
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 January 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.976078
This article is part of the Research Topic Clinical Trials, Practice and Design in Gastrointestinal Cancers View all 23 articles
 Tao Li1,2,3†
Tao Li1,2,3† Tingting Liu1,4†
Tingting Liu1,4† Lei Zhao5†
Lei Zhao5† Lu Liu1,6
Lu Liu1,6 Xuan Zheng1,2,3
Xuan Zheng1,2,3 Jinliang Wang1,7*
Jinliang Wang1,7* Fan Zhang1,2,3*
Fan Zhang1,2,3* Yi Hu1,2,3*
Yi Hu1,2,3*Purpose: Gastric cancer (GC) is one of the most frequently diagnosed cancers and one of the leading causes of cancer deaths worldwide, especially in eastern Asia and China. Anti-PD-1 immune checkpoint inhibitors, Pembrolizumab and Nivolumab, have been approved for the treatment of locally advanced or metastatic gastric or gastroesophageal junction cancer (GC/GEJC). Our study evaluated the effectiveness and safety of anti-PD-1-based treatment (monotherapy or combination therapy) in Chinese patients with advanced or metastatic GC/GEJCs in a real-world setting.
Methods: A retrospective cohort study was conducted, and 54 patients from May 31, 2015, to May 31, 2021, were included in our analysis, including 19 patients treated with anti-PD-1 monotherapy and 35 patients treated with anti-PD-1 combination therapy. Demographic and clinical information were evaluated. Clinical response, survival outcomes, and safety profile were measured and analyzed.
Results: Overall, the median overall survival (mOS) was 11.10 months (95% CI, 7.05–15.15), and the median progression-free survival (mPFS) was 3.93 months (95% CI, 2.47–5.39). Of the patients, 16.7% achieved a clinical response, and 72.2% achieved disease control. Prolonged overall survival (OS) and progression-free survival (PFS) and increased clinical response were observed in the combination group compared with the monotherapy group, although statistical significance was not reached. In subgroups with live metastases or elevated baseline neutrophil-to-lymphocyte ratio (NLR) levels, combination therapy outperformed anti-PD-1 alone in survival outcomes. Patients treated with anti-PD-1 monotherapy (n = 5, 26.3%) had fewer treatment-related adverse events (TRAEs) than those in the combination group (n = 22, 62.9%). There were also fewer patients with TRAEs of grades 3–5 with monotherapy (n = 2, 10.5%) than with combination therapy (n = 7, 20.0%). Pneumonitis in three patients was the only potential immune-related adverse event reported.
Conclusions: Anti-PD-1-based monotherapy and combination therapy showed favorable survival outcomes and manageable safety profiles in advanced or metastatic GC/GEJCs. In clinical treatment, immunotherapy should be an indispensable choice in the treatment strategy for GC/GEJC. Patients with a heavy tumor burden and more metastatic sites might benefit more from combination therapy. Elderly patients and patients with more treatment lines or high Eastern Cooperative Oncology Group (ECOG) performance scores might be more suitable for immune monotherapy, and some clinical benefits have been observed.
Gastric cancer (GC) is one of the most frequently diagnosed cancers and one of the leading causes of cancer deaths worldwide (1). It is more prevalent in eastern Asia and China. The estimated incidence and mortality of GC in 2015 were 679,100 and 498,000, respectively, ranking as the second most common cancer (2, 3). Most patients are diagnosed at an advanced stage, with a 5-year survival rate of only 33% (4). Currently, the first-line treatment for advanced GC patients is primarily platinum plus fluoropyrimidine, or trastuzumab in combination with it, for HER2-overexpressing tumors (5, 6). Preferred treatment modalities for second-line or beyond include ramucirumab plus paclitaxel or monotherapy of docetaxel, paclitaxel, irinotecan, or ramucirumab (5).
