
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 July 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.964115
This article is part of the Research Topic Clinicopathological Factors and Staging in Gastrointestinal Cancers View all 74 articles
Objectives: There is an urgent need for biomarkers that predict the survival outcome of patients diagnosed with metastatic pancreatic cancer, undergoing systemic chemotherapy. This study aimed to identify biomarkers associated with the survival of mPC patients treated with modified FOLFIRINOX (mFOLFIRINOX) as first-line chemotherapy.
Methods: This was a retrospective study of 30 patients with mPC who received mFOLFIRINOX between October 2018 and March 2021. Data on carcinoembryonic antigen (CEA), cancer antigen (CA)199, interleukin (IL)-6, C-reactive protein (CRP), neutrophils, platelets, lymphocytes, and albumin were collected and dichotomized using the upper or lower limit, as appropriate. These markers were examined for their association with progression-free survival (PFS). A receiver operating characteristic (ROC) curve analysis was used to explore a suitable model to predict mFOLFIRINOX effectiveness.
Results: IL-6 and CRP levels were associated with poor progression (P = 0.004 and P = <0.001, respectively) of mPC. The high IL-6 level was an independent poor prognostic factor for PFS (HR=4.66, 95%CI: 1.32-16.37, P=0.016) in the multivariable analysis. Patients with high IL-6 levels had a shorter PFS than those with low IL-6 levels (median PFS: 257 vs. 150 days, P=0.020). An increase in IL-6 and CRP levels during chemotherapy positively correlated with disease progression (P = <0.001 for both). The model combining IL-6 with CRP levels helped predict the outcomes of mPC patients treated with mFOLFIRINOX (AUC: 0.811, 95%CI: 0.639-0.983, P=0.003).
Conclusions: The serum levels of IL-6 and CRP might be considered as valuable biomarkers in predicting the outcomes of patients with mPC who received the mFOLFIRINOX regimen.
Pancreatic cancer (PC) is a very lethal malignancy, with an incidence rate nearly equal to its mortality rate (1). The situation is even worse in China, where 83,600 new PC cases were reported in 2017, and an estimated 85,100 patients died (2). This was because most patients were not diagnosed timely, and 80%–90% of them were in an advanced stage at diagnosis and were unable to receive curative resection (3). The reported 5-year survival rate of PC is only 8% (1).
Systemic chemotherapy is the standard of care for patients with metastatic PC (mPC). The FOLFIRINOX regimen [oxaliplatin, irinotecan, leucovorin, and 5-fluorouracil (5-FU)] significantly improves overall survival (OS) compared with gemcitabine alone in patients with mPC, but it is associated with higher toxicity (4). The modified FOFIRINOX (mFOLFIRINOX) regimen was, therefore, suggested with the intention of reducing toxicity while preserving efficacy (5–7). It was also proven to be well tolerated and effective in Chinese patients (7). The overall response rate (ORR) of mFOLFIRINOX was reported to be 30%–53.5% (5–7). Although the TNM stage and serum carbohydrate antigen 199 (CA199) levels have been demonstrated to be associated with survival, their value in predicting the efficacy of mFOLFIRINOX is low (8, 9). Therefore, biomarkers that can effectively identify patients with mPC who will benefit from mFOLFIRINOX are urgently needed to improve patient management.
Inflammation predisposes individuals to the initiation, growth, progression, and metastatic spread of cancer (10). Inflammatory markers are associated with the prognosis of PC (11–19). One study showed that elevated serum C-reactive protein (CRP) levels were significantly associated with poor clinical outcomes in patients with PC (11), while another study found that the CRP-to-albumin ratio could be a significant and promising inflammatory prognostic score (12). In addition, many studies suggested that the changes in interleukin (IL) expression were associated with poor prognosis in advanced PC (APC) (13, 14). Furthermore, high CRP and IL-6 levels were associated with a poor response in patients with PC receiving first-line gemcitabine monotherapy (15, 16). Higher levels of tumor-infiltrating neutrophils were significantly associated with shorter survival, whereas higher levels of tumor-infiltrating lymphocytes, as well as low levels of platelets (PLT), were described as being positively correlated with extended OS and progression-free survival (PFS) in PC (17–19).
Therefore, it was hypothesized that inflammatory markers, such as CRP, ILs, tumor-infiltrating neutrophils and lymphocytes, and platelets, might be potential indicators for predicting the response of patients with mPC to mFOLFIRINOX. This study aimed to investigate the impact of these inflammatory markers on the outcomes of first-line mFOLFIRINOX chemotherapy in patients with mPC. The results of this study could help improve the management of mPC by identifying the patients who might benefit the most from mFOLFIRINOX.
This was a retrospective observational single-center study that included patients treated with mFOLFIRINOX at Shanghai General Hospital between October 2018 and March 2021. All patients included had been diagnosed with mPC by pathological and imaging examinations and had received mFOLFIRINOX as first-line chemotherapy. The patients who had clinical evidence of infection or another inflammatory disease during treatment were excluded (Figure 1). The study was approved by the ethics board of Shanghai General Hospital. The requirement for informed consent was waived by the committee due to the retrospective nature of the study.
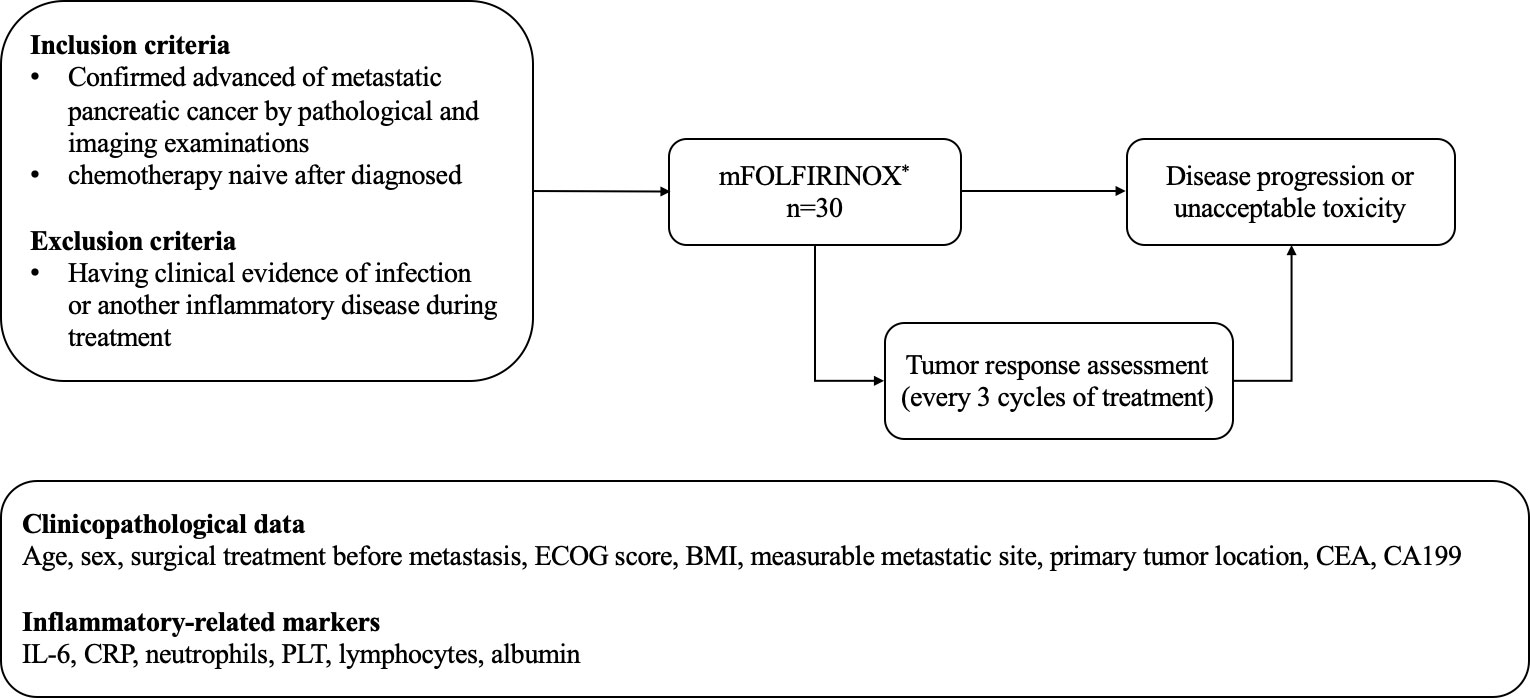
Figure 1 The mFOLFIRINOX regimen: oxaliplatin 68 mg/m2 given as a 2-h intravenous infusion, then leycovorin 400 mg/m2 delivered as a 2-h intravenous infusion, qnd finally 5-FU 2400 mg/m2 given as a continous intravenous infusion over a 46-h period. The regimen had beeb administered on day 1 and the repeatedevery 2 weeks until disease progression or patient refusal. ECOG, Easteran Cooperative Oncology Group; BMI, body mass indez; CEA, carcinoembryonic antigen; CA199, cancerantigen199; IL-6 interleukin-6; CRP, C-reactive protein; PLT, platelets.
