
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Oncol., 17 November 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.973810
This article is part of the Research TopicMethods Of Optimizing Surgical Intervention In Esophago-Gastric CancerView all 19 articles
 Qin Chuan Yang1†
Qin Chuan Yang1† Wei Dong Wang1†
Wei Dong Wang1† Zhen Chang Mo1,2†
Zhen Chang Mo1,2† Chao Yue1†
Chao Yue1† Hai Kun Zhou1
Hai Kun Zhou1 Rui Qi Gao1
Rui Qi Gao1 Juan Yu1
Juan Yu1 Dan Hong Dong1
Dan Hong Dong1 Jin Qiang Liu1
Jin Qiang Liu1 Jiang Peng Wei1
Jiang Peng Wei1 Xi Sheng Yang1
Xi Sheng Yang1 Gang Ji1*
Gang Ji1* Xiao Hua Li1*
Xiao Hua Li1*Background: Proximal gastrectomy has gradually gained more attention due to its superiority in retaining the function of part of the stomach. The inevitable loss of the antireflux barrier and postoperative complications resulting from proximal gastrectomy can severely affect the quality of life. Continuous improvements in digestive tract reconstruction after proximal gastrectomy have yielded the development of a variety of methods with antireflux functions. Recently, our center attempted the left-open single-flap technique and initiated a multicenter, prospective, randomized controlled trial for patients undergoing proximal gastrectomy to reduce the difficulty of surgical anastomosis and the incidence of perioperative complications compared with the double-flap technique. These findings will provide more evidence-based medical research for the development of clinical guidelines.
Methods/design: This study is a prospective, multicenter, randomized controlled clinical trial. We plan to recruit 250 patients who are eligible for proximal gastrectomy. After informed consent is obtained, patients will be randomly assigned to the trial group (left-open single-flap technique) and the control group (double-flap technique) in a 1:1 allocation ratio.
Discussion: Increasingly, clinical studies have focused on the improvement of reconstruction modalities after proximal gastrectomy. Among these methods, the double-flap technique is a clinically effective method. The purpose of this study is to establish a prospective randomized controlled trial to compare the efficacy of the left-open single-flap technique versus the double-flap technique after proximal gastrectomy, aiming to provide more evidence-based medical studies for digestive tract reconstruction in proximal gastrectomy.
Clinical Trial Registration: ClinicalTrials.gov, identifier [NCT05418920].
Despite the decreasing incidence and mortality of gastric cancer in recent years, the incidence and proportion of proximal early gastric cancer has markedly increased (1). However, the choice of an appropriate surgical procedure, the only radical treatment for this condition, has been controversial (2). Currently, proximal gastrectomy has gradually gained increasing attention due to its superiority in 1) maintaining the distal gastric volume; 2) preserving the fundic gland area and reducing hormonal and nutritional deficiencies; and 3) ensuring the secretion of internal factors and gastric acid, the absorption of iron ions and vitamin B12, and the maintenance of hemoglobin concentration (3–6). However, the inevitable a loss of the antireflux barrier and postoperative complications resulting from proximal gastrectomy can severely affect the quality of life (7).
Recently, continuous improvements in digestive tract reconstruction after proximal gastrectomy have yielded the development of a variety of methods with antireflux functions that contribute to retaining the function of the residual stomach and avoiding serious reflux esophagitis (8–10). Japanese guidelines indicate that popular methods for gastrointestinal reconstruction after proximal gastrectomy include esophagogastrotomy (EG), jejunal interposition (JI), and double-tract reconstruction (DTR) (11–13). In 2016, Kuroda reported a double-flap technique using the anterior gastric wall plasma muscle flap to cover the anastomosis (14). Its unidirectional flap can reconstruct the “sphincter” and reduce the incidence of reflux esophagitis and the risk of anastomotic fistula.
By comparing patients who underwent direct esophagogastrostomy, jejunal interposition, double tract reconstruction, and the double-flap technique, Shoji Y (15) and Saze Z et al. (16) found that patients undergoing double-flap anastomosis had no reflux esophagitis and a lower incidence of postoperative anastomotic stricture, which improved postoperative serum albumin ratio changes and weight maintenance. Consequently, the double-flap technique is considered to be the most effective technique for proximal gastric reconstruction (17, 18).
However, the double-flap technique is associated with shortcomings, such as a more complex suture technique, more difficult operation, longer operation time and higher incidence of postoperative anastomotic stenosis (15, 16, 19). Therefore, our center attempted the left-open single-flap technique to reduce the difficulty of surgical suturing and the incidence of complications. To further evaluate the therapeutic efficacy of this procedure, we initiated a multicenter, prospective, randomized controlled trial for patients undergoing proximal gastrectomy, which will provide more evidence-based medical research for the development of clinical guidelines.
This study is a prospective, multicenter, randomized controlled clinical trial. We plan to recruit 250 patients who are eligible for proximal gastrectomy. After informed consent is obtained, patients will be randomly assigned to the trial group (left-open single-flap technique) and the control group (double-flap technique) in a 1:1 allocation ratio, with the aim of providing more evidence-based medical outcomes for digestive tract reconstruction in proximal gastrectomy. The surgical methods applied in this study are shown in Table 1.
The objectives of this trial are as follows:
The main objective is to investigate the incidence of reflux esophagitis after 2 types of anastomosis (left-open single-flap technique vs. double-flap technique) after proximal gastrectomy.
