disease. The incidence of VTE in AIS that we reported was similar to the benign disease in the above study; Moreover, previous studies (27) have confirmed that AIS patients can be cured after complete resection, and the 10-year postoperative recurrence-free survival rate is almost 100%, which has verified the biological behavior of AIS as having low-grade malignant potential. Our study also confirmed this from the perspective of VTE, and patients with AIS have a lower risk of VTE after surgery, similar to the incidence of VTE after surgery for benign disease in thoracic surgery.
When applying the modified Caprini RAM to assess VTE risk in patients with adenocarcinoma in situ, it may be more appropriate not to score the “malignancy” item. Our study confirmed that when the “malignant tumor” item was not evaluated, the incidence of VTE in intermediate-high-risk patients was significantly increased from 7.9% to 36.4%. At this time, the modified Caprini RAM can effectively screen out the susceptible VTE population, with an excellent stratification effect. Whether the “malignancy” item is evaluated plays an important role in modified Caprini stratification in patients with AIS. The patients with AIS in this study were mainly younger female patients with fewer comorbidities, consistent with the baseline characteristics of patients in other studies (28–30), and these patients rarely have a long operation time. If the “malignancy” item remains, most postoperative patients are at intermediate risk (5-8 scores) in the modified Caprini risk stratification. The patients with AIS were further scored twice to retain and exclude the “malignant tumor” item, and then the incidence of VTE in patients at intermediate-high risk (≥5 scores) was compared between the two scores. The results showed that the “malignancy” item not being evaluated can effectively improve the accuracy of the modified Caprini RAM stratification. Therefore, in our study, it is believed that when Caprini RAM is applied to patients with AIS, excluding the “malignancy” item score will make the prediction accuracy higher.
The underlying mechanism of the lower risk of VTE in patients with AIS is unclear. However, previous studies (15, 16, 27, 31) have shown that adenocarcinoma in situ has a low-grade malignant potential with an almost 100% 5-year survival rate after surgical resection. Furthermore, studies (32, 33) have shown that the blood of patients with aggressive malignancies is hypercoagulable and prone to VTE. In this study, the preoperative and postoperative coagulation indicators of the two groups of patients after PSM were further analyzed. The results showed that the preoperative and postoperative D-dimer levels of patients with AIS were significantly lower than those of patients with stage IA adenocarcinoma. The biological behavior of AIS and malignant tumors may be different, and coagulation function is less affected; thus, patients with AIS have a significantly lower risk of VTE.
There are some limitations in this study. First, although PSM was applied to match as many variables as possible, there may still be potential confounding factors, which need to be further verified by prospective randomized controlled trials. Second, our study is a single-center study, which does not have universal applicability. Finally, monitoring for the occurrence of VTE was not continued after discharge, which may have underestimated the incidence of VTE.
Conclusion
The incidence of VTE after surgery for AIS was significantly lower than that for patients with stage IA adenocarcinoma after surgery. When using the modified Caprini RAM to assess VTE risk in patients with AIS, higher predictive accuracy was achieved when the “malignancy” item was not evaluated. Our study confirmed that from the perspective of VTE, the diagnosis of adenocarcinoma in situ removed from lung cancer may be more appropriate.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-sC collected and analyzed the patient data and were the major contributors in writing this manuscript. H-hD and X-yL participated in data collection and collation. XY and SC contributed to methodology. J-bM and Q-rC contributed to designing and critically revising the article. BH and HL contributed to the article review. All authors read and approved the final manuscript.
