
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 25 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.963094
This article is part of the Research Topic Reviews in Thoracic Oncology View all 17 articles
Most parotid metastases have been reported to come from the head and neck; however, cases metastasized from the lung are extremely rare. Missed diagnoses and misdiagnoses occurred quite a few times. Thus, accurately identifying the clinical features of parotid metastasis of lung cancer is important. However, current studies about this issue are mostly case reports, and little is known about the detailed and systematic aspects. We reported three cases of parotid metastases from lung cancer and then systematically searched similar cases through “Pub-Med” and “Web of Science”. Finally, twenty-three patients were included in the study. Eighty-three percent of which were males, and 19 patients were over 50 years old. In all cases with smoking history mentioned, 93% were smokers. The predominant pathological type was small cell lung cancer (SCLC, 13 patients, 56%). Seventeen combined with other site metastasis, while more than half of which were brain metastases. The survival time ranged from 3months-17years, and as for SCLCs, it was only 3months-40months. It can be concluded that clinical features, such as sex, age, smoking history, pathological types, and metastasis patterns, could provide valuable evidence for diagnosis. The lung seems to be the most common primary site of parotid metastases except for head and neck tumors. The two circumstances, SCLC coexisting with Warthin’s tumor and parotid small cell carcinoma with lung metastasis, should be differentiated from parotid metastasis of lung cancer with caution For cases presented as SCLC, more aggressive strategies, such as chemotherapy with immunotherapy and maintenance therapy, may be more suitable. Due to the greater tendency of brain metastasis in such diseases, whole-brain radiation therapy, stereotactic radiosurgery or prophylactic cranial irradiation should be applied to corresponding patients in time. Additionally, lung cancer parotid metastases may be a marker of poor prognosis.
Lung cancer is one of the most common malignant tumors, accounting for the leading cause of cancer death worldwide (1). Pathologically, it can be divided into small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) (2). SCLC, although accounting for only approximately 15%, is extremely malignant (3). Even after effective treatment, the median overall survival is still only approximately 1 year, especially for stage IV diseases (4). Distant metastasis is a major feature of lung cancer, but metastasis to parotid gland is really rare (5). The majority of metastatic malignant parotid diseases originate from head and neck tumors, and as a result, most of the pathological types are squamous cell carcinomas or malignant melanomas. Other pathologic types, such as small cell carcinomas, are rare (6).
Owing to the superficial location, parotid masses frequently appear as the initial symptom of lung cancer parotid metastases. Such situations often lead to parotid gland tumors being misdiagnosed as primary lesions, while ignoring the diagnosis and treatment of the real primary site (7). On the other hand, for the inherent benign impression of parotid tumors, misdiagnosis of metastatic parotid tumors also occurs quite a few, especially when lung cancer presents with parotid mass (8). Therefore, great attention should be given to when parotid pathology reveals an uncommon type. Another concern is that the treatment and prognosis of limited and extensive tumors are different, so accurately identifying features of lung cancer parotid metastases matters. However, current studies about this issue are mostly case reports, and little is known about the detailed and systematic aspects. Here, we reported three cases of parotid metastases from SCLC in our institution and reviewed cases regarding parotid metastasis of lung cancer that published previously, in order to provide some references for the management of such disease. To our knowledge, this is the first study to systematically analyze the characteristics of parotid metastasis in lung cancer.
We reported three consecutive cases of parotid metastases from lung cancer treated at our institution and then conducted a literature search with no restrictions on the year of publication. According to the following search strategies: (“bronchial carcinoma” OR “lung carcinoma” OR “lung cancer” OR “lung neoplasms” OR “lung adenocarcinoma” OR “lung squamous” OR “small cell lung cancer” OR “NSCLC” OR “SCLC”) AND (“parotid” OR “salivary”) AND (“metastasis”), the databases “Pub-Med” (http://www.ncbi.nlm.nih.gov/pubmed) and “Web of Science” (https://www.webofscience.com/wos/alldb/basic-search) were fully checked. The latest search date was July 4, 2022. All the identified relevant articles were examined independently by two investigators. Once discrepancies arose, the two reviewers discussed and analyzed the data together and reached a consensus. Studies that did not fit the topic, or were duplicated, or were not full text, or were deficient in clinical information were excluded. In addition, we checked the references within the included studies to avoid any omissions. Since all the articles involved were case reports, the risk bias assessment tool was abandoned in this study. Then, the following information was extracted: the first author, publication year, gender, age, initial symptoms, smoking history, pathological type, primary tumor lesions and size, parotid metastasis lesions and size, other accompanying metastases, treatment, and survival time. Finally, we summarized the above data and analyzed the characteristics.
A 42-year-old male came with the painless hard mass that appeared in front of his left ear. He complained that he had little discomfort except occasional cough. Based on the five-year history of heavy smoking, he was arranged for a chest computed tomography (CT), which found a 6.4 cm x 5.2 cm mass at the right hilum (Figures 1A, B), suggesting central lung cancer. Subsequent lung biopsy and immunohistochemistry confirmed it was SCLC. Further head magnetic resonance imaging (MRI) showed a preauricular mass located at the parotid gland (Figures 1C, D). It is known that tumor metastasis from the lung to the parotid is rare, so there is a big question about the nature of the parotid mass. Hematoxylin and eosin staining of biopsy of the preauricular mass revealed a large number of oat-shaped heteromorphic cells with deep nuclear staining at high magnification, suggesting metastasis of SCLC (Figures 2A–D). Additionally, head MRI and abdominal CT showed that the head and the right adrenal were also affected (Figures 1E, F). Then, he accepted a standard etoposide and cis-platinum (EP) regimen for 6 cycles, as well as the head and chest radiotherapy. Subsequent reviews showed that the patient entered a partial state of remission. Unfortunately, nine months after the completion of treatment, he died due to the progression of brain metastasis.
