
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 17 November 2022
Sec. Skin Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.975342
This article is part of the Research Topic Women in Skin Cancer Vol II: 2022 View all 10 articles
Pleomorphic dermal sarcomas (PDS) are rare neoplasms of the skin that occur in UV-exposed sites in the elderly, but represent the most common cutaneous sarcomas. Although the majority of PDS can be surgically removed, local recurrences occur in up to 28%, usually occurring within the first two years after primary excision. Metastases are diagnosed in up to 20% of cases, mainly observed in the skin, lymph nodes and lungs, preferentially affecting patients with underlying hemato-oncologic diseases. Similar to other UV-induced tumors, PDS are inflammatory and immunogenic tumors (with a high number of CD4+/CD8+ tumor-infiltrating lymphocytes (TILs) and checkpoint molecule expression such as PD-L1, LAG-3, TIGIT) with a very high mutational burden. The most common genetic alterations include UV-induced TP53 loss of function mutations, followed by alterations in the CDKN2A/B gene. Rarely, targetable genetic alterations can be detected. Compelling experimental data and clinical reports about PD-1/PD-L1-blocking antibodies in patients with PDS suggest its use as first line treatment in unresectable or metastatic tumor stages. However, individual („off-line”) patient management should be discussed in an interdisciplinary tumor board based on molecular genetic testing, mutational burden, PD-L1 expression, and evidence of tumor-infiltrating lymphocytes in addition to comorbities of the individual patient.
Pleomorphic dermal sarcomas (PDS) are rare neoplasms of the skin with a mesenchymal (fibroblastic) lineage differentiation, arising in UV-exposed locations, typically diagnosed in elderly male individuals (1–3). Although accurate incidence data do not exist, they represent the most common cutaneous sarcomas with increasing incidence due to demographic changes.
Given the similarities in clinic, histology as well as molecular genetics and epigenetics, atypical fibroxanthoma (AFX) and PDS are now considered a spectrum of one entity, but differ in terms of therapy and prognosis (4–10). Histomorphologically, AFX and PDS show similar features. The main difference is that AFX are confined to the dermis whereas PDS involves distinct portions of the subcutis and/or have necrotic tumor portions and/or perineural or lympho-vascular invasion. In AFX, the local recurrence rate after R0 resection is less than 5% (3, 11, 12). While the majority of PDS can be treated by curative excisions, local recurrences occur in up to 28% of patients. Metastases are observed in up to 20%, mainly in the skin, lymph nodes and lungs, preferentially affecting patients with underlying hemato-oncologic diseases (3, 10, 11, 13, 14).
In the last years, it could be shown that PDS are inflammatory and immunogenic tumors with a very high mutational burden. Experimental data and clinical reports indicate that PD-1/PD-L1-blocking antibodies are highly effective in patients with unresectable or metastatic PDS and suggest its use as first line treatment in these advanced tumor stages (2, 15–17).
Both, AFX and PDS have a very high mutational burden with UV signature, which is even higher than that of other UV-induced skin tumors such as cutaneous squamous cell carcinomas (cSCC) and malignant melanomas (2, 18–20). Based on very similar gene mutations, gene expressions, copy number variations as well as DNA methylation profiles, AFX and PDS are now accepted to represent a spectrum of the same tumor entity (5, 7–9). In PDS, it has been shown that the tumors exhibit the UV-induced mutation signatures 7a and 7b in almost equal proportions. In other UV-induced tumors such as cSCC, basal cell carcinoma, and melanoma, signature 7a is typically detected, whereas signature 7b is rarely detected. Signature 44, which has been associated with defective DNA mismatch repair (MMR), has been detected in a small number of our investigated PDS (3 of 28); however, is much more common in cSCC (Figure 1) (2).