The mFOLFIRINOX regimen (oxaliplatin 68 mg/m2 given as a 2-h intravenous infusion, immediately followed by irinotecan 135 mg/m2 given as a 90-min intravenous infusion, then leucovorin 400 mg/m2 delivered as a 2-h intravenous infusion, and finally 5-FU 2400 mg/m2 given as a continuous intravenous infusion over a 46-h period) had been administered on day 1 and then repeated every 2 weeks until disease progression or patient refusal. Dose modification was allowed when unacceptable toxicity occurred.
Tumor response assessment was examined every three cycles of treatment by computed tomography (CT) or magnetic resonance imaging (MRI). The efficacy outcomes included complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (20). The best clinical response during chemotherapy for each patient was recorded as the tumor response. ORR was calculated as CR + PR/all evaluated patients. The disease control rate (DCR) was calculated as CR + PR + SD/all evaluated patients.
The clinicopathological data including age, sex, surgical treatment before metastasis (yes or no), Eastern Cooperative Oncology Group (ECOG) score, body mass index (BMI), measurable metastatic site, primary tumor location, carcinoembryonic antigen (CEA), and CA199 were collected from the patients’ records. The other inflammatory-related markers, including IL-6, CRP, neutrophils, PLT, lymphocytes, and albumin, were also collected from the records. Blood samples had been obtained within 3 days before initiating treatment or during chemotherapy. CRP had been measured with an immunoturbidimetry assay. Serum IL-6, CA199, and CEA levels had been determined using electro-chemiluminescence immunoassay. As shown in Table 1, the normal reference value of IL-6 and CRP was 0–7 pg/mL and 0–10 mg/L, respectively. The other reference values were 0–40 U/mL for CA199, 0–5 ng/mL for CEA, 35–50 g/L for albumin, 85−303 × 109 for PLT, 2–7 × 109 for neutrophils, and 0.8–4 × 109 for lymphocytes. Using the upper limits of normal for IL-6 (>7 pg/mL), CRP (>10 mg/L), PLT (>303 × 109/L), neutrophils (>7 × 109/L), CA199 (>40 U/mL), and CEA (>5 ng/mL) as cutoff values, the patients were classified with low or high values for each of these markers. Using the lower limit of normal for lymphocytes (<0.8×109/L) and albumin (<35 g/L) as cutoff values, the patients were classified with low and high values. The definitions and cutoff values were based on previous reports (21–23). CEA, CA199, CRP, IL-6, neutrophils, PLT, lymphocytes, and albumin were tested at three-cycle intervals, at the same time as response evaluation. A dynamic increase was defined as two or more consecutive increases in the levels of tested markers compared with the last testing without a cutoff threshold. All markers described earlier were measured by the Department of Laboratory Medicine of Shanghai General Hospital (Shanghai Jiaotong University School of Medicine, Shanghai, China (using standard routine laboratory methods).
Survival data were obtained from the medical charts. PFS was defined as the time from the start of chemotherapy to documented disease progression or death, whichever occurred first.
Statistical analysis was performed using SPSS 20.0 (IBM Corp., NY, USA). The relationships between inflammatory markers and the clinical response were assessed using Fisher’s exact test (for categorical variables) and Student’s t test (for continuous variables). The correlations between dynamically changing inflammatory markers and the clinical response were evaluated using Spearman’s rank correlation coefficient. Survival analysis was performed using the Kaplan-Meier method, and the curves were compared using the log-rank test. Multivariate analysis was performed using a stepwise forward Cox regression (likelihood ratio, enter P < 0.05, remove P > 0.10) with significant markers from the univariate analyses (P < 0.05); the results were presented as hazard ratio (HR) and 95% confidence interval (CI). Receiving operating characteristic (ROC) curve analysis was used to explore a combination of biomarkers based on the multivariate regression model to predict the efficacy (DCR) of the mFOLFIRINOX regimen in patients with mPC. Statistical significance was set at P <0.05 (two-sided).
All the 36 patients included had stage IV PC. Six patients were excluded from data analysis: four received two or fewer cycles because of intolerable toxicity, one was lost to follow-up, and one received only one cycle for other reasons. Therefore, 30 patients were included in the evaluation of ORR and DCR.
As shown in Table 2, the median age was 63 years. Twenty-one (70.0%) patients were male, and nine (30.0%) were female. Most patients (70.0%) were ECOG 1, and one patient was ECOG 2. Eight (26.6%) patients had tumors located in the head of the pancreas, eleven (36.7%) in the body, and eleven (36.7%) in the tail. The most common metastatic sites were lymph nodes (56.7%) and the liver (56.7%). Patients received a median of 6 cycles, and 5 patients received at least 10 cycles. No patients had CR, 8 (26.7%) patients had PR, 18 (60.0%) had SD, and 4 (13.3%) had PD. The ORR was 26.7%, and the DCR was 86.7%. The median PFS was 189 (95% CI: 136–241) days (Supplementary Figure 1).
Among the inflammatory markers, IL-6 and CRP were significantly and positively associated with disease progression (r = 0.515, P = 0.004; and r = 0.711, P = <0.001, respectively) (Table 3). As shown in Figure 2, the serum IL-6 and CRP levels were higher in the PD group than in the DCR group (P = 0.019 and P = 0.003, respectively). Serum albumin levels were not different between the DCR and PD groups, and the serum levels of the other inflammatory markers were similar in the two groups (Table 3 and Supplementary Figure 2). Tumor markers, including CA199 and CEA, were not associated with the disease outcomes in this study (Table 3 and Supplementary Figure 2).