In recent years, immunotherapy has been a revolutionary treatment strategy for advanced cancers. Immune checkpoint blockades (ICBs), including antibodies to Programmed Death Receptor 1 (PD-1) or its ligand (PD-L1), are now standard therapies for a range of solid tumors as approved by the Food and Drug Administration (FDA) (7). Several clinical trials have shown that some GC patients could benefit from anti-PD-1/PD-L1 antibody therapy, indicating that ICBs are a potential treatment option for GC. For ICBs’ monotherapy as third-line, pembrolizumab (a humanized anti-PD-1 IgG4 monoclonal antibody) was approved by the FDA for the treatment of locally advanced or metastatic gastric or gastroesophageal junction cancer (GC/GEJC) patients with PD-L1 positive tumors (Combined Positive Score (CPS) ≥1). This is based on the data of KEYNOTE-059, a single-arm study (8). In ATTRACTION-2, a phase III clinical trial comparing nivolumab with a placebo in Asian patients who were heavily pretreated, nivolumab (a humanized anti-PD-1 IgG4 monoclonal antibody) led to improved overall survival (OS) and progression-free survival (PFS) and was, in general, well tolerated (9). However, in another phase III clinical trial conducted on a global scale (JAVELIN gastric 300), avelumab (a humanized anti-PD-L1 IgG1 monoclonal antibody) did not improve OS or PFS compared with chemotherapy in the total population (including 25.4% Asian patients) as a third-line treatment (10). For first- or second-line treatment, only three single-arm studies showed a 7%–22% objective response rate (ORR) from ICB monotherapy (11–13), and two phase III trials reported no significant clinical benefit from pembrolizumab monotherapy compared with chemotherapy (14, 15). Because of the modest benefit of ICB monotherapy, co-administration with another therapeutic agent provides a potential solution to enhance treatment effectiveness. However, in KEYNOTE-062, the only phase III study investigating pembrolizumab plus chemotherapy as first-line treatment of advanced GC/GEJCs, the arm given pembrolizumab plus chemotherapy did not exhibit significantly prolonged OS than those taking chemotherapy alone (15). Several phase I-II studies preliminarily showed that ICBs combined with chemotherapy, targeted, or antiangiogenic agents presented improved ORR (16–18). Most of the studies above were conducted on Caucasian patients, while little was known about how Asian GC/GEJCs patients, either treatment-naïve or previously treated, would respond to ICBs therapy. This represents an unmet medical need since Asians are known to be heavily burdened with GC/GEJCs. Moreover, the populations in the clinical trials described above are usually highly selected. It remains inconclusive whether patients with an Eastern Cooperative Oncology Group (ECOG) performance score 1, patients with brain metastases, or patients over 70 years old could benefit from ICBs’ treatment since they are usually excluded from interventional clinical trials. To explore whether ICBs could bring clinical benefit to Asian GC patients, especially in China, we conducted a real-world study to examine the safety and anti-tumor effectiveness of anti-PD-1 monotherapy and combination therapy in advanced GC patients. To our knowledge, it was one of the first real-world clinical studies of immunotherapy for GC/GEJC in China. Through this study, it was hoped that real-world studies could play a complementary role in clinical trials and provide more evidence-based medical support for immunotherapy for GC/GEJC in Asia, especially in China.
This study conducted a retrospective analysis to evaluate the effectiveness and safety profile of anti-PD-1 treatment in a real-world setting. Consecutive GC/GEJCs patients treated at the Department of Oncology, Chinese People’s Liberation Army (PLA) General Hospital from May 31, 2015, to May 31, 2021, were reviewed and screened. Inclusion criteria were: 1) pathologically confirmed GC/GEJCs; 2) advanced or metastatic disease or recurrence after curative surgery; and 3) administration of nivolumab or pembrolizumab as monotherapy or combination therapy. The ethics committee of PLA General Hospital approved this study according to the ethical standards of the Declaration of Helsinki and its subsequent amendments (Ethical approval number: S2020-284-01).
Clinicopathological characteristics were reviewed and collected. Two physicians independently extracted and recorded demographic and clinical information, followed by confirmation by a third physician in case of inconsistency. Data were collected from the following sources: 1) Chinese PLA General Hospital inpatient and outpatient records, including doctors’ notes, radiographic reports, and biopsy results; 2) patient or family interviews. Complete blood cell counts of eligible patients, covering neutrophils, lymphocytes, thrombocytes, and erythrocytes, were also collected. NLR was defined as the absolute neutrophil count divided by the entire lymphocyte count in peripheral blood, collected before the initiation of anti-PD-1 treatment. The platelet-to-lymphocyte ratio (PLR) was defined as the absolute thrombocyte count divided by the absolute lymphocyte count. Median NLR and PLR were adopted as cutoffs in our analysis.
The primary endpoint was OS, defined as the time from the first dose of anti-PD-1 to death from any cause. Secondary endpoints included PFS (defined as the time from treatment initiation to the first documented disease progression or death), ORR (defined as the proportion of patients with confirmed complete response or partial response), and disease control rate (DCR), which was defined as the proportion of patients with confirmed complete response, partial response, or stable disease, duration of response, and the maximum percentage change from baseline for the sum of diameters of target lesions. All endpoints were evaluated according to RECIST (version 1.1) guidelines (19). Follow-up imaging reports were reviewed by two radiologists independently. The director of the imaging center further verified any discrepancies. Data for patients without disease progression or death events were censored at the time of the last follow-up. Adverse events (AE) were evaluated and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) (20). Treatment-related adverse events (TRAEs) and immune-related AEs were graded and recorded. All patients were followed up until December 2021, or death.
All participants in this study signed informed consent forms. The ethics committee of the PLA General Hospital approved this study according to the ethical standards of the Declaration of Helsinki and its subsequent amendments (Ethical approval number: S2020-284-01).
