
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 29 September 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.974116
This article is part of the Research TopicState of the Art Body Composition Profiling: Advances in Imaging Modalities and Patient Outcomes.View all 9 articles
 Stefania Rizzo1,2*
Stefania Rizzo1,2* Isabel Scala2
Isabel Scala2 Angela Rodriguez Robayo3
Angela Rodriguez Robayo3 Marco Cefalì4
Marco Cefalì4 Sara De Dosso2,4
Sara De Dosso2,4 Stefano Cappio1
Stefano Cappio1 Genti Xhepa1
Genti Xhepa1 Filippo Del Grande1,2
Filippo Del Grande1,2Objectives: The objective of this systematic review was to assess associations between quantitative body composition measures extracted from imaging examinations and chemotherapy-related toxicity in pancreatic cancer patients. A secondary objective was to evaluate the different definitions of sarcopenia across included studies.
Methods: This systematic review was conducted according to the PRISMA statement. A comprehensive literature search of three electronic databases was performed by two authors. For each eligible article, information was collected concerning the clinical setting; basic study; population characteristics; technical; body composition features evaluated; CA 19.9 tumor marker levels; chemotherapy drugs administered; toxicities (hematologic, nausea/vomiting, diarrhea, neuropathy, reduction of number of cycles, overall toxicity); association of body composition values with toxicities. The overall quality of the included studies was critically evaluated.
Results: After the initial retrieval of 1137 articles, the systematic review included 12 articles (1/12 in the neo-adjuvant setting; 2/12 in the adjuvant setting; 3/12 in the metastatic setting; 2/12 in the unresectable setting; the other 4/12 included more than one clinical setting). The number of patients included ranged between 17 and 251; mean/median age ranged between 63 and 77 years; the percentage of sarcopenic patients ranged between 23 and 76%. The most frequent body composition parameter evaluated was skeletal muscle index (11/12). Chemotherapy regimens included gemcitabine (as monotherapy or in combination with other drugs); FOLFIRINOX and S-1. Among the trials including gemcitabine, 2/9 demonstrated an association with toxicity, whereas 7/9 did not; among those including FOLFIRINOX, one demonstrated associated toxicity whereas the others did not. Altogether, 4/12 papers demonstrated an association between the body composition values and the development of chemotherapy-related toxicities.
Conclusions: There is a wide variability of results about the association of body composition and chemotherapy-related toxicity in PC patients. Furthermore, cut-off values to define sarcopenia in PC patients are not yet uniformly defined.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022337753, identifier CRD42022337753.
Among the malignancies originating from the digestive system, pancreatic cancer (PC) is the second most frequent with 62,210 estimated new cases in the US in 2022, and the most lethal, with 49,830 estimated deaths (1). Complete surgical resection leads to better survival rates in PC patients. However, less than one-fifth of patients are considered resectable at the time of diagnosis (2, 3), and most patients will need to undergo chemotherapy, either in the neoadjuvant, adjuvant, or advanced setting.
In PC patients, a mix of inadequate nutritional intake, metabolic alterations due to malignancy, and malabsorption leads to a loss of muscle mass, also referred to as sarcopenia (4, 5), as well as to a change in the composition of distribution of muscle and fat in the patient’s body.
Body composition assessment may include evaluation of muscle mass by skeletal muscle area (SMA) and skeletal muscle index (SMI), as well as assessment of fat distribution by subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). Body composition has been shown to correlate with prognosis in many cancer subtypes, including ovarian (6), lung (7), bladder (8) and pancreatic malignancies (9). Furthermore, in some cancer types, sarcopenia increases the toxicity of chemotherapy (10, 11), likely because drug dosing is largely based on the body surface area, that takes into account only the patient’s height and weight but ignores the relative quantity and distribution of muscle and fat. Consequently, sarcopenic cancer patients tend to receive a higher dose of chemotherapeutic agent for a relatively small lean muscle mass and are more prone to suffer toxicity (12, 13). Unfortunately, a higher incidence of toxicity eventually leads to a higher likelihood of treatment termination and hospitalization. Cancer patients undergo numerous imaging examinations at staging and during follow-up (14–16), and since computed tomography (CT) and magnetic resonance imaging (MRI) are currently considered gold standard methods in the evaluation of human body composition (17–19), this assessment can be added to the already available imaging examinations, without the need for additional exams.