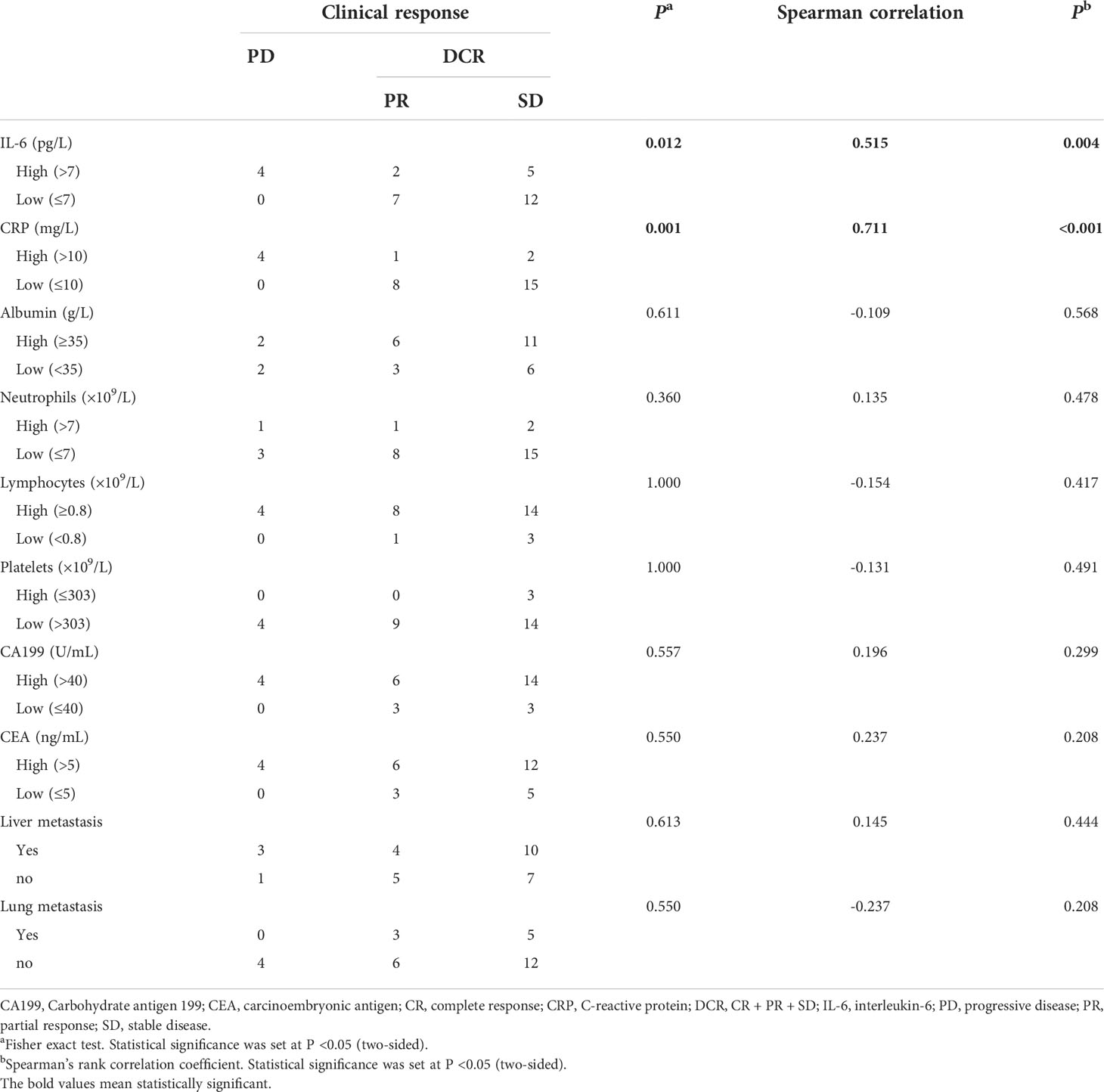
Table 3 Correlation between clinicopathological markers and clinical response in patients with mPC treated with mFOLFIRINOX.
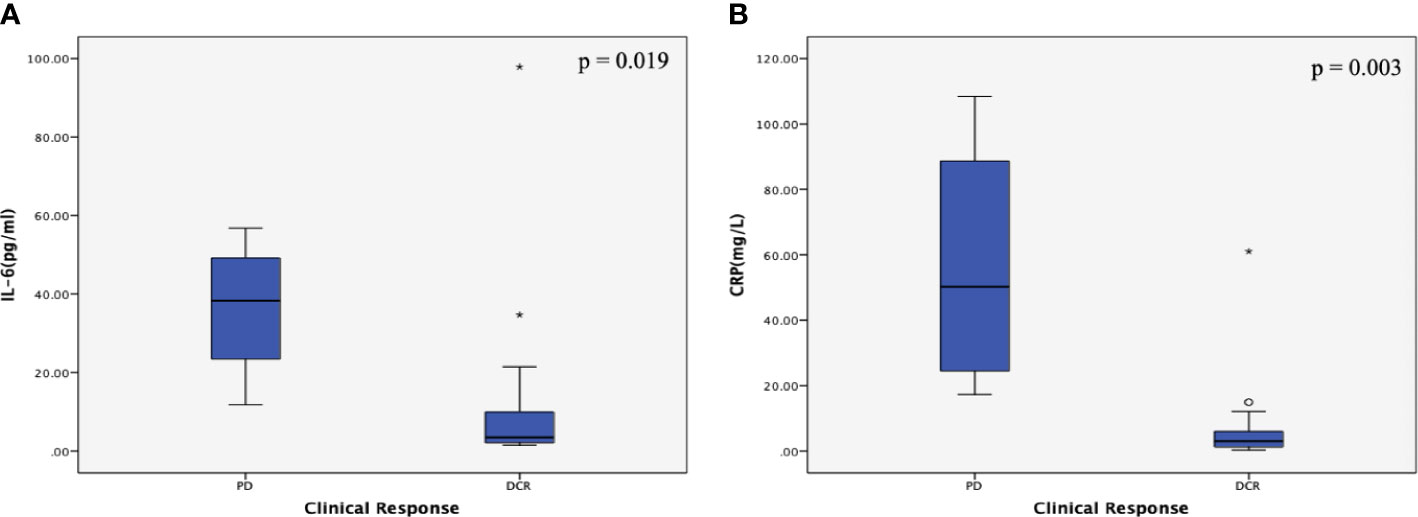
Figure 2 Correlations between inflammatory marker levels and the clinical response subgroup in patients with metastatic pancreatic cancer (mPC). (A) Correlation between interleukin (IL)-6 levels and the clinical response subgroup in patients with mPC. The serum IL-6 levels were higher in the PD group than in the DCR group (P = 0.019). (B) Correlation between C-reactive protein (CRP) levels and the clinical response subgroup in patients with mPC. The serum CRP levels were higher in the PD group than in the DCR group (P = 0.003). The P values were calculated using the Student’s t test. The symbol * means abnormal values in the boxplot.
To further describe the correlation between levels of IL-6 and CRP and clinical response, the dynamic changes in IL-6 and CRP were explored. Dynamic increases in IL-6 and CRP significantly correlated with the clinical response (P = 0.005 for IL-6; P = 0.001 for CRP) (Supplementary Table 1). As shown in Figure 3, the dynamic changes in IL-6 and CRP levels positively correlated with PD during chemotherapy (r= -0.599, P = <0.001 for IL-6; r = -0.711, P = <0.001 for CRP).
