- 1Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China
- 2Department of General Surgery, Xiangya Hospital, Central South University, Changsha, China
- 3Division of Translational Immunology, III, Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 4Department of General, Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Objective: Clear cell renal cell carcinoma may affect patients of any age. To date, there are only a limited number of large data studies on renal clear cell carcinoma in different age groups. This study assessed CCRCC risk factors in different age groups using the Surveillance Epidemiology and End Results (SEER) database.
Methods: We selected 58372 cases from the SEER database. These patients were divided into seven different age groups. Cox regression models were used to find independent risk factors for the survival of CCRCC patients. Based on independent risk factors, a nomogram was drawn with R software. Kaplan-Meier method for survival analysis and X-tile software were used to find the optimal age group for diagnosis.
Results: Univariate analysis revealed that patients’ age, sex, race, marital status, grade, TNM (tumor, node, metastasis) stage, surgery, WHO/ISUP grade were correlated with survival (P<0.01). Age was an independent risk factor for survival in patients with CCRCC according to multivariate Cox regression analysis (p<0.01). All-cause mortality and tumor-specific mortality increased according to the increasing age of the patients. The optimal cut-off values for age were defined as 58 and 76 years and 51 and 76 years, respectively, according to overall survival (OS) and cause-specific survival (CSS).
Conclusion: There is a negative correlation between age and survival of CCRCC patients. The difference in prognosis of patients in different age groups has important implications for clinical treatment. Therefore, the diagnosis and treatment plan should be based on more detailed age grouping, which is more beneficial to improving the prognosis and survival of patients.
Introduction
Renal cell carcinoma (RCC) affects >430,000 people worldwide every year (1), the incidence is about twice as high in men as in women (2).RCC includes chromophobe renal cell carcinoma (CHRCC), papillary renal cell carcinoma (PRCC), and clear cell renal cell carcinoma (CCRCC), which are three main histological subtypes with distinct molecular and genetic characteristics (3). Among them, CCRCC is the most prevalent histological subtype (approximately 80%).
Age is an indicator used to assess prognosis in many solid cancers, especially in CCRCC, and is a significant risk factor (4, 5). CCRCC may affect patients of any age, but it is more likely to affect people aged 60-70 years, with younger people rarely being affected. Meanwhile, the prognosis of CCRCC in older patients is poor, and patients are usually diagnosed at an advanced stage, which may be related to the insidious nature of CCRCC symptoms and low level of diagnosis (6–8). There have been many studies on risk factors for the prognosis of patients with CCRCC, which have revolutionized the treatment options for CCRCC. Population based data suggest that the incidence of CCRCC varies by age group. In addition, a comparison of the vascular features of patients aged <65 and >65 years has shown that age can affect the molecular features and vascular structure of CCRCC (9).
There have been several achievements regarding the role of age in patients with CCRCC. However, data on CCRCC in different age groups are limited. This study used the constructed SEER database to assess the risk factors for CCRCC in various age groups.
Materials and methods
Data Sources together with Selection Criteria CCRCC clinical data were acquired from the SEER database through employing the SEER Stat 8.3.9 software (https://seer.cancer.gov/data/). SEER is the authoritative source of the cancer statistics in the U.S., with its database covering socioeconomic status, population statistics, incidence rate and survival rate among the cancer patients. The patient data included: The patient ID, marital status, sex, grade, American Joint Committee on Cancer (AJCC) TNM stage, surgery, WHO/ISUP grade, survival (months). The database used the 7th (2010–2015) and 6th (2004–2015) TNM staging systems from 2004 to 2015, so we converted the 6th edition to the 8th edition based on CS Extension and CS Lymph Nodes.
Inclusion criteria: (1) Histologically diagnosed with CCRCC. (2) the year at time of diagnosis was 2004-2015.
Exclusion criteria: (1) Non-pathological diagnosis and patient was not CCRCC; (2) T stage=T0, Tis, Tx, NA or N stage= Nx, NA or M stage=Mx, NA; (3) patient without surgery or unknown.
Variables and results
The variables included marital status, sex, grade, T stage, N stage, M stage, Surgery, WHO/ISUP grade, Survival (months).These patients were classified as seven groups in accordance with age, that is, 0-30, 30-39, 40-49, 50-59, 60-69, 70-79, and above 80. Subgroup analysis was conducted by marital status(unmarried vs. married vs. unknown), sex(male vs. female), grade(grade I vs. grade II vs. grade III vs. grade IV), T stage(T1 vs. T2 vs. T3 vs. T4), N stage(N0 vs. N1), M stage(M0 vs. M1), surgery(no surgery vs. local tumor excision vs. partial nephrectomy vs. complete nephrectomy), WHO/ISUP grade(low grade vs. high grade vs. unknown). The survival analysis was implemented, containing cancer special survival together with overall survival.
Statistical method
With an aim of comparing the baseline features of patients in various age groups, Fisher test and Chi-square test were applied. In the univariate analysis, Kaplan Meier approach was utilized to explore the survival rate under each factor of risk. Chi-square test was employed for the comparison between groups. In univariate analysis, univariate Cox regression model was applied to assess the prognostic risk factors. In the multivariate analysis, Cox regression model was exploited to analyze the independent risk factors of survival in patients with CCRCC. R software (version 4.1.3) and SPSS 26.0 software were conducted for complete analysis. Eventually, X-tile software (version 3.6.1) was applied to classify the patients as three subgroups, namely, low risk, medium risk and high risk. No ethical statement consent was required for this study, as the SEER data were publicly available and analyzed anonymously. All authors signed the research agreement form and had access to the SEER database.