Despite different definitions and a wide variability of cut-off values for the definition of sarcopenia, this is a common condition found in PC patients (20, 21) and the assessment of muscle and fat tissues has been increasingly used in this setting. Some studies have reported poorer response to treatment and worse survival in sarcopenic patients with PC treated with chemotherapy (22–25), but these results were not consistent with other experiences, reporting association of body composition with overall survival and prognosis, but not with chemotherapy-related toxicity (26).
Therefore, the main objective of this systematic review was to collect and examine all the available literature assessing associations between quantitative body composition measures extracted from imaging examinations and chemotherapy-related toxicity in pancreatic cancer patients. A secondary objective was to evaluate the different definitions of sarcopenia across studies.
This systematic review was conducted according to the PRISMA-DTA (Preferred Reporting Items for Systematic Reviews and Meta-analysis for Diagnostic Test Accuracy) statement (27), which describes an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses of diagnostic studies.
Two authors (SR and AR) performed a comprehensive computer literature search of the electronic databases PubMed, Cochrane and Web of Science to find primary publications evaluating association between body composition and chemotherapy-related toxicities in pancreatic cancer. No beginning date limit or language restrictions were used; the literature search was last updated on May 7th 2022; and the search was expanded by also screening the references of the retrieved articles for additional potentially eligible studies.
The search terms consisted of ((pancreatic cancer) OR (pancreas carcinoma)) AND ((sarcopenia) OR (body composition) OR (muscle) OR (fat) OR (adipose tissue)) AND ((complication) OR (complications) OR (chemotherapy-related) OR (adjuvant) OR (neo-adjuvant) OR (toxicity) OR (toxicities)). Articles in which body composition assessment was based on imaging examinations (CT or MRI) in pancreatic cancer patients were obtained in full for further evaluation. Studies were excluded if they were case reports, conference abstracts, reviews or short communications because they do not provide sufficient information to assess the methodological quality. Uncertainties were resolved in consensus.
For each eligible article, information was collected concerning the clinical setting (neo-adjuvant, adjuvant, unresectable, metastatic); basic study data (authors, year of publication, country of origin, prospective or retrospective nature); population characteristics (number of patients, age, sex, sarcopenic status and cut-offs used); technical aspects (axial level for evaluation of body composition, software used for extraction); features evaluated (SMA, SMI, VAT, SAT, bone mineral density); mean/median CA 19.9 tumor marker levels (if reported); difference in sarcopenia between males and females; chemotherapy drugs administered; toxicities (hematologic, nausea/vomiting, diarrhea, neuropathy, reduction of number of cycles, overall toxicity); association of body composition values with toxicities and with older age, if any.
The overall quality of the included studies was critically evaluated based on the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2) (28). This tool comprises four domains (patient selection, index test, reference standard, and flow and timing) and each domain was assessed in terms of risk of bias, and a graph was constructed appropriately.
The initial search yielded 1137 articles, all in English. According to inclusion and exclusion criteria, 12 full-text articles were included in this systematic review (22, 26, 29–38). Details about the literature search results are reported in Figure 1.
Given the small number of papers included, and the heterogeneity of the quantitative analyses performed as well as of the results, a meta-analysis for pooled data was not performed.
Among the 12 studies included, 1 was in the neo-adjuvant setting (31), 2 in the adjuvant setting (30, 37), 3 in the metastatic setting (32, 33, 36); 2 in the unresectable setting (26, 29); the other 4 included more than one clinical setting (22, 34, 35, 38). Eight of the included studies were from Japan (22, 26, 29–32, 34, 35); 1 from South Korea (33); 1 from Canada (36); 1 from Brazil (37); 1 from Australia (38); all 12 studies were retrospective (Table 1).
The number of patients included ranged between 17 and 251, with numbers of males and females between 11 and 161 and 6 and 90, respectively; mean/median age ranged between 63 and 77 years; the percentage of sarcopenic patients ranged between 23% and 76% (Table 1). The cut-off values to define sarcopenia in males and females are summarized in Table 2. Interestingly, the assessment of differences in sarcopenia between males and females was evaluated in 9/12 papers, and among these, 5/9 indicate a significant prevalence of sarcopenia among females, whereas the other 4/9 report no difference.
