- 1Department of Anesthesiology, Taizhou People’s Hospital, Affiliated to Nanjing Medical University, Taizhou, Jiangsu, China
- 2Department of Clinical Laboratory, Taizhou People’s Hospital, Affiliated to Nanjing Medical University, Taizhou, Jiangsu, China
- 3The Center for Translational Medicine, Taizhou People’s Hospital, Affiliated to Nanjing Medical University, Taizhou, Jiangsu, China
- 4Department of Obstetrics and Gynecology, Taizhou People’s Hospital, Affiliated to Nanjing Medical University, Taizhou, Jiangsu, China
Musashi 2 (MSI2) is an RNA-binding protein that regulates mRNA translation of numerous intracellular targets and plays an important role in the development of cancer. However, the prognostic value of MSI2 in various cancers remains controversial. Herein, we conducted this meta-analysis including 21 studies with 2640 patients searched from PubMed, Web of Science, EMBASE, Chinese National Knowledge Infrastructure databases, and WanFang databases to accurately assess the prognostic significance of MSI2 in various cancers. Our results indicated that high MSI2 expression was significantly related to poor overall survival (HR = 1.84, 95% CI: 1.66-2.05, P < 0.001) and disease-free survival (HR = 1.73, 95% CI: 1.35-2.22, P < 0.001). In addition, MSI2 positive expression was associated with certain phenotypes of tumor aggressiveness, such as clinical stage, depth of invasion, lymph node metastasis, liver metastasis and tumor size. In conclusion, elevated MSI2 expression is closely correlated with poor prognosis in various cancers, and may serve as a potential molecular target for cancer patients.
Introduction
According to recently released data, there would be 19.29 million new cancer cases and 9.96 million deaths worldwide in 2020, which remains a global and growing public health problem (1). Although targeted therapy and comprehensive treatment for cancers have made remarkable progress, the therapeutic effect of most tumors is still unsatisfactory (2). The main reason is the lack of effective methods for prognosis monitoring of cancer patients (3). Thus, identification of new biomarkers with the potential to predict cancer progression and prognosis will bring new hope to cancer patients.
Posttranscriptional regulation is known to control gene expression and cell behavior (4). Accumulating evidence indicates that aberrant expression and dysfunction of RNA-binding proteins (RBPs) as posttranscriptional regulators are associated with initiation, progression, and chemoresistance of various types of tumors (5, 6). The RBP Musashi-2 (MSI2) has been characterized as a cancer-driver gene in some cancers (7). It binds and regulates the mRNA stability and translation of proteins operating in vital oncogenic signaling pathways, including NUMB/Notch, PTEN/Akt/mTOR, TGFβ/SMAD, MYC, cMET, and others (7, 8). In pancreatic cancer, Sheng et al. revealed that Msi2 promotes the occurrence and development of pancreatic cancer by downregulating Numb protein that can regulate various carcinogenic signaling pathways, including Notch, p53 and Hedgehog pathways (9). Wang et al. found that Msi2 can inhibit tumor suppressor gene PTEN and activate PDK/Akt/mTORC1 signal pathway to cause tumor (10). Jiang et al. showed that Msi2 expression regulates epithelial to mesenchymal transition (EMT) by activating transcription factors Snail and TGFβR1/Smad3 signaling, which is related to chemoresistency of glioblastoma (11). Moreover, TGFβ/Smad signaling pathway is involved in cell proliferation, differentiation, apoptosis, adhesion, invasion and cell microenvironment (8, 12). In addition, multiple other studies also showed that MSI2 protein maintains cancer stem cell populations and regulates cancer invasion, metastasis and development of more aggressive cancer phenotypes, including drug resistance (8, 13–18). Thus, MSI2 seems to be a potential prognostic biomarker and therapeutic target for cancer patients.
MSI2 has been proved to be significantly up-regulated in various cancers, such as ovarian carcinoma (OC) (19), non-small cell lung cancer (NSCLC) (20), colorectal cancer (CRC) (21), cervical cancer (CC) (16), et al. Moreover, excessive MSI2 expression is associated with poor prognosis in numerous solid tumors as well as in hematological malignancies (9, 15, 19–39), but the results are controversial (16, 32, 35, 36, 40, 41). Hence, we carried out this meta-analysis to further analyze the prognostic value of MSI2, so as to provide a theoretical basis for the prognosis and treatment of patients with cancer.
Materials and methods
Literature search
A systematic literature search of PubMed, Web of Science, EMBASE, CNKI, and Wanfang was performed through April 2022 to identify relevant papers reporting the association between MSI2 expression and survival outcomes (including overall survival [OS] and disease-free survival [DFS]) in patients with cancer. The following keywords were applied in the search: (“cancer” OR “neoplasm” OR “tumor” OR “carcinoma”) AND (“Musashi 2” OR “MSI2”) AND (“prognosis” OR “survival” OR “mortality”). An additional manual search of references cited in eligible articles was also conducted to ensure that all relevant studies were included.
Inclusion and exclusion criteria
The inclusion criteria were as follows: articles that assess the association between MSI2 expression and prognosis in patients with cancer; hazard ratio (HR) and 95% confidence interval (CI) that are provided directly or calculated with sufficient data; the expression of MSI2 in tumor tissues that are measured by immunohistochemistry (IHC), or quantitative reverse transcription polymerase chain reaction (qRT-PCR); patients that were divided into two groups according to MSI2 expression level.
The exclusion criteria were as follows: reviews, case reports, letters, and conference abstracts, etc; duplicated publication; or studies without sufficient data.
Data extraction and quality assessment
Two authors independently extracted basic data and any difference was resolved through discussion until consensus was reached. The basic information is as follows: the first author, publication year, country, duration time, cancer type, follow-up time, sample size, detection method, cut-off value, clinicopathological feature, clinical outcome, analysis method, and HR with corresponding 95% CI. For studies reporting HR values in univariate and multivariate analyses, we tend to choose the latter because of higher accuracy after adjusting for confounding factors. For articles only reporting the survival curve of OS or DFS, we estimated an HR value from the survival curve.
Two investigators independently evaluated the quality of the included articles using the Newcastle-Ottawa Scale (NOS) within the following domains: selection, 0-4; comparability, 0-2; and outcome, 0-3 (42). NOS score ≥6 was regarded as high quality (43).
Statistical analysis
Stata software version 12.0 (StataCorp, College Station, TX) was used for all statistical analyses. HRs and 95% CIs were combined to evaluate the effect of MSI2 expression on prognosis. ORs and 95% CIs were pooled to assess the association of MSI2 expression with clinicopathological characteristics. Heterogeneity across studies was measured by the Chi Squared-based Q test and I2 statistics. When P < 0 .05 or I2 > 50% indicated statistically significant heterogeneity between the studies, the random-effects model was applied for analysis. Otherwise, the fixed-effects model was used. Subgroup analysis was conducted to comprehensively evaluate the correlation between MSI2 expression and OS. Sensitivity analysis was carried out by removing one cohort at a time to prove the stability of the results. Potential publication bias was quantitatively evaluated through Begg’s and Egger’s tests and visually evaluated using funnel plots. The P < 0.05 was considered to be statistically significant.