Results
Baseline characteristics of the patients
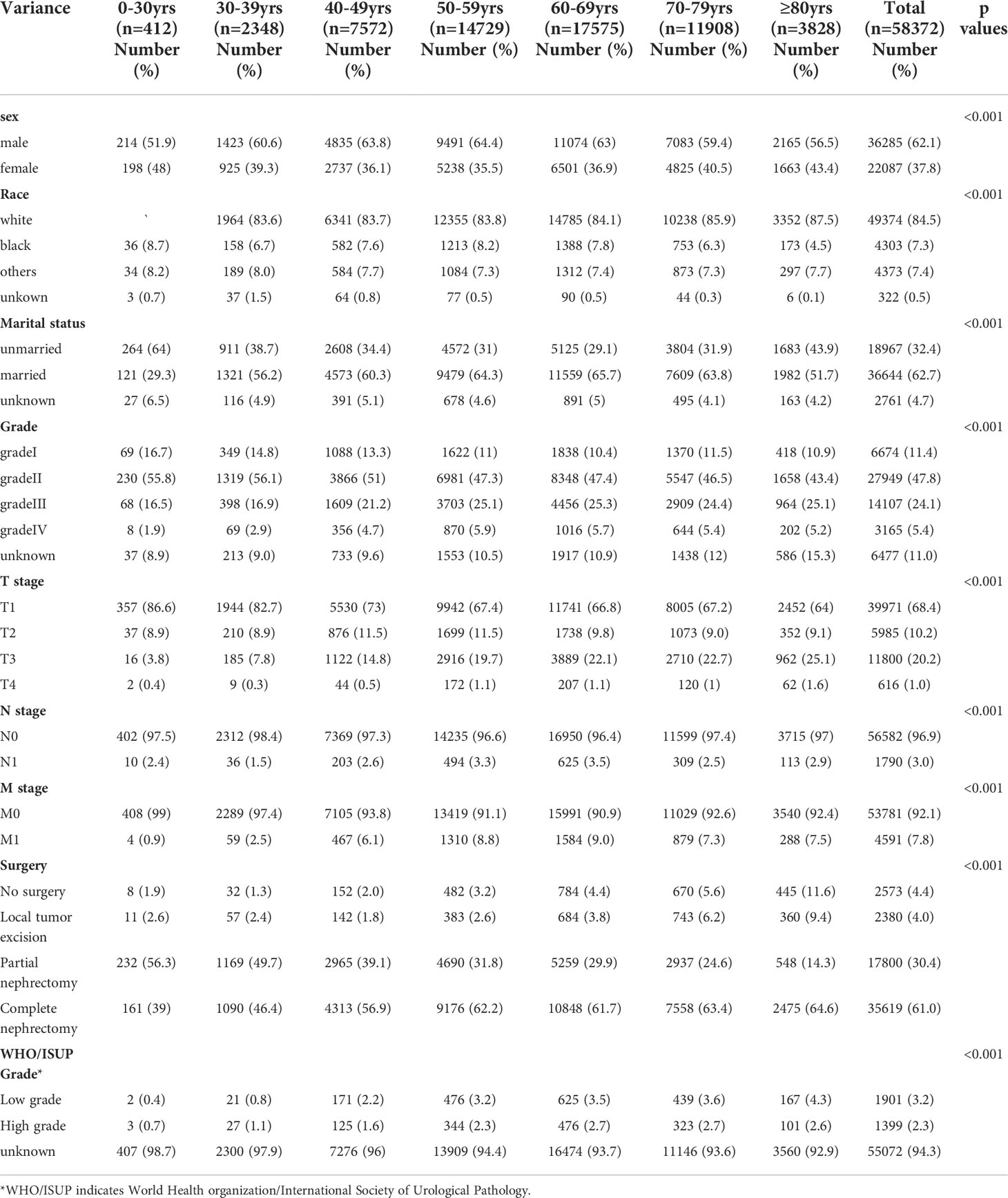
58,372 eligible patients with CCRCC were acquired (Figure 1). 412 cases, aged between 0 and 29 years; 2348 patients aged 30 to 39, 7572 patients aged between 40 and 49, and 14729 patients aged 50 to 59, 17575 patients aged 60 to 69, 11908 patients aged between 70 and 79 together with 3828 patients aged 80 and over. Compared with patients aged below 30 years, patients over 80 years of age had a higher rate of node-positive tumors (2.9% vs. 2.4%; P<0.001), gradeIII tumors (25.1% vs 16.1%; P<0.001) and gradeIV tumors (5.2% vs 1.9%; P<0.001). Meanwhile, patients aged 80 years and above less frequently harbored T1(86.4% vs. 64%), and more frequently harbored T2(8.9% vs. 9.1%), and T3 (3.8% vs. 25.1%;all P<0.001). In contrast to patients under 60 years of age, patients over 80 years of age are more frequently diagnosed at M1 (7.5% vs 0.9%). Notably, among the seven different age groups, patients aged 60-69 years had the highest percentage of node-positive tumor (3.5%), gradeIII tumors (25.3%), T4(1.1%) and M1(9.0%). The highest rates of grade IV tumors (5.9%) and T4 (1.1%) were found in patients aged 50-59 years. Patients’ data include gender, marital status, race, grade, TNM stage, surgery and WHO/ISUP grade. See (Table 1) for a summary.