Categorical characteristics and objective response were compared between treatment groups with the chi-square test or Fisher’s exact test. Continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier survival analysis was used to assess OS and PFS, and the log-rank test was used to compare groups. The Hazard Ratio (HR) was estimated using the Cox proportional hazard model. In the multivariable Cox regression model, variables with a P-value <0.10 in the univariable Cox regression or acting as clinically relevant factors were adjusted. All P-values are two-tailed, and a P-value <0.05 was considered statistically significant. The SPSS statistical package (SPSS 20, SPSS, Chicago, IL, USA) was used for all statistical analyses.
Fifty-four patients received anti-PD-1-based therapy during their course of disease and were identified and included in our analysis, including 19 patients treated with anti-PD-1 monotherapy and 35 patients treated with anti-PD-1 combined with chemotherapy (XELOX, SOX, and mFOLFOX6) targeted therapy or anti-CTLA-4 (a monoclonal antibody targeting cytotoxic T-lymphocyte-associated antigen-4). Patients’ clinicopathological characteristics are summarized in Table 1. The median age of the recruited patients was 58 years in the monotherapy group and 59 years in the combination therapy group, where 2 (10.5%) and 9 (25.7%) patients received immunotherapy at the age of over 65 in the two groups, respectively. In the two groups, 11 (57.9%) and seven patients (20.0%) had an ECOG performance score of 2-4, respectively. Fewer patients in the monotherapy group received anti-PD-1 as first-line treatment than in the combination group (5.3% vs. 37.1%, P = 0.01), while more patients in the monotherapy groups were treated with anti-PD-1 as 4th line or later therapy (36.8% vs. 11.4%, P = 0.04), respectively. Due to the long period, people initially did not realize that PD-L1 status was vital for immunotherapy when the drug was marketed, so only a small number of patients completed the test. Only 5/19 and 8/35 patients from monotherapy and combination therapy completed the PD-L1 test respectively (Table 1).
As of the data cut-off date of May 31, 2021, the median follow-up was 9.40 months, ranging from 0.80 to 43.80 months. 44 (81.5%) progression events and 37 (68.5%) deaths occurred during the follow-up period. In the overall cohort, the mOS was 11.10 months (95% CI, 7.05–15.15), and the mPFS was 3.93 months (95% CI, 2.47–5.39). Prolonged mOS was observed in the patients receiving combination therapy (11.10 months; 95% CI, 7.18–15.03), and that was over those taking anti-PD-1 alone (5.40 months; 95% CI, 2.64–8.17). However, the difference in OS between the two groups was not statistically significant, possibly due to the small sample size (HR = 0.70, 95% CI, 0.36–1.35, P = 0.29) (Figure 1). Likewise, the mPFS of the combination group at 4.07 months (95% CI, 1.83–6.03) was non-significantly longer than that of the monotherapy group at 2.93 months (95% CI, 0.85–5.01) (HR = 0.69, 95% CI, 0.37–1.26, P = 0.22) (Figure 2).
In the overall population, for patients who received anti-PD-1-based therapy as first- or second-line treatment, the mOS and mPFS were, respectively, 11.10 months (95% CI, 6.36–15.85) and 3.43 months (95% CI, 2.20–4.66). In patients treated with anti-PD-1-based therapy as the third-line or beyond, the mOS and mPFS were 9.13 months (95% CI, 2.22–16.04) and 4.20 months (95% CI, 1.60–6.80), respectively. There was no statistical difference between the monotherapy and combination groups regarding OS or PFS upon stratification by treatment lines (Supplementary Figures 1–4). The duration of treatment and outcomes for each patient were specified in Figure 3.
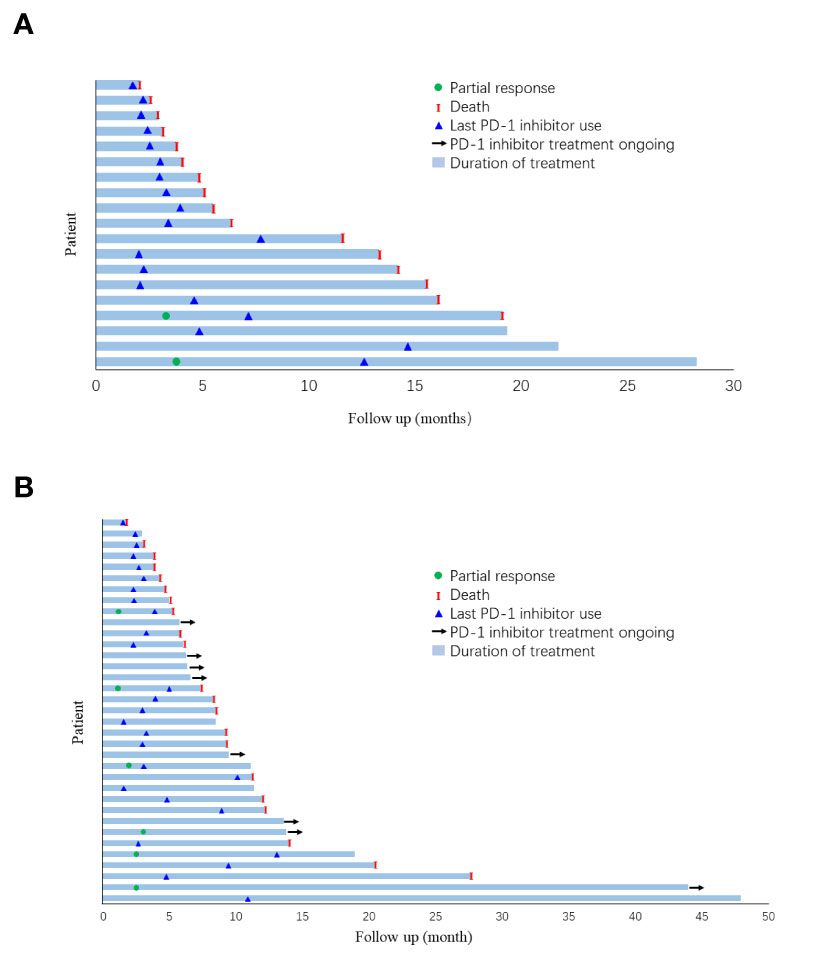
Figure 3 Duration of follow-up and outcome for each patient in (A) monotherapy group and; (B) combination group.