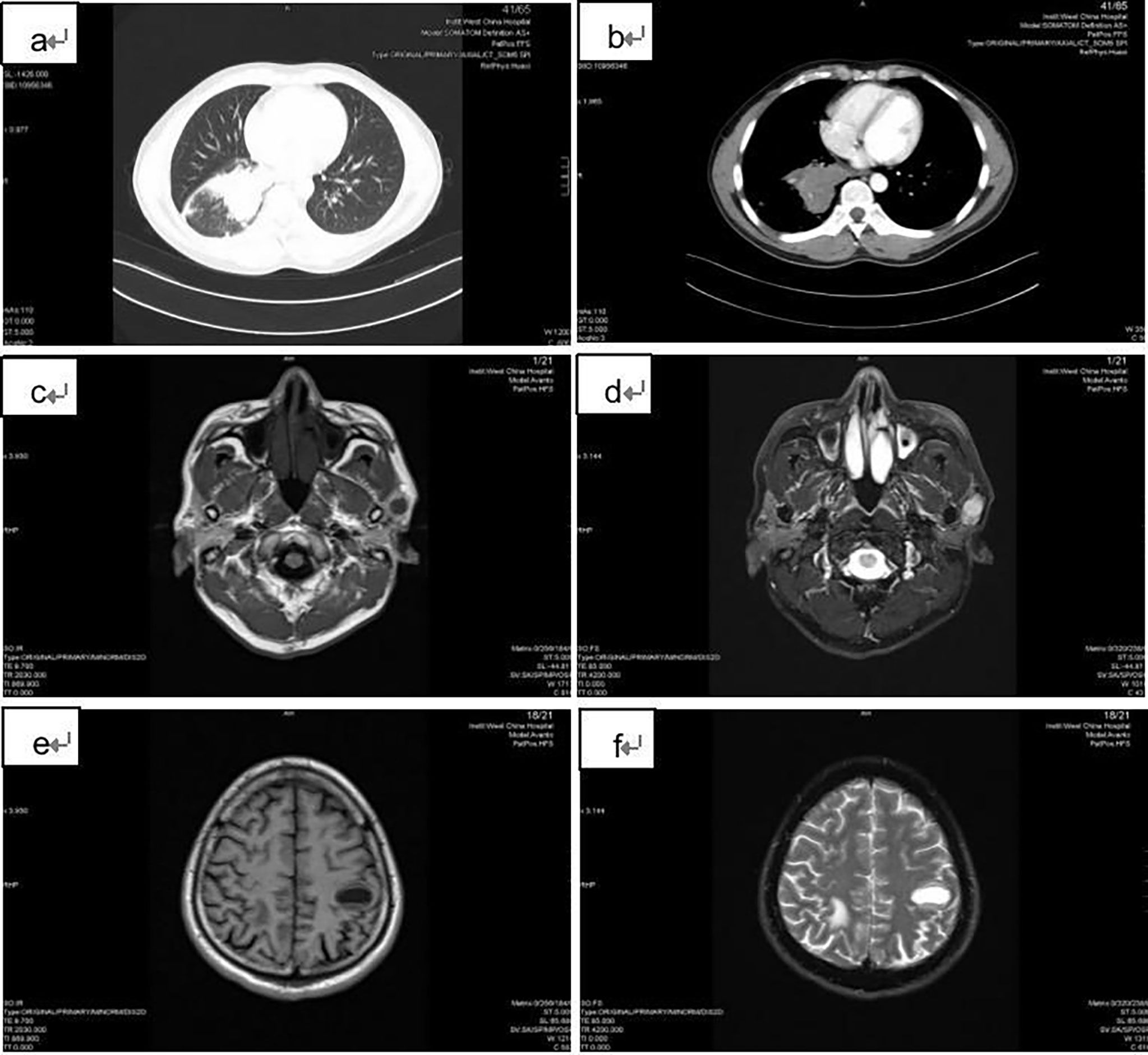
Figure 1 Imaging findings of the patient. (A) and (B): the chest CT showed a 6.4 cm x 5.2 cm mass at the right hilar; (C) and (D): T1 and T2 weighted head MRI images showed the preauricular mass located at the parotid gland; (E) and (F): T1 and T2 weighted head MRI images revealed the brain metastases.