Results
Literature search and study demographics
A total of 302 applicable records were initially identified through the database search. After removing duplicate (n=94) and obvious irrelevance (n=132) articles, 76 studies were further evaluated by scanning titles and abstracts. Then, the remaining 34 studies were further evaluated by browsing full texts. Finally, 21 articles with 2640 patients were included in the meta-analysis. The flow chart of literature search and screening process was shown in Figure 1.
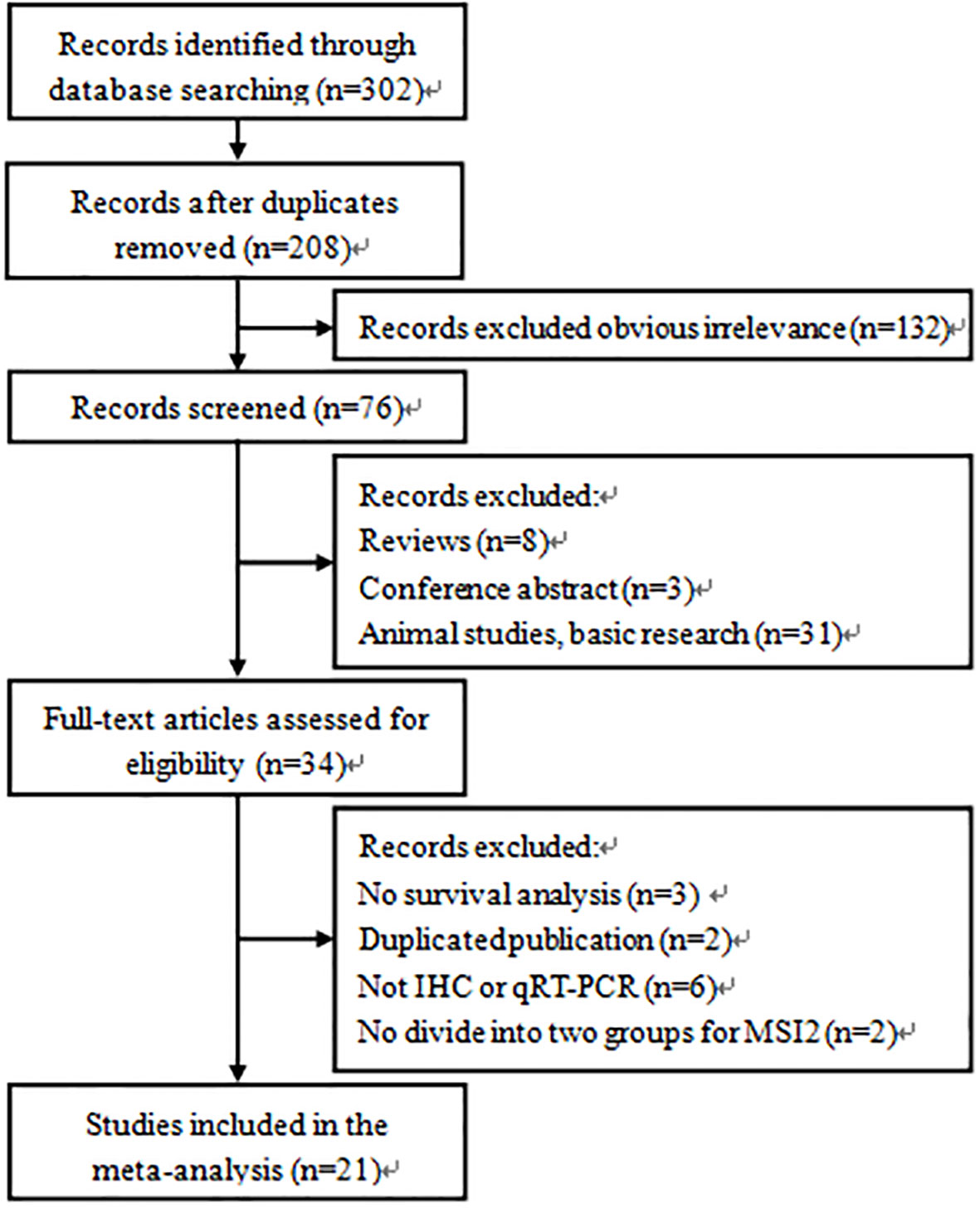
Figure 1 Flow diagram of the study selection process and specific reasons for exclusion of the studies in the meta-analysis.
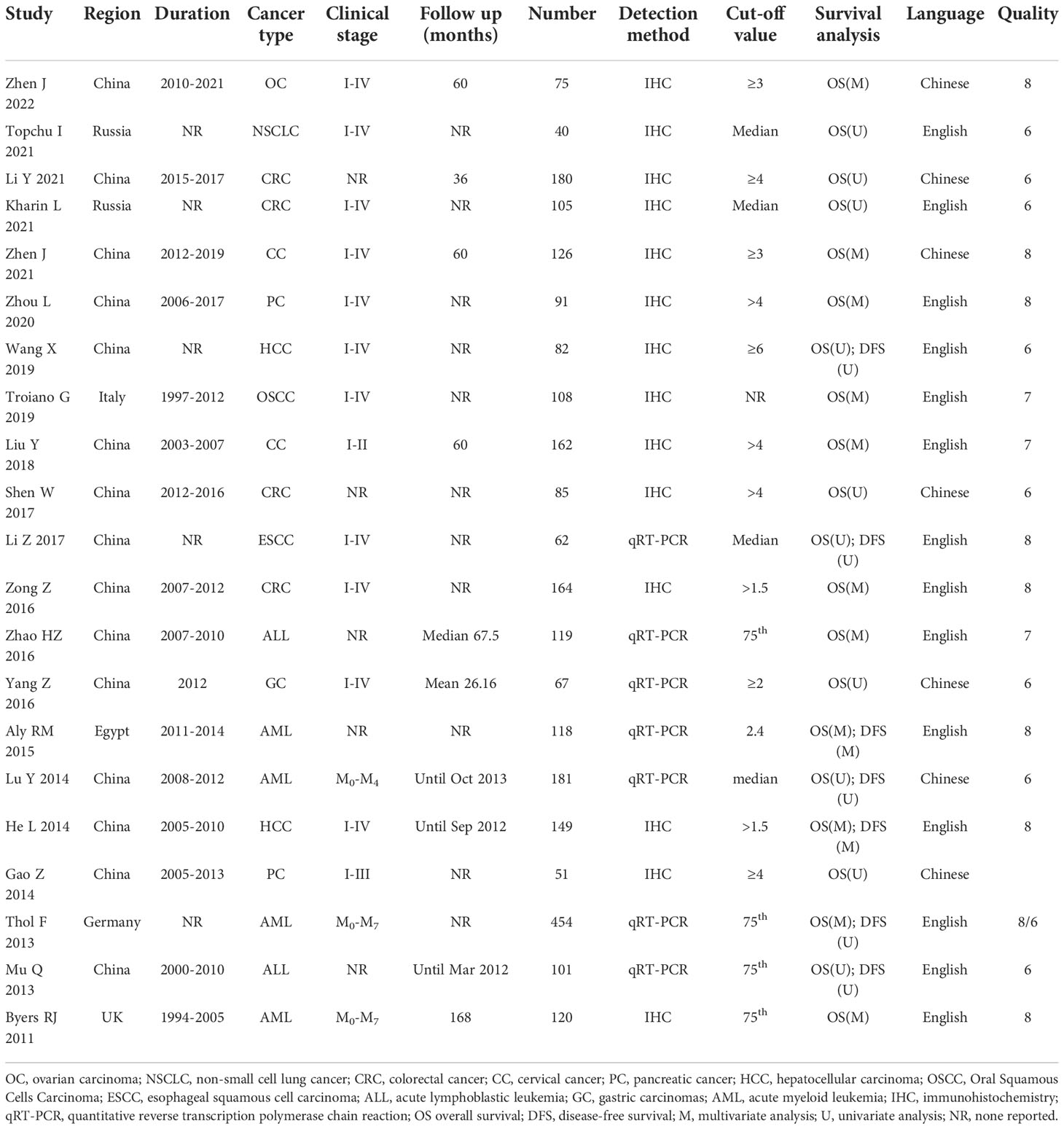
The characteristics of the included studies are summarized in Table 1. The articles included in this study were mainly from China (19, 21–30, 32–34, 36), and the rest were from Russia (16, 20), Italy (40), Egypt (31), Germany (35) and the UK (37), and were published from 2011 to 2022. The types of cancers in the enrolled studies were OC (19), NSCLC (20), CRC (16, 21, 26, 28), cervical cancer (CC) (22, 25), pancreatic cancer (PC) (23, 34), hepatocellular carcinoma (HCC) (24, 33), oral squamous cells carcinoma (OSCC) (40), esophageal squamous cell carcinoma (ESCC) (27), acute lymphoblastic leukemia (ALL) (29, 36), gastric carcinomas (GC) (30), and acute myeloid leukemia (AML) (31, 32, 35, 37). The expression levels of MSI2 were detected either by IHC (16, 19–26, 28, 33, 34, 37, 40) or by qRT-PCR (27, 29–32, 35, 36). OS (16, 19–37, 40) and DFS (24, 27, 31–33, 35, 36) were reckoned as survival outcomes. Based on NOS score, each study received a score of ≥ 6, indicating that the quality of all included studies was high.