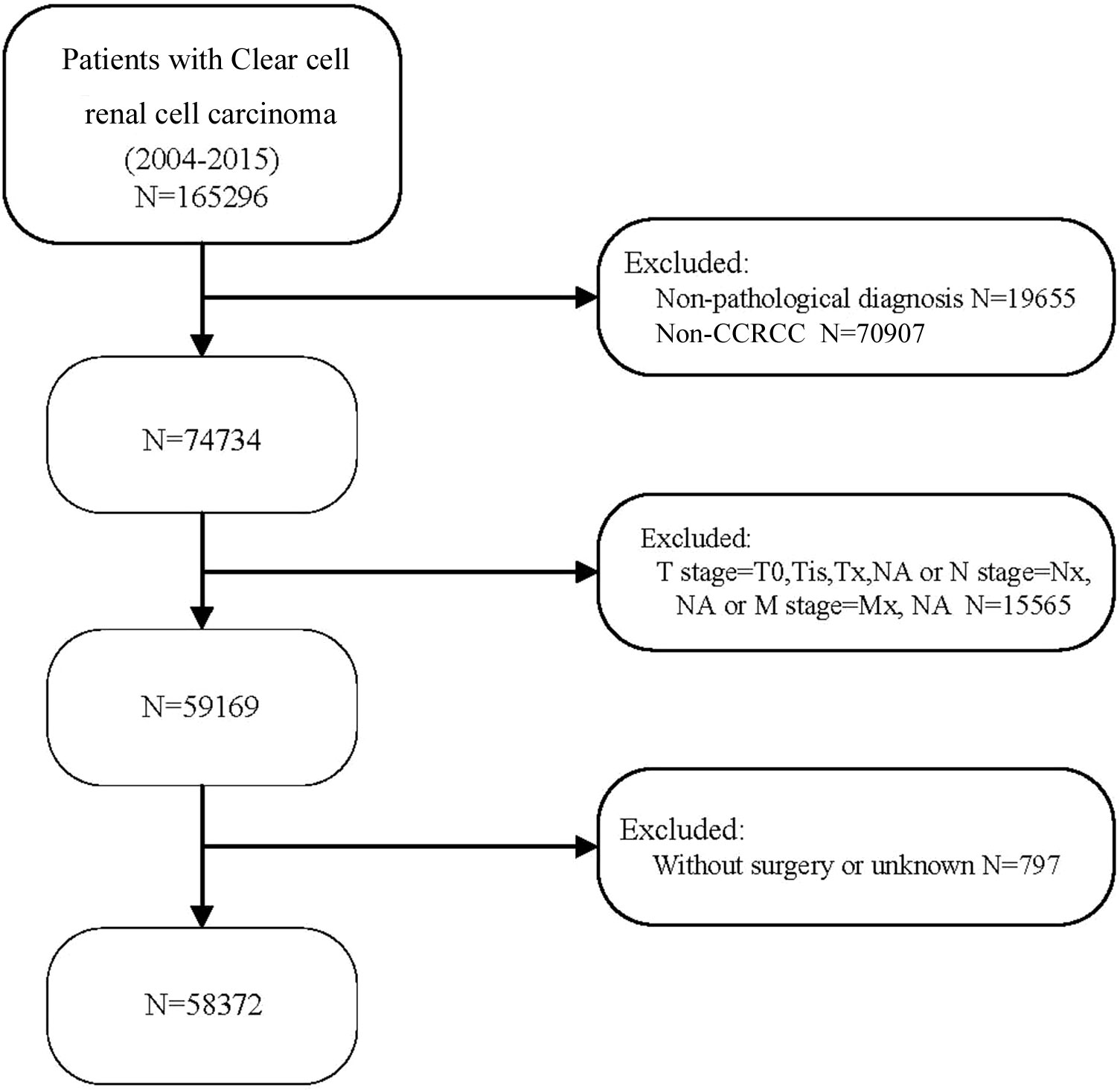
Figure 1 Flow chart for selecting patients with CCRCC. 2004-2015 CCRCC patients were selected in detail from the SEER database.
Influence of age on cancer special survival and overall survival
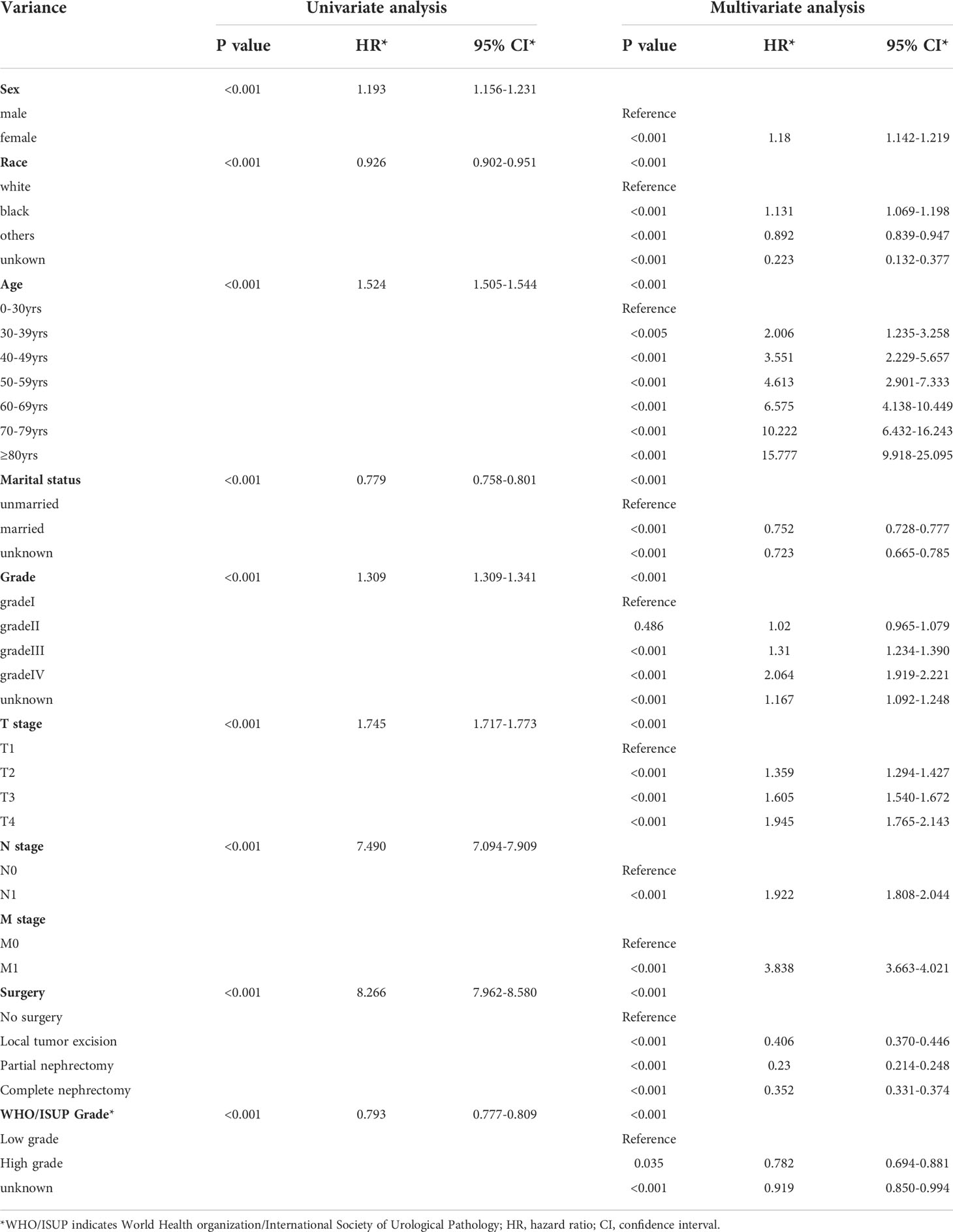
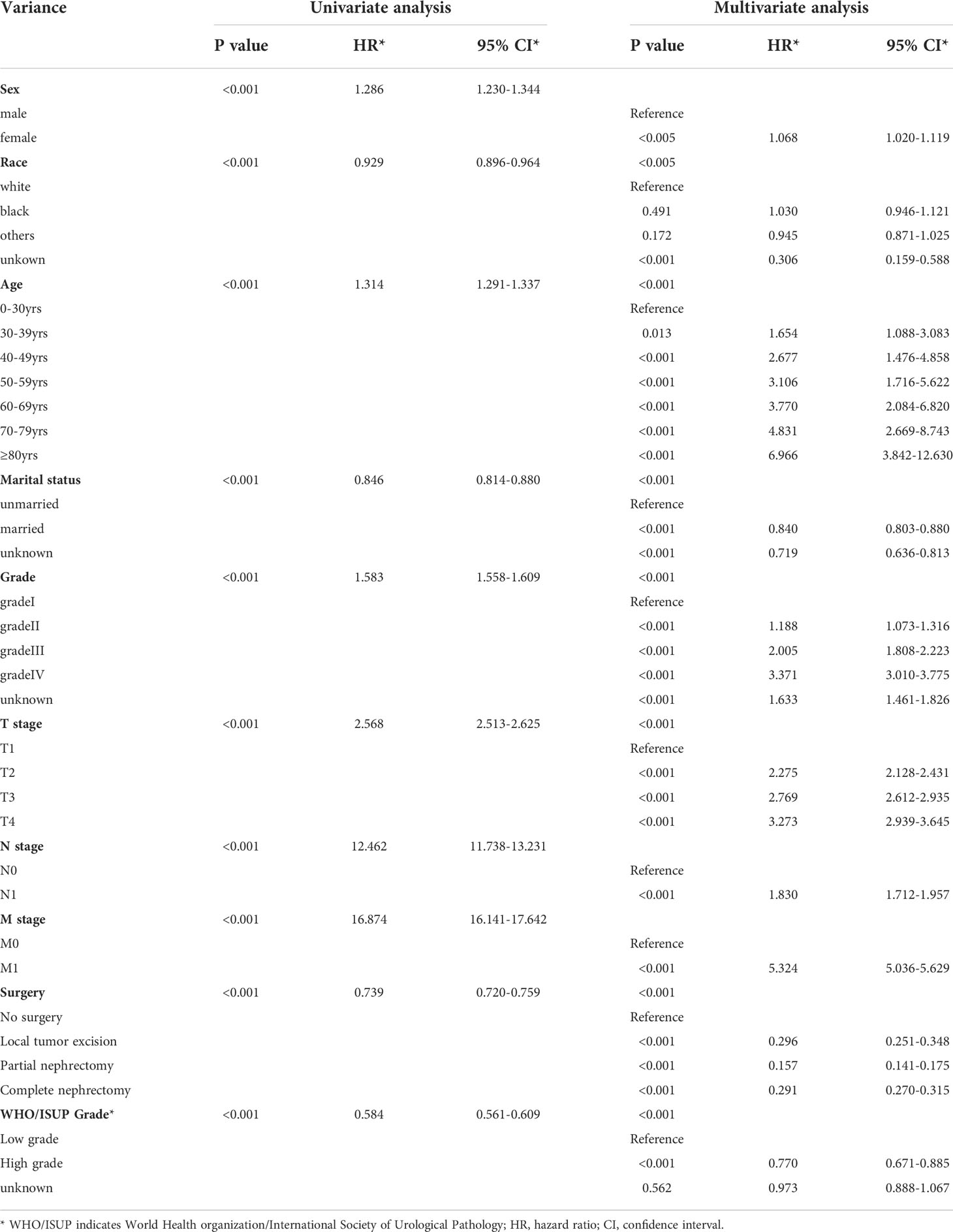
In accordance with Kaplan-Meier curves, both cancer special survival (CSS) and overall survival (OS) were revealed to decrease with age (P<0.001; Figure 2). In univariate analysis, it was found that in addition to age, gender, marital status, race, grade, TNM stage, surgery and WHO/ISUP grade were also correlated with survival (OS and CSS). Analysis of these variables using Cox regression and Kaplan-Meier curves revealed that age, gender, marital status, race, grade, TNM stage, surgery and WHO/ISUP grade were also the independent risk factors of survival (P<0.01; Tables 2, 3; Figures S1, S2).