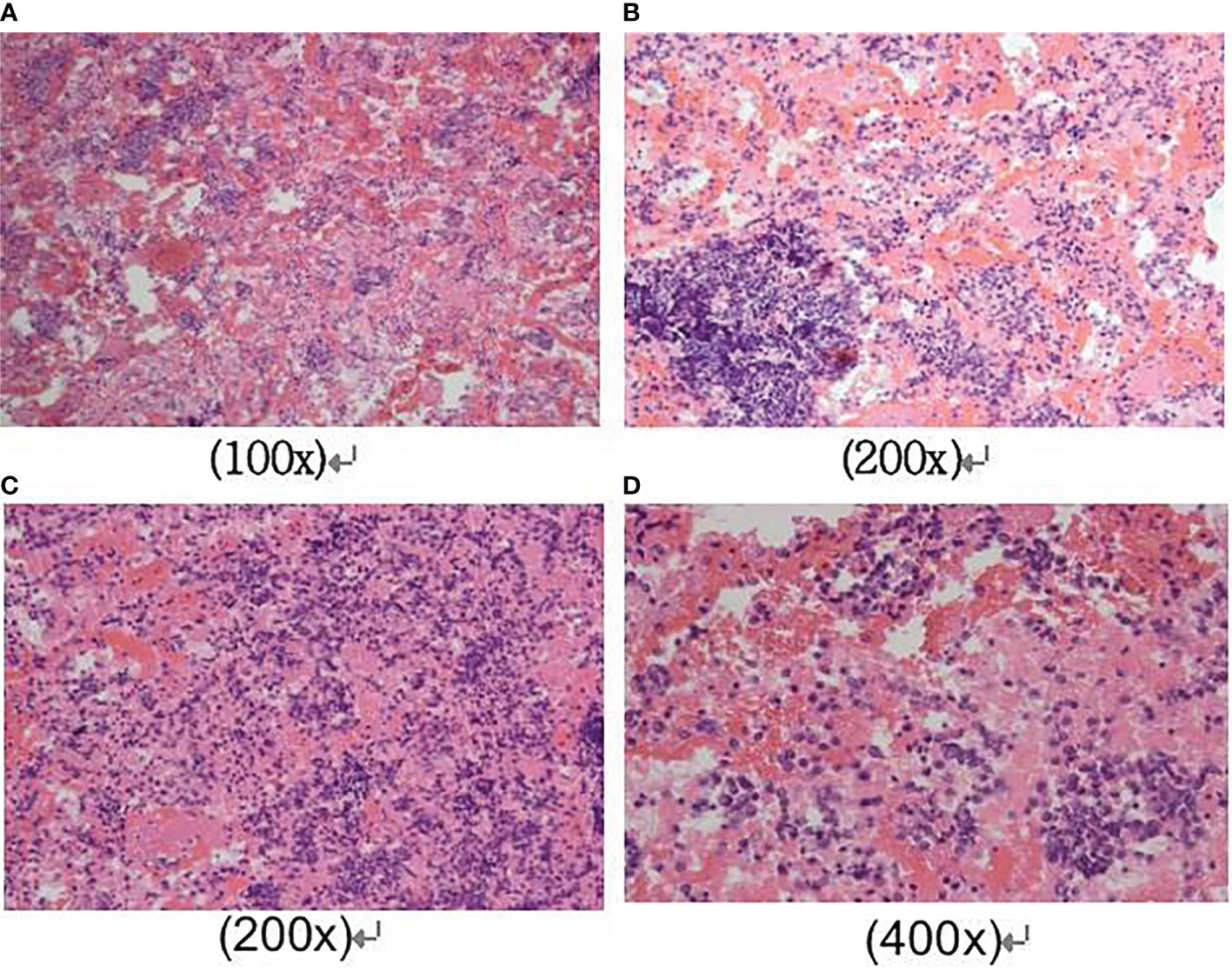
Figure 2 Cytological findings of parotid mass biopsy. Hematoxylin and eosin staining of the fine-needle aspiration biopsy of the preauricular mass revealed a large number of oat-shaped heteromorphic cells with deep nuclear staining at high magnification, distributed in the shape of chrysanthemum nests. The magnification was as follows respectively: (A) was 100x magnification; (B) and (C) were 200x magnification; (D) was 400x magnification.
A 61yearold man presented to our hospital because of persistent cough and confusion about a progressive growth mass at the left parotid. He was a heavy smoker (44-year history of 20 cigarettes per day). The chest and another neck CT showed a large mass at the left hilar and a 2.8 cm x 2.5 cm mass at the left parotid. The lung biopsy confirmed that he suffered from SCLC. Further examination found that the bilateral lung, left axillary, left adrenal and head all had metastatic nodules. The otolaryngology suggested that the parotid mass might be Warthin’s tumor, so it was not examined further. Then, he was included in a multicenter double-blind clinical study (Identifier NCT01450761) and received a standard treatment of etoposide and carboplatin with ipilimumab/placebo. After two cycles of treatment, the tumors were all shrunken, especially the parotid mass, suggesting that the parotid mass was also the metastasis of lung. Due to the previous wrong evaluation of parotid metastasis, the pathological biopsy of parotid gland was not carried out, resulting in the failure to obtain a correct diagnosis. Since the patient has already entered the extensive stage, the choice of treatment scheme has not been significantly affected. However, after four cycles of treatment, the brain metastases progressed, leading to his death. It was only three months since his diagnosis.
A 50-year-old male underwent the surgery for early left SCLC in April 2012, subsequently underwent 6 cycles of treatment with a standard EP regimen. Then, he followed up regularly. Unfortunately, the recurrence of neck lymph nodes appeared in 2014. Traditional Chinese medicine and chemotherapy (both EP and TP (paclitaxel and cis-platinum) regimen) had little effect. Thankfully, cervical lymph node radiotherapy resulted in a significant mass reduction in March 2015. However, subsequent follow-up found metastatic tumors in the head, parotid and parapharyngeal space. The biopsy of the parotid proved that it was a SCLC metastasis. Then, the patient received a single irinotecan chemotherapy regimen and whole-brain radiation therapy (WBRT). After two cycles of treatment, the tumors remained stable. However, after four cycles of treatment, the patient lost contact with us. It was only less than five months since the parotid mass was founded.