Association between MSI2 expression and prognosis
All included studies reported OS to assess the association between MSI2 expression and prognosis. A fixed effects model was applied to calculate the combined HR (95% CI) due to the absence of significant heterogeneity (I2 = 28.1%, P = 0.109). The results demonstrated that high expression levels of MSI2 were significantly associated with poorer OS in human cancers (HR = 1.84, 95% CI: 1.66-2.05, P < 0.001) (Table 2; Figure 2).
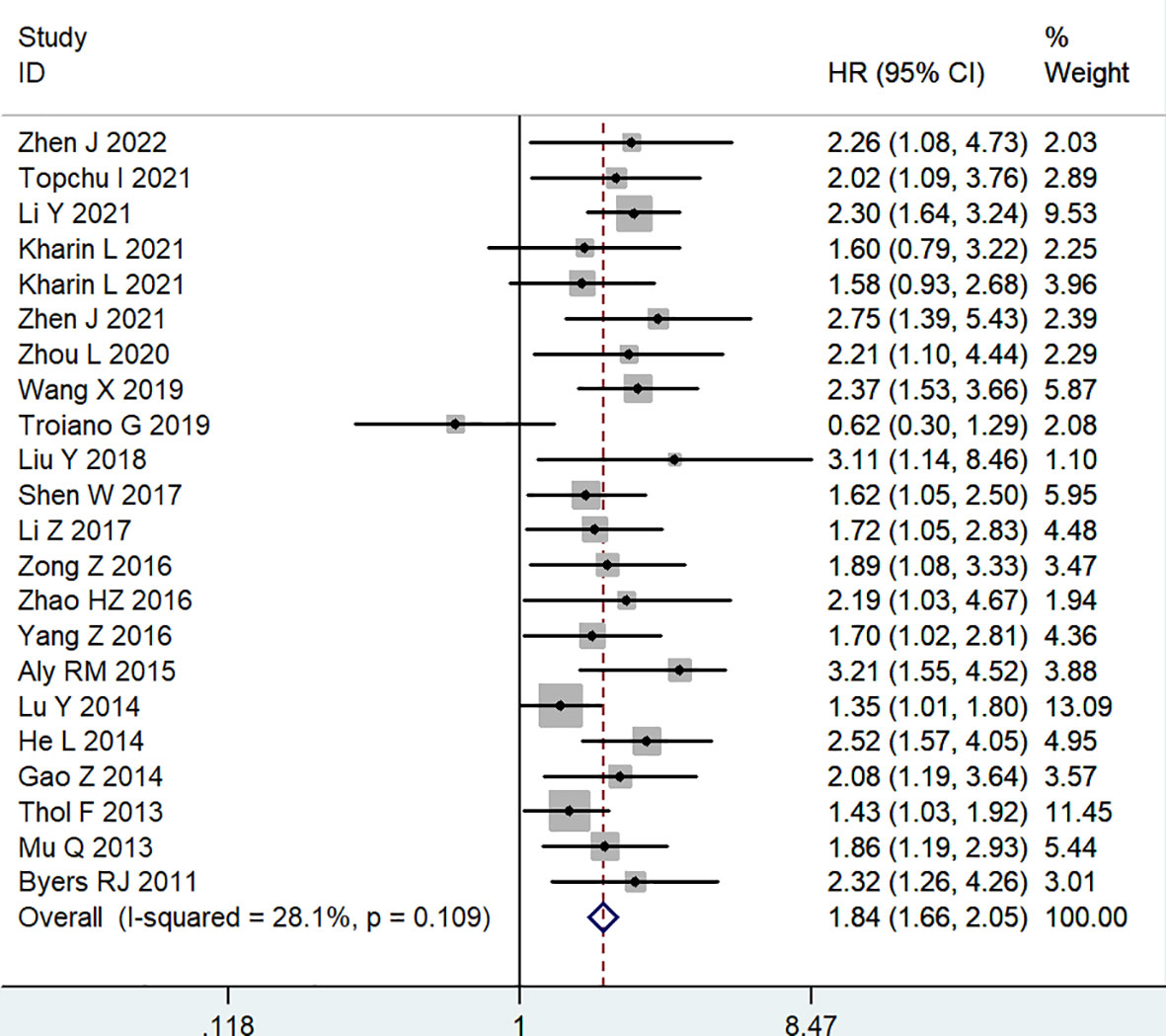
Figure 2 Forest plot of studies evaluating hazard ratios of high MSI2 expression and the overall survival of cancer patients.