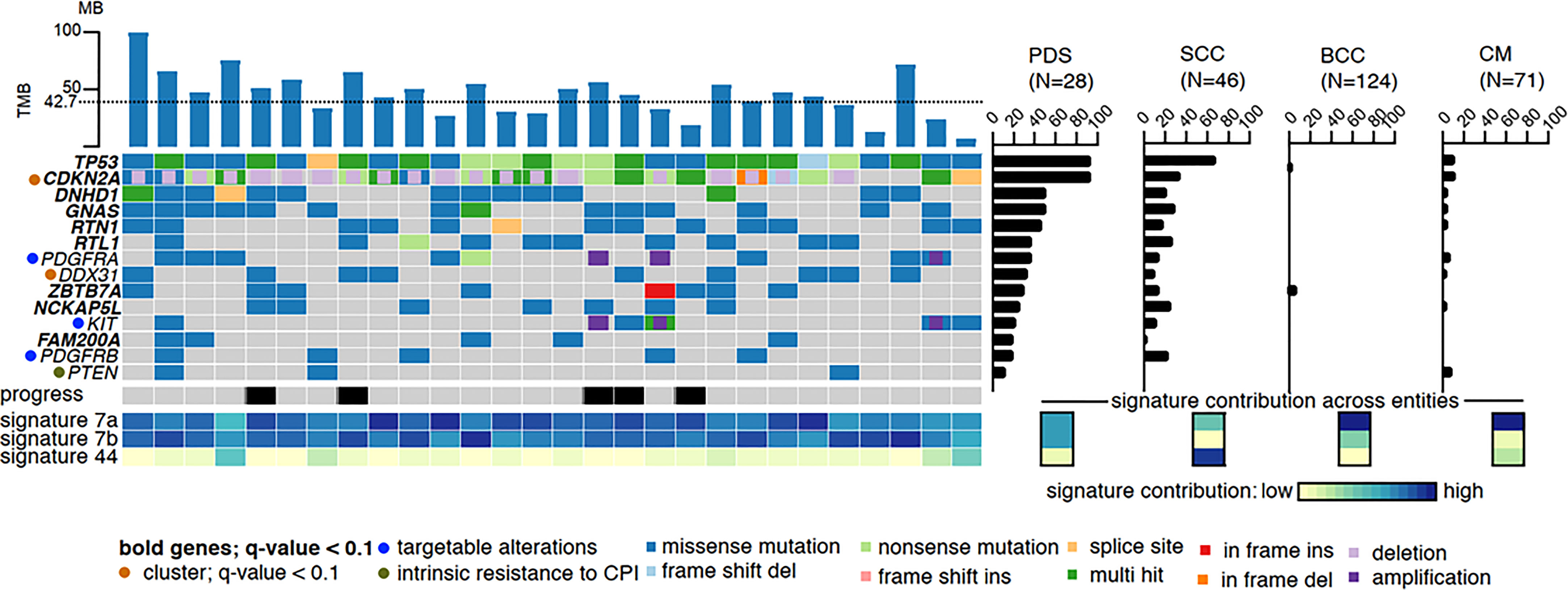
Figure 1 High mutational burden and most common mutations of PDS: The dotted line represents the average of variants per megabase. The mutational frequency of pleomorphic dermal sarcoma (PDS) is compared to cutaneous squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and cutaneous melanoma (CM), where the bars indicate their relative prevalence (percentage, right panel). Cross entity comparison of mutational signatures shown below the right panel of mutational frequencies. Clinically, locally or systemically progressed tumors are indicated with a black bar (2).
Gene mutation and gene expression analyses revealed that AFX/PDS have the highest similarity to cSCC. The most frequent genetic alterations are TP53 loss of function mutations which can be detected in all PDS, followed by genetic alterations in the CDKN2A/B gene (CDKN2A/B mutations in 68%, deletions in 71%, and both in 46%, while 7% showed even a biallelic loss.) (Figure 1) (2). Other common mutations include DNHD1, GNAS, RTN1, RTL1, ZBTB7A, NCKAP5L, FAM200A, NOTCH1/2, FAT1, and TERT promoter mutations (2, 5, 6, 8, 20, 21). In contrast, cSCC, basal cell carcinomas and malignant melanomas show a significantly lower mutation frequency of these frequently mutated genes (2). In a small proportion of PDS, amplifications of PDGFRA leading to a PDGFRA expression on protein level and mutations within the kinase domain of KIT could be detected.