Then, we reviewed previously published similar cases and summarized the characteristics. We originally identified 913 relevant articles. After removing the 323 duplicate records, 590 were left. According to the exclusion criteria, 562 studies which did not fit the topic [including 2 with parotid lymph node metastasis (9, 10)], 1 without full text, and 7 with deficient clinical information were excluded. Ultimately, 20 articles comprising 20 patients were selected for our study (the detailed filtering process is shown in Figure 3). Adding the three patients discovered at our institution, for a total of 23 [Table 1 (5, 11–26)]. Among the 23 patients, 19 (83%) were males, and 4 (17%) were females. Most patients were over 50 years old (19/23 patients, 83%), with a median age of 59 years old. Fifteen (65%) presented with parotid gland mass as their initial symptom. In all cases with smoking history mentioned, 13 (93%) were smokers and only 1 (7%) was a nonsmoker. The predominant pathological type was SCLC (13 patients, 56%), followed by adenocarcinoma (7 patients, 30%), and squamous carcinomas (3 patients, 13%). Ten of the primary sites were left lungs, and thirteen were right lungs. Twelve (52%) cases presented with left parotid metastasis, 8 (35%) cases with right parotid metastasis, 2 (9%) cases with bilateral parotid metastasis, and 1 case not mentioned. Seventeen (74%) had other site metastases, while more than half (9 patients) had brain metastases. Most patients received chemotherapy, a few combined with radiotherapy or parotidectomy, and one patient obtained lung surgery for misdiagnosis of the parotid tumor. The survival time ranged from 3 months-17 years, and for SCLCs, it was only 3 months-40 months. The above features are summarized in Table 2.
Metastatic malignant parotid diseases, accounting for only 6-8% of parotid tumors (30), mostly originate from head and neck tumors, while non-head and neck parotid metastases may originate from the gastrointestinal tract, breast, pancreas and lungs (6). Lung cancer metastasis to the parotid gland is particularly rare (5). Due to the superficial location and benign impression of the parotid (31), missed diagnosis of primary tumors or misdiagnosis of parotid metastasis tumors occurs as common (7, 8). However, there is no appropriate way to avoid the above problems thus far. With regard to different properties and stages, the treatment and prognosis of tumors are also different. To achieve better diagnosis, differential diagnosis, and more effective treatment, we analyzed the characteristics of such patients and attempted to provide reference opinions on their management.
As shown in Tables 1, 2, this disease seemed more likely to occur in elderly smoking men in general, which was consistent with the prone crowd of lung cancer (32). Most patients with parotid metastasis of lung cancer had no obvious pulmonary symptoms (5, 12–15, 21–23, 25, 27). The initial clinical manifestation was always a rapidly expanding parotid gland mass, with or without pain, but usually did not invade the skin, sometimes accompanied by facial paralysis, suggesting no specificity. Imaging examination, especially MRI, could provide evidence for the differentiation of benign and malignant tumors, such as boundary and surrounding infiltration (33). Fine needle biopsy is the most commonly used diagnostic technique (7, 34). Usually, the discovery of an unusual pathological type is the initial cause of the suspicion of metastatic parotid tumor. According to the literature reports, parotid metastases can originate from the gastrointestinal tract, breast, pancreas and lungs (6), but the lung seems to be the most common primary site except for head and neck tumors (6, 7) (34, 35). Therefore, when secondary parotid malignancy is suspected and primary lesions are not found at the head and neck, the possibility of lung origin should be considered first. Indeed, positron emission tomography CT may be a pretty choice (29).
Due to the rarity of parotid metastases of lung cancer, differential diagnosis should be made carefully. The following situations should be handled with caution: (1) Lung malignancy with parotid gland benign tumor, especially Warthin’s tumor, a common benign tumor of the parotid. The majority of parotid gland tumors are benign, but the 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) uptake values of some types, such as warthin’s tumor and pleomorphic adenoma, are pretty high, and even equivalent to that of salivary gland malignant tumors (31, 36). As a result, the misdiagnosis of secondary parotid malignancy occurs frequently. Furthermore, because smoking is an identical risk factor for lung cancer (2) and Warthin’s tumor (37), lung cancer coexisting with Wartin’s tumor is not puzzling (38–41). It is reported that there is a significant correlation between the occurrence of parotid gland warthin’s tumor and lung cancer (38, 42). About 19% of patients with warthin’s tumor in parotid gland also have lung cancer (38). False recognition of the nature of parotid tumors can lead to different tumor stages and treatment. For example, present case 2 mistook parotid metastasis as Warthin’s tumor, and the case of Yang et al. (25) reported accepted the lung cancer operation owing to the wrong judgment of the parotid mass nature. Therefore, accurately discriminating the characteristics of parotid gland masses is particularly important. Parotid benign tumors have a long course of disease and develop slowly (31). Moreover, parotid benign tumors are usually located in the superficial lobe of the parotid gland (43), with no surrounding tissue infiltration and a clear border (31). In contrast, parotid malignant tumors usually grow rapidly (31). Significantly, they are usually discovered at the deep lobe or across the superficial and deep lobe (43), with invasion of the facial nerve or surrounding tissues, and have unclear boundaries (31). Emerging imaging technologies, such as apparent diffusion coefficient (44), diffusion-weighted imaging (45), and dynamic contrast-enhanced magnetic resonance imaging (46), can provide effective help. Therefore, a detailed history and imaging studies are essential for the differential diagnosis. However, the above characteristics still cannot accurately distinguish lung cancer parotid metastasis and lung cancer coexisting with Warthin’s tumor. Thus for patients with lung mass and parotid gland mass at the same time, it is very important to perform pathological biopsy for both. (2) Primary malignant tumors of the parotid gland, especially parotid gland small cell carcinoma (PGSmCC), with lung metastasis. In the current study, small cell carcinoma accounted for the priority (65%) of lung cancer parotid metastases. Distinguishing the parotid metastases of SCLC from lung metastases of PGSmCC is the key diagnostic challenge. Primary PGSmCC, accounting for less than 1% of salivary tumors (47), has a 5-year survival rate of 37%, which is much better than that of SCLC (48). Pathologically, primary PGSmCC can be divided into ductal type, Merkel type and pulmonary type. For the ductal type and pulmonary type, cytokeratin 20 (CK20) staining is negative, but for the Merkel type, CK20 staining is strongly positive (49). In general, Servato et al. suggested that approximately 79% of PGSmCCs were CK20 positive (47). However, SCLC has no ductal morphology, and CK20 expression is negative (50). In view of the above, SCLC with parotid metastases and PGSmCC with lung metastases can be preliminarily differentiated. However, there is still no reliable method to distinguish pulmonary-type PGSmCC from SCLC parotid metastasis because the immunophenotype of the two diseases widely overlaps (50). The order of tumor occurrence may be helpful to solve this dilemma. On the other hand, a study comprising 344 cases of primary PGSmCC observed that distant metastasis in such disease was rare (rate 7.3%) (48). However, for SCLC, distant metastasis is universal. Moreover, there is no literature report of lung metastasis from PGSmCC at present. Regardless, either of the two situations should be treated more radically.