Subgroup analyses were performed according to cancer type, detection method, sample size and analysis method to comprehensively evaluate the correlation between MSI2 expression and OS (Table 2). Subgroup analysis by cancer type showed that increased expression of MSI2 was significantly related to shorter OS in patients with solid tumors (HR = 1.96, 95% CI: 1.71-2.24, P < 0.001) (including CRC (HR = 1.88, 95% CI: 1.53-2.32, P < 0.001), CC (HR = 2.86, 95% CI: 1.63-5.02, P < 0.001), PC (HR = 2.13, 95% CI: 1.38-3.29, P = 0.001), HCC (HR = 2.44, 95% CI: 1.77-3.36, P < 0.001) and Others (HR = 1.60, 95% CI: 1.23-2.08, P = 0.001)) and blood tumors (HR = 1.83, 95% CI: 1.40-2.39, P < 0.001) (including ALL (HR = 1.94, 95% CI: 1.32-2.86, P = 0.001) and AML (HR = 1.82, 95% CI: 1.26-2.63, P = 0.001)). This result was similar to that obtained by subgroup analysis of detection method, such as IHC (HR = 2.02, 95% CI: 1.75-2.33, P < 0.001) and qRT-PCR (HR = 1.64, 95% CI: 1.40-1.92, P < 0.001). In addition, the association between high expression of MSI2 and poor OS was also detected in large (HR = 1.81, 95% CI: 1.55-2.10, P < 0.001) and small (HR = 1.88, 95% CI: 1.62-2.18, P < 0.001) subgroups. What is more, MSI2 overexpression was associated with poor OS in both multivariate (HR = 2.04, 95% CI: 1.57-2.63, P < 0.001) and univariate (HR = 1.78, 95% CI: 1.56-2.04, P < 0.001) subgroups.
Meanwhile, seven articles, including 1147 patients, evaluated the correlation between MSI2 expression and DFS. Due to significant heterogeneity among studies (I2 = 66.2%, P = 0.007), a random effects model was employed to estimate the pooled HR and 95% CI of DFS. The pooled HR (HR = 1.73, 95% CI: 1.35-2.22, P < 0.001) showed that high MSI2 expression was significantly correlated with poorer DFS in patients with cancer (Figure 3).
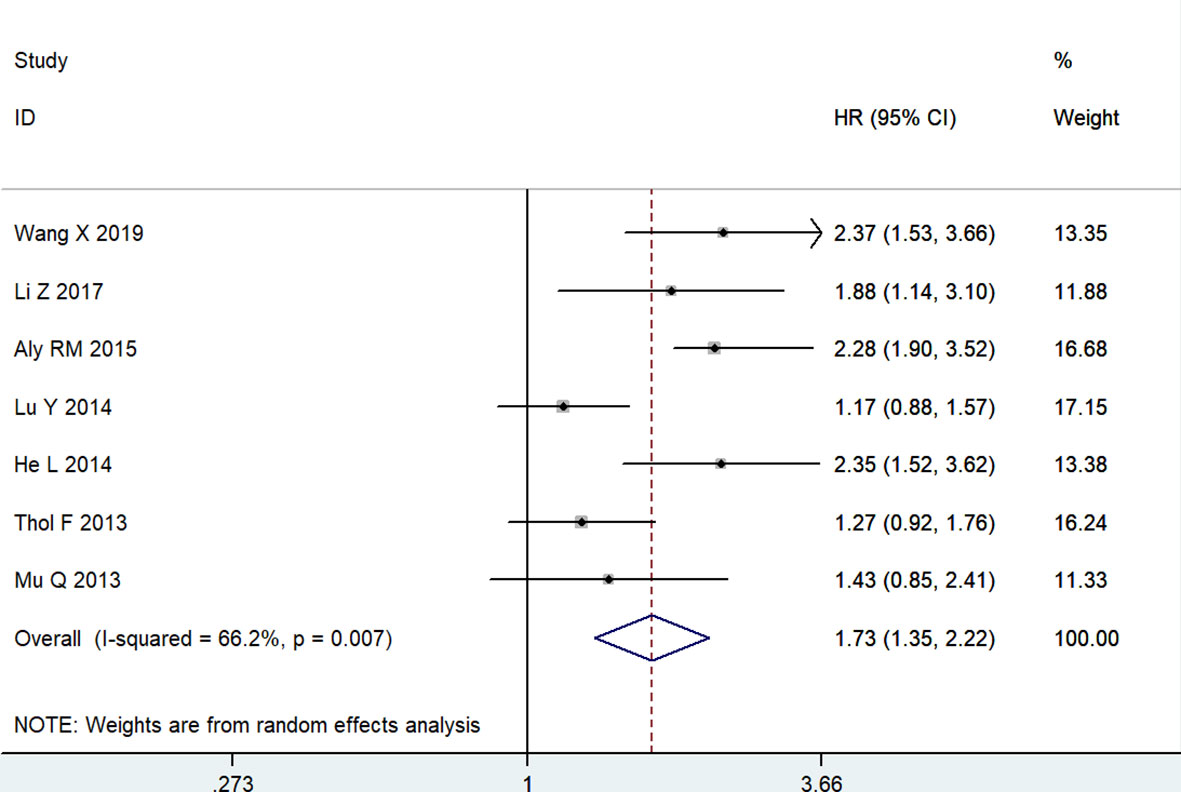
Figure 3 Forest plot of studies evaluating hazard ratios of high MSI2 expression and the disease-free survival of cancer patients.
Association between MSI2 expression and clinicopathological features