Immunohistochemical as well as mRNA expression analyses of the immune “microenvironment” have shown that the majority of PDS represent inflammatory and immunogenic tumors with a high number of CD8+ tumor-infiltrating lymphocytes (TILs) and expression of diverse checkpoint molecules such as PD-L1, TIGIT, LAG-3, and CTLA-4 (2, 15, 16).
When we classified a series of PDS tumors into immunologically hot and cold tumors (high versus low amounts of both CD4/CD8+ cells and high versus low PD-L1 expression), we did not detect a significant difference of tumor mutational burden (TMB). Nevertheless, the TMB is usually high in almost all PDS cases. Differential gene expression analysis between these immunologically hot and cold tumors revealed upregulation of TIGIT in the immunologically hot tumors (2).
In general, elevated levels of immune-related cytokines such as IL1A, IL2, as well as markers that were very recently linked to enhanced response of immunotherapy in malignant melanoma, including CD27, and CD40L have been detected in PDS tumors (22, 23). Moreover, the majority of PDS showed strong MHC-I expression and upregulated HLA class I molecules (HLA-A, HLA-B, HLA-C and HLA-E, corresponding in humans to the MHC class I) that are involved in tumor neoantigen presentation to tumor-specific CD8+ T lymphocytes leading to tumor cell apoptosis (24–28).
In CD8+high PDS cases (defined as cases with CD8 levels above median), genes such as CD74, LYZ and HLA-B were found to be differentially expressed while the remaining cases revealed enhanced levels of immunosuppressive cytokines including CXCL14 (29). In addition, the majority of PDS was infiltrated by PD-L1-, PD-1- and LAG-3-expressing immune cells and showed strong MHC-I expression on tumor cells (15).
These results imply that PDS in general, but especially those with a lot of infiltrating CD8+ and/or PD-L1- and LAG-3-expressing TILs as well as MHC-I expression, induce an adequate anti-tumor immune response, which could be enhanced by immune checkpoint inhibitors. Only a small proportion of tumors appear to develop “immune escape” mechanisms, such as downregulation of MHC-I molecules (2, 15, 16).
Radical excision followed by histopathologic workup is usually performed with curative intent as initial treatment for PDS. An appropriate safety margin should be maintained, as the risk of local recurrence or metastasis can be reduced by wide local excision (3, 12, 13, 30, 31). If this is not possible, a microscopically controlled excision should be performed. Although there are no published data on the radiation sensitivity of AFX/PDS, radiation of the tumor area may be considered if complete tumor excision is not possible. The efficacy of adjuvant radiation with respect to the prognosis of completely excised PDS has not been conclusively established. In an evaluation of a few patients who had received adjuvant postradiation, a positive tendency (fewer local recurrences and/or metastases) of this postradiation could be elicited (3).
In case of advanced stage PDS, therapy recommendations should be always discussed and issued in the context of an interdisciplinary tumor board because there is no proven standard therapy. Here, molecular genetic testing, mutational burden, PD-L1 expression, and evidence of tumor-infiltrating lymphocytes (TILs) should be incorporated into individual treatment recommendations.
Since PDS harbor a high mutational burden and mostly exhibit an inflamed, proimmunogenic tumor microenvironment, susceptibility to immune checkpoint inhibition by programmed cell death 1 (PD1)/programmed cell death ligand 1 (PD-L1) inhibitors, (e.g. pembrolizumab, nivolumab) or the anti–CTLA-4 antibody (ipilimumab), or a combination of these agents was presumed in reference to other highly mutated and immunogenic tumors including other skin tumors such as malignant melanoma and cSCC (18, 32, 33). In the meantime, the exceptionally high efficacy of the anti-PD-1 inhibitor pembrolizumab has been described in case reports or small case series (see Table 1) (2, 15–17). Until now, there are no case reports describing the use of other CPI in PDS patients. Nevertheless, larger clinical studies are needed to investigate tumor response to CPI in PDS patients.