According to the study, many patients (75%) had metastases in multiple parts of the body. Shi et al. (18) reported that this phenomenon implies the disease has progressed to extensive stage, which is a reason for the poor prognosis (32). In particular, SCLC is more malignant than any other pathological type of lung cancer (4). Thus, the survival is rarely more than one year. Therefore, for such patients, more aggressive treatment strategies should be adopted. Chemotherapy combined with immunotherapy has brought the treatment of extensive SCLC into a new era (51, 52). Many studies have confirmed that such a treatment strategy can prolong survival by 2-3 months (4, 53), implying that it might be a more suitable method for such patients in the current study. Furthermore, maintenance therapy seems to have no obvious survival benefit (54–56), but it is also worth trying (57, 58). In addition, among patients with the extensive stage, brain metastasis accounted for 53%. Oikawa et al. (59) analyzed the probability of distant metastasis sites of lung cancer and found that there was a specific pattern of lung cancer distant metastasis, that was when one site had metastasis, there was another subsequent site with a relatively high probability of metastasis (59). From the data of this study, there may be a similar relationship between parotid metastasis and brain metastasis of lung cancer. In other words, patients with lung cancer parotid metastasis seem to show a greater tendency of brain metastasis. Previous studies concluded that WBRT (60, 61), stereotactic radiosurgery (SRS) (62, 63), and prophylactic cranial irradiation (PCI) (62, 64, 65) can improve the prognosis of these patients and bring survival benefits. Therefore, for patients for whom brain metastasis already appears, WBRT or SRS should be performed as soon as possible. More importantly, for patients who have not yet developed brain metastasis, PCI should be applied in time (62). Furthermore, several studies (66, 67) have shown that parotidectomy seems to have no improvement in the prognosis of metastatic parotid tumors. However, for patients with parotid pain, parotidectomy is helpful to ameliorate their quality of life (30, 68).
Only a few studies reported the survival time in the current study, and the prognosis of SCC and AC was better than that of SCLC. Even after multiple treatments, the survival of SCLC patients with parotid metastasis is still short. Notably, as shown in present case 3, initially diagnosed at an early stage, the SCLC patient experienced a long disease-free survival after operation, but the condition deteriorated rapidly once parotid gland metastasis occurred. The case revealed the consistent viewpoint put forward by many previous studies that lung cancer parotid metastasis may be a marker of poor prognosis (13, 18, 24).
Our study has several limitations. First, due to the retrospective nature of the present study, the credibility needs to be verified. Second, for the rarity of parotid metastasis of lung cancer, the number of cases included in the current study is small; Owing to the superficial location and benign impression of parotid tumors, misdiagnosis of metastatic parotid tumors occurs quite a few, which may be another reason for the small samples; Moreover, we were unable to obtain the detailed clinical information of patients from some retrospective studies on secondary parotid metastasis, so these data were excluded from this study. Thus the current conclusion needs to be confirmed by a larger multicenter study. Third, the medical history and relevant data provided in many included cases are limited, and we cannot identify the clinical features, imaging features and survival time more specifically. Last, the metastasis of parotid gland in case 2 was not confirmed by pathological biopsy and was only a clinical diagnosis. Therefore, the value of the current study needs to be further inspected by prospective clinical research.
The lung seems to be the most common primary site of parotid metastases except for head and neck tumors. Therefore, when secondary parotid malignancy is suspected and primary lesions are not found at the head and neck, the possibility of lung origin should be considered first. Clinical features, such as sex, age, smoking history, pathological types, and metastasis patterns, could provide valuable evidence for diagnosis. The two circumstances, SCLC coexisting with Warthin’s tumor and parotid small cell carcinoma with lung metastasis, should be differentiated from parotid metastasis of lung cancer with caution. For cases presented as SCLC, more aggressive strategies, such as chemotherapy with immunotherapy and maintenance therapy, may be more suitable. Due to the greater tendency of brain metastasis in such disease, WBRT, SRS or PCI should be applied to corresponding patients in time. Additionally, lung cancer parotid metastasis may be a marker of poor prognosis.
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
(I) Guarantor of integrity of the entire study: QZ; (II) Study concepts and design: QZ; (III) Literature research: RW, TW; (IV) Clinical studies: RW, TW; (V) Experimental studies/data analysis: RW, TW; (VI) Statistical analysis: RW, TW; (VII) Manuscript preparation: All authors; (VIII) Manuscript editing: All authors. All authors contributed to the article and approved the submitted version.