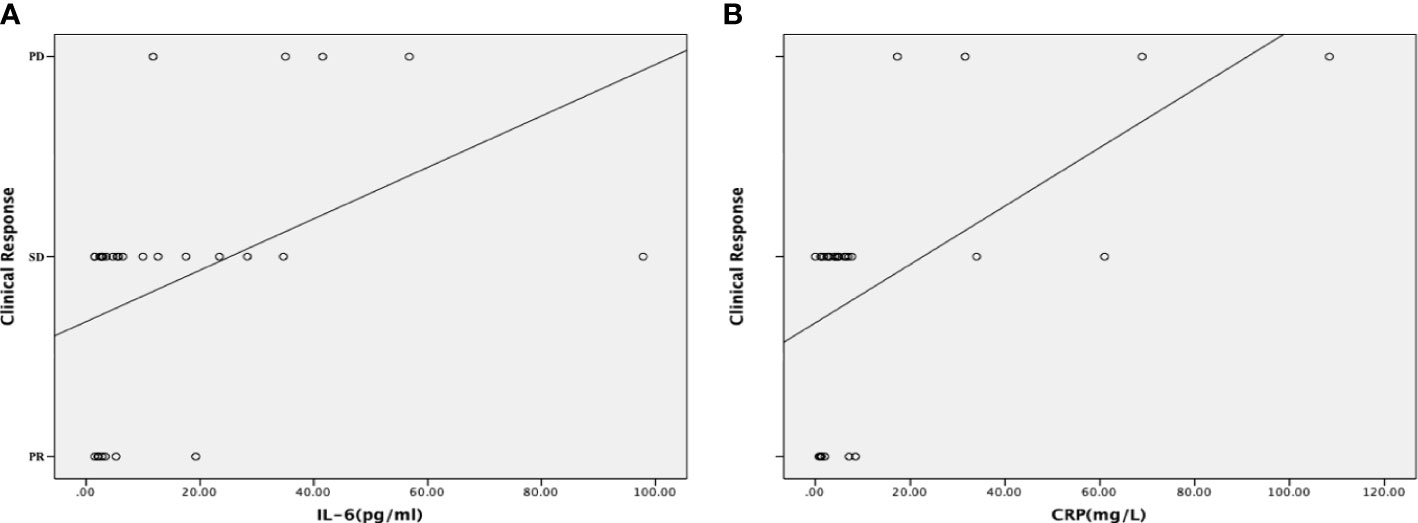
Figure 3 Correlations between the dynamic changes in inflammatory markers and the clinical response. (A) Correlation between the dynamic changes in the serum interleukin (IL)-6 level and the clinical response. The dynamic increases in the levels of IL-6 positively correlated with progressive disease (PD) during chemotherapy. (B) Correlation between the dynamic changes in the serum C-reactive protein (CRP) level and the clinical response. The dynamic increases in the levels of CRP positively correlated with progressive disease (PD) during chemotherapy. A scatter plot and a fitted curved were used to show the correlation more intuitively.
IL-6 was significantly and positively associated with CRP (r = 9.459, P = 0.004; and r = 0.562, P = 0.001, respectively) (Table 4). Since CRP is produced by the liver as part of the acute phase response, its levels correlate with cancer progression. The correlation between levels of IL-6 and CRP and the incidence of liver metastasis at baseline, as well as the correlation between the incidence of liver metastasis and the effectiveness of mFOLFIRINOX, were also explored. The correlation between levels of IL-6 and CRP and the incidence of lung metastasis was explored at the same time. The incidence of lung/liver metastasis at baseline was neither associated with baseline IL-6 or CRP levels nor with the best response to chemotherapy in this study (Table 3 and Supplementary Table 2).
The univariate analyses showed that the liver metastasis (HR = 5.10, 95% CI: 1.38-18.79, P = 0.014), high IL-6 levels (HR = 3.77, 95% CI: 1.23-11.54, P = 0.020), and high CRP levels (HR = 4.26, 95% CI: 1.04-17.46, P = 0.044) were associated with a poorer PFS, while lung metastasis was associated with a better PFS (HR = 0.16, 95% CI: 0.35-0.78, P = 0.024) (Table 5 and Figures 4A–E). The multivariate analysis revealed lung metastasis as a good independent prognostic factor (HR = 0.13, 95% CI: 0.24-0.70, P = 0.018) and high IL-6 levels as a poor independent prognostic factor for PFS (HR = 4.66, 95% CI: 1.32-16.37, P = 0.016). (Table 3). As shown in Figure 4A, the median PFS in the IL-6-low group was 257 (95% CI: 237-276) days, which was significantly higher than that in the IL-6-high group (median PFS, 150 days; 95% CI:47-252, P = 0.020). Tumor markers, including CEA and CA199, were not associated with PFS in this study (P = 0.529 and P = 0.146, respectively) (Supplementary Figures 4A, B). So far, the median OS of these patients had not been reached.
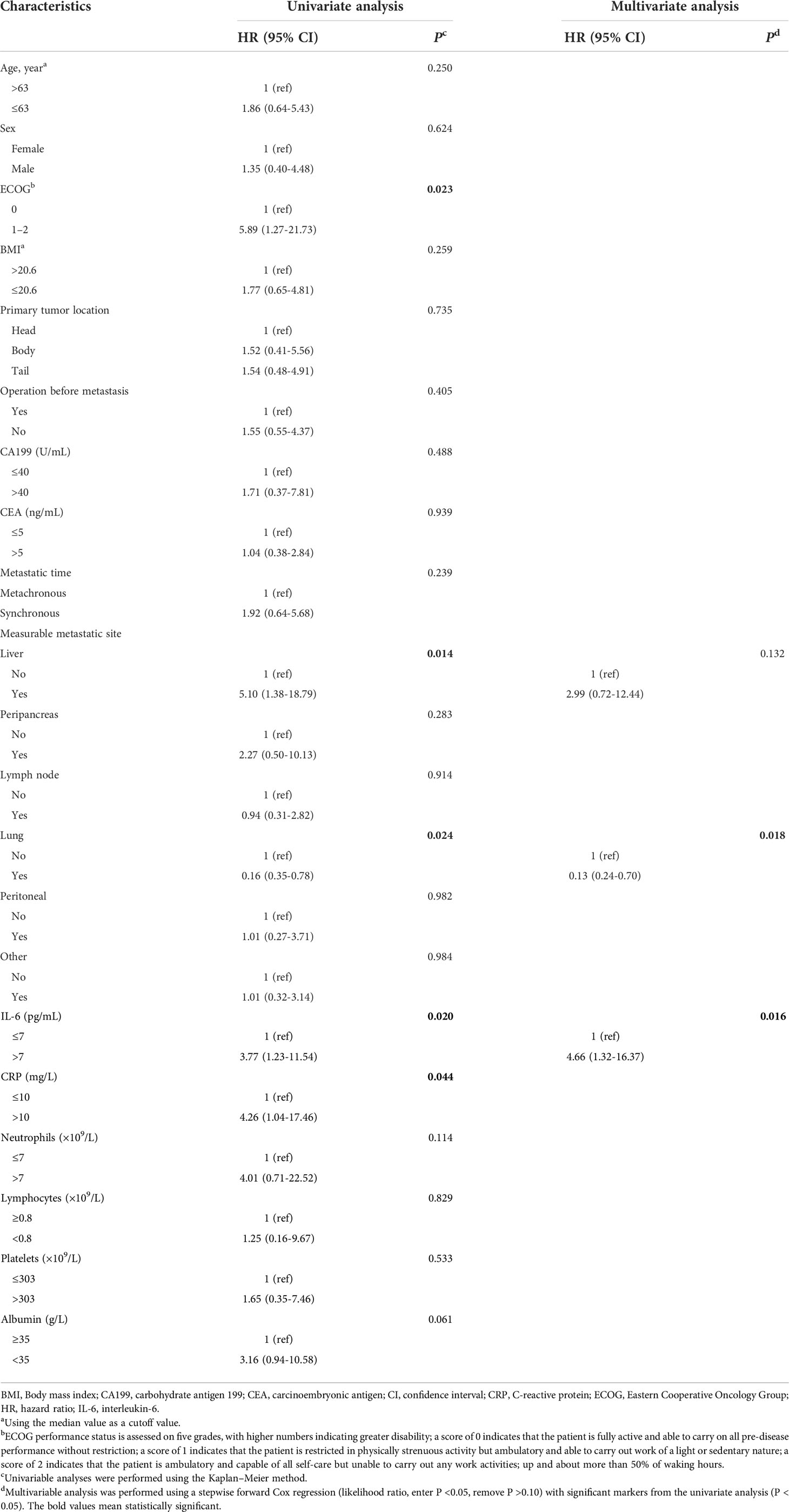
Table 5 Univariate and multivariate analyses of clinicopathological data and inflammatory markers in PFS.