Response rates for the overall population, the monotherapy group, and the combination arm were summarized in Table 2. ORR and DCR were 16.7% (95% CI, 6.4–26.9%) and 72.2% (95% CI, 59.9–84.6%) of the overall cohort, respectively. ORR tended to be higher in the combination group (20.0%, 95% CI, 8.4–36.9%) than in the monotherapy group (10.5%, 95% CI, 1.9–29.6%), but the difference was not statistically significant (P = 0.47) with the limited sample size. DCR was 63.2% and 77.1% in the monotherapy and combination groups, respectively (P = 0.35). Concerning the best overall responses, 36.8% (7/19) of the patients given monotherapy and 40.0% (14/35) of the patients on combination treatment achieved a decrease from baseline in the sum of their target lesions (Figure 4).
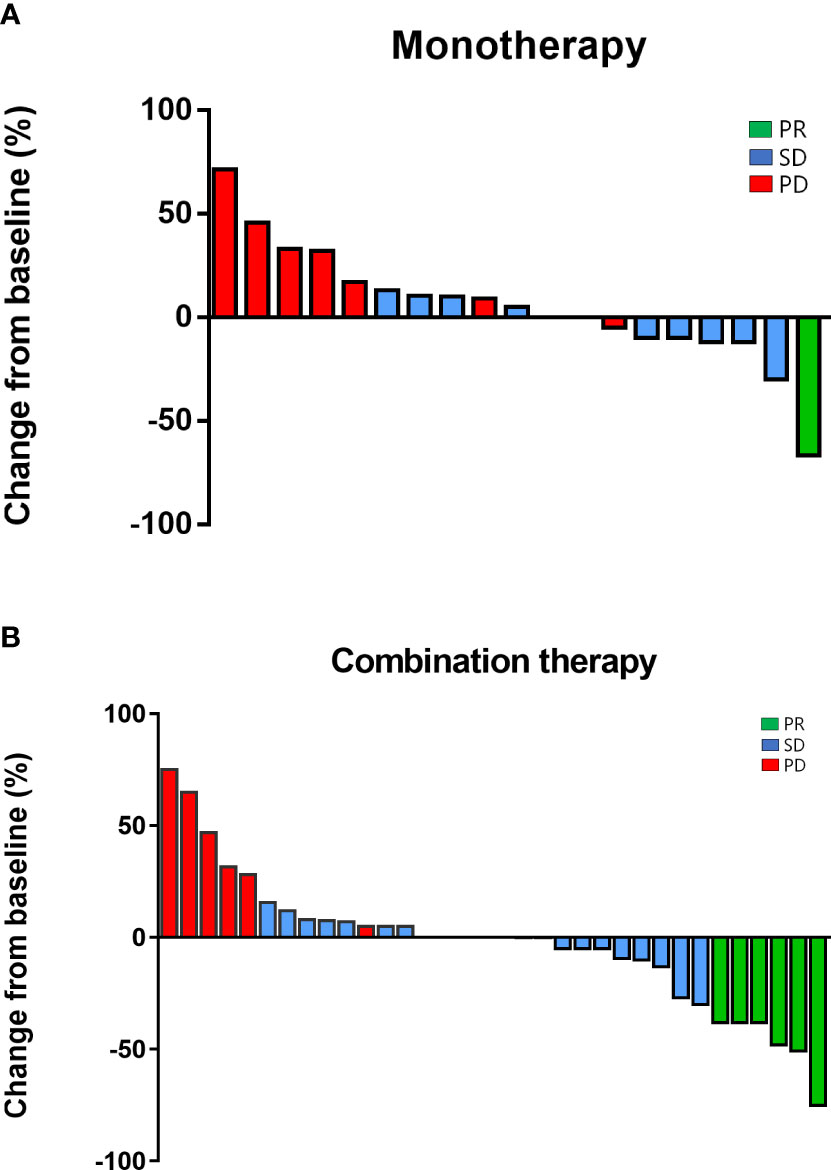
Figure 4 Regression of target lesions from baseline in each patient in (A) the monotherapy group and; (B) the combination group.