This work was supported by the Regional Innovation Cooperation Project of Sichuan Science and Technology Program, China (2021YFQ0029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Mairinger T. [Histology, cytology and molecular diagnostics of lung cancer]. Pathologe (2019) 40(6):649–61. doi: 10.1007/s00292-019-00677-8
3. Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of small cell lung cancer (SCLC). Front Oncol (2020) 10:1074. doi: 10.3389/fonc.2020.01074
4. Melosky B, Cheema PK, Brade A, McLeod D, Liu G, Price PW, et al. Prolonging survival: The role of immune checkpoint inhibitors in the treatment of extensive-stage small cell lung cancer. Oncologist (2020) 25(11):981–92. doi: 10.1634/theoncologist.2020-0193
5. MF B. Parotid gland as an initial site of metastasis. Australas Radiol 2004 (2004) 48(1):88–92. doi: 10.1111/j.1440-1673.2004.01253.x
6. Pastore A, Ciorba A, Soliani M, Di Laora A, Valpiani G, Bianchini C, et al. Secondary malignant tumors of the parotid gland: not a secondary problem! J BUON (2017) 22(2):513–8. doi: 10.1002/dc.24629
7. Horakova M, Porre S, Tommola S, Baneckova M, Skalova A, Kholova I. FNA diagnostics of secondary malignancies in the salivary gland: Bi-institutional experience of 36 cases. Diagn Cytopathol (2021) 49(2):241–51. doi: 10.1002/dc.24629
8. Ghartimagar D, Ghosh A, Shrestha MK, Thapa S, Talwar OP. Histopathologic profile of salivary gland tumors among specimens from a tertiary care hospital: A descriptive cross-sectional study. JNMA J Nepal Med Assoc (2020) 58(230):729–35. doi: 10.31729/jnma.4898
9. Imauchi Y, Nakashima M, Nigauri T. Metastasis of lung adenocarcinoma to parotid lymph node as initial clinical manifestation. Eur Arch Otorhinolaryngol (2001) 258(3):155–6. doi: 10.1007/s004050100314
10. Kumar RK, Mohan AM, Arava SA, Madan KM, Hadda VH, Guleria RG, et al. Bilateral parotid involvement as the solitary metastatic site from squamous cell lung cancer. Australas Med J (2016) 9(7):206–10. Rohit). doi: 10.4066/AMJ.2016.2626
11. Boeger D, Hocke T, Esser D. [The interesting case – case no. 68. metastasis of a small-cell bronchial carcinoma to the parotid gland]. Laryngorhinootologie (2005) 84(2):117–20. doi: 10.1055/s-2004-826220
12. Cantera JM, Hernandez AV. Bilateral parotid gland metastasis as the initial presentation of a small cell lung carcinoma. J Oral Maxillofac Surg (1989) 47(11):1199–201. doi: 10.1016/0278-2391(89)90014-1
13. Cui Y, Cui XY, Wu Y, Yin WZ, Zhu ZP. A case of metastasis of small cell lung cancer to the parotid gland: a case report and literature review. J Int Med Res (2019) 47(11):5824–30. doi: 10.1177/0300060519865645
14. Katsurago N, Shiraishi Y, Hashizume M, Miyasaka Y. [Long-term survival following multimodality treatment of metachronous metastases (parotid gland, adrenal gland, brain and mediastinal lymph node) after resection of non-small cell lung cancer; report of a case]. Kyobu Geka (2006) 59(2):168–71.
15. Lawande DJ, Monteiro MV, Kakodkar UC, Keny SJ. Metastasis to parotid gland from primary bronchogenic carcinoma: A case letter. Lung India (2017) 34(4):398–400. doi: 10.4103/0970-2113.209233
16. Lenouvel D, Bhagwat P, Warnakulasuriya S. Metastases from the lung presenting as a parotid lump. Br J Oral Maxillofac Surg (2016) 54(1):e10–2. doi: 10.1016/j.bjoms.2015.09.002
17. Shalowitz JI, Cassidy C, Anders CB. Parotid metastasis of small cell carcinoma of the lung causing facial nerve paralysis. J Oral Maxillofac Surg (1988) 46(5):404–6. doi: 10.1016/0278-2391(88)90225-X
18. Shi S, Fang QG, Liu FY, Sun CF. Parotid gland metastasis of lung cancer: a case report. World J Surg Oncol (2014) 12:119. doi: 10.1186/1477-7819-12-119
19. Ulubas B, Ozcan C, Polat A. Small cell lung cancer diagnosed with metastasis in parotid gland. J Craniofac Surg (2010) 21(3):781–3. doi: 10.1097/SCS.0b013e3181d7f186
20. Debnath CR, Shahjahan SM, Debnath MR, Alam MM, Moshwan MM, Khan MF, et al. Parotid gland metastasis - an unusual presentation of adenocarcinoma of lung. Mymensingh Med J (2015) 24(1):175–7.
21. Hisa Y, Tatemoto K. Bilateral parotid gland metastases as the initial manifestation of a small cell carcinoma of the lung. Am J Otolaryngol (1998) 19(2):140–3. doi: 10.1016/S0196-0709(98)90112-0
22. Takatsugi K, Komuta K, Hosen N, Kitada S, Iida S, Nishihara K, et al. [Metastasis of small cell lung cancer to the parotid gland as the initial clinical manifestation, followed by metastases to the pituitary gland and lumber spinal cord]. Nihon Kokyuki Gakkai Zasshi (1998) 36(3):246–50.
23. Stephen N, Raman A, Kumari S, Dineshbabu S, Gochhait D, Siddaraju N. Intraparotid metastasis from lung adenocarcinoma diagnosed by FNAC-report of a rare case. Diagn Cytopathol (2020) 48(7):662–5. doi: 10.1002/dc.24428
24. Sanchez-Legaza E, Guerrero-Cauqui R, Gallego-Gallegos R. Parotid metastasis of small cell lung carcinoma. case report. Rev ORL (2020) 11(2):237–41. doi: 10.14201/orl.19886
25. Yang C, Xiong B. Metachronous, solitary parotid gland metastasis of primary lung adenocarcinoma: a misdiagnosed case report and literature review. Int J OF Clin AND Exp Med (2017) 10(2):3906–11.