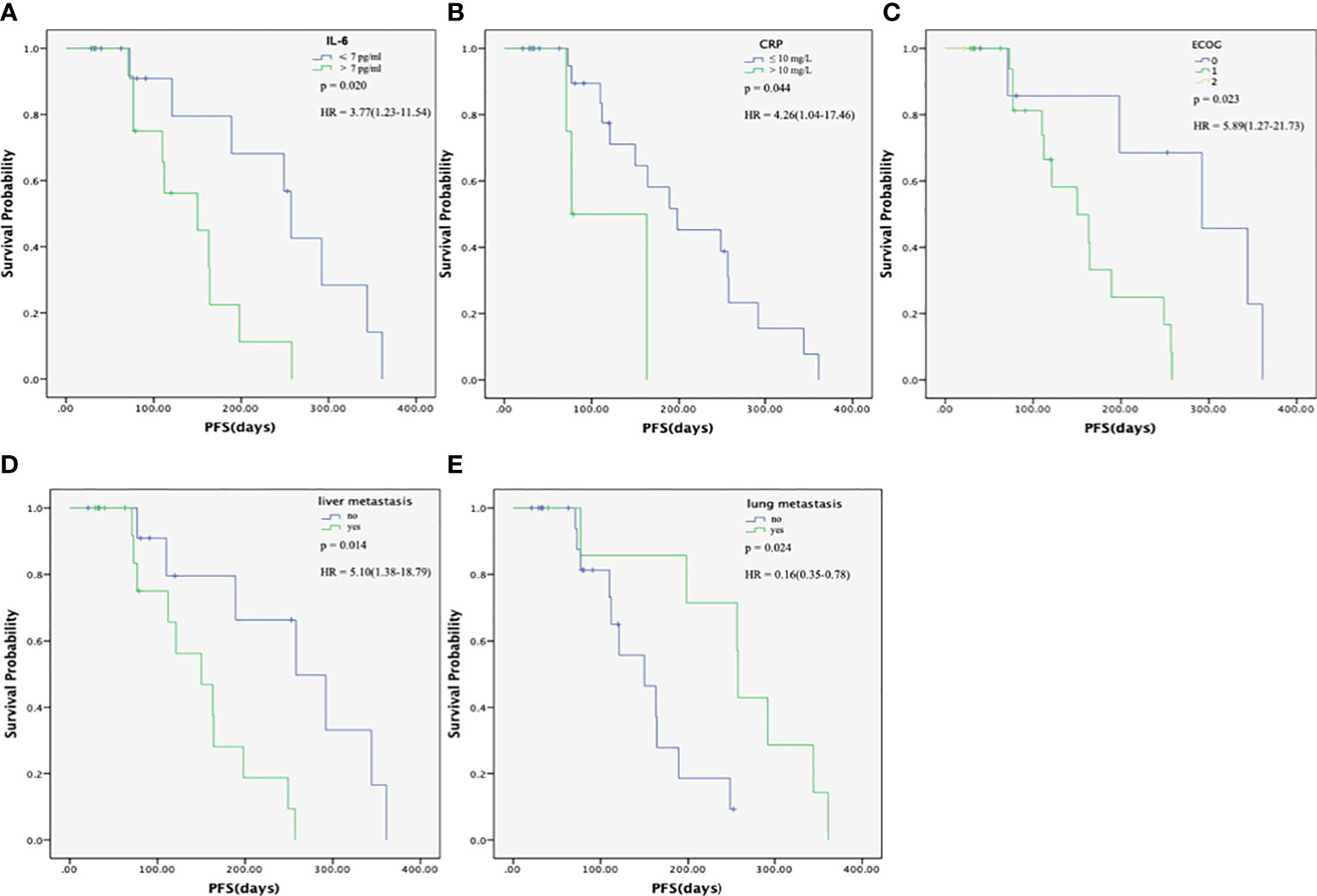
Figure 4 Progression-free survival (PFS) of patients with metastatic pancreatic cancer (mPC) treated with the modified FOLFIRINOX. (A) PFS in the interleukin (IL)-6-high and –low groups. The median PFS in the IL-6-low group was 257 (95% CI: 237-276) days, which was significantly higher than that in the IL-6-high group (median PFS, 150 days; 95% CI:47-252, P=0.020). (B) PFS in the C-reactive protein (CRP)-high and -low groups. The median PFS in the CRP-low group was 198 (95% CI: 42-353) days, which was significantly higher than that in the CRP-high group (median PFS, 163 days; 95% CI:28-297, P = 0.044). (C) PFS in the different ECOG groups. The median PFS was 292 (95% CI: 151-432) days and 150 (95% CI:81-218) days for ECOG score 0 and 1 group, respectively. (D) PFS according to the presence of liver metastasis. The median PFS in the non-liver metastasis group was 258 (95% CI:143-372) days, which was significantly higher than that in the liver metastasis group (median PFS, 150 days; 95% CI: 84-215, P = 0.014). (E) PFS, according to the presence of lung metastasis. The median PFS in the lung metastasis group was 258 (95% CI:255-260) days, which was significantly higher than that in the non-lung metastasis group (median PFS, 150 days; 95% CI: 86-213 P = 0.024). The PFS was calculated using the Kaplan-Meier method.
The inflammatory markers (IL-6 and CRP) were used for constructing a model to predict the effectiveness (DCR or not) of the mFOLFIRINOX regimen in patients with mPC. Concerning the possible predictive value of tumor biomarkers for the treatment response indicated in previous studies (8, 9, 24), CEA and CA199 were also added. As shown in Figure 5, the ROC analysis showed that the combination of IL-6 and CRP (AUC: 0.811, 95% CI: 0.639–0.983, P = 0.003) had a higher AUC compared with CRP alone (AUC: 0.767, 95% CI: 0.589–0.946, P = 0.011), IL-6 alone (AUC: 0.710, 95% CI: 0.524–0.896, P = 0.046), tumor markers (AUC: 0.640, 95% CI: 0.451–0.829, P = 0.184) alone, and their combination (IL-6 + CRP + tumor markers, AUC: 0.806, 95% CI: 0.633–0.979, P = 0.004).