1) to investigate the incidence of anastomotic leakage of 2 types of anastomosis (left-open single-flap technique vs. double-flap technique) after proximal gastrectomy
2) to investigate the incidence of anastomotic stricture of 2 types of anastomosis (left-open single-flap technique vs. double-flap technique) after proximal gastrectomy
3) to investigate the operation time of 2 types of anastomosis (left-open single-flap technique vs. double-flap technique) after proximal gastrectomy
4) to investigate the intraoperative blood loss volume of 2 types of anastomosis (left-open single-flap technique vs. double-flap technique) after proximal gastrectomy
In patients after proximal gastrectomy, the left-open single-flap technique can decrease postoperative complications and increase nutritional status compared with the double-flap technique.
The flow chart of this study is shown in Figure 1. During the routine admission of inpatients, suitable patients will be screened by the study staff according to the inclusion/exclusion criteria. Patients who are successfully selected before formal enrollment will receive study instructions from the investigator with a detailed explanation of the included documents and operations. Participants (or their legally authorized representative) will agree to sign and date the informed consent form after receiving a random serial number. All processes will strictly follow the provisions of the Ethical Review of Biomedical Research Involving Humans (Trial), the Declaration of Helsinki v.08, and the International Ethical Guidelines for Biomedical Research Involving Humans.
1) patients aged 18-80 years, regardless of sex;
2) Siewert III of esophagogastric junction adenocarcinoma: Stage I (cT1-2N0M0) or adenocarcinoma of the upper part of the stomach: Stage II (cT1-2N0M0), Stage II (cT1-2N1-3M0/cT3-4N0M0), Stage III (cT3-4aN1-3M0). All patients were selected according to the 8th AJCC clinical staging of gastric cancer.
3) primary lesion diagnosed by preoperative endoscopic end pathology: tumor diameter <4 cm and located in the upper part of the stomach (including the esophagogastric junction), histologically confirmed adenocarcinoma;
4) preoperative ASA score: I, II, or III;
5) preoperative Karnofsky physical status score: ≥ 70%; or preoperative ECOG physical status score: ≤ 2;
6) no distant metastases (confirmed by preoperative chest X-ray, abdominal ultrasound, and upper abdominal CT); No peritoneal implant metastases (confirmed by exploration surgery);
7) R0 surgical outcome was expected to be obtained with D2 lymphadenectomy in radical proximal gastrectomy;
8) patients and their families voluntarily participated in this study and signed the informed consent form after understanding the study content.
1) patients who have received any preoperative treatment, such as chemotherapy, radiotherapy, targeted therapy or immunotherapy; preoperative neoadjuvant chemotherapy recipients;
2) patients with clinical stage exceeding Siewert III of the esophagogastric junction adenocarcinoma: Stage I (cT1-2N0M0) or more than adenocarcinoma of the upper part of the stomach: Stage I (cT1-2N0M0), Stage II (cT1-2N1-3M0/cT3-4N0M0), Stage III (cT3-4aN1-3M0);
3) patients with acute infections, especially biliary tract infections;
4) patients with complications of gastric cancer (bleeding, perforation, or obstruction) requiring emergency surgery;
5) patients with uncorrectable coagulation dysfunction;
6) patients with vital organ failure, such as heart, lung, liver, brain, kidney, etc.
7) severe central nervous system disease, mental disorders, or impaired consciousness;
8) pregnant or lactating women;
9) patients with distant metastases;
10) patients with a primary tumor at another site diagnosed within the past 5 years;
11) preoperative ASA score: ≥ IV;
12) preoperative ECOG physical status score: ≥ 2;
13) history of continuous systemic corticosteroid therapy within the past 1 month;
14) history of unstable angina, myocardial infarction, cerebral infarction, or cerebral hemorrhage within the past 6 months;
15) patients with concurrent surgical treatment of other diseases;
16) patients with immunodeficiency, immunosuppression, or autoimmune diseases (organ transplant requiring immunosuppressive therapy within the past 5 years, allogeneic bone marrow transplant patients, taking immunosuppressive drugs, etc.);
17) patients with concurrent participation in other clinical studies;
18) patients refusing to sign an informed consent form to participate in this study;
19) preoperative imaging: regional fusion of enlarged lymph nodes (maximal diameter > 3 cm).
1) patients are inoperable for various reasons after recruitment;
2) the investigator considers that the patient should stop this study for safety reasons or the benefit of the patient;
3) serious complications or intolerable adverse reactions of the patient;
4) patients may request to withdraw/terminate from the trial at any time after signing the informed consent form.
1) patients with missing main observation indicators and significantly incomplete study data;
2) incomplete follow-up data;
3) patients who failed to follow the study protocol;
4) the study protocol was discontinued after the patient was judged to be a culled case. Follow-up treatment was determined by the investigator according to clinical guidelines. The excluded cases were still subject to follow-up and were included in the study analysis.
As shown in Table 2, this work is a multicenter, large-sample clinical study with six participating medical institutions (Xi-Jing Hospital, Tang-Du Hospital, First Affiliated Hospital Xi’an Jiaotong University, General Hospital of Ningxia Medical University, Henan Provincial People’s Hospital, The First Affiliated Hospital of Shanxi Medical University). The mode of enrollment is competitive. All study institutions and personnel were approved by the ethics committee and possessed extensive clinical experience in the treatment of gastric cancer.