26. Wang B, Wu C, Zhou L, Chen L. Parotid gland and cerebellum metastasis of lung cancer: a case report. Int J Clin Exp Pathol (2016) 9(2):2359–65.
27. Sankalp Singh NB, AS, PSM, AK. Non-small cell lung cancer presenting as parotid and scalp swellings: A rare clinical presentation. J Head Neck Physicians Surgeons (2020) 8(2):141–5. doi: 10.4103/jhnps.jhnps_10_20
28. Irons C, Sharma P, Dugan J, Sediqe S, Raju S. Oligometastatic lung adenocarcinoma with parotid gland metastasis. Chest (2021) 160(4):1563A–4A. doi: 10.1016/j.chest.2021.07.1429
29. Soputro NA, Asairinachan A, Prasad J. Parotid gland metastasis of lung adenocarcinoma identified on surveillance (18)F-FDG PET/CT. BMJ Case Rep (2022) 15(1):e246779. doi: 10.1136/bcr-2021-246779
30. Franzen AM, Gunzel T, Lieder A. Parotid gland metastases of distant primary tumours: A diagnostic challenge. Auris Nasus Larynx (2016) 43(2):187–91. doi: 10.1016/j.anl.2015.09.010
31. Inaka Y, Kawata R, Haginomori SI, Terada T, Higashino M, Omura S, et al. Symptoms and signs of parotid tumors and their value for diagnosis and prognosis: a 20-year review at a single institution. Int J Clin Oncol (2021) 26(7):1170–8. doi: 10.1007/s10147-021-01901-3
32. Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med (2020) 41(1):1–24. doi: 10.1016/j.ccm.2019.10.001
33. Gokce E. Multiparametric magnetic resonance imaging for the diagnosis and differential diagnosis of parotid gland tumors. J Magn Reson Imaging (2020) 52(1):11–32. doi: 10.1002/jmri.27061
34. Wang H, Hoda RS, Faquin W, Rossi ED, Hotchandani N, Sun T, et al. FNA biopsy of secondary nonlymphomatous malignancies in salivary glands: A multi-institutional study of 184 cases. Cancer Cytopathol (2017) 125(2):91–103. doi: 10.1002/cncy.21798
35. Emanuelli E, Ciorba A, Borsetto D, Cazzador D, Sarcognato S, Marino F, et al. Metastasis to parotid gland from non head and neck tumors. J BUON (2018) 23(1):163–6.
36. Minamimoto R. Proliferation PET/CT imaging of salivary gland tumor. Diagnostics (Basel) (2021) 11(11):2065. doi: 10.3390/diagnostics11112065
37. Orabona GD, Abbate V, Piombino P, Romano A, Schonauer F, Iaconetta G. Warthin's tumour: Aetiopathogenesis dilemma, ten years of our experience. J Craniomaxillofac Surg (2015) 43(4):427–31. doi: 10.1016/j.jcms.2014.11.019
38. White CK, Williams KA, Rodriguez-Figueroa J, Langer CJ. Warthin's tumors and their relationship to lung cancer. Cancer Invest (2015) 33(1):1–5. doi: 10.3109/07357907.2014.979365
39. Thomas R, Sharma N, Burke C, Maxwell D, Howlett DC. Parotid incidentaloma detected during thoracic PET imaging: how should these lesions be managed? Br J Hosp Med (Lond) (2010) 71(5):292–3. doi: 10.12968/hmed.2010.71.5.47915
40. Lardinois D, Weder W, Roudas M, von Schulthess GK, Tutic M, Moch H, et al. Etiology of solitary extrapulmonary positron emission tomography and computed tomography findings in patients with lung cancer. J Clin Oncol (2005) 23(28):6846–53. doi: 10.1200/JCO.2005.10.116
41. Arora R, Singh H, Vashistha R, Gupta RK, Jain S, Kapoor P, et al. Multiple neoplasms of the salivary gland and the lung. Indian J Chest Dis Allied Sci (1997) 39(3):183–8.
42. Zaccarini DJ, Khurana KK. Incidence of non-salivary gland neoplasms in patients with warthin tumor: A study of 73 cases. Head Neck Pathol (2020) 14(2):412–8. doi: 10.1007/s12105-019-01049-7
43. Comoglu S, Ozturk E, Celik M, Avci H, Sonmez S, Basaran B, et al. Comprehensive analysis of parotid mass: A retrospective study of 369 cases. Auris Nasus Larynx (2018) 45(2):320–7. doi: 10.1016/j.anl.2017.04.003
44. Chen P, Dong B, Zhang C, Tao X, Wang P, Zhu L. The histogram analysis of apparent diffusion coefficient in differential diagnosis of parotid tumor. Dentomaxillofac Radiol (2020) 49(5):20190420. doi: 10.1259/dmfr.20190420
45. Luna LP, Coffey W 3rd, Alvin MD, Shanechi AM, Sankaran N, Rodriguez EF, et al. Parotid warthin's tumor: novel MR imaging score as diagnostic indicator. Clin Imaging (2022) 81:9–14. doi: 10.1016/j.clinimag.2021.09.005
46. Xiang S, Ren J, Xia Z, Yuan Y, Tao X. Histogram analysis of dynamic contrast-enhanced magnetic resonance imaging in the differential diagnosis of parotid tumors. BMC Med Imaging (2021) 21(1):194. doi: 10.1186/s12880-021-00724-y
47. Servato JP, da Silva SJ, de Faria PR, Cardoso SV, Loyola AM. Small cell carcinoma of the salivary gland: a systematic literature review and two case reports. Int J Oral Maxillofac Surg (2013) 42(1):89–98. doi: 10.1016/j.ijom.2012.10.004
48. Zhan KY, Din HA, Muus JS, Nguyen SA, Lentsch EJ. Predictors of survival in parotid small cell carcinoma: A study of 344 cases. Laryngoscope (2016) 126(9):2036–40. doi: 10.1002/lary.25923