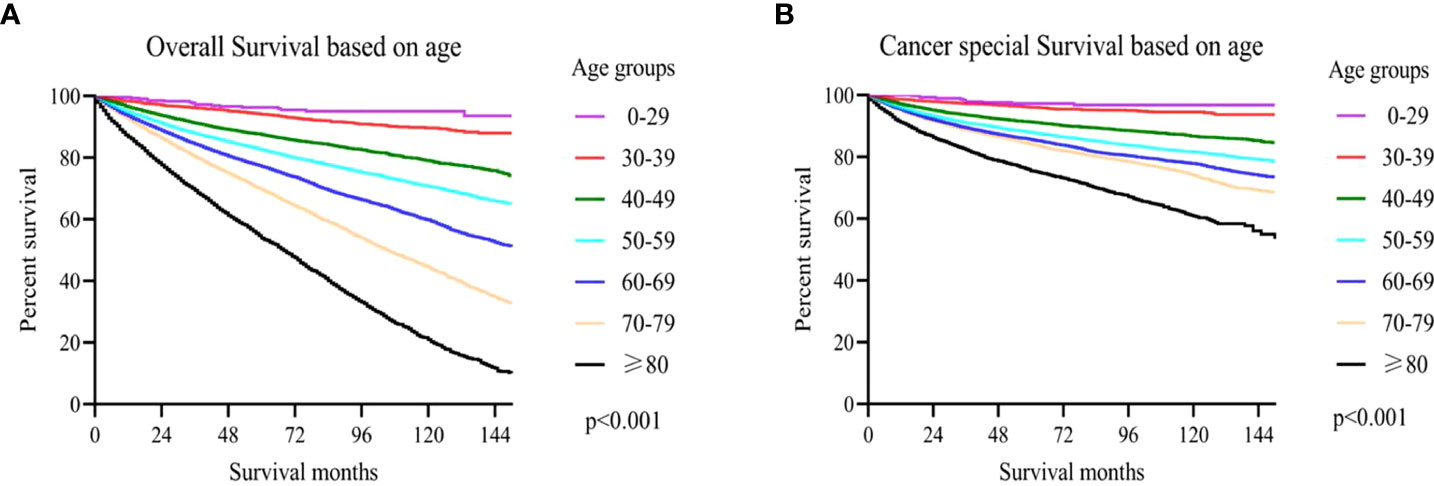
Figure 2 (A) The overall survival (OS) based on age upon diagnosis; (B) cancer-specific survival (CSS) based on age upon diagnosis.
Nomogram for forecasting OS and CSS in CCRCC patients
Nomograms are widely applied in cancer prognosis, principally owing to they can simplify the model of statistical prediction into a single numerical estimation of the event probability (recurrence or death), which is tailored to the patients’ condition. In the training set, ten variables form a nomogram. The specific phases of utilizing the nomogram are as follows: based on the classification (for instance, marital status is classified as unmarried, married and unknown) of each prognostic variable (age, gender, marital status, race, grade, TNM stage, WHO/ISUP grade and surgery), a vertical line was drawn at the top of the nomogram on the horizontal axis labeled “points”. Each prognostic variable was assigned a score at the site where vertical line crosses the “point” axis. The total score was the sum of the scores of the ten variables. Drawn a vertical line on the horizontal axis labeled “Total Score” with the 1-, 3- and 5-year OS as the axis. The intersection of the 1-year OS axis with the vertical line represents the 1-year OS rate (Figure 3).
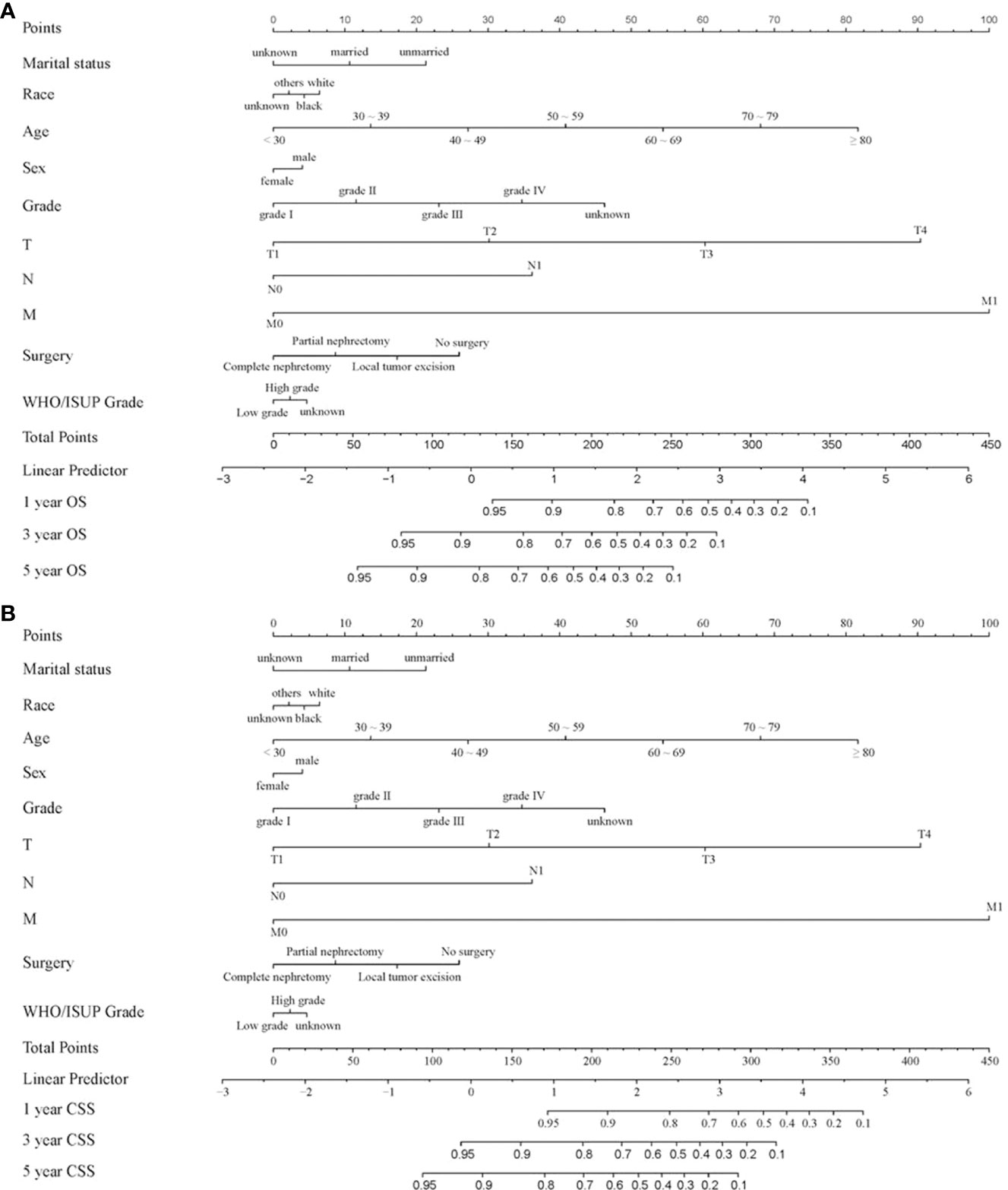
Figure 3 Nomogram for forecasting OS and CSS in CCRCC patients. (A) Nomogram forecasting 1-year, 3-year, and 5-year OS in CCRCC patients; (B) Nomogram forecasting 1-year, 3-year, and 5-year CSS in CCRCC patients.
