
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 30 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.971192
This article is part of the Research TopicCase Reports in Thoracic Oncology: 2022View all 42 articles
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) have been widely used in the treatment of locally advanced non-small cell lung cancer (NSCLC). The phenomenon of pseudoprogression in targeted therapy in EGFR-mutation NSCLC patients is rare. Here, we reported an EGFR-mutation-positive lung adenocarcinoma patient who was admitted to a hospital for cough and chest distress accompanied by shortness of breath. He underwent four cycles of chemotherapy with pemetrexed combined with carboplatin and concurrent radiotherapy in the third and fourth cycles. Then, he was treated by osimertinib maintenance therapy. After 11.5 months of osimertinib treatment, he was assessed to progressive disease by computed tomography. He underwent fiber bronchoscopy, and the biopsy pathology showed extensive necrosis without tumor cells. Until now, the patient has continued on osimertinib for 7 months without relapse or metastasis. As far as we know, we are the first to report pseudoprogression in osimertinib maintenance after definitive chemoradiation. This study reminds the clinicians to distinguish pseudoprogression from osimertinib-induced progression and avoid abandoning effective treatments.
At present, immune checkpoint inhibitors and targeted drugs have become widely used in locally advanced non-small cell lung cancer (NSCLC) (1, 2). Pseudoprogression is a special phenomenon, which is manifested by increased volume or appearance of new lesions with subsequent narrowing of the mass (3). The overall rate of pseudoprogression in immunotherapy was 5.0% (95% CI: 3.4%, 6.7%) in NSCLC patients (4). Two case reports showed three ALK-positive NSCLC patients who developed pseudoprogression by magnetic resonance imaging (MRI) after alectinib treatment (5, 6). Data are scarce on pseudoprogression in epidermal growth factor receptor (EGFR) mutation NSCLC patients associated with targeted drug monotherapy. In this study, we reported a patient with lung adenocarcinoma who developed pseudoprogression in osimertinib maintenance after definitive chemoradiation.
This patient is a 65-year-old man who was hospitalized for cough and chest distress accompanied by shortness of breath. He was a previously healthy never smoker and had no family history of tumors. No significant abnormalities were observed on physical examination. Carcinoembryonic antigen (CEA) and cytokeratin 19 fragments were high and procalcitonin was in the normal range. Chest computed tomography (CT) and positron emission tomography/computed tomography (PET/CT) showed the right lower lobe lung cancer (35 mm×60 mm) (SUVmax = 14.1) with the right hilar and mediastinal lymph node metastasis (Figures 1A–2D). Cranial-enhanced MRI showed no brain metastases. He underwent fiber bronchoscopy, and the biopsy pathology showed adenocarcinoma. The gene mutation test indicated EGFR exon 21 L858R mutation. He was diagnosed with EGFR-mutation-positive stage IIIB (cT3N2M0) lung adenocarcinoma. He underwent four cycles of chemotherapy with pemetrexed (800 mg on day 1, every 3 weeks) combined with carboplatin (500 mg on day 2, every 3 weeks) and concurrent lung tumor and metastatic lymph node radiotherapy (RT) (60 Gy/30 fractions) in the third and fourth cycles. After chemoradiation, reexamination CT showed partial response (Figure 2A). Then, he was enrolled in a clinical trial, which evaluates the efficacy and safety of osimertinib maintenance after definitive chemoradiation in unresectable EGFR mutation positive stage III NSCLC (LAURA trial) (7). During osimertinib treatment, the therapeutic effect evaluated by CT was always stable disease (SD) (Figures 2B–D). After 11.5 months of osimertinib treatment, he reviewed the chest enhanced CT showing the mass enlarged, which was inhomogeneous reinforcement (Figures 3A, B). He was assessed as having progressive disease (PD) according to the evaluation criteria of RECIST 1.1. The increased CT value after enhanced CT is 23 hounsfield unit (HU), whereas the increased value is 33 HU before osimertinib. Tumor markers were in the normal range. He underwent fiber bronchoscopy, and no tumor cells were detected by the aspiration cytology of the mediastinal lumps; the biopsy pathology showed extensive necrosis without tumor cells (Figure 3C). PET/CT showed no high fluorodeoxyglucose avidity (SUVmax = 3.4) to avoid inaccurate puncture due to tumor heterogeneity (Figures 3D, E). Therefore, the therapeutic effect was SD. Now, the patient has continued on osimertinib for 7 months without relapse or metastasis (Figure 3F). The overall course of treatment is shown in Figure 4, and the compliance of this patient was quite high.
