
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 12 December 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.969135
This article is part of the Research Topic The Management of Hematologic Malignancies in Lower-Income Countries View all 5 articles
 Matthew S. Painschab1,2*
Matthew S. Painschab1,2* Marriam Mponda1
Marriam Mponda1 Tamiwe Tomoka1
Tamiwe Tomoka1 Coxcilly Kampani1
Coxcilly Kampani1 Fred Chimzimu1
Fred Chimzimu1 Yuri Fedoriw1,2
Yuri Fedoriw1,2 Satish Gopal3
Satish Gopal3Introduction: Multicentric Castleman disease (MCD) is a lymphoproliferative disorder characterized by systemic inflammation, lymphadenopathy, and cytopenias. MCD caused by Kaposi sarcoma herpesvirus (MCD-KSHV) frequently arises in the context of HIV. It can be associated with immune reconstitution inflammatory syndrome (IRIS), but MCD-IRIS is rarely reported in sub-Saharan Africa (SSA) where HIV and KSHV infection are common.
Case description: A 36-year-old woman in Malawi with HIV on antiretroviral therapy (ART) for nine years presented with fatigue, weight loss, and lymphadenopathy. Lymph node biopsy was consistent with HIV lymphadenitis without evident KSHV-MCD and HIV RNA was 4,244 copies/mL. She switched to second-line ART and returned four months later with worsening lymphadenopathy, fever, night sweats, weight loss, and anemia. A repeat lymph node biopsy demonstrated unequivocal KSHV-MCD features not present on the original biopsy. Her repeat HIV viral load was undetectable and she received chemotherapy with subsequent remission on continued ART for 24 months.
Discussion: This is among the first reported cases of MCD-IRIS from SSA, which has implications for a region where HIV and KSHV are highly prevalent. MCD-IRIS may contribute to early mortality after ART initiation in SSA, and increased awareness alongside improved diagnostic and treatment capacity are needed.
Multicentric Castleman disease (MCD) is a lymphoproliferative disorder characterized by systemic inflammation, lymphadenopathy, anemia and thrombocytopenia (1, 2). MCD is defined by histopathologic findings but contains a spectrum of disorders, including the subtype caused by Kaposi sarcoma herpesvirus (KSHV) which primarily occurs in HIV-infected individuals (3). In immunocompetent hosts, primary KSHV infection is followed by rapid viral suppression similar to other herpesviruses. However, in immunosuppressed individuals, chronic viral reactivation can lead to Kaposi sarcoma (KS), KSHV-MCD, or primary effusion lymphoma (3, 4). KSHV seroprevalence varies widely but is highest in sub-Saharan Africa (SSA) with prevalence 40% in many countries, compared to 10% in the United States and Europe (4, 5). Our group in Malawi has previously described clinical features and outcomes for a large prospective cohort of MCD patients (6–8). Based on our experience, MCD appears to be quite common in Malawi, comprising 15% of lymphoproliferative disorders among persons living with HIV (PLWH) and 7% of all lymphoproliferative disorders, suggesting MCD may be substantially underdiagnosed in other SSA settings.
Immune reconstitution inflammatory syndrome (IRIS) is a syndrome characterized by paradoxical worsening or unmasking of any neoplastic, infectious, or inflammatory disease after initiation of antiretroviral therapy (ART). Though IRIS definitions vary, all contain some combination of a decrease in HIV viral load and worsening disease in the first few months after starting ART (9–12). A significant proportion of early deaths in patients starting ART for HIV are attributed to IRIS, especially in patients with low CD4 count (9). Although IRIS has been well described, including in SSA, reports of MCD-IRIS are rare. Here we describe in detail a patient with MCD-IRIS diagnosed and treated in Malawi.
A 36-year-old Malawian woman with HIV diagnosed nine years prior, on ART (tenofovir, lamivudine, and efaverinz), presented with a one-year history of malaise, fatigue, subjective fever, and weight loss. She had no other relevant family, medical, or social history. Over the previous eight months, she had developed progressive cervical and axillary lymphadenopathy. The largest lymph node was two cm in diameter. There was no evidence of KS on examination. Laboratory examination was significant for hemoglobin 11.4 g/dL, platelets 157 x 103/uL, HIV RNA 4,244 copies/mL and CD4 count 62 cells/mm3 (Table 1). A chest x-ray and abdominal ultrasound were normal. An excisional lymph node biopsy (Figure 1) showed non-specific histologic features compatible with HIV-lymphadenopathy, with generally uniform geminal centers and prominent paracortical hyperplasia. Scattered, small lymphoid cells showed expression of latency-associated nuclear antigen-1 (LANA-1), but neither histology nor staining pattern were typical of KSHV-MCD and plasma KSHV DNA was detectable but 500 copies/mL. Similarly, there was no evidence of lymphoma or KS. Diagnostic challenges included lack of access to advanced imaging such as CT scan and PET scan as well as access to only a limited immunohistochemical antibody profile. Given documented ART virologic failure, the patient was switched to second-line ART (zidovudine, lamivudine, atazanavir/ritonavir).