X-tile analysis determines the optimal cut-off value for age
The correlation between patient age and mortality was explored by using X-tile software. The plots were generated through randomly classifying the age as three groups: low, medium and high. The best dividing point was determined via black/white circles on the χ 2 axis. It was concluded that the optimal cut-off values for age based on overall survival (OS) were determined to be 58 and 76 years. Kaplan-Meier survival curves were formulated according to the age subgroups of OS (Figure 4A); Simultaneously, the best age cut-off depending on cause-specific survival (CSS) was 51 and 76 years and the survival curves were drawn for these age subgroups of CSS using the Kaplan Meier method (Figure 4B).
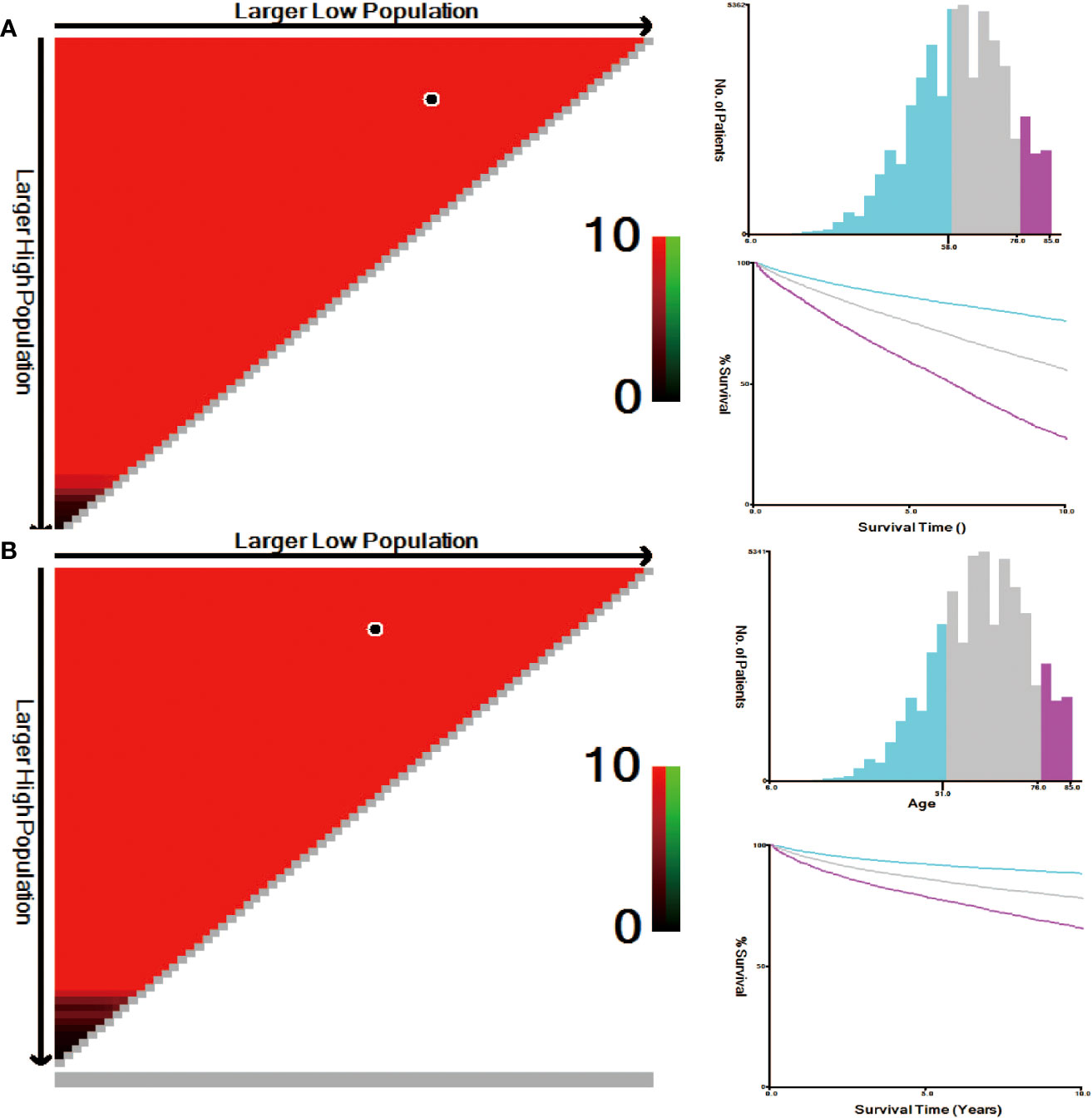
Figure 4 (A) Optimal cut-off point for OS defined with X-tile software; (B) Optimal cut-off point for CSS defined with X-tile software.