The study has an open design.
Before randomization, the oncologic evaluation will be performed based on relevant clinical parameters (vital signs, serum biochemical tests, tumor markers, CT and/or MRI, ultrasound endoscopy, etc.). Eligible participants will be informed by the investigator and required to sign an informed consent form. Patients will be randomly assigned in a 1:1 ratio to the trial group (left-open single-flap technique) and the control group (double-flap technique). The randomization sequence will be generated by a biostatistician using the SPSS 28.0.1 software. The randomization list will be sealed in an opaque envelope and placed in the custody of a dedicated person.
None of the assistants associated with the randomization process are directly involved in this study to avoid bias.
1) If the patient’s condition deteriorates between enrollment and the date of surgery, the investigator will decide whether to perform the surgery as planned. If emergency surgery or cancellation is needed, the case will be excluded according to the exclusion criteria.
2) Perioperative enteral/parenteral nutrition support will be allowed for patients with nutritional risk.
3) For high-risk patients (elderly patients, smokers, diabetic patients, obese patients, or patients with a history of chronic cardiovascular or thromboembolic disease), the perioperative administration of low-molecular heparin, lower extremity antithrombotic compression stockings, aggressive lower extremity massage and respiratory function training are recommended as prophylactic measures. Methods for other potentially high-risk complications will be determined by clinical practice routines and specific needs, but all measures need to be documented in the CRF.
4) Regarding the choice of surgical procedure performed in this study, D2 lymphadenectomy in radical proximal gastrectomy will be performed by the investigator according to the 6th edition of the Japanese Guidelines for the Treatment of Gastric Cancer (18);
5) The principles of prophylactic antibiotic use are as follows: the first intravenous drip is started 30 minutes before surgery, and the recommended choice is cephalosporin II antibiotics. The preparation, concentration, and infusion rate will be in accordance with routine clinical methods. Prophylactic use will last no longer than 3 days after surgery, and the frequency of use will be 1 time/8 hours. In cases of allergy to cephalosporins (including a history of allergy or allergy after use), other types of antibiotics will be selected according to clinical specifics, and the duration of prophylactic use will be the same as before.
6) The patient’s preoperative fasting, water fasting, and other anesthetic requirements will be implemented according to the routine anesthetic protocol;
7) The investigator will decide to leave a gastric tube or drainage tube in place based on experience and actual needs;
8) Enhanced Recovery After Surgery program: treat preoperatively anemia with intravenous or iron to diminish blood transfusion; malnutrition treatment with hyperproteic nutritional shakes; aerobic exercise daily of 30-45 minutes; anxiety treatment with mindfulness exercise and/or drugs.
1. Rules for gastric resection:
2. Routine abdominal exploration will be performed to confirm the presence of peritoneal implants, positive abdominal exfoliative cytology, or other distant metastases and to identify those who cannot be resected due to tumor;
3. Proximal gastrectomy should be performed if the tumor is confirmed to be radical;
4. For patients who require total gastrectomy or combined organ resection intraoperatively, whether to proceed to laparoscopic surgery or intermediate open surgery is decided on a case-by-case basis. These cases are not required in this study and need to be recorded in the CRF.
5. Rules for lymph node dissection: D2 lymphadenectomy in radical proximal gastrectomy will be performed according to the tumor infiltration (17, 18).
The mesenterium at the root of the esophagus is fully stripped, and the esophagus is exposed. The location of the tumor is localized according to intraoperative gastroscopy, and the sites of esophageal and gastric body dissection are further clarified (the distances between the cut edges are 5 cm and 3 cm, respectively). The esophagus is dissected, and the stomach is pulled outside the body through an adjuvant incision in the navel or epigastrium. The tumor and remnant stomach are then dissected with linear staplers. Specimens are taken at the cut edge for rapid cytopathological examination.