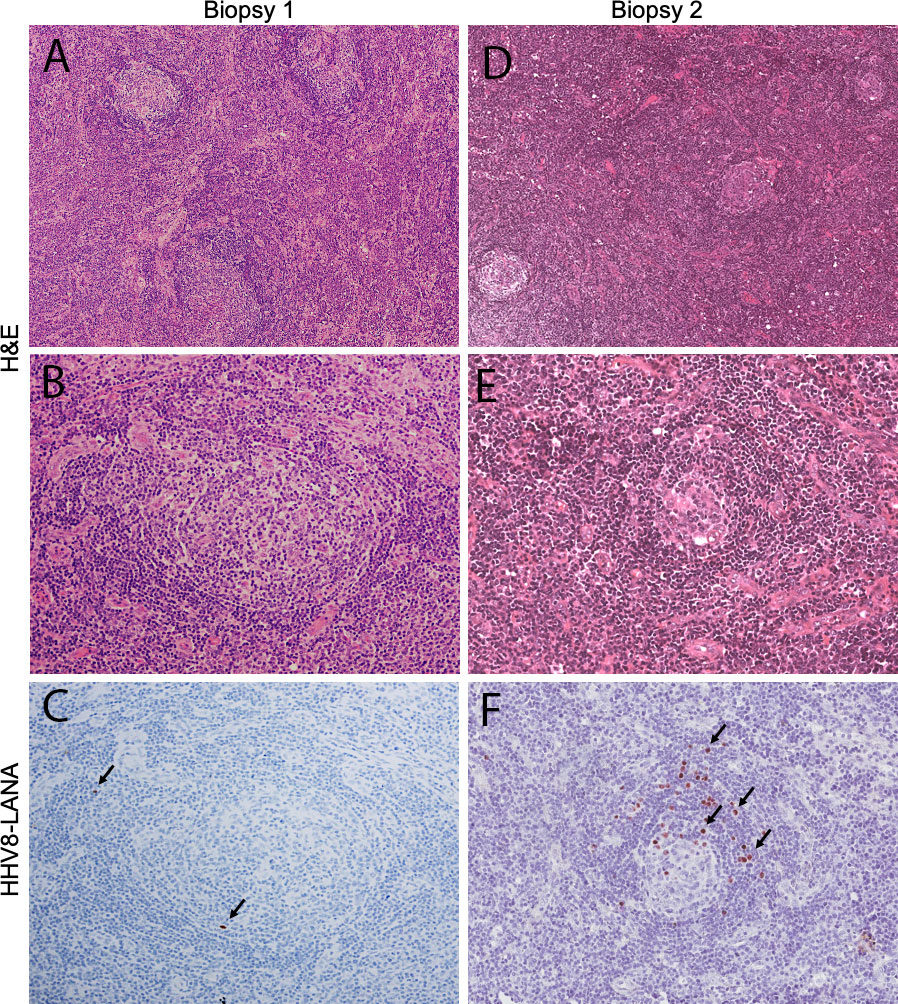
Figure 1 Histologic features of tissue biopsies. The first biopsy (left column) shows morphologic features that are expected on the spectrum of HIV lymphadenopathy, with generally uniform appearing germinal centers and paracortical hyperplasia (A; objective magnification x4), The cellular architecture of the germinal centers is preserved (B; objective magnification x10) and only rare, small lymphocytes are HHV8-LANA-positive (C; arrows; objective magnification x10). In contrast the second biopsy (right column) demonstrates small germinal centers (D; objective magnification x4), showing stromal hyalinizaiton and decreased cellularity (E; objective magnification x10). Numerous HHV8-LANA-positive plasmablasts rim the atretic follicles (F; arrows; objective magnification x10), diagnostic of MCD.