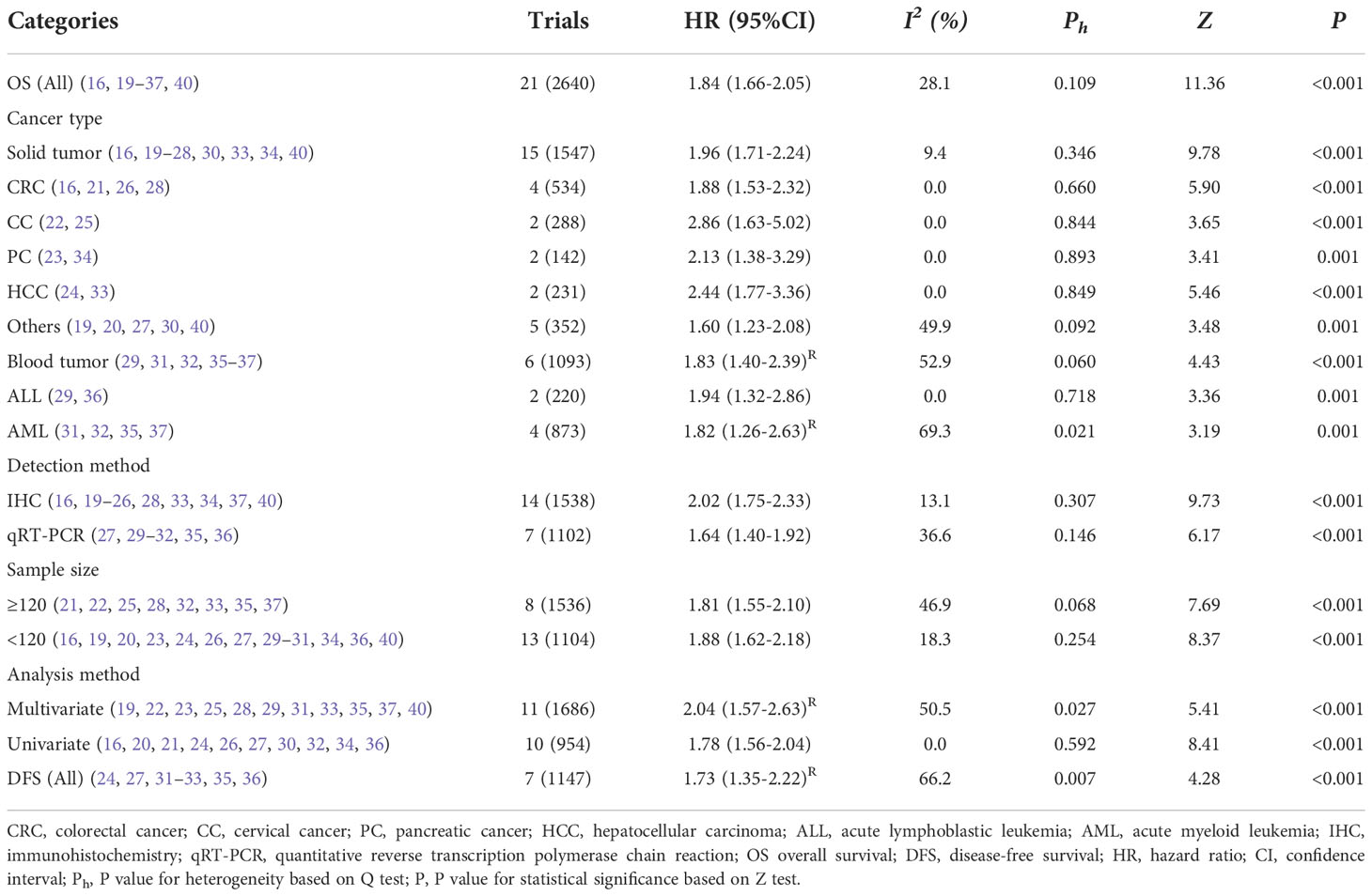
To systematically analyze the role of MSI2 expression as a biomarker in cancer, we explored the relationship between MSI2 expression and clinicopathological features (Table 3). Six studies with 619 patients described the MSI2 expression and clinical stage, and the combined result demonstrated that high expression of MSI2 was obviously associated with advanced clinical stage (OR = 2.14, 95% CI: 1.19-3.85, P = 0.011). Moreover, this significant correlation was also observed in terms of depth of invasion (OR = 2.44, 95% CI: 1.65-3.61, P < 0.001), lymph node metastasis (OR = 2.60, 95% CI: 1.75-3.85, P < 0.001), liver metastasis (OR = 2.16, 95% CI: 1.31-3.54, P = 0.002) and tumor size (OR = 1.96, 95% CI: 1.16-3.31, P = 0.013). However, MSI2 expression had no significant association with age (OR = 1.07, 95% CI: 0.86-1.34, P = 0.549), gender (OR = 0.99, 95% CI: 0.79-1.22, P = 0.899) and degree of differentiation (OR = 0.68, 95% CI: 0.27-1.72, P = 0.418).
Sensitivity analysis and publication bias
Sensitivity analysis was carried to assess the influence of each study on the meta-analysis results by omitting one study in turn. No single point estimate of the omitted individual dataset lay outside the 95% CI of the pooled analysis based on the overall HR estimate of OS (Figure 4A) and DFS (Figure 4B), indicating that the results were stable and reliable. Furthermore, all P values of Begg’s and Egger’s tests were greater than 0.05 (OS: Begg’s test, P=0.367; Egger’s test, P=0.168) (DFS: Begg’s test, P=1.000; Egger’s test, P=0.411), indicating that there was no publication bias in this meta-analysis. In addition, the symmetry of the funnel plots once again visually confirmed the absence of publication bias (Figure 5A, B).
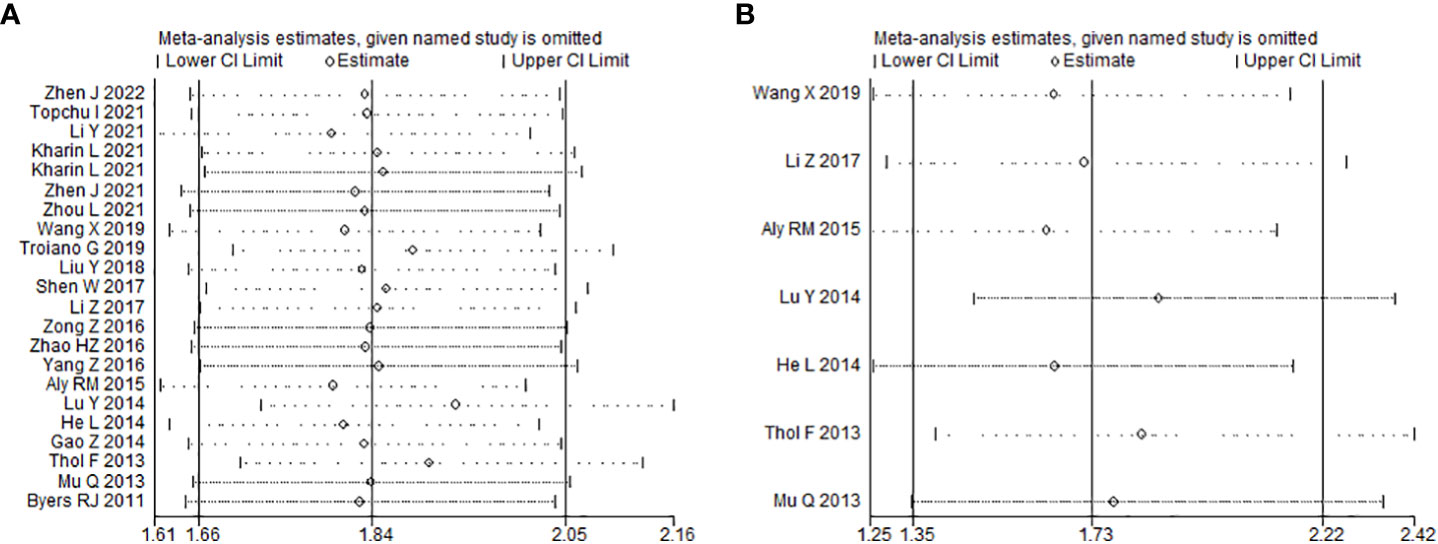
Figure 4 Effects of individual studies on pooled hazard ratios for MSI2 expression and survival of cancer patients. (A) Result of sensitivity analysis for pooled overall survival estimation. (B). Result of sensitivity analysis for pooled disease-free survival estimation.