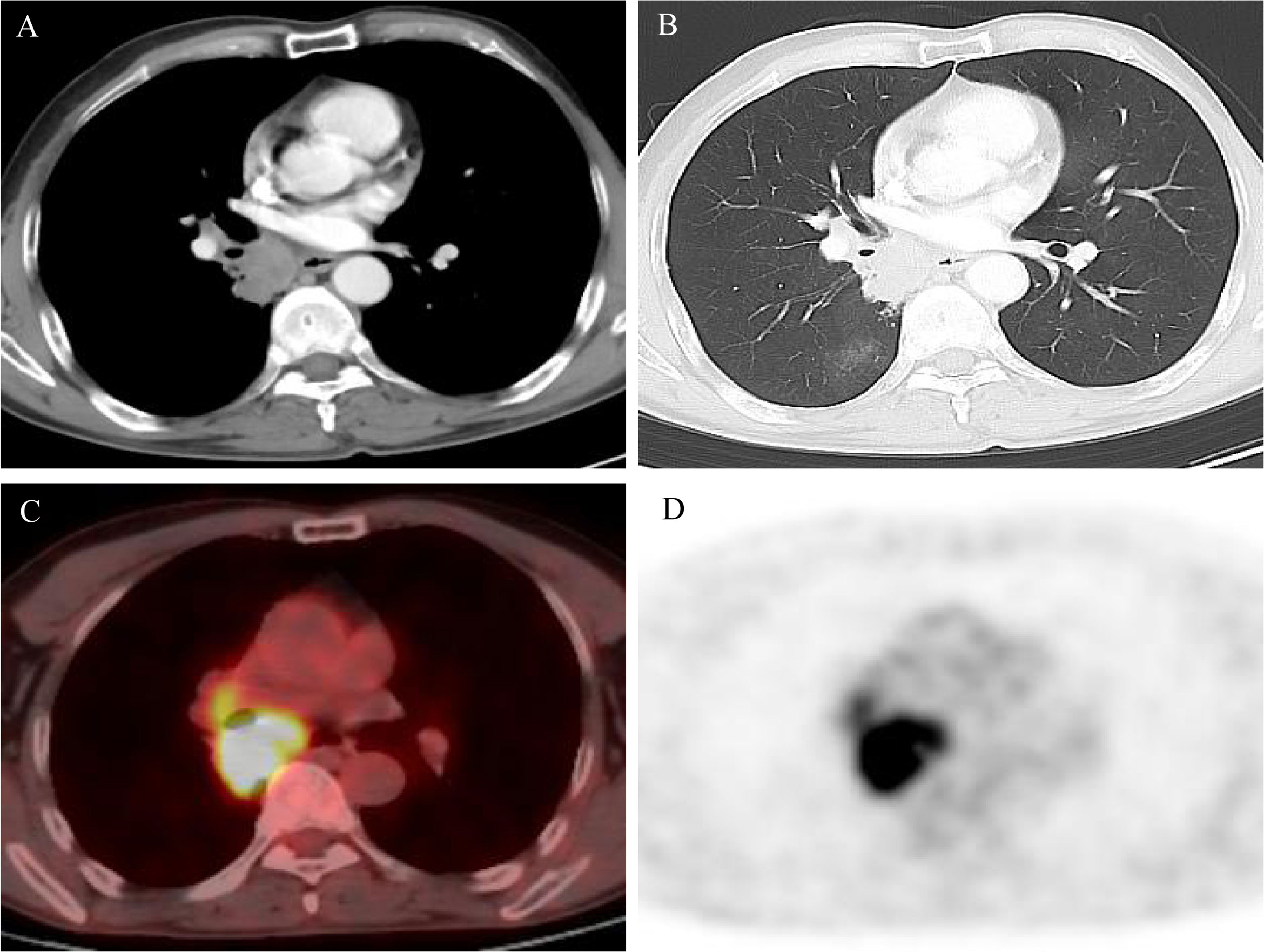
Figure 1 (A, B) Computed tomography (CT) scan at diagnosis showed a lung mass in the right lower lobe and mediastinum; (C, D) positron emission tomography/computed tomography (PET/CT) scan at diagnosis showed high fluorodeoxyglucose avidity.