We also did subgroup analysis to compare the survival outcomes of monotherapy and combination therapy according to different stratifications (Table 3). In patients with liver metastases or elevated NLR levels (NLR above the median), anti-PD-1 administered in conjunction with other medications led to a more favorable mOS compared to those on monotherapy. (The HR in the subgroup with liver metastases was 0.33, 95% CI, 0.11–1.00, P = 0.05; HR in the subgroup with elevated NLR was 0.38, 95% CI, 0.14–1.00).
Of the five patients who accepted anti-PD-1 combined with apatinib, a small molecular anti-angiogenic agent, in the combination group, they all achieved a durable, stable disease, ranging from 4.20 to 9.37 months, although no objective response was observed.
In univariate analyses with the Cox regression model, ECOG 0–1 was associated with better OS outcome (HR = 0.28, 95% CI, 0.11–0.56) (Supplementary Table 1). Patients with ascites and elevated NLR or PLR were at higher risk of death (HR for ascites was 3.27, 95% CI, 1.61–6.65, P = 0.001; HR for elevated NLR was 2.19, 95% CI, 1.18–4.05, P = 0.01; and HR for elevated PLR was 2.49, 95% CI, 1.33–4.65, P = 0.004). In light of the results from the univariate analyses, we selected the ECOG performance score, ascites, NLR, and PLR for multivariable analyses. ECOG performance score and ascites were independent risk factors associated with OS outcomes in patients treated with anti-PD-1-based therapy. NLR and PLR did not reach statistical significance in the multivariable analysis.
TRAEs of any grade occurred in 27 patients (50%) in the overall cohort (Table 4). All-grade TRAEs observed in 5% or more of patients in the overall cohort included decreased neutrophil count, nausea, vomiting, increased alanine aminotransferase, and increased alkaline phosphatase levels. Patients treated with anti-PD-1 monotherapy (n = 5, 26.3%) had fewer TRAEs than those in the combination group (n = 22, 62.9%). There were also fewer patients with TRAEs of grades 3–5 with monotherapy (n = 2, 10.5%) than with combination therapy (n = 7, 20.0%). Grade 3 to 5 events were reported in nine patients (16.7%), including oral mucositis (n = 1, 5.3%) and gastric obstruction (n = 1, 5.3%) in the anti-PD-1 monotherapy group and decreased neutrophil count (n = 4, 11.4%), gastric perforation (n = 1, 2.9%), lung infection (n = 1, 2.9%), and pneumonitis (n = 1, 2.9%) in the combination group. Only one death was attributed to treatment in the combination group (one patient with gastric perforation). No deaths related to study treatment occurred in the monotherapy group. Pneumonitis was the only potentially immune-related adverse event reported in our cohort, which was observed in three patients (one with monotherapy and two with combination therapy).
To our knowledge, this was one of the first real-world studies that evaluated the performance of an anti-PD-1-based treatment regimen in the Chinese GC/GEJC population. Anti-PD-1 monotherapy and combination therapy showed favorable survival outcomes and manageable safety profiles in advanced or metastatic GC/GEJCs. The mOS was 11.10 months (95% CI, 7.05–15.15), 5.40 months (95% CI, 2.64–8.17), and 11.1 months (95% CI, 7.17–15.03) in the overall population, the monotherapy group, and the combination group, respectively, while the mPFS was 3.93 months (95% CI, 2.47–5.39), 2.93 months (95% CI, 0.85–5.01), and 4.07 months (95% CI, 1.83–6.03). Prolonged OS and PFS were observed in the combination group compared with the monotherapy group, although statistical significance was not reached. Objective response was achieved in 16.7%, 10.5%, and 20.0% of patients in the overall population, monotherapy group, and combination group, respectively. In the overall cohort, 72.2% of the patients achieved tumor regression or disease control (95% CI, 59.9–84.6%). In patients treated with anti-PD-1/L1 monotherapy as a third-line treatment, the mOS and mPFS were reported to be 4.6–5.6 months and 1.4–2.0 months, respectively (8–10). Among the patients treated as third-line or beyond treatment in our cohort, the mOS was 6.3 months (95% CI, 0–18.43) and the mPFS was 4.13 months (95% CI, 0.86–7.40). In phase II clinical trial, anti-PD-1 as a first-line treatment showed mOS of 13.8 months (95% CI, 8.6–not evaluable) in the monotherapy cohort and 20.7 months (95% CI, 9.2–20.7) in the combination cohort (21). Of the 14 patients in our cohort who received an anti-PD-1-based regimen as a first-line treatment, their mOS was 11.10 months (95% CI, not evaluable). Among these 14 patients, 4 (28.6%) had an ECOG performance score >1. That might explain the relatively inferior survival outcome compared with the previous report. In a phase Ib/II clinical trial, 18 Chinese metastatic GC patients received anti-PD-1 plus chemotherapy as first-line treatment, and 58 received anti-PD-1 monotherapy as second-line treatment. In the former cohort, mOS was not reached and mPFS was 5.8 months, while in the latter cohort, mOS was 4.8 months and mPFS was 1.9 months (22). In our cohort, 13 patients received anti-PD-1-based combination therapy as first-line treatment, with a mOS not reached and a mPFS of 5.2 months. Of the 18 patients who progressed after at least one systematic chemotherapy and were treated with anti-PD-1 monotherapy, the mOS was 6.3 months (95% CI, 0–15.54) and the mPFS was 2.87 months (95% CI, 0–5.91). Overall, our study showed results comparable to previously published interventional studies.