As shown in Figure 2, patients in the trial group will receive the left-open single-flap technique after proximal gastrectomy, which involves the following (20):
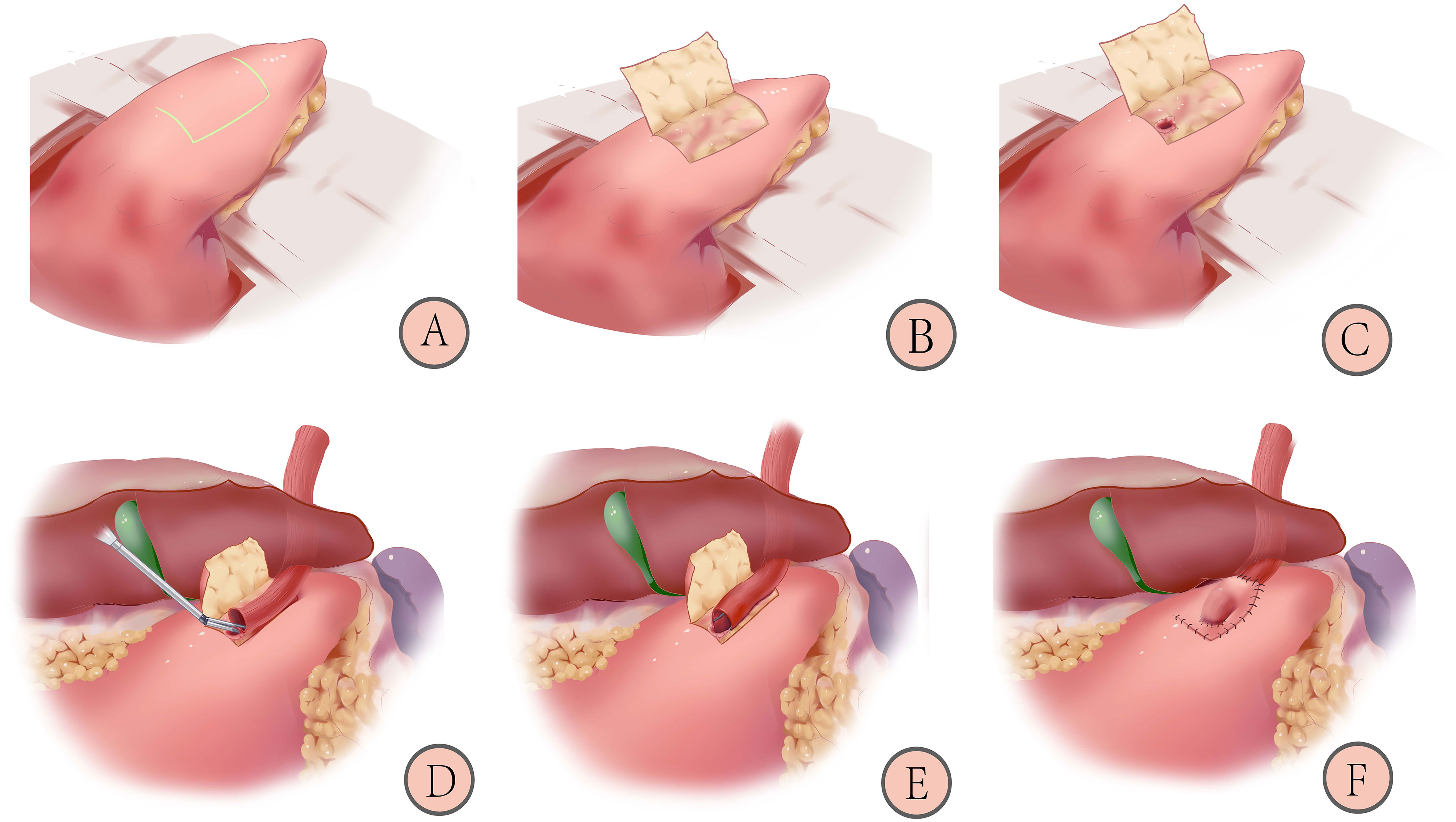
Figure 2 Schematic diagram of reconstruction after proximal gastrectomy with left-open single-flap technique: 1) Marking the “⊐”-shaped mucosal window (A); 2) Making the left-open single-flap (B); 3) Fixing the esophagus and opening a gastric mucosal window (C); 4) Anastomosing by linear staplers (D); 5) Closing of the common opening with barbed wire (E); 6) Covering the left-open single-flap and suturing with barbed wires (F) (20).
1) Marking an “⊐”-shaped mucosal window (A): The mucosal window is located on the anterior wall of the stomach near the lesser curvature, 3-4 cm from the cut edge, and a sideways “⊐” shape is marked measuring (2.5-3.5) x 3.5 cm area with methylene blue.
2) Making a left-open single flap (B): an electric knife is used to peel off the plasma membrane and muscle layers, paying attention to the protection of blood vessels and avoiding rupture of the mucosal window.
3) Fixing the esophagus and opening a gastric mucosal window (C): the posterior wall of the esophagus, approximately 5 cm from the severed end, is fixed with four sutures to the top of the anterior wall of the remnant stomach. The gastric mucosal window (upper and lower edges of the anastomosis) is made by opening the mucosal layer under the single muscle flap at the lower left side of the flap. The length of the window is determined by the caliber of the esophagus.
4) Anastomosing by linear staplers (D): the left posterior wall of the esophagus and anterior wall of the gastric mucosal window are anastomosed by linear staplers with an insertion length of 2.5-3 cm.
5) Closing of the common opening (E): the common opening is closed with a full layer of continuous sutures using a barbed wire.
6) Covering the left-open single flap and suturing with barbed wires (F): the anastomosis is covered by a left-open single flap, and the edge of the flap is continuously sutured to the anterior gastric wall with barbed wires. A “⊐”-shaped structure is formed.
As shown in Figure 3, patients in the control group will receive the double-flap technique after proximal gastrectomy, which includes the following (21):
1. 1) Marking the “H”-shaped mucosal window (A): the mucosal window is located on the anterior wall of the remnant stomach near the lesser curvature, and the “H”-shaped (2.5~3.5) cm×3.5 cm area is marked with methylene blue.
2. 2) Make a double flap (B): an electric knife is used to peel off the plasma membrane layer and muscle layer, paying attention to the protection of blood vessels and avoiding rupture of the mucosal window.
3. 3) Fixing the esophagus and opening the gastric mucosal window (C): the posterior wall of the esophagus, approximately 5 cm from the severed end, is sutured to the top of the anterior wall of the remnant stomach with 3-4 stitches to keep the anastomosis flat and prevent reflux. The gastric mucosal window is made by opening the mucosal layer below the double flap, and the width is similar to that of the esophagus.