49. Chernock RD, Duncavage EJ. Proceedings of the NASHNP companion meeting, march 18th, 2018, Vancouver, BC, Canada: Salivary neuroendocrine carcinoma-an overview of a rare disease with an emphasis on determining tumor origin. Head Neck Pathol (2018) 12(1):13–21. doi: 10.1007/s12105-018-0896-4
50. Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med (2001) 125(2):228–31. doi: 10.5858/2001-125-0228-IFTTFA
51. Horn L, Mansfield AS, Szcz휌sna A, Havel L, Krzakowski M, Hochmair MJ. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
52. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6
53. Esposito G, Palumbo G, Carillio G, Manzo A, Montanino A, Sforza V, et al. Immunotherapy in small cell lung cancer. Cancers (Basel) (2020) 12(9):2522. doi: 10.3390/cancers12092522
54. Gadgeel SM, Pennell NA, Fidler MJ, Halmos B, Bonomi P, Stevenson J, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol (2018) 13(9):1393–9. doi: 10.1016/j.jtho.2018.05.002
55. Nie K, Guo X, You Y, Zhuang X, Zhang C, Ji Y. S-1 maintenance therapy in extensive stage small-cell lung cancer-a randomized clinical study. Cancer Control (2020) 27(2):1073274820932004. doi: 10.1177/1073274820932004
56. Santo A, Pilotto S, Galetta D, Grossi F, Fasola G, Romano G, et al. Maintenance with lanreotide in small-cell lung cancer expressing somatostatine receptors: A multicenter, randomized, phase 3 trial. Lung Cancer (2019) 134:121–6. doi: 10.1016/j.lungcan.2019.06.011
57. Ai X, Pan Y, Shi J, Yang N, Liu C, Zhou J, et al. Efficacy and safety of niraparib as maintenance treatment in patients with extensive-stage SCLC after first-line chemotherapy: A randomized, double-blind, phase 3 study. J Thorac Oncol (2021) 16(8):1403–14. doi: 10.1016/j.jtho.2021.04.001
58. Yan X, Wang Q, Wang H, Li P, Zhang G, Zhang M, et al. Apatinib as maintenance therapy in extensive-stage small-cell lung cancer: results from a single-center retrospective study. J Cancer Res Clin Oncol (2019) 145(1):235–40. doi: 10.1007/s00432-018-2764-8
59. Oikawa A, Takahashi H, Ishikawa H, Kurishima K, Kagohashi K, Satoh H. Application of conditional probability analysis to distant metastases from lung cancer. Oncol Lett (2012) 3(3):629–34. doi: 10.3892/ol.2011.535
60. Li H, Xue R, Yang X, Han S, Yang W, Song X, et al. Best supportive care versus whole-brain irradiation, chemotherapy alone, or WBRT plus chemotherapy in patients with brain metastases from small-cell lung cancer: A case-controlled analysis. Front Oncol (2021) 11:568568. doi: 10.3389/fonc.2021.568568
61. Ni M, Jiang A, Liu W, Sheng Y, Zeng H, Liu N, et al. Whole brain radiation therapy plus focal boost may be a suitable strategy for brain metastases in SCLC patients: a multi-center study. Radiat Oncol (2020) 15(1):70. doi: 10.1186/s13014-020-01509-3
62. Myall NJ, Yu H, Soltys SG, Wakelee HA, Pollom E. Management of brain metastases in lung cancer: evolving roles for radiation and systemic treatment in the era of targeted and immune therapies. Neurooncol Adv (2021) 3(Suppl 5):v52–62. doi: 10.1093/noajnl/vdab106
63. Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, et al. Evaluation of first-line radiosurgery vs whole-brain radiotherapy for small cell lung cancer brain metastases: The FIRE-SCLC cohort study. JAMA Oncol (2020) 6(7):1028–37. doi: 10.1001/jamaoncol.2020.1271
64. Rusthoven CG, Kavanagh BD. Prophylactic cranial irradiation (PCI) versus active MRI surveillance for small cell lung cancer: The case for equipoise. J Thorac Oncol (2017) 12(12):1746–54. doi: 10.1016/j.jtho.2017.08.016
65. Schild SE, Sio TT, Daniels TB, Chun SG, Rades D. Prophylactic cranial irradiation for extensive small-cell lung cancer. J Oncol Pract (2017) 13(11):732–8. doi: 10.1200/JOP.2017.026765
66. Cracchiolo JR, Shaha AR. Parotidectomy for parotid cancer. Otolaryngol Clin North Am (2016) 49(2):415–24. doi: 10.1016/j.otc.2015.10.007
67. Nuyens M, Schupbach J, Stauffer E, Zbaren P. Metastatic disease to the parotid gland. Otolaryngol Head Neck Surg (2006) 135(6):844–8. doi: 10.1016/j.otohns.2006.05.010
Keywords: small cell lung cancer, metastasis, parotid, feature, diagnose, treatment
Citation: Wang R, Wang T and Zhou Q (2022) Parotid metastases from primary lung cancer: Case series and systematic review of the features. Front. Oncol. 12:963094. doi: 10.3389/fonc.2022.963094
Received: 07 June 2022; Accepted: 08 August 2022;
Published: 25 August 2022.
Edited by:
Nikolaos I Kanellakis, University of Oxford, United KingdomReviewed by:
Guang-yan Yu, Peking University Hospital of Stomatology, ChinaCopyright © 2022 Wang, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Zhou, cHJvZl9xaF96aG91QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.