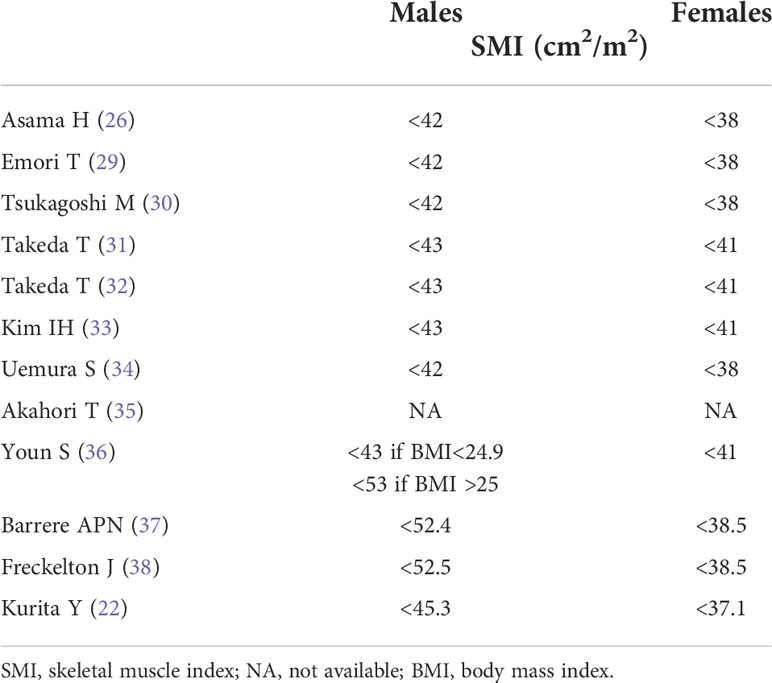
Table 2 Definition of sarcopenia according to skeletal muscle index in males and females within each included study.
All the articles included evaluated the body composition values at the level of the third lumbar vertebra (L3); the software used was Slice-o-matic (Tomovision) in 5 studies (22, 26, 36–38) and SYN-APSE Vincent in 7 studies (29–35). As shown in Table 3, body composition parameters evaluated were: SMI (derived from SMA) in 11/12 studies; VAT in 6/12 studies; SAT in 7/12 studies; SMD was evaluated in 3/12 articles; bone mineral density was never evaluated.
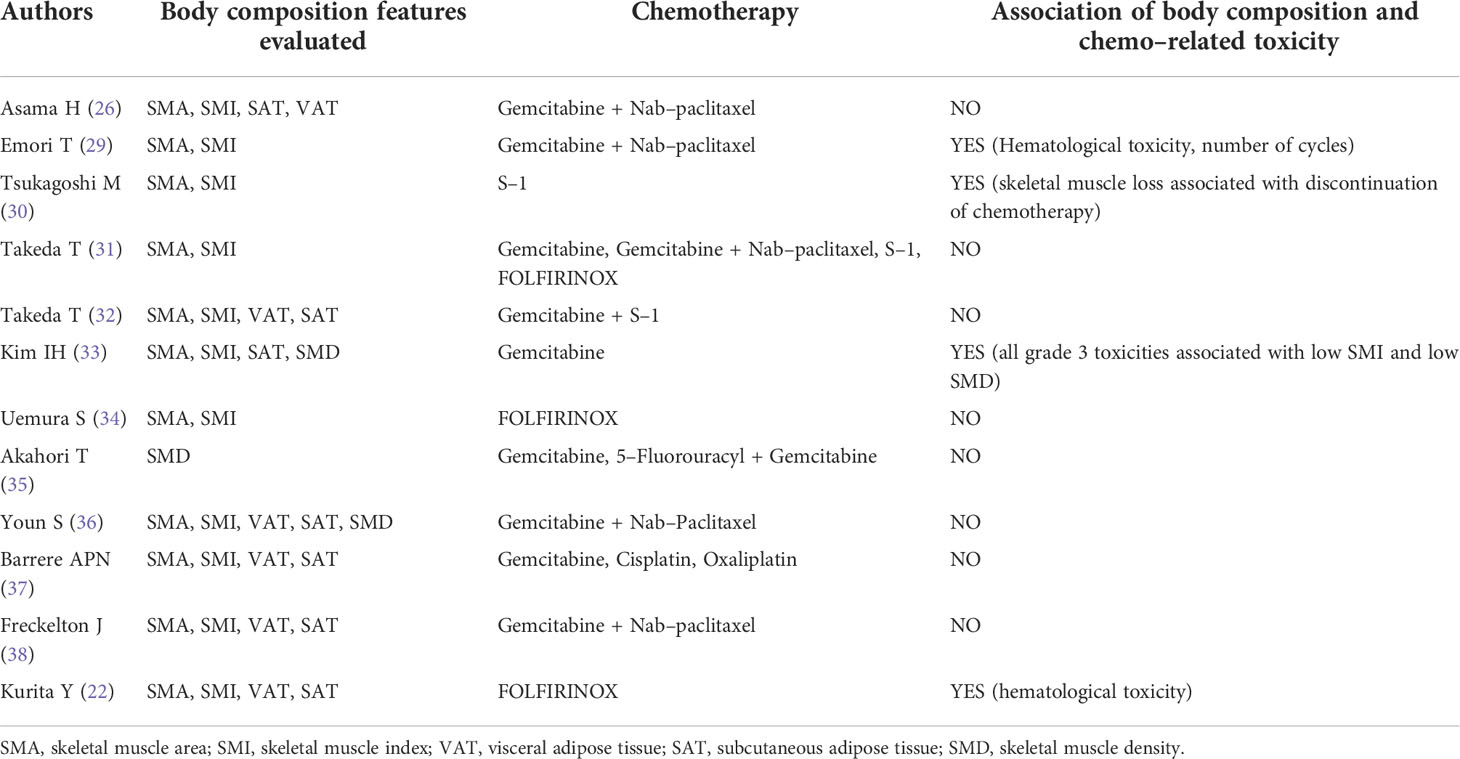
Table 3 Body composition features evaluated, chemo–related toxicities encountered and their association (if any).
Chemotherapy regimens included gemcitabine (as monotherapy or in combination with either nab-paclitaxel, cisplatin, oxaliplatin, 5-fluorouracyl or the fluoropyrimidine S-1), in 9/12 studies; FOLFIRINOX (5-fluorouracyl, irinotecan, oxaliplatin) in 3/12 studies (Table 3); and S-1 as monotherapy in 2/12 studies. Among the trials including gemcitabine, 2/9 demonstrated an association with toxicity, whereas 7/9 did not; among those including FOLFIRINOX, one demonstrated associated toxicity whereas the others did not. Altogether, 4/12 papers demonstrated an association between the body composition values and the development of chemotherapy-related toxicities (1 between low SMI and hematological toxicity and reduction of cycles number (29); 1 between loss of skeletal muscle and discontinuation of chemotherapy (30); 1 between low SMI and low SMD with all grade 3 toxicities (33); 1 with hematological toxicity (22)). Associations of body composition values and chemo-related toxicities are summarized in Table 3. Among the included articles, only 3/12 performed a specific analysis for older patients, with 2/3 study reporting no association between age and the advent of chemotherapy-related toxicity (26, 29), 1/3 reporting a significant association of chemo-related toxicity for octuagenarian patients (30).
The overall quality assessment of the studies is reported in Figure 2.
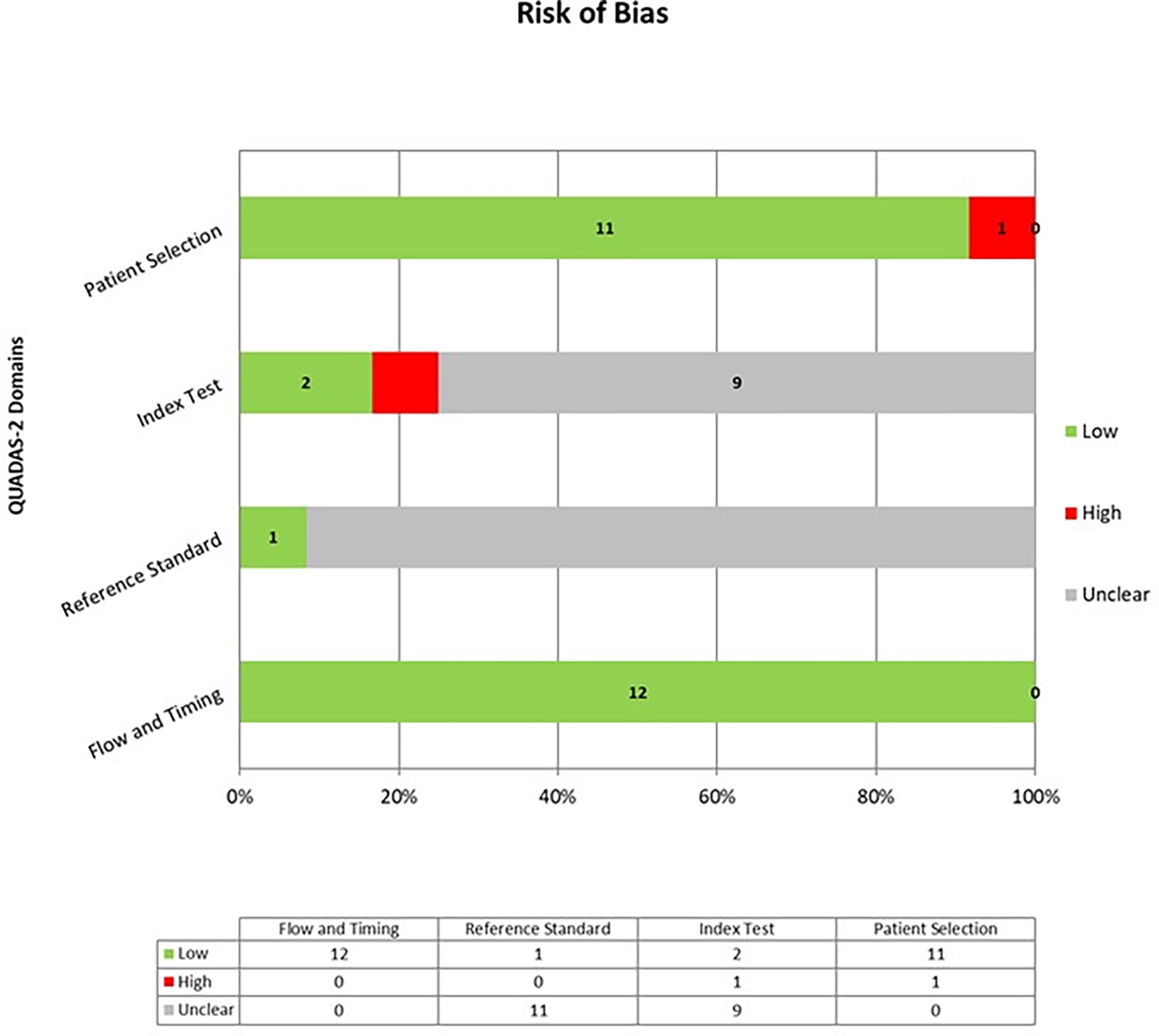
Figure 2 Overall Quality Assessment of the Studies Included in the Systematic Review, according to the QUADAS–2 Tool.
This systematic review demonstrated that the association between body composition and chemo-related toxicity in PC is still uncertain. Indeed, 4/12 studies demonstrated the presence of a significant association, but 8/12 did not. Furthermore, Rollins et al, in a paper that we excluded because it combined patients affected by pancreatic adenocarcinoma along with patients affected by distal cholangiocarcinoma, demonstrated no significant association between sarcopenia and chemo-related toxicity in a sub-group analysis performed on 98 patients treated with chemotherapy (39).