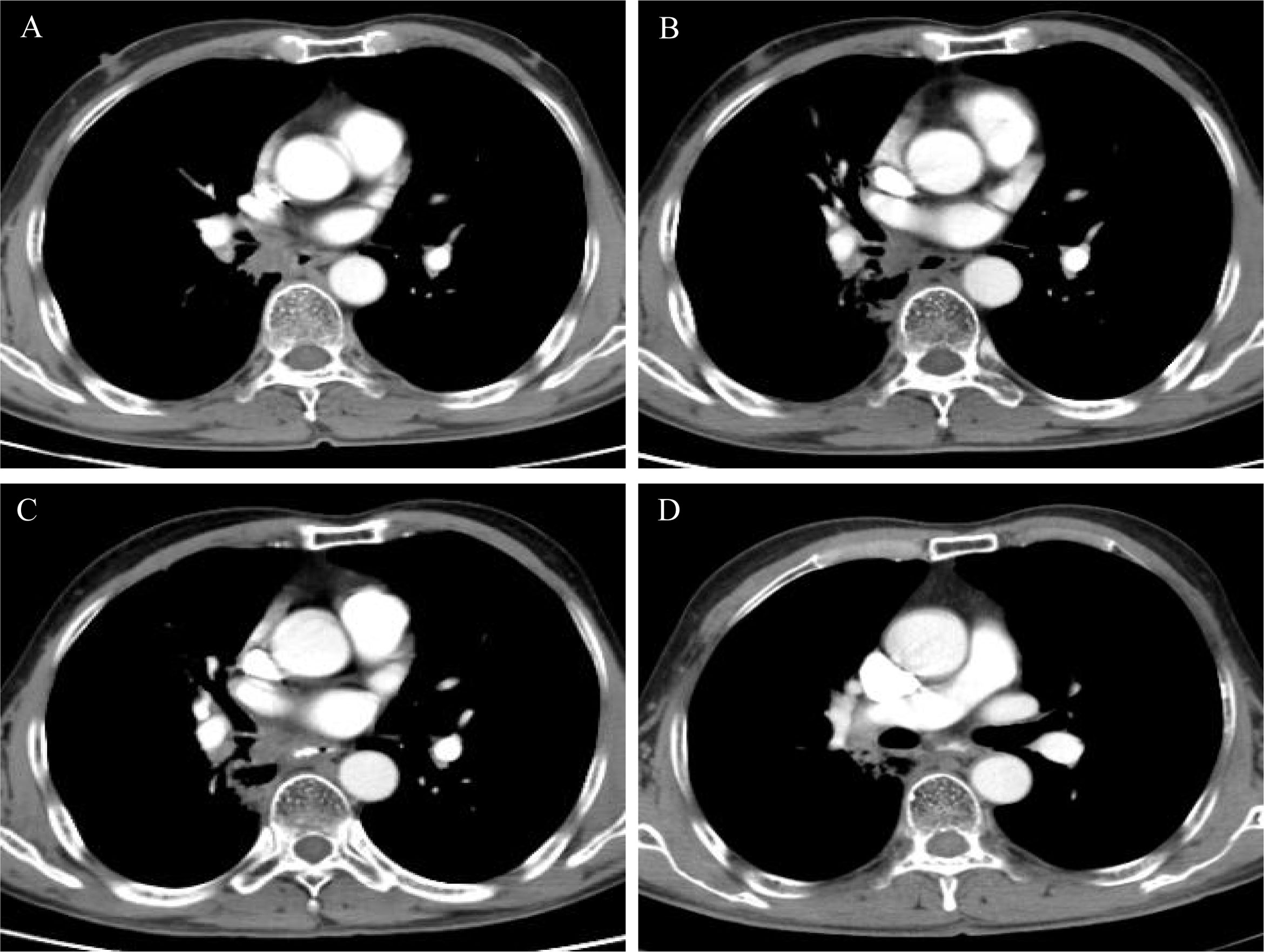
Figure 2 (A) Computed tomography (CT) scan after chemoradiation showed partial response; (B–D) CT scan showed stable disease during osimertinib treatment.