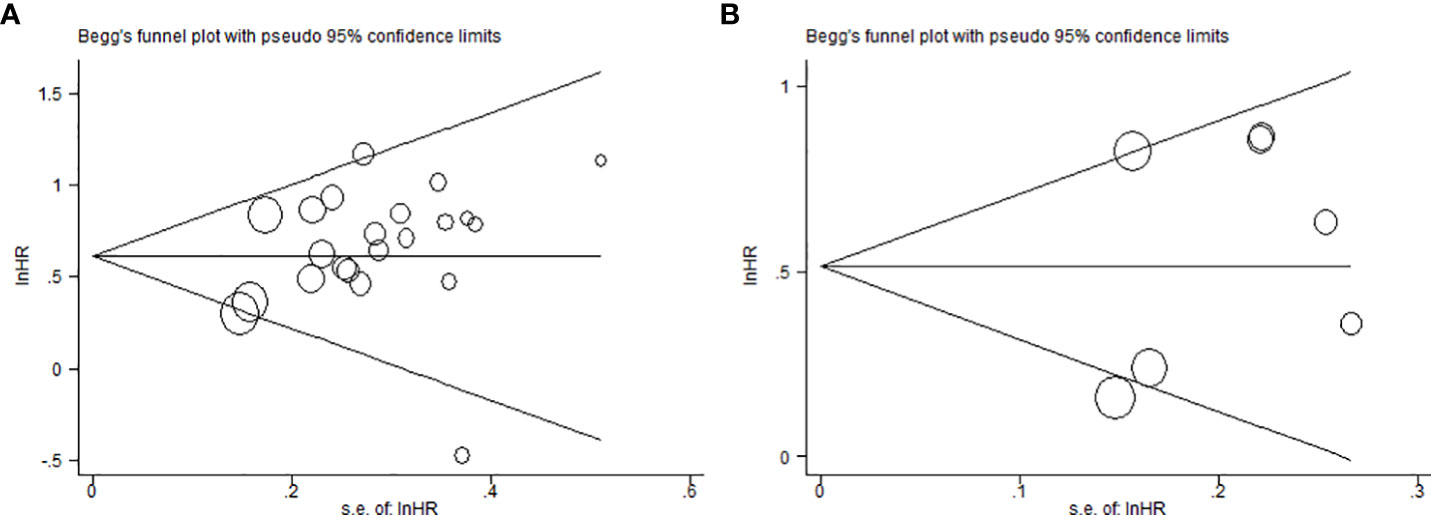
Figure 5 Begg’s funnel plots for assessment of potential publication bias in studies of MSI2 in cancer patients. (A) Funnel plot of publication bias for overall survival. (B) Funnel plot of publication bias for disease-free survival.
Discussion
MSI-2 has been shown to be involved in numerous solid and blood malignancies, and its expression is higher than in normal tissues and correlated with prognosis. However, its prognostic role in patients with cancer is inconsistent and unclear. Thus, we reviewed published literature and conducted a meta-analysis to evaluate the association between MSI2 expression and the risk of cancer mortality and relapse. Twenty-one studies including 2640 patients were included in the meta-analysis. The results demonstrated that high MSI2 expression was significantly associated with poor prognosis, with results of poor OS (HR = 1.84, 95% CI: 1.66-2.05, P < 0.001), and poor DFS (HR = 1.73, 95% CI: 1.35-2.22, P < 0.001). In addition, the association remained significant in subgroups of OS based on cancer type, detection method, sample size and analysis method. Moreover, sensitivity analysis and publication bias showed that the results were stable and reliable. Furthermore, MSI2 positive expression was associated with certain phenotypes of tumor aggressiveness. Thus, increased MSI2 expression was associated with poor survival.
The evolutionarily conserved translation regulatory protein MSI2 is a member of the Musashi family of RBP (20). It regulates mRNA translation of many intracellular targets and maintains the properties of stem cells, thereby controlling cell proliferation and differentiation (24, 44). Thus, it is widely expressed in various tumors, and the level of expression is associated with poor prognosis of the disease (8, 39). Moreover, MSI2 was identified as a metastatic driver that supported the protein expression associated with epithelial-mesenchymal transition, including E-cadherin, the tight junction protein ZO1, the cytokine TGFβ1, the small mothers against decapentaplegic homolog 3, and the zinc finger proteins SNAI1 and SNAI2 and down-regulated expression of properties-related proteins, including claudin (claudin 3, claudin 5 and claudin 7) (16, 45). Furthermore, MSI2 plays an important role in drug resistance (11, 17, 18, 46, 47). For example, increased MSI2 expression enhances resistance to epidermal growth factor receptor tyrosine kinase inhibitors that are effective in patients with NSCLC harboring EGFR mutations (17).
Based on the fact that high expression of MSI2 can predict poor prognosis in cancer patients, and the relevant regulatory mechanisms of MSI2 in tumors, therapy targeting MSI2 may have considerable potential. In addition, small molecule inhibitors of MSI2 have been shown to be effective in vivo or in vitro. Lan et al. found that Gn, a natural inhibitor of MSI1, can similarly disrupt the binding of MSI2 to Numb RNA, like MSI1, so it is considered a dual inhibitor of MSI1 and MSI2 (48). Furthermore, the use of MSI1/MSI2 dual inhibitors such as Gn in colorectal patients with MSI overexpression can achieve better efficacy (49). In addition, Lan et al. also found that Aza-9, a derivative of secondary metabolites from Aspergillus nidulans, is a dual Msi1/2 inhibitor that can inhibit MSI2-RNA interaction in cells (50). Moreover, Aza-9-liposome inhibits proliferation, induces apoptosis and autophagy, and down-regulates Notch and Wnt signaling in colon cancer cell lines (50). Wang et al. confirmed that the small compound largazole can bind to MSI2 and may be a potential MSI2 inhibitor (51). Largazole significantly reduces MSI2 protein and mRNA levels and inhibits its downstream mammalian rapamycin signaling pathway targets (51). Largazole also inhibits proliferation and induces apoptosis in NSCLC and chronic myeloid leukemia cells (51). Thus, the MSI2 inhibitor largazole is promising as a treatment for these malignancies. Overall, the development of MSI2 inhibitors is still in the early stage, and the development of effective and highly specific MSI2 inhibitors will provide a new strategy for precise targeted therapy of tumors (8).
Although this meta-analysis comprehensively assessed the prognostic value of MSI2 expression in cancer, some limitations should be considered. First, most of the patients included in this study were from China, which to some extent affected the applicability of the results. Second, this study only included articles in Chinese and English, missing important studies published in other languages, which resulted in a certain language bias. Third, the expression level of MSI2 was not detected by a unified method, and its grouping criteria did not adopt a consistent cut-off value, which might have some effect on the results. Fourth, several HRs were extracted from the survival curves, rather than directly obtained from the article, which can cause bias.
Conclusion
In conclusion, the present meta-analysis demonstrated that high MSI2 expression was significantly associated with poor prognosis in various cancer patients. Thus, MSI2 can be used as a great biomarker for the prognosis of various cancers, and therapy targeting MSI2 is worthy of further study.
Data availability statement
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available within the article.