Acknowledgments
We want to thank all staff and patients enrolled in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.976988/full#supplementary-material
References
1. Klemen ND, Feingold PL, Hashimoto B, Wang M, Kleyman S, Brackett A, et al. Mortality risk associated with venous thromboembolism: A systematic review and Bayesian meta-analysis. Lancet Haematol (2020) 7(8):e583–93. doi: 10.1016/S2352-3026(20)30211-8
2. Rokosh RS, Ranganath N, Yau P, Rockman C, Sadek M, Berland T, et al. High prevalence and mortality associated with upper extremity deep venous thrombosis in hospitalized patients at a tertiary care center. Ann Vasc Surg (2020) 65:55–65. doi: 10.1016/j.avsg.2019.10.055
3. Mahajan A, Brunson A, White R, Wun T. The Epidemiology of Cancer-Associated Venous Thromboembolism: An Update. Semin Thromb Hemost (2019) 45(4):321–5. doi: 10.1055/s-0039-1688494
4. Vitale C, D'Amato M, Calabro P, Stanziola AA, Mormile M, Molino A. Venous thromboembolism and lung cancer: A review. Multidiscip Respir Med (2015) 10(1):28. doi: 10.1186/s40248-015-0021-4
5. Song C, Shargall Y, Li H, Tian B, Chen S, Miao J, et al. Prevalence of venous thromboembolism after lung surgery in China: A single-centre, prospective cohort study involving patients undergoing lung resections without perioperative venous thromboembolism prophylaxisdagger. Eur J Cardiothorac Surg (2019) 55(3):455–60. doi: 10.1093/ejcts/ezy323
6. Hachey KJ, Hewes PD, Porter LP, Ridyard DG, Rosenkranz P, McAneny D, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg (2016) 151(1):37–44.e1. doi: 10.1016/j.jtcvs.2015.08.039
7. Li H, Jiang G. [Perioperative venous thromboembolism (VTE) prophalaxis in thoracic cancer patients: chinese experts consensus]. Zhongguo Fei Ai Za Zhi (2018) 21(10):739–52. doi: 10.3779/j.issn.1009-3419.2018.10.03
8. Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2012) 141(2 Suppl):e227S–77S. doi: 10.1378/chest.141.5.1369b
9. Li X, Ren F, Wang S, He Z, Song Z, Chen J, et al. The epidemiology of ground glass opacity lung adenocarcinoma: a network-based cumulative meta-analysis. Front Oncol (2020) 10:1059. doi: 10.3389/fonc.2020.01059
10. Infante M, Lutman RF, Imparato S, Di Rocco M, Ceresoli GL, Torri V, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J (2009) 33 p(4):821–7. doi: 10.1183/09031936.00047908
11. Pedersen JH, Saghir Z, Wille MM, Thomsen LH, Skov BG, Ashraf H. Ground-Glass Opacity Lung Nodules in the Era of Lung Cancer CT Screening: Radiology, Pathology, and Clinical Management. Oncol (Williston Park) (2016) 30(3):266–74.
12. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol (2015) 10(9):1240–2. doi: 10.1097/JTO.0000000000000663
13. Tsao MS, Nicholson AG, Maleszewski JJ, Marx A, Travis WD. Introduction to 2021 WHO classification of thoracic tumors. J Thorac Oncol (2022) 17(1):e1–4. doi: 10.1016/j.jtho.2021.09.017
14. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest (2017) 151p (1):193–203. doi: 10.1016/j.chest.2016.10.010
15. Ishida H, Shimizu Y, Sakaguchi H, Nitanda H, Kaneko K, Yamazaki N, et al. Distinctive clinicopathological features of adenocarcinoma In Situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer (2019) 129:16–21. doi: 10.1016/j.lungcan.2018.12.020
16. Jia M, Yu S, Cao L, Sun PL, Gao H. clinicopathologic features and genetic alterations in adenocarcinoma In Situ and minimally invasive adenocarcinoma of the lung: long-term follow-up study of 121 asian patients. Ann Surg Oncol (2020) 27(8):3052–63. doi: 10.1245/s10434-020-08241-y
17. Cao L, Wang Z, Gong T, Wang J, Liu J, Jin L, et al. Discriminating between bronchiolar adenoma, adenocarcinoma In Situ and minimally invasive adenocarcinoma of the lung with CT. Diagn Interv Imaging (2020) 101(12):831–7. doi: 10.1016/j.diii.2020.05.005
18. Li Q, Dai J, Zhang P, Jiang G. Management of pulmonary ground glass nodules: Less is more. Ann Thorac Surg (2021) 112(1):1–2. doi: 10.1016/j.athoracsur.2021.01.077
19. Salla E, Dimakakos EP, Tsagkouli S, Giozos I, Charpidou A, Kainis E, et al. Venous thromboembolism in patients diagnosed with lung cancer. Angiology (2016) 67(8):709–24. doi: 10.1177/0003319715614945