Discussion
Despite many studies on CCRCC, limited studies have reported novel findings on age as a prognostic factor for CCRCC and many studies have reported widely divergent findings. To our knowledge, no studies have independently analyzed the prognostic differences between different age groups of patients with CCRCC using a large database. Various studies have shown that children and adults show different features and outcomes of CCRCC. Age is a significant prognostic factor in patients with CCRCC (10–12). The current studies have focused on children, adults, or a particular age point and ignored the differences between age groups (6, 10, 11, 13). Several studies have shown that younger patients with RCC have better pathological characteristics and histological subtypes as well as significantly lower disease-specific mortality than older patients (13–16). Notably, in the treatment decision-making of patients with CCRCC, older patients should be provided with better treatment options and more attention. In contrast to young people, older patients have worse treatment results, lower survival rates, and a greater deterioration risk. This is probably associated with the poor physical activity or underlying diseases in older patients (6). Many studies have found differences between young and older people, but no studies have reported differences between age groups and their impact on the prognosis of patients with CCRCC (15, 17, 18). Several studies comparing mortality rates in older and younger cohorts have found that mortality rates were significantly higher in older adults than in younger adults. Therefore, it has been hypothesized that mortality in patients with CCRCC increases with age (17, 19). This hypothesis is supported by the outcomes of our study, revealing an evident increase in both tumor-specific and all-cause mortality with age in seven different age subgroups of patients with CCRCC, analyzed using univariate and multivariate analyses. Therefore, we recommend individualized treatment for patients with CCRCC to achieve better survival.
The present study included 58,372 patients with CCRCC from the SEER database. According to our age grouping, we found that the TNM stage gradually increased with age. However, patients aged 60-69 years had the highest percentage of node-positive tumor, gradeIII tumors, T4 and M1. The highest rates of grade IV tumors and T4 were found in patients aged 50-59 years. Moreover, 51 and 76 years were used as the cutoff age according to cancer-specific survival rates. The results of the survival analysis suggested that younger age was an independent predictor of CCRCC.
Several studies have suggested a higher stage, smaller size and lower nuclear grade in older patients aged over 50 years with RCC (11). A cutoff age of 40 years to categorize young and older patients with CCRCC. Taccoen et al. observed that age <40 years is a CCRCC-independent prognostic factor, and the prognosis is better (12). Aziz et al. discovered a significantly lower rate of all etiologies, morbidity, and mortality in young patients aged <40 years with RCC (20). Kang et al. also discovered that young age was related to good pathological features, although it was not an independent prognostic factor for survival in patients with surgically treated RCC. Nevertheless, the outcomes of Kaplan–Meier analysis indicated that the younger group aged <40 years had significantly better CCS rates than the middle-aged group aged 40–60 years and older group aged ≥60 years (13). Kim et al. found a more favorable histological subtype for young patients with RCC aged 20–39 years than for those aged 40–79 years (17). Komai et al. have reported that 45 years is the best critical cutoff age to categorize patients into the older and younger groups. Although younger patients with RCC have relapse-free survival rates similar to those of older patients, they have higher CSS rates (15). Jung et al. reported that young age was an independent predictor of prognosis according to multivariate analysis. Moreover, 55 years is a critical cutoff age for distinguishing between older and young patients with CCRCC (11). In this study, we evaluated all possible ages using X-tile plots and selected 51 and 76 years as the cutoff age based on cancer-specific survival. The younger group had better overall survival and CSS than the older group.
However, our study has some limitations. First, in the SEER database, studies utilizing population databases have inherent limitations due to heterogeneity in clinical practice at the participating centers. Second, important treatment information is missing from the SEER database, such as the use of systemic therapy, chemotherapy regimens, metastasectomy, dose of radiation therapy, completeness of surgery, or any other information related to treatment intensity. In addition, information on socioeconomic status and other clinical variables is lacking. The major known risk factors for CCRCC are hypertension, obesity, and smoking (21–26). Nonetheless, owing to the lack of associated data in the renal cancer database, we could not analyze these factors. Third, there may be selection bias due to the retrospective nature of this analysis. Therefore, a prospective randomized trial should be designed to test our study hypothesis and to validate or refute any of the above explanations for the observed relationship between age and prognosis in patients with CCRCC. Despite these limitations, our study was a population-based study in which a large number of patients with CCRCC were included and the results were convincing.
Conclusion
Negative correlation between age and survival of CCRCC patients was found through SEER database. In other words, the older the patient was, the lower the survival rate. Discovering differences in the patients’ prognosis with CCRCC in various age groups has important implications for clinical treatment. Therefore, individualized treatment of patients with CCRCC is imperative. It is unreasonable to distinguish the clear cell renal cell carcinoma only in accordance with the situation of the children, adults and elderly, the diagnosis and treatment plan should be based on more detailed age grouping, which is more beneficial to improving the prognosis and survival rate of the patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: www.seer.cancer.gov.
Author contributions
ZL, HG and ZP designed the study. DW, NS, and YX contributed to data selection and assembly. ZL, HG, and ZP analyzed data. ZL and HG were involved in drafting the manuscript. ZP critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.975779/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier curves for OS according to age for (A) grade; (B) T stage; (C) N stage; (D) M stage; (E) surgery.
Supplementary Figure 2 | Kaplan-Meier curves for CSS according to age for (A) grade; (B) T stage; (C) N stage; (D) M stage; (E) surgery.