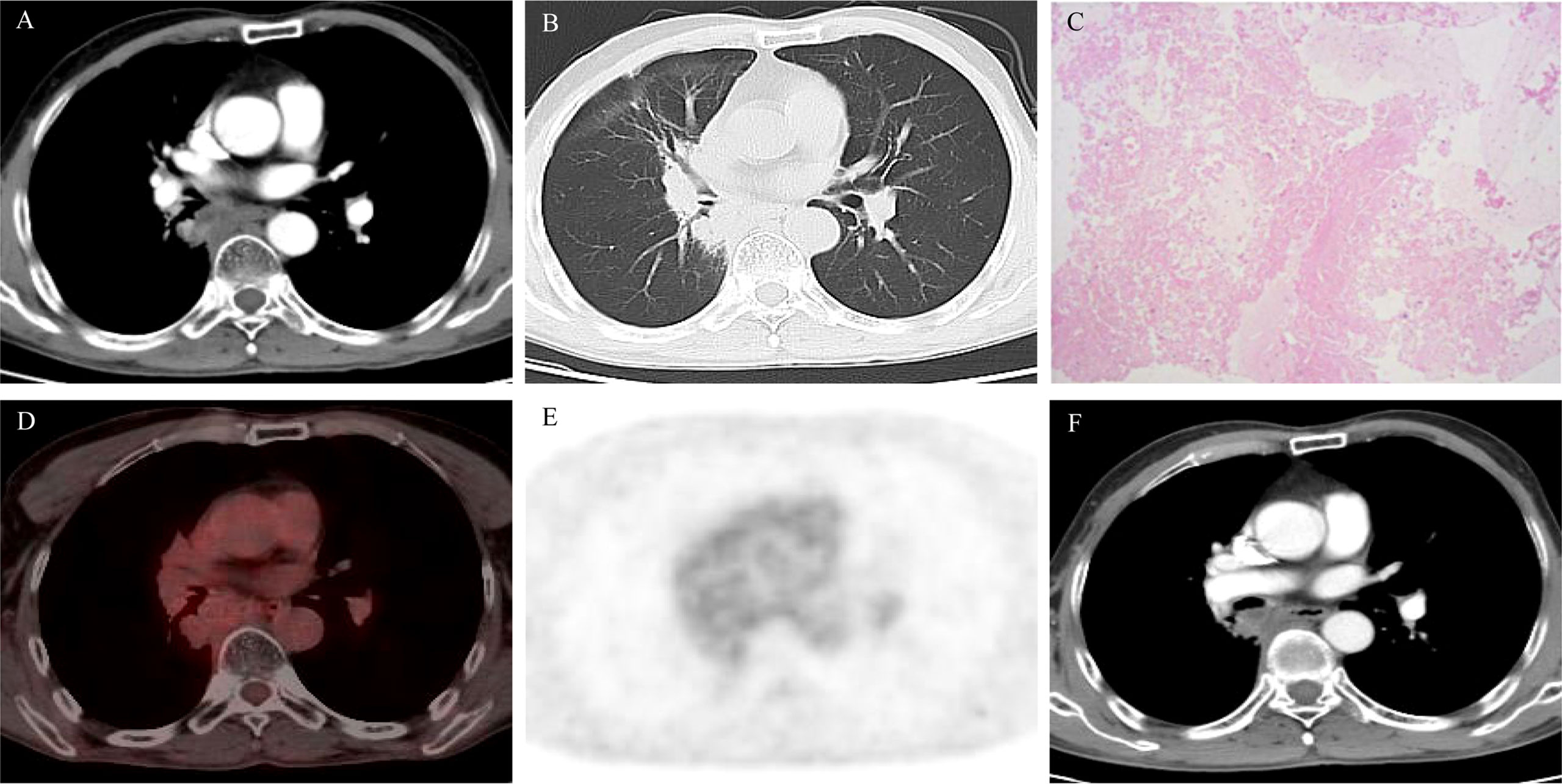
Figure 3 (A, B) Computed tomography (CT) scan after 11.5 months of osimertinib therapy showed enlarged primary mass; (C) hematoxylin and eosin (H&E) stain of the mediastinal biopsy tissue sample demonstrating extensive necrosis without tumor cells; (D, E) PET/CT scan after 11.5 months of osimertinib therapy showed no fluorodeoxyglucose avidity; (F) CT scan after 18.5 months of osimertinib treatment showed no relapse or metastasis.
In our study, we are the first to report pseudoprogression in osimertinib maintenance after definitive chemoradiation. However, it is not clear whether the necrosis was caused by RT or osimertinib treatment or both. Radiation therapy can cause vascular endothelial damage, leading to the increase in vascular permeability and the release of some pro-inflammatory cytokines, concomitant with the upregulation of vascular endothelial growth factor (VEGF), and ultimately facilitating7 radioactive injury lesion expansion (8). A case report showed a stage IIIA adenocarcinoma patient who developed pseudoprogression at 4 months after stereotactic body radiotherapy (SBRT) (9). Michael C Stauder et al. reported that the pseudoprogression occurred at 12 months after stereotactic ablative RT (10). Another study showed that the median time for developing an enlarged lesion in the area of SBRT was 12 months in patients without tumor recurrence (11). This suggests that the possibility of radionecrosis can occur at several months to one year after RT.
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI), is now the first-line treatment for EGFR-mutation-positive advanced NSCLC (1, 2). VEGF and EGFR have many overlapping and parallel downstream pathways, and the activation of EGFR can upregulate the expression of VEGF and VEGFR and facilitate VEGFR activating (12). The combination therapy of anti-angiogenic drugs and EGFR-TKIs can simultaneously block the VEGFR/EGFR pathway, which has synergistic effects to enhance the antitumor effect (13, 14). In a clinical trial, osimertinib combined with bevacizumab showed that the overall response rate was 80% (95% CI, 67–91%), and the median progression free survival (PFS) was 19 months (95% CI, 15–24 months) (15). However, VEGF expression is affected by many factors, including cytokines (e.g., tumor necrosis factor-α [TNF-α], transforming growth factor-β [TGF-β], EGF, fibroblast cytokines, and interleukin-1), oncogene expression (e.g., EGFR, erbB2/human EGFR 2 [HER2], ras, and src), and hypoxia (16). The EGF/EGFR pathway is one of the many factors that regulate VEGF expression. EGFR inhibition alone does not block VEGF, thereby allowing tumor angiogenesis (17). We cannot make the certain claim that pseudo-progression was due to endothelial injury.