4. 4) Completing continuous hand suture (D and E): the entire esophageal wall is sutured to the gastric mucosa using a barbed wire with a complete continuous inversion. The point of entry is the left side of the upper edge of the gastric mucosa. The direction of entry is entering the gastric mucosal layer and penetrating from the esophageal plasma membrane layer. The direction of the suture is from left to right until the left side of the gastric mucosa upper edge. The direction of the suture at the lower edge is the opposite.
5. 5) Covering the double flap and suturing with barbed wires (F): the anastomosis is covered by a double flap, and the lower edge of the flap is continuously sutured to the anterior gastric wall with barbed wires. The double flap is obliquely reinforced to the esophageal epithelium without mutual sutures, and a “Y”-shaped collar-like structure is formed.
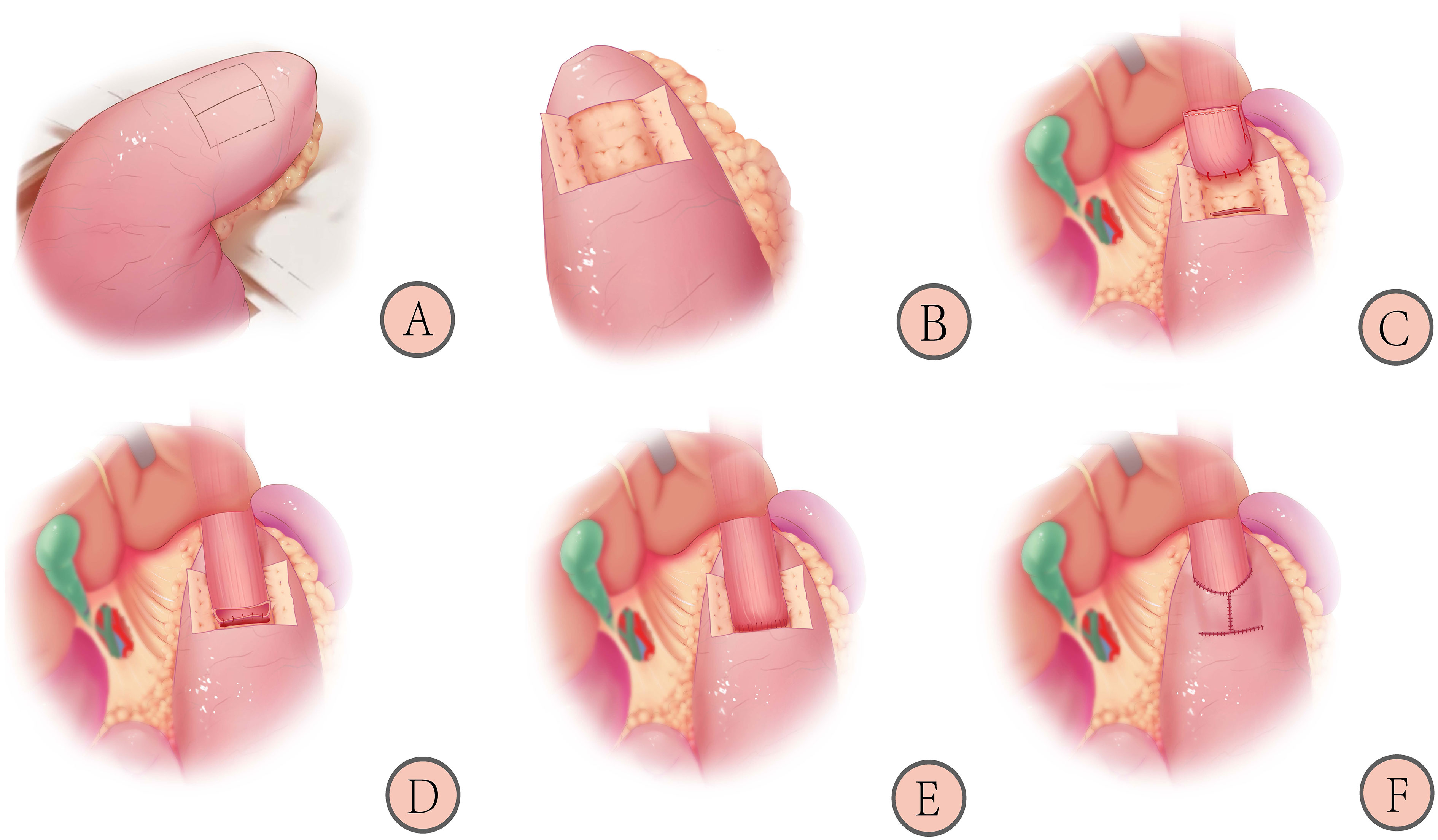
Figure 3 Schematic diagram of reconstruction after proximal gastrectomy with the double-flap technique: 1) Marking the “H”-shaped mucosal window (A); 2) Making the double-flap (B); 3) Fixing the esophagus and opening the gastric mucosal window (C); 4) Completing continuous hand suture (D, E); 5) Covering the double-flap and suturing with barbed wires (F) (21).