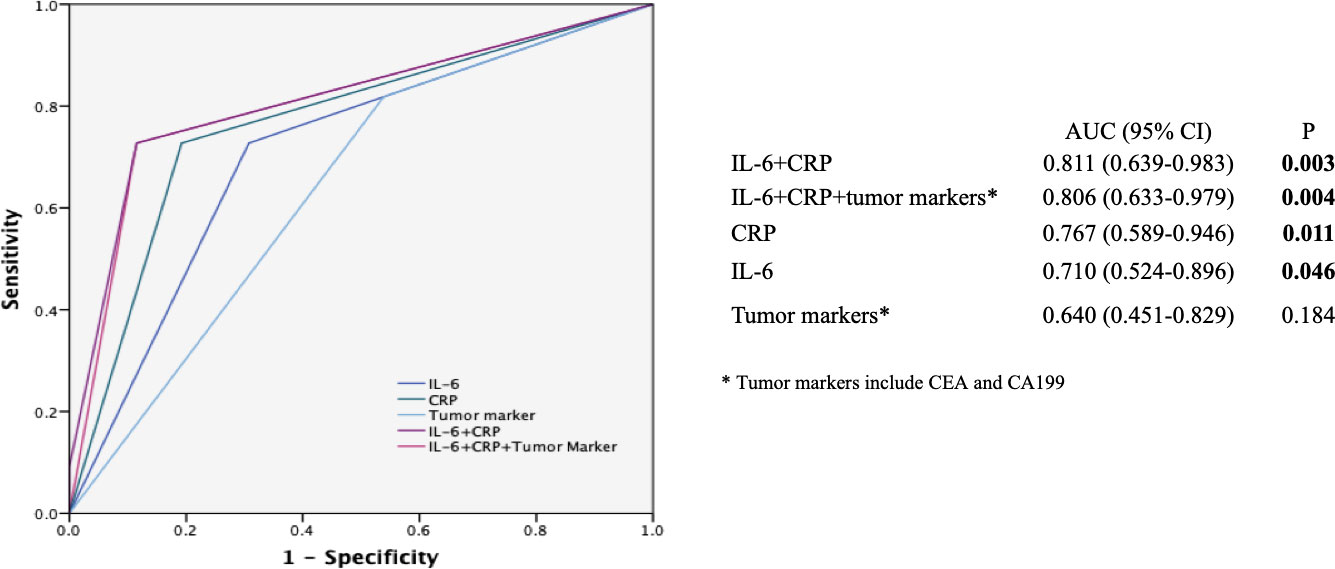
Figure 5 Receiver operating characteristic (ROC) curves for predicting the efficacy of the mFOLFIRINOX regimen. ROC analyses of the prediction of the efficacy of mFOLFIRINOX using the inflammatory marker model, the tumor marker model, and the combined inflammatory and tumor marker model. The ROC analysis showed that the combination of IL-6 and CRP (AUC: 0.811, 95% CI: 0.639-0.983, P = 0.003) had a higher AUC compared with CRP alone (AUC: 0.767, 95% CI: 0.589-0.946, P = 0.011), IL-6 alone (AUC: 0.710, 95% CI: 0.524-0.896, P = 0.046), tumor markers (AUC: 0.640, 95% CI: 0.451-0.829. P = 0.184) alone, and their combination (IL-6 + CRP + tumor markers, AUC: 0. 806, 95% CI: 0.633-0.979, P = 0.004). AUC, Area under the curve. The symbol * is the annotation of tumor markers.
In this single-institution retrospective analysis, the median PFS was 189 days and the DCR was 86.7% among 30 patients with mPC treated with mFOLFIRINOX, which were comparable with previously reported findings (4–7). High levels of IL-6 and CRP were associated with poor treatment response. The dynamic increase in IL-6 and CRP levels during treatment was associated with a poor progression of mPC. High IL-6 was a predictor of poor PFS, while lung metastasis was a predictor of better PFS in the multivariate analysis. The model combining IL-6 with CRP showed a better value in predicting the outcomes of patients with mPC treated with mFOLFIRINOX. Furthermore, this study was novel in reporting the associations between the dynamic changes in serum IL-6 and CRP levels and clinical response.
CRP is synthesized by the hepatocytes and is one of the most commonly used markers to reflect systemic inflammation. In this study, the serum CRP levels were associated with the tumor response, which was consistent with previous findings. Indeed, Nurmi et al. (25) described the combination of CRP and CA19-9 as a useful prognostic marker in evaluating disease-specific survival of surgically treated patients with PC. Liu et al. (12) revealed an elevated CRP/albumin ratio as an independent factor for poor prognosis with the cutoff value of 0.180 in PC patients. The prognostic significance of the CRP/albumin ratio existed in Stages III and IV PC patients and had no connection with the primary tumor location. Mitsunaga et al. (16) investigated the serum levels of CRP and clinical outcomes in patients with APC treated with first-line gemcitabine monotherapy; low, intermediate, and high CRP levels were associated with a good, intermediate, and poor response to chemotherapy. Besides, the elevated CRP level was found to be statistically significant in patients with mPC receiving first-line chemotherapy, including FOLFIRINOX, monotherapy with gemcitabine, and doublet chemotherapy with gemcitabine in combination with cisplatin or oxaliplatin; it was also significantly associated with shorter PFS and OS (26, 27). These results implied that an underlying inflammatory state might play a role in resistance to systemic therapy or in increased tumor aggressiveness, thus supporting the conclusion of the present study that CRP was a possible biomarker predicting the efficacy of the mFOLFIRINOX regimen. This should be further explored in future studies.
IL-6 regulates the secretion of vascular endothelial growth factor (VEGF) in PC cells, thereby stimulating angiogenesis and tumor vascularization, resulting in lymphatic and distant metastasis and disease progression. High IL-6 levels predict poor PFS and poor response to gemcitabine monotherapy in patients with APC (15). A phase II trial reported that higher serum IL-6 levels before treatment were associated with a reduced response to therapy and worse OS in patients with APC treated with gemcitabine and curcumin (28). This study suggested that the serum levels of IL-6 were closely related to poor response and prognosis of mPC treated with mFOLFIRINOX, which was supported by the aforementioned studies. In consideration of the complexity of the low half-life of IL-6, it may not be an ideal biomarker in clinical practice. A high serum level of CRP was reported to be related to a high serum IL-6 level in patients with treatment-naive APC (29). CRP may be a better substitute marker for IL-6 in predicting the efficacy of the mFOLFIRINOX regimen and PFS in patients with mPC. In the present study, IL-6 was significantly and positively associated with CRP in mPC patients who received the mFOLFIRINOX regimen as first-line chemotherapy. High levels of IL-6 and CRP were associated with poor treatment response, and they were also proved to be a predictor in the univariate analyses. The AUC of CRP alone was also higher than that of IL-6 alone. However, CRP was not associated with PFS in the multivariate analysis. The small sample size might have played a role in this result. The relationship between CRP and IL-6 in predicting PFS in patients with mPC should be further explored in future studies.
IL-6 induces metastasis through programming hematopoietic stem and progenitor cells toward tumor-supporting metastatic cells (30). Additionally, IL-6 affects monocyte-dendritic progenitors to differentiate into metastasis-promoting cells, thereby promoting tumor aggressiveness (30). An elevated CRP level may reflect a non-specific inflammatory response to tumor necrosis, which in turn indicates the levels of inflammatory cytokines such as IL-6. The production of inflammatory cytokines leads to the promotion of adhesion of circulating tumor cells to the vascular endothelium of distant organs by enhancing the E-selectin expression. This results in a microenvironment that favors tumor metastases (31, 32).The most common sites of metastasis in mPC are the liver, lung, regional lymph nodes, and peritoneum. Different metastatic patterns may involve different tumor biology and prognosis (33).
Kurokawa et al. (32) showed that liver metastasis occurred more frequently in high serum levels of CRP group in patients with pT2-T4 gastric cancer who underwent R0 resection. Kim et al. (34)revealed that IL-6 was significantly direct correlated with CRP, and it was an independent risk factor for progression to extensive hepatic metastasis in patients with PC. The correlation between lung metastasis and IL-6/CRP levels has not been found yet in published studies. In our study, IL-6 was significantly and positively associated with CRP in mPC patients, which was in line with Kim et al’s study. However, the incidence of lung/liver metastasis at baseline was neither associated with baseline IL-6 or CRP levels nor with the best response to chemotherapy. The small sample size might have played a role in this result. The relationship between the lung/liver metastasis and CRP/IL-6 levels in predicting chemotherapy efficacy in mPC patients should be further explored in future studies.
Liver metastasis was a poor prognostic factor in this study, as supported by the MPACT study (35), while the presence of lung metastasis was considered as a good prognostic factor, which was also observed in other studies, but the mechanisms remain unclear.