In a recent phase Ia/b clinical trial, 41 GC/GEJC patients whose disease progressed after one or two lines of systemic therapy were treated with pembrolizumab plus ramucirumab (an IgG1 VEGFR-2 monoclonal antibody). Of these patients, 21 (51%) achieved disease control, including 3 (7%) partial responses and 18 (44%), stable disease (23). In our cohort, five patients were given anti-PD-1 (three with pembrolizumab and two with nivolumab) combined with apatinib, and all five patients obtained clinical benefits with durable disease control. De-novo or acquired resistance to ICBs is complex and could be attributed to several factors, such as an immunosuppressive tumor microenvironment, a lack of PD-L1 expression, and T-cell exclusion (24–26). Anti-angiogenesis therapy could prevent immunotherapy resistance by increasing T-cell trafficking, migration across vascular endothelium, and infiltration into tumor tissue (27). Further studies with larger sample sizes are needed to verify the synergistic effects of anti-angiogenesis therapy with ICBs.
Patients recruited into randomized clinical trials are usually highly selected. Inclusion criteria typically include an ECOG performance score of <2 and no symptomatic brain metastases or other unfavorable physical conditions (28, 29). Randomized clinical trials provide an optimal way to evaluate the effectiveness and safety of a specific treatment. However, such studies are intrinsically less amenable to extrapolation. An ECOG performance score of >1 was previously reported as an independent risk factor for unfavorable survival upon treatment with ICBs in real-world studies (30, 31). In our cohort, of 18 patients with a baseline ECOG performance score of 2–4, only 5.6% (95% CI, 0–17.3%) obtained a clinical response. In contrast, for patients with a baseline ECOG of 0–1, the ORR was 22.2% (95% CI, 8.0–36.5%), and the mPFS was 5.20 months (95% CI, 2.03–8.37). Patients with ECOG 2–4 also had worse overall survival than those with ECOG 0–1 (median OS, 4.80 months vs. 13.23 months; HR = 3.54, 95% CI, 1.80–6.97). Consistent with previous studies, the ECOG performance score was validated as an independent OS risk factor (Table 4). Patients with brain metastases are usually excluded from randomized studies (9, 23). In our cohort, four patients had brain metastases at the onset of anti-PD-1 therapy; their mPFS was 1.30 months (95% CI, 1.05–1.56) and mOS was 8.37 months (95% CI, 0.98–15.75). In addition to the particular subpopulations mentioned above, there were nine patients in our study aged ≥70, among whom 10% responded to the treatment, and their mPFS and mOS were 3.07 months (95% CI, 2.66–3.48) and 5.17 months (95% CI, 4.58–5.75), respectively. In populations that usually do not meet the inclusion criteria of randomized studies, anti-PD-1-based therapy also showed favorable survival outcomes.
Various responses to ICBs by different tumor types mainly arise from the diversity of tumor immune microenvironments (32, 33). One of the well-investigated and commonly used biomarkers for systemic inflammatory response is circulating white blood cells, including neutrophils and lymphocytes (34, 35). Recent studies investigated the predictive value of NLR (neutrophil-to-lymphocyte ratio) and PLR for the treatment of checkpoint inhibitors. Low baseline NLR levels were associated with prolonged PFS and OS in metastatic melanomas following treatment with ipilimumab (CTLA-4) (36). In non-small cell lung cancers treated with anti-PD-1 inhibitors, low NLR and PLR levels were also reported to be associated with better PFS, OS, and response rate (37, 38). In our cohort, elevated NLR and PLR were predictive markers for OS in univariate analyses but not in multivariable analyses when considering clinical factors. This could have been partly explained by the small sample size, which might limit the statistical power.