Four months later, she returned with worsening diffuse lymphadenopathy, fatigue and myalgias. Her body weight had decreased from 60.4 kg to 56.5 kg. Her hemoglobin had decreased to 8.7 g/dL and her platelet count was 189 x 103/uL (Table 1). Her HIV viral load was now undetectable. Repeat chest x-ray and abdominal ultrasound were normal. A differential diagnosis of IRIS secondary to tuberculosis, disseminated fungal infection, or lymphoma was considered. We also considered Kaposi sarcoma inflammatory cytokine syndrome (KICS), an inflammatory syndrome resembling MCD driven by KSHV, but KICS would require an absence of other pathology on lymph node biopsy. A repeat excisional biopsy (Figure 1) showed features strongly consistent with KSHV-MCD including variably involuted germinal centers rimmed by LANA-1 positive plasmablasts and did not demonstrate tuberculosis, fungal infection, or other lymphoma.
The patient was diagnosed with MCD-IRIS and treated with eight cycles of cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2, and prednisone 60 mg/m2, every three weeks. She had no serious adverse events during treatment and tolerated treatment well. After eight cycles, lymphadenopathy and systemic symptoms completely resolved, weight increased to 63.0 kg, hemoglobin increased to 12.0 g/dL, platelets increased to 233 x 103/uL, HIV viral load remained undetectable, and CD4 count increased to 191 cells/uL (Table 1). KSHV viral load was undectable after chemotherapy. The patient has subsequently remained in remission for 24 months.
After informed consent, the patient was enrolled in the Kamuzu Central Hospital Lymphoma Study (NCT02835911) prospective cohort described previously (6). All diagnoses were pathologically confirmed by histology and immunohistochemistry supported by real-time telepathology between pathologists in Malawi and the United States.
We describe MCD-IRIS occurring after second-line ART in Malawi, a phenomenon which has been infrequently reported in SSA. Strengths of this case study are the prospective, comprehensive patient evaluation and follow-up available. Weaknesses include lack of access to diagnosic testing including real time KSHV viral load testing. Aspects of this case have broader implications for the control of KSHV-associated diseases in SSA.
First, KSHV-MCD may become more and more common across SSA as ART access improves. KS tends to develop in patients with severe immunosuppression characterized by low CD4 T-cell counts and poorly controlled HIV, or T-cell dysfunction from immunosuppressive medications in solid organ transplant recipients. However, KSHV-MCD tends to present in patients with HIV on ART for many years, with well-preserved CD4 counts, and some studies have suggested increasing MCD incidence among HIV-infected individuals with increasing access and earlier application of ART (4, 13, 14).
Next, to our knowledge, this is among the first documented cases of MCD-IRIS from SSA (15–18) and adds to an emerging literature related to KSHV-MCD in the region (6, 19). Given the frequency of HIV and KSHV infection throughout SSA, our report raises the possibility that MCD-IRIS may contribute to continued excess early mortality after ART initiation, including for patients without clinically evident KSHV-associated disease manifestations before ART initiation. For example, a study in Botswana found 28% mortality and 9% lost to follow-up in the first year after ART initiation (20). These deaths likely have multifactorial causes but 12% were attributed to anemia/pancytopenia and an additional 29% of deaths were from “unknown cause.” Our report suggests that some early deaths may be attributable to unrecognized MCD-IRIS or KICS, a closely related disease characterized by cytokine storm in the presence of KS but without pathologic findings of MCD (21). Supporting this hypothesis, a recent study found a significantly higher risk of mortality from KS-IRIS among KS patients in South Africa compared to the United Kingdom with risk being especially high among those with an elevated blood KSHV load (11, 22). KSHV-MCD and KICS are likely not diagnosed in many SSA settings given protean systemic manifestations with more limited access to lymph node biopsy and LANA immunohistochemistry. More information is needed on the early causes of death after ART in SSA in order to develop comprehensive evidence-based strategies to address the early death rate.
Finally, MCD may emerge as an important complication in the use of checkpoint inhibitor immunotherapy for cancer in PLWH. Recent development and clinical testing of immune checkpoint inhibitors have led to numerous indications in high-income countries (23). Access to such agents in SSA remains extremely limited, and registrational trials in high-income countries have typically excluded patients with HIV and/or been conducted in settings where KSHV prevalence is low (24). However, despite the small number of PLWH who have been treated on clinical trials, MCD has been reported after checkpoint inhibitors in patients with HIV-associated KS, including a patient who died of this complication (25). In SSA where KSHV infection is often co-occurring with HIV, MCD may therefore be observed as an important treatment-related toxicity for cancer patients receiving immune checkpoint inhibitor therapy as access to these agents increases. This highlights the continued need for rigorous clinical evaluation of anticancer medicines when applied in previously unstudied populations and contexts, even when these agents have received regulatory approvals in the United States or Europe.