For PDS cases with low levels of CD8+ TILs, interventions to increase the infiltration of these inflammatory cells in general need to be explored as a future direction for successful treatment with CPI. As shown in other tumor entities, a dual blockade of CTLA-4 and PD-1 or PD-1/PDL-1 and LAG-3 could probably enhance the efficacy of CPI monotherapies, also by rescuing CD8+ T cells more vigorously from exhaustion than single signaling blockade (34, 35).
In case of contraindications for a CPI treatment and if oncogenic alterations are detected, targeted therapies should be discussed, although there is no experience with targeted therapies in PDS to date (2, 7, 8). In relation to this, rarely detected PDGFRA or KIT amplifications/mutations could be of interest as several drugs have proven to induce long-term remissions in PDGFR-expressing cancers, such as gastrointestinal stromal tumors,dermatofibrosarcoma protuberans, or myeloid malignancies (36–40). Furthermore, it has been shown that tumors with a loss of CDKN2A/B may benefit from CDK4/6 inhibitors, such as palbociclib, abemaciclib or ribociclib, all approved for the treatment of metastasized breast cancer (41–44).
In patients with advanced stage PDS treated with CPI, further investigation of predictors is still needed. However, all existing studies suggest a high efficacy of immune checkpoint blockade in inoperable or metastatic PDS patients.
All authors contributed to the article and approved the submitted version.
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GL declared a shared affiliation with the author SK to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg (2007) 33(6):693–6. doi 10.1111/j.1524-4725.2007.33145.x
2. Klein S, Quaas A, Noh KW, Cartolano M, Abedpour N, Mauch C, et al. Integrative analysis of pleomorphic dermal sarcomas reveals fibroblastic differentiation and susceptibility to immunotherapy. Clin Cancer Res (2020) 26(21):5638–45. doi: 10.1158/1078-0432.CCR-20-1899
3. Persa OD, Loquai C, Wobser M, Baltaci M, Dengler S, Kreuter A, et al. Extended surgical safety margins and ulceration are associated with an improved prognosis in pleomorphic dermal sarcomas. J Eur Acad Dermatol Venereol (2019) 33(8):1577–80. doi: 10.1111/jdv.15493
4. Withers AH, Brougham ND, Barber RM, Tan ST. Atypical fibroxanthoma and malignant fibrous histiocytoma. J Plast Reconstr Aesthet Surg (2011) 64(11):e273–8. doi: 10.1016/j.bjps.2011.05.004
5. Griewank KG, Wiesner T, Murali R, Pischler C, Muller H, Koelsche C, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma harbor frequent NOTCH1/2 and FAT1 mutations and similar DNA copy number alteration profiles. Mod Pathol (2018) 31(3):418–28. doi: 10.1038/modpathol.2017.146
6. Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, Sucker A, et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol (2014) 27(4):502–8. doi: 10.1038/modpathol.2013.168
7. Helbig D, Quaas A, Mauch C, Merkelbach-Bruse S, Buttner R, Emberger M, et al. Copy number variations in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget (2017) 8(65):109457–67. doi: 10.18632/oncotarget.22691
8. Helbig D, Ihle MA, Putz K, Tantcheva-Poor I, Mauch C, Buttner R, et al. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget (2016) 7(16):21763–74. doi: 10.18632/oncotarget.7845
9. Koelsche C, Stichel D, Griewank KG, Schrimpf D, Reuss DE, Bewerunge-Hudler M, et al. Genome-wide methylation profiling and copy number analysis in atypical fibroxanthomas and pleomorphic dermal sarcomas indicate a similar molecular phenotype. Clin Sarcoma Res (2019) 9:2. doi: 10.1186/s13569-019-0113-6