In our cohort, combination therapy presented better survival outcomes in patients with liver metastases and elevated baseline NLR levels compared with anti-PD-1 monotherapy. The differences in PFS and OS between the treatment groups were no longer significant after adjusting according to clinical characteristics. In a recent update on the phase III study KEYNOTE-062, pembrolizumab combined with chemotherapy in gastric cancers as first-line therapy did not improve PFS or OS compared with pembrolizumab alone (15). In non-small cell lung cancer (NSCLC), anti-PD-1/L1 combined with chemotherapy showed better survival benefits than anti-PD-1/1 alone (39, 40). Tumor PD-L1 expression and the tumor immune microenvironment were previously reported to be affected by cytotoxic agents, which could explain the synergistic effects of the combination of anti-PD-1/L1 inhibitors and chemotherapy (41, 42). However, in the GC population, combination therapy’s synergistic effects were relatively modest. Significant spatial heterogeneity of genomic alterations and tumor immune microenvironment in GC might account for inconsistent results and should be considered in future clinical trial designs (43, 44). There are several limitations to our study. Firstly, the sample size was limited. However, to our knowledge, this is one of the first real-world studies to explore the effectiveness and safety of anti-PD-1/L1 inhibitor monotherapy and combination therapy as first-line or second-line treatment in GC, especially in Asian patients. Second, this is a retrospective cohort study and could have been potentially biased. However, a retrospective real-world cohort allows us to investigate whether patients with higher ECOG scores or more advanced GC could benefit from immunotherapies, which is usually not feasible with prospective trials. Moreover, patients were recruited consecutively in an effort to minimize bias, and comprehensive clinicopathological information was collected to enable adjustment in multivariable regression analyses. Third, the combination regimens were heterogeneous, and a specific combination regimen should be considered when designing prospective studies in the future. Furthermore, the real-world study was very different from prospective clinical trials, and the subjects included in the real-world study were highly heterogeneous. For patients with GC, the heterogeneity of the tumor itself and its impact on the patient’s physical condition, as well as their different physical status, treatment willingness, previous treatment history, economic status, drug availability, and other factors, affect the final treatment effect and survival outcomes. At the same time, due to the impact of the COVID-19 epidemic, the treatment of patients would be more or less affected, including whether they could come to the hospital to receive treatment during the prescribed time and the accessibility of drugs. The uncertainty of immunotherapy and the different methods of combination therapy also determined the heterogeneity and persistence of treatment.
On the other hand, real-world data played a vital role in perfecting and supplementing clinical research data. At the same time, we have seen whether immune monotherapy or combination therapy plays an important role, and the significance of this cannot be ignored in GC. Anti-PD-1 monotherapy and combination therapy showed promising survival outcomes and manageable safety profiles in advanced or metastatic GC/GEJCs in a real-world setting. Prolonged OS and PFS and increased ORR were observed in the combination group compared with the monotherapy group, although statistical significance was not reached. In some subgroups, such as patients with live metastases or elevated baseline NLR levels, combination therapy outperformed anti-PD-1 alone regarding survival outcomes. Treatment regimens involving anti-PD-1 in GC warrant further prospective investigations with larger sample sizes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All participants in this study signed informed consent forms. The ethics committee of the People’s Liberation Army General Hospital approved this study according to the ethical standards of the Declaration of Helsinki and its subsequent amendments(Ethical approval number:S2020-284-01). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TaL, TiL, and LZ served as co-first authors, with primary contributions to the manuscript. LL, XZ, and JW contributed to the acquisition, analysis, and interpretation of data. Study supervision: YH, FZ. All authors contributed to the article and approved the submitted version.
We are grateful to all the patients included in this study. My deepest gratitude goes first and foremost to Professor Hu for his guidance and coaching. I am also greatly indebted to Dr. Zhang, who has instructed and helped me a lot in the past years.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.976078/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier plot of overall survival in patients treated as first and second-line therapy.
Supplementary Figure 2 | Kaplan-Meier plot of overall survival in patients treated as third-line or beyond therapy.
Supplementary Figure 3 | Kaplan-Meier plot of progression-free survival in patients treated as first and second-line therapy.
Supplementary Figure 4 | Kaplan-Meier plot of progression-free survival in patients treated as third-line or beyond therapy.
Supplementary Table 1 | Univariate analysis and multivariate analysis for overall survival
AE, Adverse events; DCR, Disease control rate; ECOG, Eastern Cooperative Oncology Group; FDA, Food and Drug Administration; GC, Gastric cancer; HR, Hazard Ratio; ICB, Immune checkpoint blockades; NLR, Neutrophil-to-lymphocyte ratio; NSCLC, Non-small cell lung cancer; ORR, Objective response rate; OS, Overall survival; PFS, Progression-free survival; PLA, People’s Liberation Army; PLR, Platelet-to-lymphocyte ratio; SPSS, SPSS statistical; TRAE, Treatment-related adverse events
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
4. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin (2019) 69:363–85. doi: 10.3322/caac.21565
5. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw version 2.2022 (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
6. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
7. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
8. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
9. Kang YK, Boku N, Satoh T, Ryu M, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
10. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN gastric 300. Ann Oncol (2018) 29:2052–60. doi: 10.1093/annonc/mdy264
11. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol (2016) 17:717–26. doi: 10.1016/S1470-2045(16)00175-3
12. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol (2018) 36:2836–44. doi: 10.1200/JCO.2017.76.6212
13. Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, et al. Avelumab (anti–PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: Phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer (2019) 7:30. doi: 10.1186/s40425-019-0508-1
14. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
15. Josep Tabernero EVC, Bang Y-J, Fuchs CS, Fuchs CS, Wyrwicz L, Lee KW, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37(suppl; abstr LBA4007):LBA4007. doi: 10.1200/JCO.2019.37.18_suppl.LBA4007
16. Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol (2020) 21(8):1066–76. doi: 10.1016/S1470-2045(20)30326-0
17. Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res (2020) 26(4):846–54. doi: 10.1158/1078-0432.CCR-19-2443
18. Bang YJ, Muro K, Fuchs CS, Golan T, Geva R, Hara H, et al. KEYNOTE-059 cohort 2: Safety and effectiveness of pembrolizumab plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(15_suppl):4012.