In summary, with increasing access to ART across SSA, increased awareness of MCD is needed, including as a possible IRIS manifestation after ART initiation. Expanded diagnostic and treatment capacity are also needed to mitigate this potentially unrecognized and emerging contributor to morbidity and mortality in several distinct patient populations in SSA.
“When I was diagnosed with cancer almost at the same time I was diagnosed with HIV, I just accepted the situation and I did not have any worries. Doctors explained to me about my situation and I was ready to start treatment. During my course of treatment with ARVs and cancer medications, I was experiencing different symptoms like weight loss, dizziness and loss of appetite. Along the way my ARVs got switched to a new regimen because I was having some blood derangements. Other than these, there were no general things affecting my life.
After finishing cancer treatment, everything got improved. I no longer experience the above symptoms. I am able to do house chores except that sometimes I feel short of breath after walking a long distance. In general, my life and health got improved greatly after receiving chemotherapy. I would like to encourage others that if they get diagnosed with this type of cancer they should not be worried because it can get cured.
Lastly, I would like to thank you doctors to continue helping other patients the same way you helped me so that they can also be cured and be happy as I am.”
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Malawi National Health Sciences Research Committee University of North Carolina Institutional Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MP conceived and designed the study, collected data, and wrote the paper. MM, YF, CK, FC, and TT collected data. SG conceived and designed the study. All authors approved the final version of the manuscript.
This study was supported by funds from Lineberger Comprehensive Cancer Center, University of North Carolina Institute for Global Health and Infectious Diseases, and the National Institutes of Health (K01TW011470, U54CA25464, and U54CA190152).
We appreciate the assistance of Dr. Patricio Cano and Dr. Dirk Dittmer, both of UNCH Lineberger Comprehensive Cancer Center, for KSHV viral load testing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study reflects work done while Dr. SG was employed at the University of North Carolina at Chapel Hill. The opinions expressed in this article are the authors own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fajgenbaum DC, Shilling D. Castleman disease pathogenesis. Hematol Oncol Clin North Am (2018) 32(1):11–21. doi: 10.1016/j.hoc.2017.09.002
2. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer (1956) 9(4):822–30. doi: 10.1002/1097-0142(195607/08)9:4<822::AID-CNCR2820090430>3.0.CO;2-4
3. Oksenhendler E, Boutboul D, Galicier L. Kaposi sarcoma-associated herpesvirus/human herpesvirus 8-associated lymphoproliferative disorder. Blood (2019) 133(11):1186–90. doi: 10.1182/blood-2018-11-852442
4. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers (2019) 5(1):9. doi: 10.1038/s41572-019-0060-9
5. Etta EM, Alayande DP, Mavhandu-Ramarumo LG, Gachara G, Bessong PO. HHV-8 seroprevalence and genotype distribution in Africa, 1998(-)2017: A systematic review. Viruses (2018) 10(9):458. doi: 10.3390/v10090458
6. Tomoka T, Painschab MS, Montgomery ND, Seguin R, Mulenga M, Kaimila B, et al. A prospective description of HIV-associated multicentric castleman disease in Malawi. Haematologica (2018). doi: 10.3324/haematol.2018.204479
7. Montgomery ND, Liomba NG, Kampani C, Krysiak R, Stanley CC, Tomoka T, et al. Accurate real-time diagnosis of lymphoproliferative disorders in Malawi through clinicopathologic teleconferences: A model for pathology services in Sub-Saharan Africa. Am J Clin Pathol (2016) 146(4):423–30. doi: 10.1093/ajcp/aqw118
8. Gopal S, Liomba NG, Montgomery ND, Moses A, Kaimila B, Nyasosela R, et al. Characteristics and survival for HIV-associated multicentric castleman disease in Malawi. J Int AIDS Soc (2015) 18:20122. doi: 10.7448/IAS.18.1.20122
9. Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect Dis (2010) 2010(10):251–61. doi: 10.1016/S1473-3099(10)70026-8
10. Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in south Africa: a prospective study. AIDS (2008) 22:601–10. doi: 10.1097/QAD.0b013e3282f4a607
11. Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, et al. Immune reconstitution inflammatory syndrome associated with kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS (2013) 27(10):1603–13. doi: 10.1097/QAD.0b013e328360a5a1
12. Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune reconstitution inflammatory syndrome associated with kaposi's sarcoma. J Clin Oncol (2005) 23(22):5224–8. doi: 10.1200/JCO.2005.14.597
13. Bower M, Newsom-Davis T, Naresh K, Merchant S, Lee B, Gazzard B, et al. Clinical features and outcome in HIV-associated multicentric castleman's disease. J Clin Oncol (2011) 29(18):2481–6. doi: 10.1200/JCO.2010.34.1909
14. Powles T, Stebbing J, Bazeos A, Hatzimichael E, Mandalia S, Nelson M, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric castleman's disease. Ann Oncol (2009) 20(4):775–9. doi: 10.1093/annonc/mdn697
15. Micallef D, Attard J, Mallia-Azzopardi C, Boffa MJ. Lymphadenopathy after initiating HAART in an HIV-positive patient with kaposi sarcoma: a case of multicentric castleman disease. Eur J Case Rep Internal Med (2015). doi: 10.12890/2015_000251
16. Siegel MO, Ghafouri S, Ajmera R, Simon GL. Immune reconstitution inflammatory syndrome, human herpesvirus 8 viremia, and HIV-associated multicentric castleman disease. Int J Infect Dis (2016) 48:49–51. doi: 10.1016/j.ijid.2016.05.005
17. Zietz C, Bogner JR, Goebel FD, Lohrs U. An unusual cluster of cases of castleman’s disease during highly active antiretroviral therapy for AIDS. N Engl J Med (1999) 340(24):1923–4. doi: 10.1056/NEJM199906173402415
18. Kurukumbi M, Steiner S, Dunlap S, Chapman S, Solieman N, Jayam-Trouth A. A rare case of immune reconstitution inflammatory syndrome development in an immunocompromised patient with progressive multifocal leukoencephalopathy and multicentric castleman's disease. Case Rep Neurol Med (2013) 2013:460701. doi: 10.1155/2013/460701
19. Patel M, Philip V, Lakha A, et al. Multicentric castleman’s disease. Immunopathol Immunomodulation (2015). doi: 10.5772/61709
20. Steele KT, Steenhoff AP, Newcomb CW, Rantleru T, Nthobatsang R, Lesetedi G, et al. Early mortality and AIDS progression despite high initial antiretroviral therapy adherence and virologic suppression in Botswana. PloS One (2011) 6(6):e20010. doi: 10.1371/journal.pone.0020010
21. Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Marshall V, Wang V, et al. Clinical features and outcomes of patients with symptomatic kaposi sarcoma herpesvirus (KSHV)-associated inflammation: Prospective characterization of KSHV inflammatory cytokine syndrome (KICS). Clin Infect Dis (2016) 62(6):730–8. doi: 10.1093/cid/civ996
22. Mezger NCS, Feuchtner J, Griesel M, Hammerl L, Seraphin TP, Zietsman A, et al. Clinical presentation and diagnosis of adult patients with non-Hodgkin lymphoma in Sub-Saharan Africa. Br J Haematol (2020) 190(2):209–21. doi: 10.1111/bjh.16575
23. Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol (2016) 21(3):462–73. doi: 10.1007/s10147-016-0959-z
24. Patel A, Goldstein DA, Tannock IF. Improving access to immunotherapy in low- and middle-income countries. Ann Oncol (2022) 33(4):360–1. doi: 10.1016/j.annonc.2022.01.003
Keywords: multicentric castleman disease, HIV, IRIS, KSHV, malawi, africa
Citation: Painschab MS, Mponda M, Tomoka T, Kampani C, Chimzimu F, Fedoriw Y and Gopal S (2022) Case report: Multicentric Castleman disease as a manifestation of immune reconstitution inflammatory syndrome in Malawi. Front. Oncol. 12:969135. doi: 10.3389/fonc.2022.969135
Received: 14 June 2022; Accepted: 24 November 2022;
Published: 12 December 2022.
Edited by:
Sung-Hsin Kuo, National Taiwan University, TaiwanReviewed by:
Sonikpreet Aulakh, West Virginia University, United StatesCopyright © 2022 Painschab, Mponda, Tomoka, Kampani, Chimzimu, Fedoriw and Gopal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew S. Painschab, cGFpbnNjaGFibUBtZWQudW5jLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.