According to the privacy policy, only the researcher will know the patient’s identity and various information. Clinical information will be recorded by the investigators in the case report form and on the web platform (http://www.medresman.org.cn). The patients’ clinical data will include general information, previous medical history, previous surgical history, laboratory findings (preoperative and postoperative blood tests, biochemical indicators, and tumor markers), upper gastrointestinal endoscopy, imaging findings, the incidence of postoperative reflux esophagitis, the incidence of anastomotic leakage, the incidence of anastomotic stricture, operative time and intraoperative blood loss. The schedule of data collection in this study is shown in Table 3.
In this study, blood samples will be collected from subjects to monitor blood biochemical indicators and tumor markers. After testing, all samples will be destroyed in strict accordance with laboratory regulations.
The left-open single-flap technique after proximal gastrectomy is a new and improved procedure in our center, and national or international studies comparing clinical outcomes after reconstruction with the double-flap technique have not been conducted. Kuroda S et al. (22) showed that the incidence of reflux esophagitis, performed by endoscopy at 1.0 years (median) after the double flap technique, was 10.6% for all grades. This finding was considered more in line with “real‐world data”.
According to the database of this study, we designed a noninferiority study with a noninferiority margin of 10% (α=0.05, β=0.20, δ =0.10, 80% power, 10% dropout rate). The test statistic used is the one-sided Z test (unpooled) by PASS 15.0.5 software.
The result is N = 250. Therefore, 125 patients will be enrolled in each of the 2 groups.
The postoperative complications are classified according to the Clavien–Dindo grading system.
1) Incidence of reflux esophagitis (22);
1) Incidence of anastomotic stricture;
2) Overall postoperative complication rate;
3) Incidence of anastomotic fistula;
4) Operation time;
5) Blood loss volume;
6) Postoperative mortality rate;
7) R0 resection rate;
8) Overall resection rate;
9) Intraoperative complication rate;
10) Postoperative severe morbidity rate;
11) Postoperative recovery course;
12) 3-year overall survival rate;
13) 3-year disease-free survival rate;
14) Recurrence pattern;
15) The length of ICU stay;
16) Lengths of admission;
17) Length of post operational stays;
18) Nutritional status.
The start time of this study was August 1, 2022. The preliminary completion time is July 31, 2024. Follow-up will be planned for three years, and the study completion time will be July 31, 2027.
Esophagogram fluoroscopy will be performed 6 days after surgery to evaluate anastomotic complications. Subsequent follow-ups will be performed at 1, 3, 6, 12, 24 and 36 months. The follow-up will include questioning, physical examination, gastrointestinal endoscopy, blood examination items (peripheral blood routine, serum iron, vitamin B12, folic acid, blood biochemistry and serum tumor markers), and imaging items (chest imaging, esophagogram fluoroscopy, and whole abdomen enhanced CT). All examinations will be recorded to evaluate the presence of tumor recurrence or metastasis, the survival status of patients and the occurrence of complications. Other assessment tools will be used according to the specific situation, such as color ultrasound of other sites, whole-body bone scan, PET-CT, etc. The follow-up schedule in this study is shown in Table 3.
The diagnosis of postoperative reflux symptoms is based on a combination of symptom presentation, endoscopic evaluation of esophageal mucosa, reflux monitoring, and response to therapeutic intervention (23–25). Heartburn and regurgitation remain the typical symptoms of postoperative reflux symptoms. The control steps of postoperative reflux symptoms are shown in the Table 4.
In this study, the patients should be reexamined at the hospital where the surgery was performed, but cases of outside hospital examination will not be excluded (outside hospital reexamination should be conducted at a tertiary care hospital). A follow-up specialist will follow up and record the results of each examination.
All patients refuse to be followed up according to the above protocol will be recorded as lost cases and analyzed together with cases meeting the study criteria at the end of the study.
The informed consent process was approved by the internal review board/independent ethics committee. If any changes occur during the study, they will be resubmitted for review.
Informed consent procedures will be implemented in strict compliance with relevant Chinese laws. Original informed consent will be retained in writing by the investigator.
We will rigorously protect patient privacy: The collection, transmission, process and storage of participant data will comply with data security and privacy protection regulations.
The study protocol, which was submitted to the ethical review committee of the health administration department, is in line with the relevant regulations in China and the Measures for Ethical Review of Biomedical Research Involving Human Beings (2007).
We will retain video recordings and unedited image files of all patients throughout the surgery. The organizers will review and monitor the quality of the surgery. The main objectives will be performed for these purposes: 1) to confirm the rationality of the surgical approach, the extent of lymph node dissection and the minimally invasive nature of the incision; 2) to verify the original data of all subjects to confirm consistency with the CRF; and 3) to regularly assess the progress of the study at each center to ensure that it was carried out according to the plan.
Patients and the public will not be involved in our process of design, recruitment, clinical treatment, measurement of outcomes and analysis of the data.
For patients diagnosed with Siewert III esophagogastric junction adenocarcinoma and early-stage upper gastric cancer, current clinical guidelines recommend proximal gastrectomy (17, 18). Compared with total gastrectomy, proximal gastrectomy can maintain part of the storage and digestive function of the stomach, which has greater advantages for nutrient absorption and weight maintenance (5–8). Therefore, this approach is more commonly used in East Asia.
Unavoidably, patients’ postoperative quality of life is severely affected by postoperative complications (7). Wang S et al. (8) found that proximal gastrectomy is also associated with many complications, most notably reflux esophagitis, which causes heartburn, chest pain, acid reflux, and anorexia. These postoperative complications can severely reduce the quality of life after surgery.