Author contributions
Concept and design: LJ and CZ. Literature search and extracting of data: SX, JX, JW and CZ. Analyzing and interpretation of data: XF and CZ. Drafting of the manuscript: LJ. Critical revision of the manuscript: LJ and CZ. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Taizhou People’s Hospital Medical Innovation Team Foundation (CXTDA201901) and Taizhou People’s Hospital Mandatory Project (ZL201929, ZL202020 and ZL202029).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MSI2, Musashi 2; RBP, RNA-binding protein; OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; IHC, immunohistochemistry; qRT-PCR, quantitative reverse transcription polymerase chain reaction; NOS, Newcastle-Ottawa Scale; OC, ovarian carcinoma; NSCLC, non-small cell lung cancer; CRC, colorectal cancer; CC, cervical cancer; PC, pancreatic cancer; HCC, hepatocellular carcinoma; OSCC, oral squamous cells carcinoma; ESCC, esophageal squamous cell carcinoma; ALL, acute lymphoblastic leukemia; GC, gastric carcinomas; AML, acute myeloid leukemia.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin (2019) 69(5):363–85. doi: 10.3322/caac.21565
3. Cai H, Ke ZB, Dong RN, Chen H, Lin F, Zheng WC, et al. The prognostic value of homeobox A9 (HOXA9) methylation in solid tumors: a systematic review and meta-analysis. Transl Cancer Res (2021) 10(10):4347–54. doi: 10.21037/tcr-21-765
4. Li M, Li AQ, Zhou SL, Lv H, Wei P, Yang WT. RNA-Binding protein MSI2 isoforms expression and regulation in progression of triple-negative breast cancer. J Exp Clin Cancer Res (2020) 39(1):92. doi: 10.1186/s13046-020-01587-x
5. Das CP, Baroni M, Brassesco MS, Tone LG. Interplay between the RNA binding-protein musashi and developmental signaling pathways. J Gene Med (2020) 22(1):e3136. doi: 10.1002/jgm.3136
6. Xu C, Chen X, Zhang X, Zhao D, Dou Z, Xie X, et al. RNA-Binding protein 39: a promising therapeutic target for cancer. Cell Death Discovery (2021) 7(1):214. doi: 10.1038/s41420-021-00598-7
7. Kudinov AE, Karanicolas J, Golemis EA, Boumber Y. Musashi RNA-binding proteins as cancer drivers and novel therapeutic targets. Clin Cancer Res (2017) 23(9):2143–53. doi: 10.1158/1078-0432.CCR-16-2728
8. Yang Y, Zhao M, Ding T, Ni C, Zheng Q, Li X. [Advances in research of Musashi2 in solid tumors]. Nan Fang Yi Ke Da Xue Xue Bao (2022) 42(3):448–56. doi: 10.12122/j.issn.1673-4254.2022.03.20
9. Sheng W, Dong M, Chen C, Li Y, Liu Q, Dong Q. Musashi2 promotes the development and progression of pancreatic cancer by down-regulating numb protein. Oncotarget (2017) 8(9):14359–73. doi: 10.18632/oncotarget.8736
10. Wang S, Li N, Yousefi M, Nakauka-Ddamba A, Li F, Parada K, et al. Transformation of the intestinal epithelium by the MSI2 RNA-binding protein. Nat Commun (2015) 6:6517. doi: 10.1038/ncomms7517
11. Jiang X, Tan J, Wen Y, Liu W, Wu S, Wang L, et al. MSI2-TGF-beta/TGF-beta R1/SMAD3 positive feedback regulation in glioblastoma. Cancer Chemother Pharmacol (2019) 84(2):415–25. doi: 10.1007/s00280-019-03892-5
12. Meulmeester E, Ten DP. The dynamic roles of TGF-beta in cancer. J Pathol (2011) 223(2):205–18. doi: 10.1002/path.2785
13. Sun J, Sheng W, Ma Y, Dong M. Potential role of musashi-2 RNA-binding protein in cancer EMT. Onco Targets Ther (2021) 14:1969–80. doi: 10.2147/OTT.S298438
14. Yang K, Guo W, Ren T, Huang Y, Han Y, Zhang H, et al. Knockdown of HMGA2 regulates the level of autophagy via interactions between MSI2 and Beclin1 to inhibit NF1-associated malignant peripheral nerve sheath tumour growth. J Exp Clin Cancer Res (2019) 38(1):185. doi: 10.1186/s13046-019-1183-2
15. Veronez LC, Das CP, Correa C, Baroni M, Da SK, Nagano LF, et al. MSI2 expression in adrenocortical carcinoma: Association with unfavorable prognosis and correlation with steroid and immune-related pathways. J Cell Biochem (2021) 122(12):1925–35. doi: 10.1002/jcb.30153
16. Kharin L, Bychkov I, Karnaukhov N, Voloshin M, Fazliyeva R, Deneka A, et al. Prognostic role and biologic features of musashi-2 expression in colon polyps and during colorectal cancer progression. PloS One (2021) 16(7):e252132. doi: 10.1371/journal.pone.0252132
17. Yiming R, Takeuchi Y, Nishimura T, Li M, Wang Y, Meguro-Horike M, et al. MUSASHI-2 confers resistance to third-generation EGFR-tyrosine kinase inhibitor osimertinib in lung adenocarcinoma. Cancer Sci (2021) 112(9):3810–21. doi: 10.1111/cas.15036
18. Yang H, Hu J, Chen J, Chen Z, Jiao F, Cui J, et al. RNA-Binding protein Musashi2 regulates hippo signaling via SAV1 and MOB1 in pancreatic cancer. Med Oncol (2020) 37(9):84. doi: 10.1007/s12032-020-01384-8
19. Zhen J, Liu Y, Song C, Wu S, Jiao R. Expression of MSI2 protein in ovarian carcinoma and its correlation with clinicopathological factors. Chin J Clin Exp Pathology (2022) 38(1):40–5. doi: 10.13315/j.cnki.cjcep.2022.01.008
20. Topchu I, Karnaukhov N, Mazitova A, Yugai V, Voloshin M, Tikhomirova M, et al. Musashi 2 (MSI2) expression as an independent prognostic biomarker in non-small cell lung cancer (NSCLC). J Thorac Dis (2021) 13(3):1370–9. doi: 10.21037/jtd-20-2787
21. Li Y, Zhang D, Qian Y, Fu Y, Yang B, Liu C. Expression of PEA15 and MSI-2 in colorectal cancer tissues and its relationship with liver metastasis and prognosis. Chin J Difficult Complicated Cases (2021) 20(9):924–928, 947. doi: 10.3969/j.issn.1671-6450.2021.09.013
22. Zhen J, Liu Y, Song C, Wu S, Bi X. The relationship between the expression of RNA-binding protein Musashi2 and cervical squamous-cell carcinoma. Tianjin Med J (2021) 49(8):842–6. doi: 10.11958/20210292
23. Zhou L, Sheng W, Jia C, Shi X, Cao R, Wang G, et al. Musashi2 promotes the progression of pancreatic cancer through a novel ISYNA1-p21/ZEB-1 pathway. J Cell Mol Med (2020) 24(18):10560–72. doi: 10.1111/jcmm.15676
24. Wang X, Wang R, Bai S, Xiong S, Li Y, Liu M, et al. Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway. J Exp Clin Cancer Res (2019) 38(1):505. doi: 10.1186/s13046-019-1508-1
25. Liu Y, Fan Y, Wang X, Huang Z, Shi K, Zhou B. Musashi-2 is a prognostic marker for the survival of patients with cervical cancer. Oncol Lett (2018) 15(4):5425–32. doi: 10.3892/ol.2018.8077