Many different factors can explain the discrepancy of these results; for instance, the chemotherapy agent administered. Indeed, the efficacy of FOLFIRINOX and of gemcitabine/nab-paclitaxel chemotherapy has been demonstrated, but gemcitabine/nab-paclitaxel tended to cause less toxicity than FOLFIRINOX (40, 41). Accordingly, the European Society of Medical Oncology recommend FOLFIRINOX as the first adjuvant therapeutic option after resection of pancreatic cancer in selected and fit patients, in view of survival outcomes and associated toxicity profile (I, A; ESMO-Magnitude of Clinical Benefit Scale (MCBS) v1.1 score: A); gemcitabine/capecitabine as an option in less fit patients (age > 70, Eastern Cooperative Oncology Group performance status 2, or patients who have any contraindication to the drugs used in FOLFIRINOX) (I, B; ESMO-MCBS v1.1 score A); gemcitabine alone only in frail patients (42). Therefore, if gemcitabine/nab-paclitaxel is considered more appropriate than FOLFIRINOX for use as first-line chemotherapy in patients with sarcopenia and frail patients, the comparison between different populations treated with different agents may be biased.
Another possible cause of discrepancy across studies may be the segmentation method used. Indeed, the areas of muscle and fat at the level of L3 were extracted by using different software programs (SYNAPSE VINCENT or Slice-o-matic) set up at the same Hounsfield Units levels for muscle segmentation (-29 +150), but the articles did not specify whether the segmentation was semi-automatic or automatic (the semi-automatic being generally more precise but less reproducible).