10. Helbig D, Ziemer M, Dippel E, Erdmann M, Hillen U, Leiter U, et al. S1-guideline atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS). J Dtsch Dermatol Ges (2022) 20(2):235–43. doi: 10.1111/ddg.14700
11. Helbig D. Hemato-oncological diseases as risk factor for recurrence or metastasis of pleomorphic dermal sarcoma. Front Oncol (2022) 12:873771. doi: 10.3389/fonc.2022.873771
12. Tolkachjov SN, Kelley BF, Alahdab F, Erwin PJ, Brewer JD. Atypical fibroxanthoma: Systematic review and meta-analysis of treatment with mohs micrographic surgery or excision. J Am Acad Dermatol (2018) 79(5):929–34.e6. doi: 10.1016/j.jaad.2018.06.048
13. Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma: Adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol (2012) 36(9):1317–26. doi: 10.1097/PAS.0b013e31825359e1
14. Tardio JC, Pinedo F, Aramburu JA, Suarez-Massa D, Pampin A, Requena L, et al. Pleomorphic dermal sarcoma: A more aggressive neoplasm than previously estimated. J Cutan Pathol (2016) 43(2):101–12. doi: 10.1111/cup.12603
15. Klein S, Mauch C, Wagener-Ryczek S, Schoemmel M, Buettner R, Quaas A, et al. Immune-phenotyping of pleomorphic dermal sarcomas suggests this entity as a potential candidate for immunotherapy. Cancer Immunol Immunother (2019) 68(6):973–82. doi: 10.1007/s00262-019-02339-3
16. Klein S, Persa OD, Mauch C, Noh KW, Pappesch R, Wagener-Ryczek S, et al. First report on two cases of pleomorphic dermal sarcoma successfully treated with immune checkpoint inhibitors. Oncoimmunology (2019) 8(12):e1665977. doi: 10.1080/2162402X.2019.1665977
17. Klein O, McTigue C, Wong ZW, Syme DB, Hunter-Smith DJ. Complete response of metastatic pleomorphic dermal sarcoma to anti-PD-1 therapy. Br J Dermatol (2020) 183(6):e189. doi: 10.1111/bjd.19309
18. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol (2020) 21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4
19. Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer (2020) 8(1):1–8. doi: 10.1136/jitc-2020-000775
20. Lai K, Harwood CA, Purdie KJ, Proby CM, Leigh IM, Ravi N, et al. Genomic analysis of atypical fibroxanthoma. PLoS One (2017) 12(11):e0188272. doi: 10.1371/journal.pone.0188272
21. Sakamoto A, Oda Y, Itakura E, Oshiro Y, Tamiya S, Honda Y, et al. H-, K-, and n-ras gene mutation in atypical fibroxanthoma and malignant fibrous histiocytoma. Hum Pathol (2001) 32(11):1225–31. doi: 10.1053/hupa.2001.28953
22. Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med (2018) 24(10):1545–9. doi: 10.1038/s41591-018-0157-9
23. Wasiuk A, Testa J, Weidlick J, Sisson C, Vitale L, Widger J, et al. CD27-mediated regulatory T cell depletion and effector T cell costimulation both contribute to antitumor efficacy. J Immunol (2017) 199(12):4110–23. doi: 10.4049/jimmunol.1700606
24. Chowell D, Krishna S, Becker PD, Cocita C, Shu J, Tan X, et al. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc Natl Acad Sci U.S.A. (2015) 112(14):E1754–62. doi: 10.1073/pnas.1500973112
25. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348(6230):124–8. doi 10.1126/science.aaa1348
26. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med (2014) 371(23):2189–99. doi: 10.1056/NEJMoa1406498
27. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (2015) 350(6257):207–11. doi: 10.1126/science.aad0095
28. Qian J, Luo F, Yang J, Liu J, Liu R, Wang L, et al. TLR2 promotes glioma immune evasion by downregulating MHC class II molecules in microglia. Cancer Immunol Res (2018) 6(10):1220–33. doi: 10.1158/2326-6066.CIR-18-0020
29. Starnes T, Rasila KK, Robertson MJ, Brahmi Z, Dahl R, Christopherson K, et al. The chemokine CXCL14 (BRAK) stimulates activated NK cell migration: Implications for the downregulation of CXCL14 in malignancy. Exp Hematol (2006) 34(8):1101–5. doi: 10.1016/j.exphem.2006.05.015