Abbreviations
CCRCC: Clear cell renal cell carcinoma; PRCC: papillary renal cell carcinoma; CHRCC: chromophobe renal cell carcinoma; KC: kidney cancer; RCC: renal cell cancer; TCC: transitional cell cancer; SEER: Surveillance, Epidemiology, and End Result; OS: overall survival; CSS: cancer special survival.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol (2015) 67:85–97. doi: 10.1016/j.eururo.2014.04.029
4. Meehan B, Appu S, St Croix B, Rak-Poznanska K, Klotz L, Rak J, et al. Age-related properties of the tumour vasculature in renal cell carcinoma. BJU Int (2011) 107:416–24. doi: 10.1111/j.1464-410X.2010.09569.x
5. Takada S, Namiki M, Takahara S, Matsumiya K, Kondoh N, Kokado Y, et al. Serum HGF levels in acute renal rejection after living related renal transplantation. Transplant Int (1996) 9:151–4. doi: 10.1007/BF00336393
6. Tang F, Lu Z, He C, Zhang H, Wu W, He Z. 53 years old is a reasonable cut-off value to define young and old patients in clear cell renal cell carcinoma: a study based on TCGA and SEER database. BMC Cancer (2021) 21(1):638. doi: 10.1186/s12885-021-08376-5
7. Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med (2005) 353(23):2477–90. doi: 10.1056/NEJMra043172
8. Yusim I, Mermershtain W, Neulander E, Eidelberg I, Gusakova I, Kaneti J, et al. Influence of age on the prognosis of patients with renal cell carcinoma (RCC). Onkologie (2002) 25(6):548–50. doi: 10.1159/000068626
9. Al-Janabi O, Wach S, Nolte E, Weigelt K, Rau TT, Stohr C, et al. Piwi-like 1 and 4 gene transcript levels are associated with clinicopathological parameters in renal cell carcinomas. Biochim Biophys Acta (2014) 1842(6):686–90. doi: 10.1016/j.bbadis.2014.01.014
10. Thoroddsen A, Einarsson GV, Hardarson S, Petursdottir V, Magnusson J, Jonsson E, et al. Renal cell carcinoma in young compared to older patients: comparison of clinicopathological risk factors and survival. Scand J Urol Nephrol (2008) 42(2):121–5. doi: 10.1080/00365590701571555
11. Jung EJ, Lee HJ, Kwak C, Ku JH, Moon KC. Young age is independent prognostic factor for cancer-specific survival of low-stage clear cell renal cell carcinoma. Urology (2009) 73(1):137–41. doi: 10.1016/j.urology.2008.08.460
12. Taccoen X, Valeri A, Descotes JL, Morin V, Stindel E, Doucet L, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer-specific survival. Eur Urol (2007) 51(4):980–7. doi: 10.1016/j.eururo.2006.10.025
13. Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. Impact of young age at diagnosis on survival in patients with surgically treated renal cell carcinoma: a multicenter study. J Korean Med Sci (2016) 31(12):1976–82. doi: 10.3346/jkms.2016.31.12.1976
14. Hupe MC, Merseburger AS, Lokeshwar VB, Eggers H, Rott H, Wegener G, et al. Age–an independent prognostic factor of clinical outcome in renal malignancies: results of a large study over two decades. World J Urol (2014) 32(1):115–21. doi: 10.1007/s00345-013-1164-6
15. Komai Y, Fujii Y, Iimura Y, Tatokoro M, Saito K, Otsuka Y, et al. Young age as favorable prognostic factor for cancer-specific survival in localized renal cell carcinoma. Urology (2011) 77(4):842–7. doi: 10.1016/j.urology.2010.09.062
16. Karakiewicz PI, Jeldres C, Suardi N, Hutterer GC, Perrotte P, Capitanio U, et al. Age at diagnosis is a determinant factor of renal cell carcinoma-specific survival in patients treated with nephrectomy. Can Urol Assoc J (2008) 2(6):610–7. doi: 10.5489/cuaj.978
17. Kim JH, Park YH, Kim YJ, Kang SH, Byun SS, Hong SH, et al. Is there a difference in clinicopathological outcomes of renal tumor between young and old patients? a multicenter matched-pair analysis. Scand J Urol (2016) 50(5):387–91. doi: 10.1080/21681805.2016.1204621
18. Cai M, Wei J, Zhang Z, Zhao H, Qiu Y, Fang Y, et al. Impact of age on the cancer-specific survival of patients with localized renal cell carcinoma: martingale residual and competing risks analysis. PloS One (2012) 7(10):e48489. doi: 10.1371/journal.pone.0048489
19. Panian J, Lin X, Simantov R, Derweesh I, Choueiri TK, McKay RR, et al. The impact of age and gender on outcomes of patients with advanced renal cell carcinoma treated with targeted therapy. Clin Genitourin Cancer (2020) 18(5):e598–609. doi: 10.1016/j.clgc.2020.03.010
20. Aziz A, May M, Zigeuner R, Pichler M, Chromecki T, Cindolo L, et al. Do young patients with renal cell carcinoma feature a distinct outcome after surgery? a comparative analysis of patient age based on the multinational CORONA database. J Urol (2014) 191(2):310–5. doi: 10.1016/j.juro.2013.08.021
21. Kroeger N, Klatte T, Birkhauser FD, Rampersaud EN, Seligson DB, Zomorodian N, et al. Smoking negatively impacts renal cell carcinoma overall and cancer-specific survival. Cancer (2012) 118(7):1795–802. doi: 10.1002/cncr.26453
22. Patel NH, Attwood KM, Hanzly M, Creighton TT, Mehedint DC, Schwaab T, et al. Comparative analysis of smoking as a risk factor among renal cell carcinoma histological subtypes. J Urol (2015) 194(3):640–6. doi: 10.1016/j.juro.2015.03.125
23. Golabek T, Bukowczan J, Szopinski T, Chlosta P, Lipczynski W, Dobruch J, et al. Obesity and renal cancer incidence and mortality–a systematic review of prospective cohort studies. Ann Agric Environ Med (2016) 23(1):37–43. doi: 10.5604/12321966.1196850
24. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol (2014) 10(8):455–65. doi: 10.1038/nrendo.2014.94
25. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol (2010) 7(5):245–57. doi: 10.1038/nrurol.2010.46
Keywords: Clear cell renal cell carcinoma, seer, age, prognosis, all-cause mortality, tumor-specific mortality
Citation: Liao Z, Wang D, Song N, Xu Y, Ge H and Peng Z (2022) Prognosis of clear cell renal cell carcinoma patients stratified by age: A research relied on SEER database. Front. Oncol. 12:975779. doi: 10.3389/fonc.2022.975779
Received: 22 June 2022; Accepted: 21 September 2022;
Published: 12 October 2022.
Edited by:
Vadim S. Koshkin, University of California San Francisco, United StatesReviewed by:
Stefano Luzzago, European Institute of Oncology (IEO), ItalyRahul Mannan, University of Michigan, United States
Copyright © 2022 Liao, Wang, Song, Xu, Ge and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhangzhe Peng, bHpuODI5QGZveG1haWwuY29t; Heming Ge, Z2VoZW1pbmdAb3V0bG9vay5jb20=
 Zhouning Liao1
Zhouning Liao1 Dang Wang
Dang Wang Zhangzhe Peng
Zhangzhe Peng