Furthermore, among the studies included there is a wide variability in the proportion of sarcopenic patients. Originally, the term ‘sarcopenia’ was used to describe age-related decreases in muscle mass, but the European Working Group on Sarcopenia in Older People later defined sarcopenia as a syndrome characterized by decrease in skeletal muscle mass and strength, associated with physical disability, poor quality of life, and high mortality (4). Since the values of SMI in that definition were based on bioelectrical impedance analysis method, Martin et al, in a large cohort of lung and gastro-intestinal cancer patients (n=1473), proposed sex-specific cut-offs for lumbar SMI extracted from CT images, associated with mortality in obese and non-obese patients. Nonetheless, they included only a very small proportion of pancreatic cancer patients (9% of males and 33% of females) and therefore the values proposed may not be generalizable for all cancer patients (43). Accordingly, in 2015 the Japan Society of Hepatology decided to establish its own assessment criteria for sarcopenia in patients affected by liver disease (44). This variability in the definition of sarcopenia may cause differences in the results of different studies, and indeed this evaluation was included as a secondary objective in this study. The Asian studies referred to cut-off values defined for the Asian population, but some used slightly lower cut-off values (26, 29, 30, 34) than others (31–33). On the other hand, other studies (36–38) referred either to values elaborated in North America (43) or defined in post-hoc analyses (22). These discrepancies leave space for larger studies to define a proper definition for sarcopenia in PC patients, possibly according to ethnicity, in order to further assess whether an association between sarcopenia and chemo-related toxicity does exist.
This systematic review certainly has some limitations. The first is the lack of randomized trials that would clarify the role of the drug according to the sarcopenic status. However, such a study is difficult to obtain and, so far, most of the published studies, including those on other cancer types, are based on retrospective evaluations. Secondly, we did not include in this review the data about survival and general prognosis, even when available. However, since the literature search was focused on chemo-related toxicity, an evaluation of survival only in the 12 included articles would have been incomplete and even misleading, because many articles specifically dedicated to survival were excluded. Thirdly, we aimed to evaluate many body composition features, but only SMI was present in all but one (35) of the included papers, whereas data about the distribution of fat tissue was not always present or included in the statistical analysis. Nonetheless, recent advances in the extraction of body composition values such as opportunistic information from imaging studies are leading to an increasing number of dedicated studies, and we may expect more data to be published in the near future.
In conclusion, we demonstrated that there is a wide variability of results about the association of body composition and chemotherapy-related toxicity in PC patients, and more studies, hopefully prospective and including cohorts of patients treated with pre-defined agents, are warranted to better understand this association. Furthermore, uniform cut-off values to define sarcopenia in PC patients should ideally be defined, leading to more consistent results and allowing for cross-trial comparisons to a degree.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conception and design: all authors. Data extraction from included studies: SR, IS, AR, MC, SC, SD. Analysis and interpretation of data: SR, MC, AR, SD. Manuscript writing: all authors. All authors contributed to the article and approved the submitted version.
Open access funding provided by Università della Svizzera italiana.
The English text was revised by Susan West.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin (2013) 63:318–48. doi: 10.3322/caac.21190
3. Huang L, Jansen L, Balavarca Y, Molina-Montes E, Babaei M, van der Geest L, et al. Resection of pancreatic cancer in Europe and USA: An international large-scale study highlighting large variations. Gut. (2019) 68:130–9. doi: 10.1136/gutjnl-2017-314828
4. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Writing group for the European working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing (2019) 48:16–31. doi: 10.1093/ageing/afy169
5. Chan MY, Chok KSH. Sarcopenia in pancreatic cancer - effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol (2019) 11(7):527–37. doi: 10.4251/wjgo.v11.i7.527
6. Del Grande M, Rizzo S, Nicolino GM, Colombo I, Rossi L, Manganaro L, et al. Computed tomography-based body composition in patients with ovarian cancer: Association with chemotoxicity and prognosis. Front Oncol (2021) 11:718815. doi: 10.3389/fonc.2021.718815
7. Rizzo S, Petrella F, Bardoni C, Bramati L, Cara A, Mohamed S, et al. CT-derived body composition values and complications after pneumonectomy in lung cancer patients: Time for a sex-related analysis? Front Oncol (2022) 12:826058. doi: 10.3389/fonc.2022.826058
8. Sanchez A, Kissel S, Coletta A, Scott J, Furberg H. Impact of body size and body composition on bladder cancer outcomes: Risk stratification and opportunity for novel interventions. Urol Oncol (2020) 38(9):713–8. doi: 10.1016/j.urolonc.2020.03.017
9. Basile D, Corvaja C, Caccialanza R, Aprile G. Sarcopenia: looking to muscle mass to better manage pancreatic cancer patients. Curr Opin Support Palliat Care (2019) 13(4):279–85. doi: 10.1097/SPC.0000000000000455
10. Sjøblom B, Grønberg BH, Benth JŠ, Baracos VE, Fløtten Ø, Hjermstad MJ, et al. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer (2015) 90:85–91. doi: 10.1016/j.lungcan.2015.07.001
11. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res (2009) 15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525
12. Hopkins JJ, Sawyer MB. A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol (2017) 10:947–56. doi: 10.1080/17512433.2017.1347503
13. Cousin S, Hollebecque A, Koscielny S, Mir O, Varga A, Baracos VE, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs (2014) 32:382–7. doi: 10.1007/s10637-013-0053-6
14. Bellomi M, Rizzo S, Travaini LL, Bazzi L, Trifirò G, Zampino MG, et al. Role of multidetector CT and FDG-PET/CT in the diagnosis of local and distant recurrence of resected rectal cancer. Radiol Med (2007) 112(5):681–90. doi: 10.1007/s11547-007-0172-2
15. Rietjens M, Villa G, Toesca A, Rizzo S, Raimondi S, Rossetto F, et al. Appropriate use of magnetic resonance imaging and ultrasound to detect early silicone gel breast implant rupture in postmastectomy reconstruction. Plast Reconstr Surg (2014) 134(1):13e–20e. doi: 10.1097/PRS.0000000000000291
16. Genovese E, Canì A, Rizzo S, Angeretti MG, Leonardi A, Fugazzola C. Comparison between MRI with spin-echo echo-planar diffusion-weighted sequence (DWI) and histology in the diagnosis of soft-tissue tumours. Radiol Med (2011) 116(4):644–56. doi: 10.1007/s11547-011-0666-9
17. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab (2008) 33(5):997–1006. doi: 10.1139/H08-075
18. Huber FA, Del Grande F, Rizzo S, Guglielmi G, Guggenberger R. MRI In the assessment of adipose tissues and muscle composition: how to use it. Quant Imaging Med Surg (2020) 10(8):1636–49. doi: 10.21037/qims.2020.02.06
19. Zaffina C, Wyttenbach R, Pagnamenta A, Grasso RF, Biroli M, Del Grande F, et al. Body composition assessment: comparison of quantitative values between magnetic resonance imaging and computed tomography. Quant Imaging Med Surg (2022) 12(2):1450–66. doi: 10.21037/qims-21-619
20. Di Sebastiano KM, Yang L, Zbuk K, Wong RK, Chow T, Koff D, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br J Nutr (2013) 109:302–12. doi: 10.1017/S0007114512001067
21. Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg (2012) 16:1478–86. doi: 10.1007/s11605-012-1923-5
22. Kurita Y, Kobayashi N, Tokuhisa M, Goto A, Kubota K, Endo I, et al. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology (2019) 19:127–35. doi: 10.1016/j.pan.2018.11.001
23. Park I, Choi SJ, Kim YS, Ahn HK, Hong J, Sym SJ, et al. Prognostic factors for risk stratification of patients with recurrent or metastatic pancreatic adenocarcinoma who were treated with gemcitabine-based chemotherapy. Cancer Res Treat (2016) 48:1264–73. doi: 10.4143/crt.2015.250
24. Kays JK, Shahda S, Stanley M, Bell TM, O'Neill BH, Kohli MD, et al. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J Cachexia Sarcopenia Muscle (2018) 9:673–84. doi: 10.1002/jcsm.12307
25. Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol (2015) 22:2416–23. doi: 10.1245/s10434-014-4285-2