30. Lonie S, Yau B, Henderson M, Gyorki D, Angel C, Webb A. Management of pleomorphic dermal sarcoma. ANZ J Surg (2020) 90(11):2322–4. doi: 10.1111/ans.15909
31. Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: Atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol (2017) 18(8):50. doi: 10.1007/s11864-017-0489-6
32. Krieger T, Pearson I, Bell J, Doherty J, Robbins P. Targeted literature review on use of tumor mutational burden status and programmed cell death ligand 1 expression to predict outcomes of checkpoint inhibitor treatment. Diagn Pathol (2020) 15(1):6. doi: 10.1186/s13000-020-0927-9
33. Maubec E, Boubaya M, Petrow P, Beylot-Barry M, Basset-Seguin N, Deschamps L, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol (2020) 38(26):3051–61. doi: 10.1200/JCO.19.03357
34. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med (2016) 374(26):2542–52. doi: 10.1056/NEJMoa1603702
35. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
36. Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother (2014) 15(14):1979–89. doi: 10.1517/14656566.2014.937707
37. Metzgeroth G, Walz C, Erben P, Popp H, Schmitt-Graeff A, Haferlach C, et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: A phase-II study. Br J Haematol (2008) 143(5):707–15. doi: 10.1111/j.1365-2141.2008.07294.x
38. Rutkowski P, Klimczak A, Lugowska I, Jagielska B, Wagrodzki M, Debiec-Rychter M, et al. Long-term results of treatment of advanced dermatofibrosarcoma protuberans (DFSP) with imatinib mesylate - the impact of fibrosarcomatous transformation. Eur J Surg Oncol (2017) 43(6):1134–41. doi: 10.1016/j.ejso.2017.03.011
39. Ugurel S, Mentzel T, Utikal J, Helmbold P, Mohr P, Pfohler C, et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: a multicenter phase II DeCOG trial with long-term follow-up. Clin Cancer Res (2014) 20(2):499–510. doi: 10.1158/1078-0432.CCR-13-1411
40. Fu Y, Kang H, Zhao H, Hu J, Zhang H, Li X, et al. Sunitinib for patients with locally advanced or distantly metastatic dermatofibrosarcoma protuberans but resistant to imatinib. Int J Clin Exp Med (2015) 8(5):8288–94.
41. Eilers G, Czaplinski JT, Mayeda M, Bahri N, Tao D, Zhu M, et al. CDKN2A/p16 loss implicates CDK4 as a therapeutic target in imatinib-resistant dermatofibrosarcoma protuberans. Mol Cancer Ther (2015) 14(6):1346–53. doi: 10.1158/1535-7163.MCT-14-0793
42. Shah A, Bloomquist E, Tang S, Fu W, Bi Y, Liu Q, et al. FDA Approval: Ribociclib for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer. Clin Cancer Res (2018) 24(13):2999–3004. doi: 10.1158/1078-0432.CCR-17-2369
43. Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol (2017) 35(25):2875–84. doi: 10.1200/JCO.2017.73.7585
Keywords: Immune checkpoint inhibitor, PD-1, PD-L1, pleomorphic dermal sarcoma (PDS), unresectable, metastasized
Citation: Helbig D and Klein S (2022) Immune checkpoint inhibitors for unresectable or metastatic pleomorphic dermal sarcomas. Front. Oncol. 12:975342. doi: 10.3389/fonc.2022.975342
Received: 22 June 2022; Accepted: 03 November 2022;
Published: 17 November 2022.
Edited by:
Pamela Bond Cassidy, Oregon Health and Science University, United StatesReviewed by:
Georg Christian Lodde, University Hospital of Essen, GermanyCopyright © 2022 Helbig and Klein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doris Helbig, ZG9yaXMuaGVsYmlnQHVrLWtvZWxuLmRl; Sebastian Klein, c2ViYXN0aWFuLmtsZWluQHVrLWVzc2VuLmRl
†ORCID: Doris Helbig, orcid.org/0000-0002-5841-4631
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.