26. Sheng W, Dong M, Wang Z, Zhou J, Li Y, Kong F, et al. Clinicopathological significance of musashi 2 expression in human colorectal cancer. Chin J Gen Surgery (2017) 32(9):783–6. doi: 10.3760/cma.j.issn.1007-631X.2017.09.018
27. Li Z, Jin H, Mao G, Wu L, Guo Q. Msi2 plays a carcinogenic role in esophageal squamous cell carcinoma via regulation of the wnt/beta-catenin and hedgehog signaling pathways[J]. Exp Cell Res (2017) 361(1):170–7. doi: 10.1016/j.yexcr.2017.10.016
28. Zong Z, Zhou T, Rao L, Jiang Z, Li Y, Hou Z, et al. Musashi2 as a novel predictive biomarker for liver metastasis and poor prognosis in colorectal cancer. Cancer Med (2016) 5(4):623–30. doi: 10.1002/cam4.624
29. Zhao HZ, Jia M, Luo ZB, Cheng YP, Xu XJ, Zhang JY, et al. Prognostic significance of the musashi-2 (MSI2) gene in childhood acute lymphoblastic leukemia. Neoplasma (2016) 63(1):150–7. doi: 10.4149/neo_2016_018
30. Yang Z, Guo X, Shi Y, Li C, Li L. Expression and clinical significance of MSI2 in gastric cancer. Chin J Bases Clinics Gen Surgery (2016) 23(1):52–5. doi: 10.7507/1007-9424.20160014
31. Aly RM, Ghazy HF. Prognostic significance of MSI2 predicts unfavorable outcome in adult b-acute lymphoblastic leukemia. Int J Lab Hematol (2015) 37(2):272–8. doi: 10.1111/ijlh.12284
32. Lu Y, Yu M, Mu Q, Pei R, Wang Q, Chen Z, et al. Expression of Musashi2 gene in de novo acute myeloid leukemia and its clinical implications. Chin J Med Genet (2014) 31(6):713–8. doi: 10.3760/cma.j.issn.1003-9406.2014.06.007
33. He L, Zhou X, Qu C, Hu L, Tang Y, Zhang Q, et al. Musashi2 predicts poor prognosis and invasion in hepatocellular carcinoma by driving epithelial-mesenchymal transition. J Cell Mol Med (2014) 18(1):49–58. doi: 10.1111/jcmm.12158
34. Gao Z, Guo K, Song S. Clinicopathological significance of MSI2 expression in human pancreatic cancer. Chin J Pancreatology (2014) 14(6):392–5. doi: 10.3760/cma.j.issn.1674-1935.2014.06.008
35. Thol F, Winschel C, Sonntag AK, Damm F, Wagner K, Chaturvedi A, et al. Prognostic significance of expression levels of stem cell regulators MSI2 and NUMB in acute myeloid leukemia. Ann Hematol (2013) 92(3):315–23. doi: 10.1007/s00277-012-1637-5
36. Mu Q, Wang Y, Chen B, Qian W, Meng H, Tong H, et al. High expression of musashi-2 indicates poor prognosis in adult b-cell acute lymphoblastic leukemia. Leuk Res (2013) 37(8):922–7. doi: 10.1016/j.leukres.2013.05.012
37. Byers RJ, Currie T, Tholouli E, Rodig SJ, Kutok JL. MSI2 protein expression predicts unfavorable outcome in acute myeloid leukemia. Blood (2011) 118(10):2857–67. doi: 10.1182/blood-2011-04-346767
38. Palacios F, Yan XJ, Ferrer G, Chen SS, Vergani S, Yang X, et al. Musashi 2 influences chronic lymphocytic leukemia cell survival and growth making it a potential therapeutic target. Leukemia (2021) 35(4):1037–52. doi: 10.1038/s41375-020-01115-y
39. Kharas MG, Lengner CJ, Al-Shahrour F, Bullinger L, Ball B, Zaidi S, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med (2010) 16(8):903–8. doi: 10.1038/nm.2187
40. Troiano G, Caponio V, Botti G, Aquino G, Losito NS, Pedicillo MC, et al. Immunohistochemical analysis revealed a correlation between musashi-2 and cyclin-D1 expression in patients with oral squamous cells carcinoma. Int J Mol Sci (2019) 21(1):121. doi: 10.3390/ijms21010121
41. Kang MH, Jeong KJ, Kim WY, Lee HJ, Gong G, Suh N, et al. Musashi RNA-binding protein 2 regulates estrogen receptor 1 function in breast cancer. Oncogene (2017) 36(12):1745–52. doi: 10.1038/onc.2016.327
42. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
43. Guo J, Min K, Deng L. Potential value of tripartite motif-containing 59 as a biomarker for predicting the prognosis of patients with lung cancer: A protocol for systematic review and meta-analysis. Med (Baltimore) (2021) 100(32):e26868. doi: 10.1097/MD.0000000000026868
44. Sakakibara S, Nakamura Y, Yoshida T, Shibata S, Koike M, Takano H, et al. RNA-Binding protein musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci U S A (2002) 99(23):15194–9. doi: 10.1073/pnas.232087499
45. Kudinov AE, Deneka A, Nikonova AS, Beck TN, Ahn YH, Liu X, et al. Musashi-2 (MSI2) supports TGF-beta signaling and inhibits claudins to promote non-small cell lung cancer (NSCLC) metastasis[J]. Proc Natl Acad Sci U S A (2016) 113(25):6955–60. doi: 10.1073/pnas.1513616113
46. Sheng W, Dong M, Chen C, Wang Z, Li Y, Wang K, et al. Cooperation of musashi-2, numb, MDM2, and P53 in drug resistance and malignant biology of pancreatic cancer. FASEB J (2017) 31(6):2429–38. doi: 10.1096/fj.201601240R
47. Fang T, Lv H, Wu F, Wang C, Li T, Lv G, et al. Musashi 2 contributes to the stemness and chemoresistance of liver cancer stem cells via LIN28A activation. Cancer Lett (2017) 384:50–9. doi: 10.1016/j.canlet.2016.10.007
48. Lan L, Liu H, Smith AR, Appelman C, Yu J, Larsen S, et al. Natural product derivative gossypolone inhibits musashi family of RNA-binding proteins. BMC Cancer (2018) 18(1):809. doi: 10.1186/s12885-018-4704-z
49. Li N, Yousefi M, Nakauka-Ddamba A, Li F, Vandivier L, Parada K, et al. The msi family of RNA-binding proteins function redundantly as intestinal oncoproteins. Cell Rep (2015) 13(11):2440–55. doi: 10.1016/j.celrep.2015.11.022
50. Lan L, Liu J, Xing M, Smith AR, Wang J, Wu X, et al. Identification and validation of an aspergillus nidulans secondary metabolite derivative as an inhibitor of the musashi-RNA interaction. Cancers (Basel) (2020) 12(8):2221. doi: 10.3390/cancers12082221
Keywords: MSI2, meta-analysis, prognosis, clinicopathological features, cancer
Citation: Jiang L, Xue S, Xu J, Fu X, Wei J and Zhang C (2022) Prognostic value of Musashi 2 (MSI2) in cancer patients: A systematic review and meta-analysis. Front. Oncol. 12:969632. doi: 10.3389/fonc.2022.969632
Received: 21 June 2022; Accepted: 02 November 2022;
Published: 01 December 2022.
Edited by:
Zhengming Chen, Cornell University, United StatesReviewed by:
Melanie C. MacNicol, University of Arkansas for Medical Sciences, United StatesShilpita Karmakar, JAX Cancer Center, United States
Copyright © 2022 Jiang, Xue, Xu, Fu, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanshan Xue, MjQ1MTE2OTk5QHFxLmNvbQ==; Chuanmeng Zhang, dHp5eXpjbUBuam11LmVkdS5jbg==
 Lin Jiang1
Lin Jiang1 Chuanmeng Zhang
Chuanmeng Zhang