19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. TP. new response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
20. Common terminology criteria for adverse events (CTCAE). v.5.0 (2017). Available at: https://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm#ctc_50.
21. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer (2019) 22:828–37. doi: 10.1007/s10120-018-00909-5
22. Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, effectiveness and tumor mutational burden as a biomarker of overall survival benefit in chemorefractory gastric cancer treated with toripalimab, a PD1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol (2019) 30:1479–86. doi: 10.1093/annonc/mdz197
23. Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): A multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol (2019) 20:1109–23. doi: 10.1016/S1470-2045(19)30458-9
24. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, et al. Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discovery (2019) 9:1124–41. doi: 10.1158/2159-8290.CD-19-0074
25. Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol (2017) 18:e731–41. doi: 10.1016/S1470-2045(17)30607-1
26. Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol Res (2019) 145:104258. doi: 10.1016/j.phrs.2019.104258
27. Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun (2016) 7:12624. doi: 10.1038/ncomms12624
28. Shah MA, Xu RH, Bang YJ, Hoff PM, Liu T, Herráez-Baranda LA, et al. HELOISE: Phase IIIb randomized multicenter study comparing standard-of-Care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol (2017) 35:2558–67. doi: 10.1200/JCO.2016.71.6852
29. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (2014) 383:31–9. doi: 10.1016/S0140-6736(13)61719-5
30. Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer (2018) 126:217–23. doi: 10.1016/j.lungcan.2017.11.015
31. Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer (2018) 119:14–20. doi: 10.1016/j.lungcan.2018.02.017
32. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell (2018) 175:313–26. doi: 10.1016/j.cell.2018.09.035
33. Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res (2019) 7:737–50. doi: 10.1158/2326-6066.CIR-18-0436
34. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol (2019) 5:1481–5. doi: 10.1001/jamaoncol.2019.1747
35. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med (1999) 340:448–54. doi: 10.1056/NEJM199902113400607
36. Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer (2015) 112:1904–10. doi: 10.1038/bjc.2015.180
37. Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother (2018) 67:459–70. doi: 10.1007/s00262-017-2092-x
38. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
39. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
40. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol (2016) 17:1497–508. doi: 10.1016/S1470-2045(16)30498-3
41. Tsai TF, Lin JF, Lin YC, Chou KY, Chen HE, Ho CY, et al. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci Rep (2019) 39(9):BSR20190362. doi: 10.1042/BSR20190362
42. Chiang SF, Huang CY, Ke TW, Chen TW, Lan YC, You YS, et al. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol Immunother (2019) 68:283–96. doi: 10.1007/s00262-018-2275-0
43. Lim B, Kim JH, Kim M, Kim SY. Genomic and epigenomic heterogeneity in molecular subtypes of gastric cancer. World J Gastroenterol (2016) 22:1190–201. doi: 10.3748/wjg.v22.i3.1190
Keywords: anti-PD-1, gastric cancer, real-word study, Chinese, efficacy and safety analyses
Citation: Li T, Liu T, Zhao L, Liu L, Zheng X, Wang J, Zhang F and Hu Y (2023) Effectiveness and safety of anti-PD-1 monotherapy or combination therapy in Chinese advanced gastric cancer: A real-world study. Front. Oncol. 12:976078. doi: 10.3389/fonc.2022.976078
Received: 23 June 2022; Accepted: 19 December 2022;
Published: 05 January 2023.
Edited by:
Alberto Puccini, Ospedale Policlinico San Martino IRCCS, ItalyReviewed by:
Lin Wu, Hunan Cancer Hospital, Xiangya School of Medicine, Central South University, ChinaCopyright © 2023 Li, Liu, Zhao, Liu, Zheng, Wang, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinliang Wang, d2FuZ2ppbmxpYW5nMzAxQDEyNi5jb20=; Fan Zhang, emhhbmdmYW5AMzAxaG9zcGl0YWwuY29tLmNu; Yi Hu, aHV5aTMwMXpseGJAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.