Consequently, the choice of a reasonable approach to reconstruct the digestive tract after proximal gastrectomy and addressing complications, such as reflux esophagitis, remain a challenge for clinicians (10–13). Therefore, an increasing number of clinical studies have focused on improving reconstruction modalities after proximal gastrectomy, such as esophagogastrotomy (EG), jejunostomy (JI), jejunal pouch placement (JPI) and double tract reconstruction (DTR).
Among various gastrointestinal reconstruction methods, the double-flap technique is clinically effective, wrapping around and increasing the pressure of anastomosis through a unidirectional flap. This structure is similar to the “reconstructed cardia” structure, which can improve the antireflux effect. Saze Z et al. (16) found that the double-flap technique did not result in postoperative reflux esophagitis and had the smallest postoperative weight change ratio and the lowest prevalence of gastric residual at 12 months after surgery compared with direct esophagogastrostomy, jejunostomy and double tract reconstruction.
However, double-flap technique is also associated with shortcomings, such as a complicated surgical suture technique, difficult operation, strict surgical indications and high incidence of postoperative anastomotic stenosis (26–28).
To address the shortcomings of the above procedure, we established a multicenter, prospective, randomized controlled trial to modify the flap-making procedure for the double-flap technique: a left-open single flap will be used instead of a double flap to cover the anastomosis, acting as a “sphincter” and providing a tunneling effect. Preliminary data from our center showed that patients have excellent postoperative results. We will explore and summarize our initial clinical experience and apply it to the development of the treatment protocol. If this study meets the expected results of the trial protocol, it will bridge the gap between the complicated operative procedure and longer operative time of the double-flap reconstruction style and further improve the postoperative quality of life of patients. These outcomes will be landmark improvements in patient prognosis and provide additional high-level research evidence for the standardization of gastrointestinal reconstruction protocols after proximal gastrectomy.Strengths and limitations of this study
Strengths: This was a prospective, large sample, multicenter randomized controlled trial that systematically compared the efficacy of 2 methods of gastrointestinal reconstruction after proximal gastrectomy. In previous studies, the left-open single-flap technique had not been reported.
Limitations: Japanese guidelines recommended that the common methods of gastrointestinal reconstruction after proximal gastrectomy include esophagogastrotomy (EG), jejunostomy placement (JI), jejunal pouch placement (JPI) and double-tract reconstruction (DTR). Nevertheless, we compared only 2 of these reconstruction methods in our study.
All authors agree to publish and all participants will sign a data release consent form.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Chinese Ethics Committee of Registering Clinical Trials. The patients/participants provided their written informed consent to participate in this study.
Concept Proposal: XL; Survey and Data Summary: QY, WW, RG, CY, JY, and DD; Data Collection, analysis and statistics: QY, WW, ZM, CY, HZ, and JW; Specific scheme implementation: GJ, XL, QY, WW, ZM, CY, RG, DD, HZ, JW, JL, and XY; Research Regulatory: GJ and XL; Writing – Draft: QY and WW; Writing-Proofreading and Editing: XL and QY. All authors approved the final version of this manuscript.
This study was funded in part by grants from the Scientific and Technological Innovation Team Plan of Shaanxi Province (2021TD-43) by GJ.
The authors thank all the medical staff and patients who contribute to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GC, gastric cancer; RCT, randomized controlled study; EGC, early gastric cancer; CRF, Case Report Form; EG, esophagogastrotomy; DFT, double-flap technique; LOSF, left-open single-flap.
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Kosuga T, Tsujiura M, Nakashima S, Masuyama M, Otsuji E. Current status of function-preserving gastrectomy for gastric cancer. Ann Gastroenterol Surg (2021) 5(3):278–86. doi: 10.1002/ags3.12430
3. Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, et al. Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc (2017) 31(9):3664–72. doi: 10.1007/s00464-016-5403-y
4. Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery (1998) 123(2):127–30. doi: 10.1016/S0039-6060(98)70248-X
5. Kano Y, Ohashi M, Ida S, Kumagai K, Nunobe S, Sano T, et al. Oncological feasibility of laparoscopic subtotal gastrectomy compared with laparoscopic proximal or total gastrectomy for cT1N0M0 gastric cancer in the upper gastric body. Gastric Cancer (2019) 22(5):1060–8. doi: 10.1007/s10120-019-00947-7
6. Lee I, Oh Y, Park SH, Kwon Y, Park S. Postoperative nutritional outcomes and quality of life-related complications of proximal versus total gastrectomy for upper-third early gastric cancer: a meta-analysis. Sci Rep (2020) 10(1):21460. doi: 10.1038/s41598-020-78458-0
7. Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer (2015) 18(2):407–16. doi: 10.1007/s10120-014-0377-8