The patient’s enhanced CT demonstrated the mediastinum invasion by lung tumor with unclear pericardial demarcation. He was diagnosed as EGFR-mutation-positive unresectable locally advanced lung adenocarcinoma. The standard treatment of unresectable locally advanced NSCLC is concurrent chemoradiotherapy (CCRT) (18). In a retrospective study of EGFR mutation positive of stage IIIB lung adenocarcinoma, there were no statistically significant differences in the 5-year overall survival (OS) rates between TKIs and CCRT groups (30% vs. 26%) (19). Phase I trial of erlotinib combined with CCRT and the SWOG S0023 trial also failed to prove any benefit of TKI addition (20, 21). In unresectable EGFR-mutated positive stage III NSCLC patients, the initial results indicated that the median PFS of EGFR TKI and CRT was significantly longer than that of CRT alone (26.1 months vs. 6.9 months, log-rank P = 0.023) (22). In the RECEL trial, compared with etoposide/cisplatin concurrent RT, the median PFS with erlotinib concurrent RT was significantly longer (24.5 vs. 9.0 months [P < 0.001]) (23). The LAURA trial will assess the efficacy of osimertinib after definitive chemoradiation in EGFR mutation NSCLC patients (7).
In conclusion, this is the first report of pseudoprogression in osimertinib maintenance after definitive chemoradiation. The mechanism of pseudoprogression after osimertinib treatment is still unclear, but the significance of this study suggests that clinicians should not easily interrupt osimertinib treatment when imaging progression occurs during osimertinib, and further determine pathological examination and PET-CT before making decisions. There are very few relevant studies about the mechanism and possibility of whether EGFR-TKI targeted therapy combined with RT increases pseudoprogression compared with RT alone; it deserves further exploration and discussion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong Cancer Hospital and Institute. The patients/participants provided their written informed consent to participate in this study.
FR performed the literature search and wrote the original draft, FR and YW collected and analyzed the data, and XM and YG revised the manuscript critically for important scientific content. All authors contributed to the article and approved the submitted version.
This work was supported by CSCO-Haosen Cancer Research Fund (No. Y-HS202102-0089).
We thank the patient and his family for giving consent to this case report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol (2020) 17(5):300–12. doi: 10.1038/s41571-019-0316-z
2. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30(2):171–210. doi: 10.1093/annonc/mdy554
3. Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med (2019) 16(4):655–70. doi: 10.20892/j.issn.2095-3941.2019.0144
4. Park HJ, Kim KW, Pyo J, Suh CH, Yoon S, Hatabu H, et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: A systematic review and meta-analysis. Radiology (2020) 297(1):87–96. doi: 10.1148/radiol.2020200443
5. Ou SH, Klempner SJ, Azada MC, Rausei-Mills V, Duma C. Radiation necrosis presenting as pseudoprogression (PsP) during alectinib treatment of previously radiated brain metastases in ALK-positive NSCLC: Implications for disease assessment and management. Lung Cancer (2015) 88(3):355–9. doi: 10.1016/j.lungcan.2015.03.022
6. Ou SH, Weitz M, Jalas JR, Kelly DF, Wong V, Azada MC, et al. Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer (2016) 96:15–8. doi: 10.1016/j.lungcan.2016.03.008
7. Lu S, Casarini I, Kato T, Cobo M, Özgüroğlu M, Hodge R, et al. Osimertinib maintenance after definitive chemoradiation in patients with unresectable EGFR mutation positive stage III non-small-cell lung cancer: LAURA trial in progress. Clin Lung Cancer (2021) 22(4):371–5. doi: 10.1016/j.cllc.2020.11.004
8. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer (2019) 18(1):21. doi: 10.1186/s12943-019-0950-1
9. Frechette KM, Brown LC, Aubry MC, Wigle DA, Olivier KR. Pseudoprogression after stereotactic body radiotherapy. J Thorac Oncol (2014) 9(4):e29–30. doi: 10.1097/JTO.0000000000000067
10. Stauder MC, Rooney JW, Neben-Wittich MA, Garces YI, Olivier KR. Late tumor pseudoprogression followed by complete remission after lung stereotactic ablative radiotherapy. Radiat Oncol (2013) 8:167. doi: 10.1186/1748-717X-8-167
11. Dunlap NE, Yang W, McIntosh A, Sheng K, Benedict SH, Read PW, et al. Computed tomography-based anatomic assessment overestimates local tumor recurrence in patients with mass-like consolidation after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2012) 84(5):1071–7. doi: 10.1016/j.ijrobp.2012.01.088
12. Hirata A, Ogawa S, Kometani T, Kuwano T, Naito S, Kuwano M, et al. ZD1839 (Iressa) induces antiangiogenic effects through inhibition of epidermal growth factor receptor tyrosine kinase. Cancer Res (2002) 62(9):2554–60.