26. Asama H, Ueno M, Kobayashi S, Fukushima T, Kawano K, Sano Y, et al. Sarcopenia: Prognostic value for unresectable pancreatic ductal adenocarcinoma patients treated with gemcitabine plus nab-paclitaxel. Pancreas (2022) 51(2):148–52. doi: 10.1097/MPA.0000000000001985
27. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accu-racy studies: The PRISMA-DTA statement. JAMA (2018) 319(4):388–96. doi: 10.1001/jama.2017.19163
28. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
29. Emori T, Itonaga M, Ashida R, Tamura T, Kawaji Y, Hatamaru K, et al. Impact of sarcopenia on prediction of progression-free survival and overall survival of patients with pancreatic ductal adenocarcinoma receiving first-line gemcitabine and nab-paclitaxel chemotherapy. Pancreatology (2022) 22(2):277–85. doi: 10.1016/j.pan.2021.12.013
30. Tsukagoshi M, Harimoto N, Araki K, Kubo N, Watanabe A, Igarashi T, et al. Skeletal muscle loss and octogenarian status are associated with s-1 adjuvant therapy discontinuation and poor prognosis after pancreatectomy. Cancers (Basel) (2021) 13(16):4105. doi: 10.3390/cancers13164105
31. Takeda T, Sasaki T, Suzumori C, Mie T, Furukawa T, Yamada Y, et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol (2021) 26(7):1293–303. doi: 10.1007/s10147-021-01912-0
32. Takeda T, Sasaki T, Mie T, Furukawa T, Yamada Y, Kasuga A, et al. The impact of body composition on short-term outcomes of neoadjuvant chemotherapy with gemcitabine plus s-1 in patients with resectable pancreatic cancer. Jpn J Clin Oncol (2021) 51(4):604–11. doi: 10.1093/jjco/hyaa247
33. Kim IH, Choi MH, Lee IS, Hong TH, Lee MA. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer (2021) 21(1):77. doi: 10.1186/s12885-020-07753-w
34. Uemura S, Iwashita T, Ichikawa H, Iwasa Y, Mita N, Shiraki M, et al. The impact of sarcopenia and decrease in skeletal muscle mass in patients with advanced pancreatic cancer during FOLFIRINOX therapy. Br J Nutr (2021) 125(10):1140–7. doi: 10.1017/S0007114520003463
35. Akahori T, Sho M, Kinoshita S, Nagai M, Nishiwada S, Tanaka T, et al. Prognostic significance of muscle attenuation in pancreatic cancer patients treated with neoadjuvant chemoradiotherapy. World J Surg (2015) 39(12):2975–82. doi: 10.1007/s00268-015-3205-3
36. Youn S, Chen A, Ha V, Chambers C, Eurich DT, McCall M, et al. An exploratory study of body composition as a predictor of dose-limiting toxicity in metastatic pancreatic cancer treated with gemcitabine plus nab-paclitaxel. Clin Nutr (2021) 40(8):4888–92. doi: 10.1016/j.clnu.2021.06.026
37. BarrÈre APN, Piovacari SMF, UsÓn Junior PLS, Gansl RC, Pereira AZ, Hamerschlak N. Body composition impact on survival and toxicity of treatment in pancreatic cancer: cross-sectional pilot study. Arq Gastroenterol (2020) 57(3):278–82. doi: 10.1590/S0004-2803.202000000-52
38. Freckelton J, Croagh D, Holt DQ, Fox A, Wong R, Lee M, et al. Body composition adjusted dosing of gemcitabine-Nab-Paclitaxel in pancreatic cancer does not predict toxicity compared to body surface area dosing. Nutr Cancer (2019) 71(4):624–8. doi: 10.1080/01635581.2018.1542011
39. Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr (2016) 35(5):1103–9. doi: 10.1016/j.clnu.2015.08.005
40. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med (2018) 379(25):2395–406. doi: 10.1056/NEJMoa1809775
41. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
42. ESMO Guidelines Committee. eUpdate – cancer of the pancreas treatment recommendations (2019). Available at: https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/pancreatic-cancer/eupdate-cancer-of-the-pancreas-treatment-recommendations (Accessed June 1st).
43. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol (2013) 31(12):1539–47. doi: 10.1200/JCO.2012.45.2722
Keywords: pancreatic cancer, chemotherapy, body composition, sarcopenia, toxicity
Citation: Rizzo S, Scala I, Robayo AR, Cefalì M, De Dosso S, Cappio S, Xhepa G and Del Grande F (2022) Body composition as a predictor of chemotherapy-related toxicity in pancreatic cancer patients: A systematic review. Front. Oncol. 12:974116. doi: 10.3389/fonc.2022.974116
Received: 20 June 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Marco Rengo, Sapienza University of Rome, ItalyReviewed by:
Giovanni Mauri, University of Milan, ItalyCopyright © 2022 Rizzo, Scala, Robayo, Cefalì, De Dosso, Cappio, Xhepa and Del Grande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Rizzo, c3RlZmFuaWEucml6em9AZW9jLmNo;cml6em9zQHVzaS5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.