8. Wang S, Lin S, Wang H, Yang J, Yu P, Zhao Q, et al. Reconstruction methods after radical proximal gastrectomy: A systematic review. Med (Baltimore) (2018) 97(11):e0121. doi: 10.1097/MD.0000000000010121
9. Shen J, Ma X, Yang J, Zhang JP. Digestive tract reconstruction options after laparoscopic gastrectomy for gastric cancer. World J Gastrointest Oncol (2020) 12(1):21–36. doi: 10.4251/wjgo.v12.i1.21
10. Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-assisted proximal gastrectomy with the hinged double flap method. World J Surg (2016) 40(10):2419–24. doi: 10.1007/s00268-016-3510-5
11. Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, et al. Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: Double tract versus jejunal interposition. World J Surg Oncol (2014) 12:20. doi: 10.1186/1477-7819-12-20
12. Kumamoto T, Sasako M, Ishida Y, Kurahashi Y, Shinohara H. Clinical outcomes of proximal gastrectomy for gastric cancer: A comparison between the double-flap technique and jejunal interposition. PloS One (2021) 16(2):e0247636. doi: 10.1371/journal.pone.0247636
13. Yu B, Park KB, Park JY, Lee SS, Kwon OK, Chung HY, et al. Double tract reconstruction versus double flap technique: short-term clinical outcomes after laparoscopic proximal gastrectomy for early gastric cancer. Surg Endosc (2022) 36(7):5243–56. doi: 10.1007/s00464-021-08902-3
14. Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, et al. Double-flap technique as an antireflux procedure in esophagogastrostomy after proximal gastrectomy. J Am Coll Surg (2016) 223(2):e7–e13. doi: 10.1016/j.jamcollsurg.2016.04.041
15. Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T, et al. Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer (2019) 22(5):1036–43. doi: 10.1007/s10120-019-00940-0
16. Saze Z, Kase K, Nakano H, Yamauchi N, Kaneta A, Watanabe Y, et al. Functional benefits of the double flap technique after proximal gastrectomy for gastric cancer. BMC Surg (2021) 21(1):392. doi: 10.1186/s12893-021-01390-1
17. Wang F-H, Zhang X-T, Li Y-F, Tang L, Qu X-J, Ying J-E, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (2021) 41:747–95. doi: 10.1002/cac2.12193
18. Japanese Gastric Cancer A. Japanese Gastric cancer treatment guidelines. 6th. Berlin, Germany: Springer (2021).
19. Hosoda K, Washio M, Mieno H, Moriya H, Ema A, Ushiku H, et al. Comparison of double-flap and OrVil techniques of laparoscopy-assisted proximal gastrectomy in preventing gastroesophageal reflux: a retrospective cohort study. Langenbecks Arch Surg (2019) 404(1):81–91. doi: 10.1007/s00423-018-1743-5
20. Wang WD, Wei JP, Gao RQ, Yu PF, Gao XX, Yang XS, et al. [Preliminary experience of laparoscopic proximal gastrectomy with esophagogastrostomy single flap technique]. Zhonghua Wei Chang Wai Ke Za Zhi (2022) 25(5):462–5. doi: 10.3760/cma.j.cn441530-20211027-00440
21. Wang WD, Gao RQ, Chen T, Dong DH, Yang QC, Zhou HK, et al. Protocol for comparing the efficacy of three reconstruction methods of the digestive tract (Kamikawa versus double-tract reconstruction versus tube-like stomach) after proximal gastrectomy. Front Surg (2022) 9:891693. doi: 10.3389/fsurg.2022.891693
22. Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A, et al. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP study). Ann Gastroenterol Surg (2018) 3(1):96–103. doi: 10.1002/ags3.12216
23. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol (2022) 117(1):27–56. doi: 10.14309/ajg.0000000000001538
24. Yadlapati R, Gyawali CP, Pandolfino JE. CGIT GERD Consensus Conference Participants. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review., Clin Gastroenterol Hepatol (2022) 20(5):984–94.e1. doi: 10.1016/j.cgh.2022.01.025
25. Jung HK, Tae CH, Song KH, Kang SJ, Park JK, Gong EJ. Korean Society of neurogastroenterology and motility. 2020 Seoul consensus on the diagnosis and management of gastroesophageal reflux disease. J Neurogastroenterol Motil (2021) 27(4):453–81. doi: 10.5056/jnm21077
26. Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol (2017) 24(6):1635–42. doi: 10.1245/s10434-017-5782-x
27. Kano Y, Ohashi M, Ida S, Kumagai K, Sano T, Hiki N, et al. Laparoscopic proximal gastrectomy with double-flap technique versus laparoscopic subtotal gastrectomy for proximal early gastric cancer. BJS Open (2020) 4(2):252–9. doi: 10.1002/bjs5.50241
Keywords: gastric cancer, double-flap technique, left-open single-flap technique, proximal gastrectomy, study protocol
Citation: Yang QC, Wang WD, Mo ZC, Yue C, Zhou HK, Gao RQ, Yu J, Dong DH, Liu JQ, Wei JP, Yang XS, Ji G and Li XH (2022) Study protocol for comparing the efficacy of left-open single-flap technique versus double-flap technique after proximal gastrectomy: A multicenter randomized controlled trial. Front. Oncol. 12:973810. doi: 10.3389/fonc.2022.973810
Received: 27 June 2022; Accepted: 26 October 2022;
Published: 17 November 2022.
Edited by:
Fausto Rosa, Catholic University of the Sacred Heart, Rome, ItalyReviewed by:
Zhi Zhu, China Medical University, ChinaCopyright © 2022 Yang, Wang, Mo, Yue, Zhou, Gao, Yu, Dong, Liu, Wei, Yang, Ji and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Ji, SmlnYW5nQGZtbXUuZWR1LmNu; Xiao Hua Li, eGp5eWxpeGlhb2h1YUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.