13. Perdrizet K, Leighl NB. The role of angiogenesis inhibitors in the era of immune checkpoint inhibitors and targeted therapy in metastatic non-small cell lung cancer. Curr Treat Options Oncol (2019) 20(3):21. doi: 10.1007/s11864-019-0617-6
14. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res (2009) 15(10):3484–94. doi: 10.1158/1078-0432.CCR-08-2904
15. Yu HA, Schoenfeld AJ, Makhnin A, Kim R, Rizvi H, Tsui D, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: A phase 1/2 single-group open-label trial. JAMA Oncol (2020) 6(7):1048–54. doi: 10.1001/jamaoncol.2020.1260
16. Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res (2007) 5(3):203–20. doi: 10.1158/1541-7786.MCR-06-0404
17. Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene (2002) 21(13):2000–8. doi: 10.1038/sj.onc.1205260
18. Jiang L, Meng X, Zhao X, Xing L, Yu J. Perspective on treatment for unresectable locally advanced non-small cell lung cancer with oncogene-driven mutation: a narrative review. Transl Lung Cancer Res (2020) 9(5):2137–44. doi: 10.21037/tlcr-20-722
19. Hsia TC, Liang JA, Li CC, Chien CR. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR-tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR. Thorac Cancer (2018) 9(11):1398–405. doi: 10.1111/1759-7714.12847
20. Choong NW, Mauer AM, Haraf DJ, Lester E, Hoffman PC, Kozloff M, et al. Phase I trial of erlotinib-based multimodality therapy for inoperable stage III non-small cell lung cancer. J Thorac Oncol (2008) 3(9):1003–11. doi: 10.1097/JTO.0b013e31818396a4
21. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol (2008) 26(15):2450–6. doi: 10.1200/JCO.2007.14.4824
22. Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol (2021) 16(6):1030–41. doi: 10.1016/j.jtho.2021.01.1628
23. Xing L, Wu G, Wang L, Li J, Wang J, Yuan Z, et al. Erlotinib versus Etoposide/Cisplatin with radiation therapy in unresectable stage III epidermal growth factor receptor mutation-positive non-small cell lung cancer: A multicenter, randomized, open-label, phase 2 trial. Int J Radiat Oncol Biol Phys (2021) 109(5):1349–58. doi: 10.1016/j.ijrobp.2020.11.026
Keywords: pseudoprogression, osimertinib, EGFR mutation, NSCLC, case report
Citation: Ren F, Wang Y, Gao Y and Meng X (2022) Pseudo-progression with osimertinib after definitive chemoradiation in unresectable epidermal growth factor receptor mutation positive of stage III non-small cell lung cancer: A case report. Front. Oncol. 12:971192. doi: 10.3389/fonc.2022.971192
Received: 16 June 2022; Accepted: 05 August 2022;
Published: 30 August 2022.
Edited by:
Yuan-Zheng Xia, China Pharmaceutical University, ChinaReviewed by:
Fubing Wu, Nanjing Medical University, ChinaCopyright © 2022 Ren, Wang, Gao and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangjiao Meng, bWVuZ3hpYW5namlhb0AxMjYuY29t; Yongsheng Gao, Z3lzLTc3N0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.