20. Gong CY, Li ZW, Zhou DX, Yan H, Bao J, Ye RJ, et al. [Risk factors of lung cancer complicated with symptomatic venous thromboembolism]. Zhonghua Jie He He Hu Xi Za Zhi (2016) 39(6):454–8. doi: 10.3760/cma.j.issn.1001-0939.2016.06.010
21. Lee YG, Kim I, Lee E, Bang SM, Kang CH, Kim YT, et al. Risk factors and prognostic impact of venous thromboembolism in Asian patients with non-small cell lung cancer. Thromb Haemost (2014) 111(6):1112–20. doi: 10.1160/TH13-11-0956
22. Cui S, Chen S, Li H, Ke L, Liu Y, Jiang R, et al. Risk factors for venous thromboembolism and evaluation of the modified Caprini score in patients undergoing lung resection. J Thorac Dis (2020) 12(9):4805–16. doi: 10.21037/jtd-20-1279
23. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol (2011) 6(2):244–85. doi: 10.1097/JTO.0b013e318206a221
24. Weichert W, Warth A. Early lung cancer with lepidic pattern: adenocarcinoma In Situ, minimally invasive adenocarcinoma, and lepidic predominant adenocarcinoma. Curr Opin Pulm Med (2014) 20(4):309–16. doi: 10.1097/MCP.0000000000000065
25. Takashima S, Maruyama Y, Hasegawa M, Yamanda T, Honda T, Kadoya M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol (2003) 180(3):817–26. doi: 10.2214/ajr.180.3.1800817
26. Yang X, Yu L, Yu T, Li F, Zhang Y, Yu Z, et al. Venous thromboembolism after adult thymus or thymic tumor resection: A single-center experience. Thorac Cancer (2020) 11(8):2291–6. doi: 10.1111/1759-7714.13543
27. Yotsukura M, Asamura H, Motoi N, Kashima J, Yoshida Y, Nakagawa K, et al. Long-term prognosis of patients with resected adenocarcinoma In Situ and minimally invasive adenocarcinoma of the lung. J Thorac Oncol (2021) 16(8):1312–20. doi: 10.1016/j.jtho.2021.04.007
28. Choi SH, Chae EJ, Shin SY, Kim EY, Kim JE, Lee HJ, et al. Comparisons of clinical outcomes in patients with and without a preoperative tissue diagnosis in the persistent malignant-looking, ground-glass-opacity nodules. Med (Baltimore) (2016) 95(34):e4359. doi: 10.1097/MD.0000000000004359
29. Yang X, Ye X, Lin Z, Jin Y, Zhang K, Dong Y, et al. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacity-Lung adenocarcinoma: A pilot study. J Cancer Res Ther (2018) 14(4):764–71. doi: 10.4103/jcrt.JCRT_269_18
30. Xue L, Fan H, Shi W, Ge D, Zhang Y, Wang Q, et al. Preoperative 3-dimensional computed tomography lung simulation before video-assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J Thorac Dis (2018) 10(12):6598–605. doi: 10.21037/jtd.2018.10.126
31. Jiang L, Yin W, Peng G, Wang W, Zhang J, Liu Y, et al. Prognosis and status of lymph node involvement in patients with adenocarcinoma In Situ and minimally invasive adenocarcinoma-a systematic literature review and pooled-data analysis. J Thorac Dis (2015) 7(11):2003–9. doi: 10.3978/j.issn.2072-1439.2015.11.48
32. Hammouda A, Souilah S, Ferhat-Hamida MY, Amir ZC, Aouichat-Bouguerra S, Hariti G. [Activation of coagulation in patients with lung cancer]. Ann Biol Clin (Paris) (2019) 77(3):272–80. doi: 10.1684/abc.2019.1445
Keywords: adenocarcinoma in situ (AIS), venous thromboembolism (VTE), propensity score matching (PSM), modified caprini risk assessment model (Caprini RAM), world health organization (WHO)
Citation: Cai Y-s, Dong H-h, Li X-y, Ye X, Chen S, Hu B, Li H, Miao J-b and Chen Q-r (2022) Incidence of venous thromboembolism after surgery for adenocarcinoma in situ and the validity of the modified Caprini score: A propensity score-matched study. Front. Oncol. 12:976988. doi: 10.3389/fonc.2022.976988
Received: 24 June 2022; Accepted: 12 August 2022;
Published: 02 September 2022.
Edited by:
Lizza E.L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Fabrizio Minervini, University of Lucerne, SwitzerlandTomasz Urbanek, Medical University of Silesia, Poland
Copyright © 2022 Cai, Dong, Li, Ye, Chen, Hu, Li, Miao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-bai Miao, miaojinbai@ccmu.edu.cn; Qi-rui Chen, doctor_cqr@sina.com
 Yong-sheng Cai
Yong-sheng Cai