Lung metastasis as the primary recurrence was reported as a favorable prognostic factor in patients with relapsed PC (36). Isolated lung metastases were demonstrated as a better prognosticator for OS in stage IV PC treated with palliative chemotherapy (37). Kruger et al. showed that limited disease (defined as metastatic disease of fewer than 10 metastases) confined to one lung might predict favorable outcomes in patients with PC without metastases to other organs (38). In addition, Lovecek et al. examined patients with PC who developed metachronous pulmonary metastases as the first site of recurrence after the curative-intent surgery. His result showed that patients with pulmonary metastases, including isolated pulmonary oligometastases, isolated pulmonary multiple metastases, and pulmonary metastases accompanied by other metastases, had prior DFS and OS compared with patients with nonpulmonary metastases (39). Patients with lung metastasis from PC had a better prognosis compared with those with other site metastases, which is still a controversial issue. Lung metastasis might confer less clinical-related complications compared with the complications that might be caused by local recurrence or liver metastasis, including biliary obstruction or gastric outlet obstruction. It might explain the better PFS or OS in patients with lung metastases. Patients with lung metastasis as the primary recurrence usually had lower pT category and less vascular invasion compared with patients with other metastases, and it might influence the prognosis (36). In the present study, all of the patients with lung metastasis also had other metastases. Patients with lung metastasis accompanied by other metastases had longer PFS compared with patients with non-lung metastasis. The difference between isolated lung metastasis and lung metastasis accompanied by other metastases in PFS of patients with mPC should be further studied in the future.
Circulating neutrophils (innate immune system) are thought to play a tumor-promoting role, while lymphocytes (adaptive immune system) play an anti-tumor role. High neutrophil counts were associated with a worse prognosis in patients with various cancers (40, 41). Angiogenesis is one of the most important causes of tumor proliferation and metastasis. Platelets contribute to tumor vascular growth through a number of platelet-derived angiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, and hepatocyte growth factor. High platelet counts were associated with a worse prognosis in PC (42) and other cancer types (23, 43). Serum albumin is an important indicator of nutritional status. Hypoalbuminemia usually occurs in combination with poor performance status, weight loss, and nutritional deficiency, which negatively affect the prognosis of cancer patients. The relationship between hypoalbuminemia and poor survival may also depend on the systemic inflammatory response (44). A low serum albumin level was reported as a significant independent predictive factor for PFS in cancer patients (45–47). CEA and CA199 have been demonstrated to be predictive markers for the response to chemotherapy in PC (8, 48), with elevated serum values leading to poor OS (49, 50). Several studies showed that CA199 reduction during chemotherapy was a potential predictor of efficacy (51, 52). However, in this study, neutrophils, lymphocytes, platelets, albumin, CEA, and CA199 did not correlate with the clinical response to mFOLFIRINOX or with PFS. In this study, the cutoff values were determined using the normal ranges, while previous studies used statistical methods to determine the cutoff. The sample size, as well as the type of therapy, might also play a role.
In the present study, the results suggested that IL-6 and CRP could effectively predict the clinical response to mFOLFIRINOX in mPC. CEA and CA199 were added to the predictive model because they are known to be markers of treatment response in PC (51, 52), but they did not improve the predictive value of IL-6 and CRP; the combination of IL-6 and CRP showed the best predictive value. This was supported by the fact that high IL-6 and CRP levels were associated with poorer PFS and that increases in IL-6 and CRP levels during treatment were associated with poorer prognosis. Nevertheless, models remain to be determined and improved for the prognosis of mPC. For now, the results suggested that patients with low IL-6 and low CRP levels before treatment might be those who would respond to mFOLFIRINOX. Additional studies are needed to refine these results.
This study had some limitations. The small sample size of this study might have prevented achieving sufficient power to detect survival differences between subgroups, which might be regarded as a major limitation. Additional data from other databases should be validated as well to provide a more powerful conclusion to avoid bias. However, no comparable published studies support a meta-analysis for external effectiveness in chemotherapy-naive mPC patients treated with the mFOLFIRINOX regimen as first-line chemotherapy. The results need to be validated using more samples in prospective studies. In addition, the retrospective nature of the study limited the analyzable data to those contained in the charts. A prospective cohort study may answer many questions regarding the markers of good response to mFOLFIRINOX.
In conclusion, higher IL-6 or CRP levels before or during chemotherapy positively correlated with PD. Higher serum levels of IL-6 and CRP might be predictors of a poor response to mFOLFIRINOX chemotherapy in patients with mPC. The combination of IL-6 and CRP might predict the response to mFOLFIRINOX. The relationship of inflammatory markers with the therapeutic effect of mFOLFIRINOX in patients with mPC deserves to be further investigated in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
(I) Conception and design, FZ; (II) Administrative support, none; (III) Provision of study materials or patients, FZ and QL; (IV) Collection and assembly of data, FS, WZ, CL, SH, FW, and JW; (V) Data analysis and interpretation, FS; (VI) Manuscript writing, all authors; (VII) Final approval of manuscript, all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.964115/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Xu XH, Zeng XY, Wang LJ, Liu YN, Liu JM, Qi JL, et al. The disease burden of pancreatic cancer in China in 1990 and 2017. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. (2019) 40:1084–8. doi: 10.3760/cma.j.issn.0254-6450.2019.09.012
4. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med (2011) 364(19):1817–25. doi: 10.1056/NEJMoa1011923
5. Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas (2013) 42(8):1311—1315. doi: 10.1097/mpa.0b013e31829e2006
6. Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. (2016) 114(7):737–43. doi: 10.1038/bjc.2016.45
7. Bai X, Su R, Ma T, Shen S, Li G, Lou J, et al. Modified FOLFIRINOX for advanced pancreatic cancer: a tertiary center experience from China. Zhonghua Wai Ke Za Zhi. (2016) 54(4):270–5. doi: 10.3760/cma.j.issn.0529-5815.2016.04.006
8. Morris-Stiff G, Taylor MA. Ca19-9 and pancreatic cancer: Is it really that good? J Gastrointest Oncol (2012) 3(2):88–9. doi: 10.3978/j.issn.2078-6891.2012.016
9. Mitsunaga S, Hasebe T, Iwasaki M, Kinoshita T, Ochiai A, Shimizu N. Important prognostic histological parameters for patients with invasive ductal carcinoma of the pancreas. Cancer Sci (2005) 96(12):858–65. doi: 10.1111/j.1349-7006.2005.00128.x
10. Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
11. Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, et al. Validation of c-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. (2014) 110(1):183–8. doi: 10.1038/bjc.2013.701
12. Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol (2017) 24(2):561–8. doi: 10.1245/s10434-016-5579-3
13. Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, et al. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J Int Med Res (2018) 46(12):5228–36. doi: 10.1177/0300060518800588
14. Ramsey ML, Talbert E, Ahn D, Bekaii-Saab T, Badi N, Bloomston PM, et al. Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology (2019) 19(1):80–7. doi: 10.1016/j.pan.2018.11.002
15. Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. (2013) 108(10):2063–9. doi: 10.1038/bjc.2013.174
16. Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Okuyama H, et al. C-reactive protein level is an indicator of the aggressiveness of advanced pancreatic cancer. Pancreas (2016) 45(1):110–6. doi: 10.1097/MPA.0000000000000465
17. Miksch R, Schoenberg M, Weniger M, Bösch F, Ormanns S, Mayer B, et al. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils on survival of patients with upfront resection of pancreatic cancer. Cancers (2019) 11:39. doi: 10.3390/cancers11010039
18. Chen Y, Wang YR, Deng GC, Dai GH. CA19–9 decrease and survival according to platelet level in patients with advanced pancreatic cancer. BMC Cancer. (2019) 19(1):860. doi: 10.1186/s12885-019-6078-2
19. Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. (2013) 108(4):914–23. doi: 10.1038/bjc.2013.32
20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
21. Babic A, Schnure N, Neupane NP, Zaman MM, Rifai N, Welch MW, et al. Plasma inflammatory cytokines and survival of pancreatic cancer patients. Clin Transl Gastroenterol (2018) 9(4):145–5. doi: 10.1038/s41424-018-0008-5
22. Djaladat H, Bruins HM, Miranda G, Cai J, Skinner EC, Daneshmand S. The association of preoperative serum albumin level and American society of anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int (2014) 113(6):887–93. doi: 10.1111/bju.12240
23. Huszno J, Kolosza Z, Mrochem-Kwarciak J, Rutkowski T, Skladowski K. The role of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and platelets in the prognosis of metastatic renal cell carcinoma. Oncology (2019) 97(1):7–17. doi: 10.1159/000498943
24. Gebhardt C, Meyer W, Reichel M, Wünsch PH. Prognostic factors in the operative treatment of ductal pancreatic carcinoma. Langenbecks Arch Surg (2000) 385(1):14–20. doi: 10.1007/s004230050004
25. Nurmi AM, Mustonen HK, Stenman UH, Seppänen HE, Haglund CH. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci Rep (2021) 11(1):781–1. doi: 10.1038/s41598-020-80778-0
26. Schlick K, Magnes T, Huemer F, Ratzinger L, Weiss L, Pichler M, et al. C-reactive protein and Neutrophil/Lymphocytes ratio: Prognostic indicator for doubling overall survival prediction in pancreatic cancer patients. J Clin Med (2019) 8(11):1791. doi: 10.3390/jcm8111791
27. Markus M, Abendroth A, Noureddine R, Paul A, Breitenbuecher S, Virchow I, et al. Combined systemic inflammation score (SIS) correlates with prognosis in patients with advanced pancreatic cancer receiving palliative chemotherapy. J Cancer Res Clin Oncol (2021) 147(2):579–91. doi: 10.1007/s00432-020-03361-0
28. Pastorelli D, Fabricio ASC, Giovanis P, D'Ippolito S, Fiduccia P, Soldà C, et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol Res (2018) 132:72–9. doi: 10.1016/j.phrs.2018.03.013
29. Miura T, Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, et al. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas (2015) 44(5):756–63. doi: 10.1097/MPA.0000000000000335
30. Magidey-Klein K, Cooper TJ, Kveler K, Normand R, Zhang T, Timaner M, et al. IL-6 contributes to metastatic switch via the differentiation of monocytic-dendritic progenitors into prometastatic immune cells. J Immunother Cancer. (2021) 9(6):e002856. doi: 10.1136/jitc-2021-002856
31. Kim JM, Kwon CHD, Joh JW, Ko JS, Park JB, Lee JH, et al. C-reactive protein may be a prognostic factor in hepatocellular carcinoma with malignant portal vein invasion. World J Surg Oncol (2013) 11(1):92. doi: 10.1186/1477-7819-11-92
32. Kurokawa Y, Yamashita K, Kawabata R, Fujita J, Imamura H, Takeno A, et al. Prognostic value of postoperative c-reactive protein elevation versus complication occurrence: a multicenter validation study. Gastric Cancer. (2020) 23(5):937–43. doi: 10.1007/s10120-020-01073-5
33. Hani O, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A surveillance epidemiology and end results database analysis. World J Gastroenterol (2017) 23:1872. doi: 10.3748/wjg.v23.i10.1872
34. Kim HW, Lee JC, Paik KH, Kang J, Kim J, Hwang JH. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Med (Baltimore). (2017) 96(5):e5926–6. doi: 10.1097/MD.0000000000005926
35. Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. (2015) 20(2):143–50. doi: 10.1634/theoncologist.2014-0394
36. Zheng B, Ohuchida K, Yan Z, Okumura T, Ohtsuka T, Nakamura M. Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and postoperative recurrence. World J Surg (2017) 41(11):2858–66. doi: 10.1007/s00268-017-4068-6
37. Liu KH, Hung CY, Hsueh SW, Chang PH, Chen YY, Lu CH, et al. Lung metastases in patients with stage IV pancreatic cancer: Prevalence, risk factors, and survival impact. J Clin Med (2019) 8(9):1402. doi: 10.3390/jcm8091402
38. Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, et al. Isolated pulmonary metastases define a favorable subgroup in metastatic pancreatic cancer. Pancreatology (2016) 16(4):593–8. doi: 10.1016/j.pan.2016.03.016
39. Lovecek M, Skalicky P, Chudacek J, Szkorupa M, Svebisova H, Lemstrova R, et al. Different clinical presentations of metachronous pulmonary metastases after resection of pancreatic ductal adenocarcinoma: Retrospective study and review of the literature. World J Gastroenterol (2017) 23(35):6420–8. doi: 10.3748/wjg.v23.i35.6420
40. Fankhauser CD, Sander S, Roth L, Gross O, Eberli D, Sulser T, et al. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br J Cancer. (2018) 118(6):825–30. doi: 10.1038/bjc.2017.467
41. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol (2018) 18(1):148. doi: 10.1186/s12876-018-0877-9
42. Wang H, Gao J, Bai M, Liu R, Li H, Deng T, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets (2013) 25. doi: 10.3109/09537104.2013.827782
43. Kizer NT, Hatem H, Nugent EK, Zhou G, Moore K, Heller P, et al. Chemotherapy response rates among patients with endometrial cancer who have elevated serum platelets. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc (2015) 25(6):1015–22. doi: 10.1097/IGC.0000000000000453
44. Crumley ABC, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg (2010) 34(10):2393–8. doi: 10.1007/s00268-010-0641-y
45. Fiala O, Pesek M FINEK J, Racek J, Minarik M, Benesova L, et al. Serum albumin is a strong predictor of survival in patients with advanced-stage non-small cell lung cancer treated with erlotinib. Neoplasma (2016) 63:471–6. doi: 10.4149/318_151001N512
46. Stenman M, Laurell A, Lindskog M. Prognostic significance of serum albumin in patients with metastatic renal cell carcinoma. Med Oncol (2014) 31(3):841. doi: 10.1007/s12032-014-0841-7
47. Fan L, Chi C, Guo S, Wang Y, Cai W, Shao X, et al. Serum pre-albumin predicts the clinical outcome in metastatic castration-resistant prostate cancer patients treated with abiraterone. J Cancer. (2017) 8(17):3448–55. doi: 10.7150/jca.21134
48. Xu HX, Li S, Wu CT, Qi ZH, Wang WQ, Jin W, et al. Postoperative serum CA19-9, CEA and CA125 predicts the response to adjuvant chemoradiotherapy following radical resection in pancreatic adenocarcinoma. Pancreatology (2018) 18(6):671–7. doi: 10.1016/j.pan.2018.05.479
49. Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J (2013) 54(3):643–9. doi: 10.3349/ymj.2013.54.3.643
50. Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, et al. Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: A retrospective cohort study. Pancreatology (2016) 16(5):859–64. doi: 10.1016/j.pan.2016.05.007
51. Ishii H, Okada S, Sato T, Wakasugi H, Saisho H, Furuse J, et al. CA 19-9 in evaluating the response to chemotherapy in advanced pancreatic cancer. Hepatogastroenterology (1997) 44(13):279—283. doi: 10.1111/j.1523-5378.1997.tb00090.x
Keywords: C-reactive protein, interleukin-6, inflammatory markers, metastatic pancreatic cancer, modified FOLFIRINOX
Citation: Shen F, Liu C, Zhang W, He S, Wang F, Wang J, Li Q and Zhou F (2022) Serum levels of IL-6 and CRP can predict the efficacy of mFOLFIRINOX in patients with advanced pancreatic cancer. Front. Oncol. 12:964115. doi: 10.3389/fonc.2022.964115
Received: 08 June 2022; Accepted: 05 July 2022;
Published: 29 July 2022.
Edited by:
Ravindra Deshpande, Wake Forest School of Medicine, United StatesReviewed by:
Vipin Rawat, University of Illinois at Chicago, United StatesCopyright © 2022 Shen, Liu, Zhang, He, Wang, Wang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Zhou, 13816837501@sina.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.